Abstract
Background
Infection of rhesus macaques (RMs) of Indian origin with SIV or SHIV provided powerful tools to study HIV-1 transmission and disease, and for testing the efficacy of novel drugs, vaccines and prevention strategies. In developing alternative nonhuman primate AIDS models for the CCR5 (R5)-tropic SHIVSF162P3N, we characterized virus transmission and infection in Chinese origin RMs.
Methods
Virologic, immunologic and pathogenic evaluations of R5 SHIVSF162P3N infection in Chinese RMs challenged intrarectally (ir) or intravaginally (ivg) were performed and compared to those previously observed in Indian origin rhesus exposed to the same inoculum dose and via similar route.
Results
R5 SHIVSF162P3N transmits efficiently across mucosal surfaces in Chinese RMs. The magnitude and kinetics of early virus dissemination following intrarectal inoculation in the Chinese macaques were similar to those observed in Indian rhesus, but a trend towards increased SHIVSF162P3N vaginal infectivity and rapid virus spread was seen in the Chinese macaques compared to the Indian origin animals. Once infected, however, set-point viremia in the ir- and ivg-infected Chinese rhesus was significantly lower and the animals survived longer compared with infected Indian rhesus.
Conclusions
The R5 SHIVSF162P3N/Chinese rhesus macaque infection model is suitable for studies of mucosal HIV-1 transmission and protection, but the high frequency of spontaneous control of chronic viremia and reduced virulence with SHIVSF162P3N in this macaque subspecies may limit its utility in studying HIV-1 pathogenesis and in evaluating vaccines and antiretrovirals that rely on reduction in chronic viral load or AIDS development as an experimental endpoint.
Keywords: SHIV, rhesus macaque subspecies, mucosal transmission, AIDS
Introduction
Studies in nonhuman primates are recognized as playing a critical role in advancing our understanding of HIV-1 transmission, pathogenesis, as well as basic vaccine, prevention and treatment concepts1–4. Experimental infection of Asian macaques with simian or simian-human immunodeficiency viruses (SIV and SHIV, respectively) have provided important information on the early host events and kinetics of virus transmission and replication, the dynamics of CD4+ T cell homeostasis during virus infection, the mechanisms of disease induction and host immune responses5–9. In particular, we have used infection of Indian rhesus macaques (Macaca mulatta) with pathogenic CXCR4 (X4) and CCR5 (R5) tropic SHIVs to study the impact of tropism on AIDS pathogenesis10–14, and have evaluated the ability of topical microbicides used alone or in combination with vaccines to prevent virus transmission using this model15,16. Because the majority of HIV-1 transmitted/founder viruses are CCR5-tropic, with neutralization susceptibility profiles that are typical of primary viruses17,18, we focused on developing an R5 SHIV infection model that recapitulates key features of HIV-1 infection in humans. We showed that infection of Indian origin RMs via the intravenous (iv), intrarectal (ir) or intravaginal (ivg) route with R5 SHIVSF162P3N resulted in acute CD4+ T cell depletion in the gut, uncontrolled replication and progression to AIDS, with switch in coreceptor preference towards CXCR4 in ~50% of iv- and ir-infected animals19–21. This model therefore mimics the type of transmission route in the majority of HIV-1 infected patients, allowing studies of viral selection through mucosal transmission, and late stage coreceptor switch that is also seen in chronically infected HIV individuals not on treatment. Furthermore, development of giant cell encephalitis (SIVE) was observed in ~30% of monkeys with symptoms of AIDS, with neuropathology mirroring that of HIV-1 associated encephalitis (HIVE) in infected patients (unpublished observations), providing an important model to study neuropathogenesis.
Several features of infection in Indian RMs however differ from that of HIV-1 infected individuals. These include a faster rate of progression to AIDS22, high plasma virus levels and a rapid and sustained loss of circulating and mucosal CD4+ CCR5+ memory T cells that is not seen in HIV-1 infected patients23–25. This raises the concern that the Indian RM infection models may not fully reproduce the immunopathogenic events occurring during HIV-1 infection. Accordingly, and because Indian RMs were often in short supply, alternative macaque species such as cynomolgous, pig-tailed or rhesus macaques from different geographic origin were used as models of HIV infection and AIDS26. Several groups have reported that the slower course of infection and reduced risk of progression to AIDS in SIV/SHIV-infected Chinese origin RMs are closer to HIV-1 infections in untreated adult humans than infection of Indian RMs27–31. Moreover, similar to HIV-1 infected patients, the levels of immune activation in SIV-infected Chinese RMs are markers of disease progression32,33. These findings led to the suggestion that SIV/SHIV infection of Chinese origin rhesus is a more relevant model of AIDS outcomes than Indian RMs28,34.
In the present study, we evaluated mucosal transmissibility and pathogenesis of R5 SHIVSF162P3N in Chinese RMs and compared infection outcomes with those observed in Indian RMs. We found that the transmission efficiency, magnitude and kinetics of early virus dissemination were similar in the two macaque subspecies following intrarectal inoculation, but transmission and early virus spread with intravaginal challenge appeared to be more efficient in the Chinese than Indian RMs. Consistent with findings for SIV, mucosal infection of Chinese RMs with R5 SHIVSF162P3N resulted in attenuated pathogenicity when compared to infection in Indian RMs, as evidenced by lower levels of set-point viremia and prolonged survival. Understanding these subspecies differences in response to R5 SHIVSF162P3N should guide the use of this virus in NHP studies of HIV-1 transmission and prevention, resistance to infection and progression to pathology.
Methods
Animal inoculation and clinical assessments
All intrarectal (ir) or intravaginal (ivg) inoculations were carried out in adult rhesus monkeys (Macaca mulatta) of Chinese origin housed at the Tulane National Primate Research Center (TNRPC) in compliance with the Guide for the Care and use of Laboratory Animals. Chinese origin rhesus were 5 – 10 years old and were confirmed to be serologically and virus negative for simian type D retrovirus, and serologically negative for SIV and simian T-cell lymphotropic virus prior to infection. The males were born and raised at TNRPC whereas the females were purchased from an outside vendor, the latter were used without Depo-provera treatment and randomized with regard to the stage of the menstrual cycle at the time of ivg challenge. Macaques received a single 104 50% tissue culture infectious dose (TCID50) of the cell free challenge stock SHIVSF162P3N10. Whole blood from the inoculated animals was collected weekly for the first eight to eleven weeks, biweekly for another 16 weeks, and monthly thereafter. Surgery was performed during acute [2–3 weeks post-infection (wpi)] and chronic (12–18 wpi) phase of infection for tissue collection. These include the colonic, mesenteric, iliac and/or inguinal lymph nodes for lymphoid cell isolation and immunohistological examination, as well as ~20cm of the jejunum for processing of laminar propria lymphocytes (LPL), with ileum and colon wedge biopsies for immunohistological analysis. Animals were euthanized at end of study period (~50 weeks for ivg and >60 weeks for ir) by intramuscular administration of telazol and buprenorphine followed by an overdose of sodium pentobarbital. Euthanasia was considered to be AIDS related if the animal exhibited peripheral blood CD4+ T-cell depletion (<200/mm3), greater than 25% loss of body weight, or combinations of the following conditions: diarrhea unresponsive to treatment, opportunistic infections, peripheral lymph node atrophy, and abnormal hematology. Plasma viremia was quantified by branched DNA analysis (Siemens Medical Solutions Diagnostic Clinical Lab, Emeryville, CA) and absolute CD4+ and CD8+ cell counts were monitored in TruCount tubes (BD Biosciences, Palo Alto, CA). The percentages of CD4+ T cells in the tissue cells were analyzed by flow cytometry (FACScalibur) using CD3-fluorescein isothiocyanate (FITC), CD4-phycoerythrin (PE) and CD8-peridinin chlorophyll protein (PerCP) antibodies. Except for CD3-FITC (BioSource, Camarillo, CA), all antibodies were obtained from BD Biosciences. Comparative data from Indian-origin rhesus monkeys were obtained from other pathogenesis studies in which the animals received the same virus inoculum dose21. The cohort of Indian female rhesus monkeys used was similarly randomized with respect to the menstrual cycle.
Statistical Analyses
Plasma virus loads were transformed to log10 copies/ml before all analysis. Peak viral load was the highest recorded value (2–4wpi), and baseline CD4+ T cell count was measured at pre-infection. Plateau (set-point) viremia and CD4+ T cell counts were calculated as the median of all values between days 56 and 168 post-infection, with differences examined using Mann-Whitney U-tests. Changes in the percentage of tissue CD4+ T lymphocytes over time were also determined using Mann-Whitney U-tests. Disease-free survival curves for the intrarectal and intravaginal infected macaques were estimated using the Kaplan-Meier method, and statistical significance of the differences in the survival curves was determined by log-rank test. A P value less than 0.05 was considered statistically significant.
Results
Efficient mucosal transmission of R5 SHIVSF162P3N in Chinese RMs
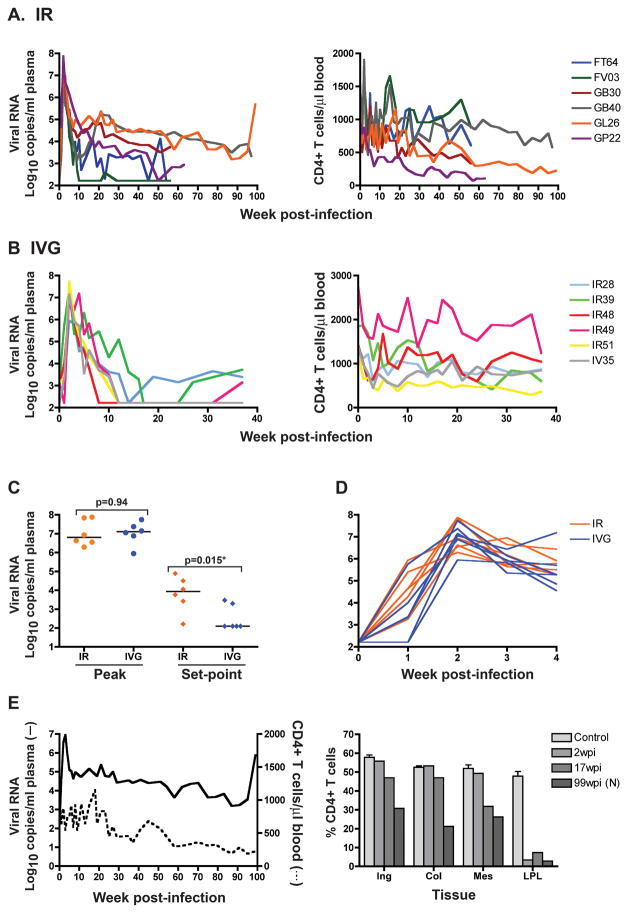
To determine mucosal transmissibility and pathogenicity of R5 SHIVSF162P3N in rhesus macaques of Chinese origin, we inoculated six males intrarectally and six females intravaginally with 10,000 TCID50 virus. All animals in the ir- and ivg-inoculated groups became infected, with seroconversion at 4–5 weeks post-infection (wpi). Peak viremia of 6–8 log10 RNA copies/ml plasma was detected in both ir- and ivg-infected macaques (Figures 1A and B), with no significant difference in magnitude between the two groups (Figure 1C). The kinetics of virus spread was also comparable among the two inoculation groups, with plasma viremia detected at 1 wpi in six of six ir- and four of six ivg-infected RMs, reaching peak one week later in all except GL26, an ir-inoculated animal that peaked at 3 wpi (Figure 1D). Viral load subsequently declined, with greater control in the ivg- than the ir-infected macaques. Virus replication reached undetectable levels (< 165 RNA copies/ml plasma) in all six ivg-infected Chinese RMs between 8–16wpi, with partial rebound to <4 log10 RNA copies/ml plasma in three of the six animals. In comparison, only one ir-infected Chinese RM (FV03) suppressed plasma viremia below the level of detection. Accordingly, viral set-point was significantly higher in the ir- than the ivg-infected Chinese origin RMs (Figure 1C, p=0.015). The difference in viral control between the two inoculation groups suggests a route-dependent effect on SHIVSF162P3N replication in Chinese RMs, and is consistent with our previous findings of a route-dependency in infection outcome of Indian origin rhesus monkeys with this virus21.
Figure 1.
Viral load and peripheral CD4+ T cell count in ir- (A, n=6) and ivg- (B, n=6) inoculated Chinese RMs. Comparison of peak and set-point viral load (C), and kinetics of virus dissemination within the first four weeks of infection (D) in the ir- and ivg-infected Chinese RMs. (E) Plasma viremia, peripheral CD4+ T count during the course of infection, and tissue CD4 T cells at time of necropsy in macaque GL26. Horizontal bars in (C) indicate median values, and baseline tissue CD4+ T cell count shown for reference in (E) were generated from 3–4 macaques (control). N, necropsy.
The infected animals all experienced transient peripheral CD4+ T cell loss with the onset of viremia. The absolute number of this T-cell subset decreased by approximately 15% during the first two weeks of infection in the ir-infected animals (from a median baseline value of 1037 at d0 to 877 CD4+cells/μl blood) and by approximately 30% in the ivg-infected monkeys (from a median baseline value of 1409 at d0 to 998 CD4+cells/μl blood) (Figures 1A and B). Peripheral CD4+ T cell count stabilized or fluctuated thereafter in six of the six ivg- and three of the six ir-infected animals. The exceptions were the ir-infected rhesus GL26, GB30 and GP22 where a gradual decline in CD4+ T lymphocytes was seen despite a viral load that is <104 RNA copies/ml plasma in the latter two animals. We concluded therefore that R5 SHIVSF162P3N transmits efficiently across mucosal surfaces in Chinese RMs, but infection is frequently controlled. Because the level of set-point viremia is a strong predictor for disease progression in HIV-1 infected humans and SIV-infected Indian RMs35,36, the two ir-infected Chinese macaques with sustained viremia > 104 RNA copies/ml plasma for over 40 weeks of infection (GL26, GB40) were followed for AIDS development.
Disease progression in ir-infected Chinese RM
GB40 was euthanized at 97 wpi for AIDS-unrelated causes. Viral load in this animal at the time of euthanasia was ~3 log10 copies/ml plasma, with a peripheral CD4+ T cell count of 578 cells/ul blood. In contrast, GL26 developed clinical symptoms consistent with AIDS, including chronic diarrhea and weight loss, and was euthanized at 99 wpi with a CD4+ T cell count of 226 cells/ul blood. Histological examination revealed secondary and mycobacterial (M. Avium) infection. Peak viremia in this macaque reached 6–7 log10 RNA copies/ml plasma but dropped ~2 log thereafter, reaching a plateau of 4–5 log10 RNA copies/ml (Figure 1E). A rise in viremia to near peak plasma viral load level however was seen toward end-stage disease. Examination of mucosal and lymph node CD4+ T cells during the course of infection showed severe acute depletion of gut CD4+ T cells (90%; 2wpi), with minimal loss in the lymph node compartments. Gut CD4+ T cell loss was sustained during chronic infection (17wpi) and at the time of necropsy (99wpi), with ~50% preservation of this lymphocyte subset in the lymph node compartments at end-stage disease. R5 SHIVSF162P3N, therefore, can induce AIDS in Chinese origin rhesus in a manner similar to HIV-1 infection in humans.
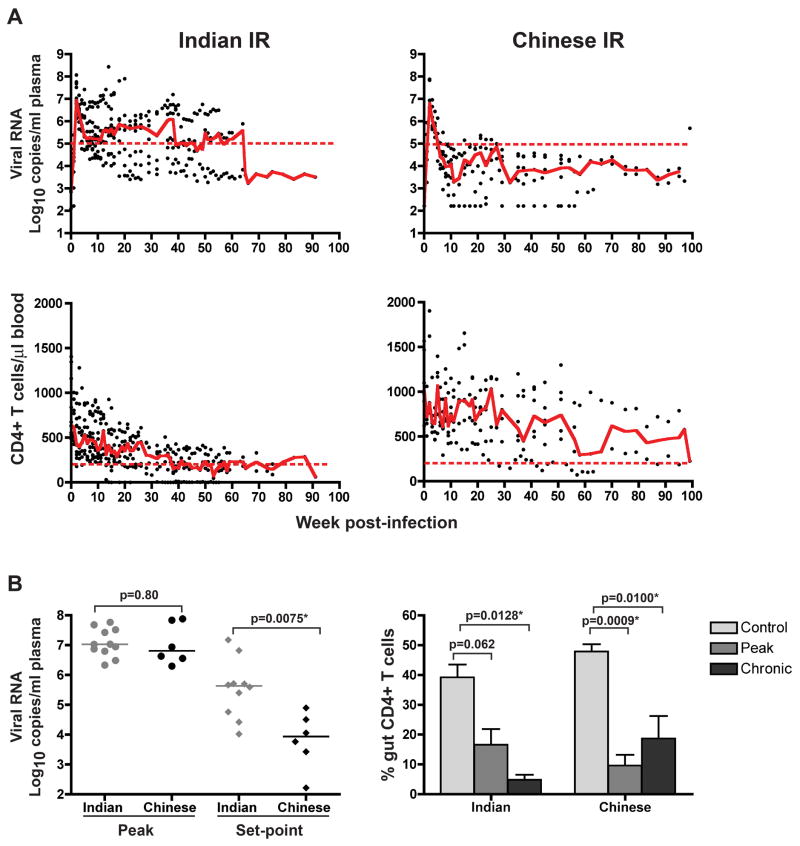
R5 SHIVSF162P3N infection is attenuated in mucosally infected Chinese RMs in comparison to Indian RMs
SIV infection in Chinese RMs had been reported to be more attenuated compared to RMs of Indian origin27–31. Since an objective of this study is to assess the utility of R5 SHIVSF162P3N infection of Chinese origin RMs in studies of HIV-1 transmission and pathogenesis, we compared the virologic and immunologic parameters in ir- and ivg-infected Chinese RMs to those observed previously in Indian RMs21. We found that the peak viremia was of similar magnitude in the Chinese (n=6) and Indian (n=11) ir-infected RMs (Figures 2A and B, p>0.05). However, while a range in set-point viremia was seen in both subspecies hosts, the median steady-state viremia was significantly lower in the Chinese rhesus than in the Indian origin monkeys (Figure 2B, p=.0075). This result is consistent with findings with SIV that the differences between the subspecies appear in the chronic phase.
Figure 2.
Comparison of the changes in viral load (VL) and CD4+ T cell levels over time (A), and peak, set-point and gut CD4+ T cell depletion (B) in Indian and Chinese RMs infected intrarectally with R5 SHIVSF162P3N. Trend lines in (A) were created using the median VL or CD4 count values at each time point, and the dashed lines marked a set-point of 5 log10 RNA copies/ml plasma or a CD4+ T cell count of 200 cells/ul blood. The horizontal bars in (B) represent median values, and the control gut CD4+ T cell counts were generated from 3 and 12 uninfected Indian and Chinese origin rhesus respectively, with error bars indicating mean and standard deviations.
There was no significant difference in baseline peripheral CD4+ T cell counts between the Chinese and Indian origin ir-infected animals (p>0.05), but the Indian origin ir-infected RMs displayed greater loss of peripheral CD4+ T cells during acute infection than the Chinese origin ir-inoculated monkeys (Figure 2A). The Indian ir-infected animals suffered a 33% drop in median peripheral CD4+ T cell count (from 635 to 427 CD4+cells/μl blood at 2 wpi), while the ir-infected Chinese rhesus only lost 15% of this lymphocyte subset (from 1037 to 877 CD4+cells/μl blood) at the corresponding time post-infection. The peripheral CD4+ T cell loss of the two subspecies remained divergent during the chronic phase of infection, consistent with the differences in viral load. CD4+ T lymphocyte count declined steadily in the ir-infected Indian but varied widely among the ir-infected Chinese RMs. Severe depletion of CD4+ T lymphocytes in the lamina propria (LPL) of the gut occurred in both Indian and Chinese origin ir-infected RMs (Figure 2B). However, the dynamics of this loss varied between the two macaque subspecies. CD4+ T cells constituted less than 10% of total gut CD3+ T lymphocytes in ir-infected Chinese RMs as compared to 20% in the ir-infected Indian RMs at 2 wpi, despite similar peak viremia. Accordingly, the depletion of CD4+ T lymphocytes in the LPL was highly significant during peak viremia (p=0.0009) in the Chinese and only approached significance in the Indian RMs (p=0.062). Further analysis of the percentage of CD4+ T lymphocytes in the LPL during the chronic phase of infection (12 –18wpi) revealed continued diminution of CD4+ T cells in the Indian RMs, resulting in a loss that is now significant. The percentage of CD4+ T lymphocytes in the LPL of the Chinese ir-infected RMs rose during the chronic phase, suggestive of gut CD4+ T cell reconstitution37, but is still significantly lower than the uninfected controls.
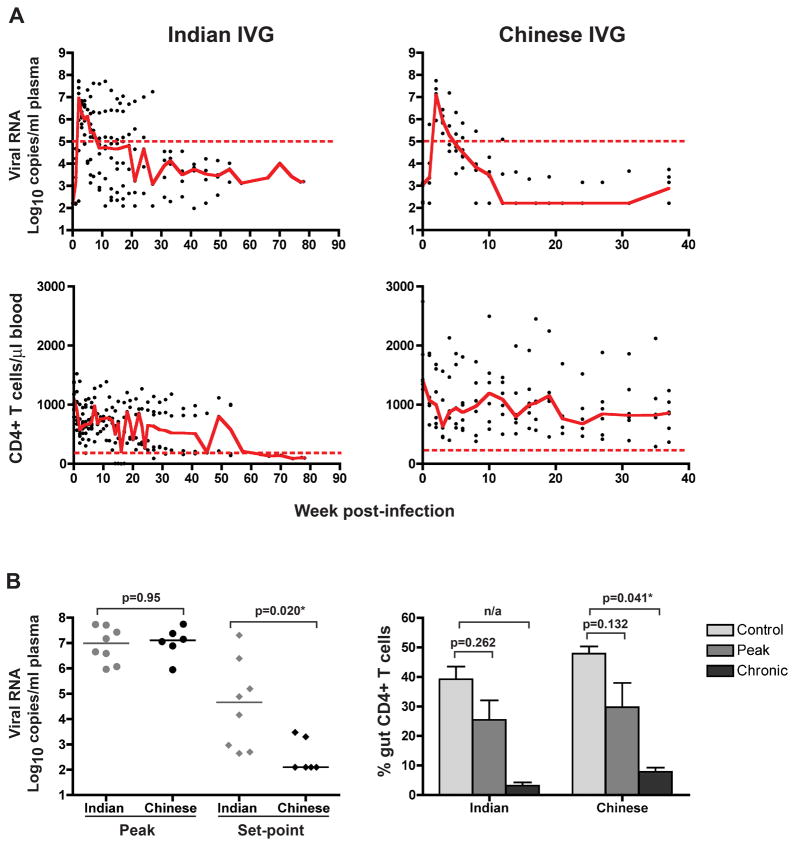
As in the ir-inoculated RMs, peak viremia was of similar magnitude in the Chinese (n=6) and Indian (n=8) origin RMs exposed intravaginally to the same inoculum dose (Figure 3A, p>0.05). But while a greater range in set-point plasma viral load was found among the Indian origin ivg-infected animals, the median set-point viremia in this subspecies host was significantly higher compared to animals of Chinese origin (Figure 3B, p=0.02). The subspecies difference in SHIVSF162P3N chronic phase viremia therefore is route independent. With the onset of viremia, peripheral CD4+ T counts declined substantially in both Indian and Chinese origin ivg-inoculated RMs (Figure 3A). The median peripheral CD4+ T cell count dropped by 30% in the Chinese ivg-infected RMs (from 1409 to 998 CD4+cells/μl blood) and by 47% in the ivg-infected Indian rhesus monkeys (from 1097 to 576 CD4+cells/μl blood) during peak viremia. However, five of the six ivg-infected Chinese RMs were able to recover their blood CD4+ T cell loss post-peak, while only four of eight ivg-inoculated Indian RMs showed a rebound in peripheral CD4+ T cell levels. Unlike the ir-infected RMs, both macaque subspecies infected by the intravaginal route experienced only moderate gut CD4+ T cell loss during acute infection (30–40%; Figure 3B), and only during the chronic phase (12 –18wpi) did the percentages of CD4+ T cells plummet, with more severe depletion in the Indian (>90%) than the Chinese origin RMs (>75%).
Figure 3.
Comparison of the changes in viral load (VL) and CD4+ T cell levels over time (A), and peak, set-point and gut CD4+ T cell depletion (B) in Indian and Chinese RMs infected intravaginally with R5 SHIVSF162P3N. Trend lines in (A) were created using the median VL or CD4 count values at each time point, and the dashed lines marked a set-point of 5 log10 RNA copies/ml plasma or a CD4+ T cell count of 200 cells/ul blood. The horizontal bars in (B) represent median values, and the control gut CD4+ T cell counts were generated from 3 and 12 uninfected Indian and Chinese origin rhesus respectively, with error bars indicating mean and standard deviations. n/a, statistics analysis for the ivg-infected Indian RMs at set-point viremia could not be determined because data from only two animals were available.
R5 SHIVSF162P3N is minimally pathogenic in Chinese rhesus monkeys
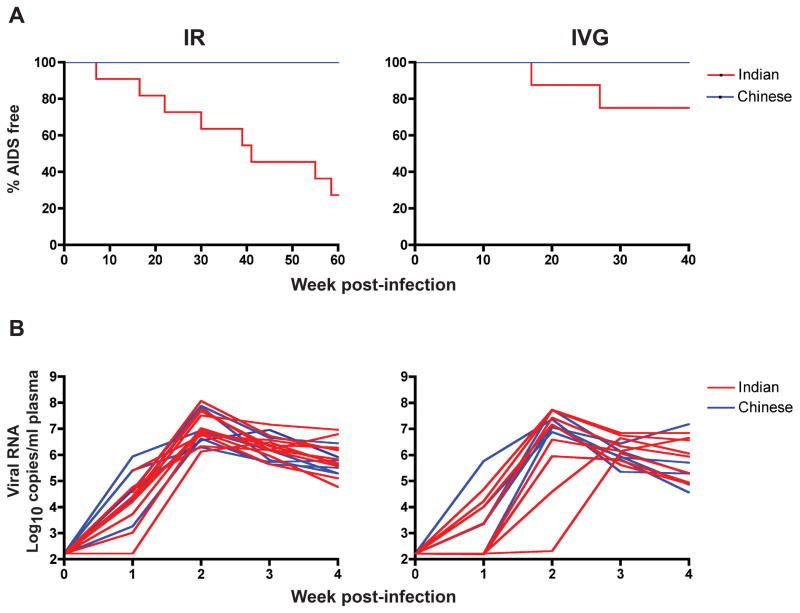
Ten of the eleven ir-infected Indian origin RMs (91%) progressed to disease over a 1–1.5 year infection period as compared to one of six ir-infected Chinese RMs (16.7 %), with a RP phenotype in four of the eleven ir-infected Indian RMs (36.4%) and none of the ir-infected macaques of Chinese origin (Table 1). Kaplan-Meier analysis of disease progression showed statistically significant difference in the rate of disease progression between the two macaque subspecies (Figure 4A, p= 0.0009; log-rank test). The percentage of animals AIDS-free at 60wpi was 27.3% for the ir-infected Indian origin macaques and 100% for the ir-infected macaques of Chinese origin. In comparison, there was no statistical significant difference in the rate of disease progression between the ivg-infected Chinese and Indian RMs (p>0.05), with 75% and 100% of animals AIDS-free at 40wpi in the ivg-infected Indian and Chinese origin rhesus respectively. However, whereas all six of the Chinese origin RMs were infected following a single high-dose ivg exposure, only 8 of the 12 Indian RMs challenged intravaginally with the same inoculum dose established systemic infection (Table 1). Furthermore, the observation that the kinetics of early virus spread in the ivg-and ir- infected Chinese RMs was similar (Figure 1D) contrasts with our findings with this virus in the Indian RMs21, prompting us to compare early SHIVSF162P3N dissemination in the two subspecies hosts. We found that the time to peak 1viremia overlapped in the ir-infected Indian and Chinese RMs, but varied by geographical origin for the ivg-infected animals (Figure 4B). Peak virema was w2 in all six ivg-infected Chinese rhesus, but it took 3–4 weeks to reach peak viremia in 2 of the 8 ivg-infected Indian origin macaques, with one showing no evidence of systemic infection until 3 wpi. These results of ivg inoculation suggest that the Chinese origin monkeys may be more susceptible to SHIVSF162P3N vaginal infection than Indian origin animals.
Table 1.
Infection outcome in Chinese and Indian rhesus macaques inoculated intrarectally (ir) or intravaginally (ivg) with R5 SHIVSF162P3N.
| IR-inoculated | IVG-inoculated | |||
|---|---|---|---|---|
|
| ||||
| Indian (n=11) | Chinese (n=6) | Indian (n=12) | Chinese (n=6) | |
|
| ||||
| % Infected | 100 | 100 | 66.7 | 100 |
| % infected with AIDS | 91 | 16.7 | 25 | 0 |
| % infected with RP phenotype | 36.4 | 0 | 25 | 0 |
Figure 4.
(A) Kaplan-Meier disease-free survival curves for ir- and ivg-infected Chinese and Indian RMs. AIDS development within a 60 and 40 week infection period in the ir- and ivg-infected macaques respectively is shown. (B) Kinetics of early virus spread in the ir- and ivg-infected Chinese and Indian RMs. Plasma RNA levels within the first four weeks of infection in the ir-infected Indian (n=11) and Chinese (n=6), and ivg-infected Indian (n=8) and Chinese (n=6) rhesus macaques are shown.
Discussion
In developing AIDS models of HIV-1 infection using different nonhuman primate species or rhesus macaques of different geographic origin with SIVs or SHIVs, it is important to understand the characteristics of infection with each virus in the various animal hosts to guide the rational design and optimal use of the models. In this study, we established that R5 SHIVSF162P3N transmits efficiently across the rectal and vaginal/cervical mucosa of Chinese origin rhesus macaques, modeling the type of transmission route in the majority of HIV-1 patients. This model therefore allows for studies of viral selection though mucosal transmission as well as evaluation of the effectiveness of vaccines and pharmacological agents to block virus acquisition. However, a large number of the SHIVSF162P3N ir- and ivg- infected Chinese origin animals controlled their infection, with undetectable plasma viremia found more frequently in the ivg- than the ir-infected animals, and AIDS development in only one of six ir-infected Chinese rhesus after 99 weeks of infection. The variability in chronic viremia and limited pathogenicity of SHIVSF162P3N in Chinese origin rhesus may restrict the utility of this model in understanding the mechanisms of HIV-1 disease development, in dissecting cellular and humoral immune responses over the course of infection from the time of inoculation to the development of fatal immunodeficiency, and in evaluating vaccines and antiretrovirals that aim at reducing viral loads post-infection and delaying AIDS progression.
Consistent with reports with SIV27–31, peak viremia was similar but viral load post-infection was lower and survival was longer in the SHIVSF162P3N-infected Chinese RMs compared to Indian origin macaques. The lack of a rapid progressor phenotype and sustained peripheral CD4+ T cell levels in the infected Chinese origin monkeys provided further evidence that the clinical course of SHIVSF162P3N infection differs in the two macaque subspecies. Several hypotheses have been proposed to explain the divergent outcome of SIV/SHIV chronic infection in rhesus macaques of Chinese and Indian origin. It has been suggested that because the inoculating virus was passaged and recovered from Indian RMs, it is better adapted and replicate more successfully in the Indian RM immune environment than in the “foreign” Chinese RM immune milieu28,30. SHIVSF162P3N was also passaged and recovered from Indian RMs, providing a plausible explanation for the differences in pathogenesis we observed between the two macaque subpopulations. Genetic differences including factors governing CCR5 expression, cellular molecules that restrict viral replication and adaptive immunity could also affect the biological consequences of viral infections in the two monkey subspecies34,38,39, 40. The animals used in this study were genotyped for TRIM5α, with results showing that genetic polymorphism at this locus cannot explain the difference in R5 SHIVSF162P3N infection of Indian and Chinese RMs. 33.3% of the male (2 of 6) as well as female (2 of 6) Chinese origin macaques used in the current study expressed the restrictive homozygous TFP/TFP allele compared to 72.2% of the male (8 of 11) and 37.5% of the female (3 of 8) Indian rhesus monkeys (data not shown). CD8 T cell mediated immunity has been suggested to play a role in the spontaneous control of chronic viremia in Chinese origin rhesus37. Very little is known about the degree to which class I alleles in the Chinese rhesus population confer protection for SIV/SHIV challenges. Recent advances in identifying major histocompatibility complex (MHC) alleles for Chinese-origin macaques41–45 will be important for studying cellular immunology in this monkey subpopulation and its impact on SIV/SHIV replication.
All six Chinese origin macaques not treated with progesterone were infected with one high dose SHIVSF162P3N intravaginal challenge, a rate of infection that is similar to that achieved following ir inoculation (Figures 1A and B). This finding contrasts early studies in Indian RMs using nonphysiological high doses of SIVs and SHIVs, including SHIVSF162P3N, showing that the infection rate was lower after inoculation by the ivg than by ir route21,46–50. Moreover, the kinetics of early virus spread was similar in Chinese RMs infected by the ir and ivg routes (Figure 1D), differing from observations of slower kinetics of virus dissemination and greater variability in RNA levels in Indian origin monkeys infected by the ivg route than those infected by the ir route48,51,52. Baseline peripheral CD4+ T cell counts were significantly higher in the ivg-inoculated Chinese RMs than the Indian RMs (p=0.0080). If this observation in peripheral blood translates into the genital mucosa, the greater number of CD4+ T cells and targets could provide a possible explanation for the successful ivg transmission and rapid spread of virus in the Chinese origin animals. Alternatively, and because the differences in the rate of infection and kinetics of early dissemination in the two macaque subspecies were only seen with ivg and not ir inoculation, differential host response to exposure at the genital mucosa with an Indian RM-adapted virus may have favored vaginal transmissibility and the initial wave of viral replication in the Chinese RMs. Studies in a larger number of animals will be required to confirm and understand the differences in SHIVSF162P3N vaginal transmission in Chinese and Indian RMs.
In summary, our findings with R5 SHIVSF162P3N in Chinese and Indian origin rhesus macaques further illustrate that the subspecies differences in pathogenicity after SIV/SHIV infection is independent of the route of inoculation and the virus strain used. The ease of mucosal transmission with SHIVSF162P3N in Chinese RMs suggests that this model will be suitable for prevention, early host events and kinetics of virus replication studies. In particular, it will be of interest to determine if transmission of different viral variants and/or local host responses influenced the observed subspecies differences in SHIVSF162P3N genital mucosal infection. However, the finding that a significant fraction of the SHIVSF162P3N-infected Chinese origin animals, especially those infected intravaginally controlled virus replication to levels that are intermittent or below conventional detection highlights the limitation of this model for vaccine and therapeutic trials aimed at reducing set-point viremia and HIV-1 disease progression.
Acknowledgments
Supported by the National Institute of Health grant RO1 AI084765 and by Primate Center Base grant P51-OD011104-51
We thank Wendy Chen for help with the graphics. This work was supported by NIH grant RO1 AI084765 to C.C.M. and by the Tulane Primate Center Base grant P51-OD011104-51.
Footnotes
The authors have no conflicts of interest to disclose
Conceived and designed the experiments: A.G., J.B. and C.C.M.; Performed the experiments: A.M., A.G.; Analyzed the data: A.M., C.C.M.; Wrote the article: A.M., C.C.M., Critical review of article: A.G., J.B.
References
- 1.Hirsch VM, Lifson JD. Simian immunodeficiency virus infection of monkeys as a model system for the study of AIDS pathogenesis, treatment, and prevention. Advances in Pharmacology (San Diego, Calif) 2000;49:437–477. doi: 10.1016/s1054-3589(00)49034-4. [DOI] [PubMed] [Google Scholar]
- 2.Morgan C, Marthas M, Miller C, et al. The use of nonhuman primate models in HIV vaccine development. PLoS Medicine. 2008 Aug 12;5(8):e173. doi: 10.1371/journal.pmed.0050173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Veazey RS, Shattock RJ, Johan Klasse P, Moore JP. Animal Models for Microbicide Studies. Curr HIV Res. 2012;10(1):79–87. doi: 10.2174/157016212799304715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Rompay KKA. The Use of Nonhuman Primate Models of HIV Infection for the Evaluation of Antiviral Strategies. AIDS Res Hum Retroviruses. 2012;28(1):16–35. doi: 10.1089/aid.2011.0234. [DOI] [PubMed] [Google Scholar]
- 5.Grossman Z, Picker LJ. Pathogenic mechanisms in simian immunodeficiency virus infection. Curr Opin HIV AIDS. 2008;3(3):380–386. doi: 10.1097/COH.0b013e3282fbaae6. 310.1097/COH.1090b1013e3282fbaae1096. [DOI] [PubMed] [Google Scholar]
- 6.Haase AT. Targeting early infection to prevent HIV-1 mucosal transmission. Nature. 2010;464(7286):217–223. doi: 10.1038/nature08757. [DOI] [PubMed] [Google Scholar]
- 7.Letvin NL. Progress and obstacles in the development of an AIDS vaccine. Nat Rev Immunol. 2006;6(12):930–939. doi: 10.1038/nri1959. [DOI] [PubMed] [Google Scholar]
- 8.Picker LJ, Hansen SG, Lifson JD. New Paradigms for HIV/AIDS Vaccine Development. Annu Rev Med. 63(1):95–111. doi: 10.1146/annurev-med-042010-085643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sodora DL, Silvestri G. Immune activation and AIDS pathogenesis. AIDS. 2008;22(4):439–446. doi: 10.1097/QAD.0b013e3282f2dbe7. 410.1097/QAD.1090b1013e3282f1092dbe1097. [DOI] [PubMed] [Google Scholar]
- 10.Harouse JM, Gettie A, Tan RC, Blanchard J, Cheng-Mayer C. Distinct pathogenic sequela in rhesus macaques infected with CCR5 or CXCR4 utilizing SHIVs. Science. 1999;284(5415):816–819. doi: 10.1126/science.284.5415.816. [DOI] [PubMed] [Google Scholar]
- 11.Harouse JM, Gettie A, Eshetu T, et al. Mucosal transmission and induction of simian AIDS by CCR5-specific simian/human immunodeficiency virus SHIV(SF162P3) J Virol. 2001;75(4):1990–1995. doi: 10.1128/JVI.75.4.1990-1995.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harouse JM, Buckner C, Gettie A, et al. CD8+ T cell-mediated CXC chemokine receptor 4-simian/human immunodeficiency virus suppression in dually infected rhesus macaques. Proc Natl Acad Sci U S A. 2003 Sep 16;100(19):10977–10982. doi: 10.1073/pnas.1933268100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tasca S, Tsai L, Trunova N, et al. Induction of potent local cellular immunity with low dose X4 SHIVSF33A vaginal exposure. Virology. 2007;367(1):196–211. doi: 10.1016/j.virol.2007.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trunova N, Tsai L, Tung S, et al. Progestin-based contraceptive suppresses cellular immune responses in SHIV-infected rhesus macaques. Virology. 2006;352(1):169–177. doi: 10.1016/j.virol.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 15.Boadi T, Schneider E, Chung S, et al. Cellulose acetate 1,2-benzenedicarboxylate protects against challenge with pathogenic X4 and R5 simian/human immunodeficiency virus. AIDS. 2005;19(15):1587–1594. doi: 10.1097/01.aids.0000186020.24426.62. [DOI] [PubMed] [Google Scholar]
- 16.Cheng-Mayer C, Huang Y, Gettie A, et al. Delay of simian human immunodeficiency virus infection and control of viral replication in vaccinated macaques challenged in the presence of a topical microbicide. AIDS. 2011;25(15):1833–1841. doi: 10.1097/QAD.0b013e32834a1d94. 1810.1097/QAD.1830b1013e32834a32831d32894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keele BF, Giorgi EE, Salazar-Gonzalez JF, et al. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc Natl Acad Sci U S A. 2008 May 27;105(21):7552–7557. doi: 10.1073/pnas.0802203105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salazar-Gonzalez JF, Salazar MG, Keele BF, et al. Genetic identity, biological phenotype, and evolutionary pathways of transmitted/founder viruses in acute and early HIV-1 infection. The Journal of Experimental Medicine. 2009 Jun 8;206(6):1273–1289. doi: 10.1084/jem.20090378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ho SH, Tasca S, Shek L, et al. Coreceptor switch in R5-tropic simian/human immunodeficiency virus-infected macaques. J Virol. 2007 Aug;81(16):8621–8633. doi: 10.1128/JVI.00759-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ren W, Tasca S, Zhuang K, Gettie A, Blanchard J, Cheng-Mayer C. Different Tempo and Anatomic Location of Dual-Tropic and X4 Virus Emergence in a Model of R5 Simian-Human Immunodeficiency Virus Infection. J Virol UPDATE. 2010 Jan 1;84(1):340–351. doi: 10.1128/JVI.01865-09. Epub ahed of print Apr 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shakirzyanova M, Tsai L, Ren W, Gettie A, Blanchard J, Cheng-Mayer C. Pathogenic Consequences of Vaginal Infection with CCR5-Tropic Simian-Human Immunodeficiency Virus SHIVSF162P3N. J Virol UPDATE. 2012 Sep 1;86(17):9432–9442. doi: 10.1128/JVI.00852-12. Epub ahed of print Apr 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stephen M, Smith BH, Russo Christine, Dailey Peter J, Marx Preston A, Connor Ruth I. Retrospective Analysis of Viral Load and SIV Antibody Responses in Rhesus Macaques Infected with Pathogenic SIV: Predictive Value for Disease Progression. AIDS Res Hum Retroviruses. 1999;15(18):1691–1701. doi: 10.1089/088922299309739. [DOI] [PubMed] [Google Scholar]
- 23.Mattapallil JJ, Douek DC, Hill B, Nishimura Y, Martin M, Roederer M. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature. 2005 Apr 28;434(7037):1093–1097. doi: 10.1038/nature03501. [DOI] [PubMed] [Google Scholar]
- 24.Ostrowski MA, Justement SJ, Catanzaro A, et al. Expression of chemokine receptors CXCR4 and CCR5 in HIV-1-infected and uninfected individuals. J Immunol. 1998 Sep 15;161(6):3195–3201. [PubMed] [Google Scholar]
- 25.Reynes J, Portales P, Segondy M, et al. CD4 T cell surface CCR5 density as a host factor in HIV-1 disease progression. AIDS. 2001;15(13):1627–1634. doi: 10.1097/00002030-200109070-00004. [DOI] [PubMed] [Google Scholar]
- 26.Baroncelli S, Negri DRM, Michelini Z, Cara A. Macaca mulatta, fascicularis and nemestrina in AIDS vaccine development. Expert Rev Vaccines. 2008;7(9):1419–1434. doi: 10.1586/14760584.7.9.1419. [DOI] [PubMed] [Google Scholar]
- 27.Joag SV, Stephens EB, Adams RJ, Foresman L, Narayan O. Pathogenesis of SIVmac infection in Chinese and Indian rhesus macaques: effects of splenectomy on virus burden. Virology. 1994 May 1;200(2):436–446. doi: 10.1006/viro.1994.1207. [DOI] [PubMed] [Google Scholar]
- 28.Ling B, Veazey RS, Luckay A, et al. SIV(mac) pathogenesis in rhesus macaques of Chinese and Indian origin compared with primary HIV infections in humans. AIDS. 2002 Jul 26;16(11):1489–1496. doi: 10.1097/00002030-200207260-00005. [DOI] [PubMed] [Google Scholar]
- 29.Marthas ML, Lu D, Penedo MC, Hendrickx AG, Miller CJ. Titration of an SIVmac251 stock by vaginal inoculation of Indian and Chinese origin rhesus macaques: transmission efficiency, viral loads, and antibody responses. AIDS Res Hum Retroviruses. 2001 Oct 10;17(15):1455–1466. doi: 10.1089/088922201753197123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reimann KA, Parker RA, Seaman MS, et al. Pathogenicity of simian-human immunodeficiency virus SHIV-89.6P and SIVmac is attenuated in cynomolgus macaques and associated with early T-lymphocyte responses. J Virol. 2005 Jul;79(14):8878–8885. doi: 10.1128/JVI.79.14.8878-8885.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trichel AM, Rajakumar PA, Murphey-Corb M. Species-specific variation in SIV disease progression between Chinese and Indian subspecies of rhesus macaque. Journal of Medical Primatology. 2002;31(4–5):171–178. doi: 10.1034/j.1600-0684.2002.02003.x. [DOI] [PubMed] [Google Scholar]
- 32.Monceaux V, Fang RHT, Cumont MC, Hurtrel B, Estaquier J. Distinct Cycling CD4+-and CD8+-T-Cell Profiles during the Asymptomatic Phase of Simian Immunodeficiency Virus SIVmac251 Infection in Rhesus Macaques. J Virol UPDATE. 2003 Sep 15;77(18):10047–10059. doi: 10.1128/JVI.77.18.10047-10059.2003. Epub ahed of print Apr 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Monceaux Vr, Viollet L, Petit Fdr, et al. CD8+ T Cell Dynamics during Primary Simian Immunodeficiency Virus Infection in Macaques: Relationship of Effector Cell Differentiation with the Extent of Viral Replication. The Journal of Immunology. 2005 Jun 1;174(11):6898–6908. doi: 10.4049/jimmunol.174.11.6898. [DOI] [PubMed] [Google Scholar]
- 34.Monceaux V, Viollet L, Petit F, et al. CD4+ CCR5+ T-Cell Dynamics during Simian Immunodeficiency Virus Infection of Chinese Rhesus Macaques. J Virol UPDATE. 2007 Dec 15;81(24):13865–13875. doi: 10.1128/JVI.00452-07. Epub ahed of print Apr 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lifson JD, Nowak MA, Goldstein S, et al. The extent of early viral replication is a critical determinant of the natural history of simian immunodeficiency virus infection. J Virol. 1997 Dec;71(12):9508–9514. doi: 10.1128/jvi.71.12.9508-9514.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mellors JW, Rinaldo CR, Gupta P, White RM, Todd JA, Kingsley LA. Prognosis in HIV-1 Infection Predicted by the Quantity of Virus in Plasma. Science. 1996 May 24;272(5265):1167–1170. doi: 10.1126/science.272.5265.1167. [DOI] [PubMed] [Google Scholar]
- 37.Ling B, Veazey RS, Hart M, et al. Early restoration of mucosal CD4 memory CCR5 T cells in the gut of SIV-infected rhesus predicts long term non-progression. AIDS. 2007;21(18):2377–2385. doi: 10.1097/QAD.0b013e3282f08b32. 2310. 1097/QAD.2370b2013e3282f2308b2332. [DOI] [PubMed] [Google Scholar]
- 38.Degenhardt JD, de Candia P, Chabot A, et al. Copy Number Variation of CCL3-like Genes Affects Rate of Progression to Simian-AIDS in Rhesus Macaques (Macaca mulatta) PLoS Genet. 2009;5(1):e1000346. doi: 10.1371/journal.pgen.1000346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xia H-J, Zhang G-H, Ma J-P, et al. Dendritic cell subsets dynamics and cytokine production in SIVmac239-infected Chinese rhesus macaques. Retrovirology. 7(1):102. doi: 10.1186/1742-4690-7-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kirmaier A, Wu F, Newman RM, et al. TRIM5 suppresses cross-species transmission of a primate immunodeficiency virus and selects for emergence of resistant variants in the new species. PLoS Biol. 2010;8(8):e1000462. doi: 10.1371/journal.pbio.1000462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karl J, Wiseman R, Campbell K, et al. Identification of MHC class I sequences in Chinese-origin rhesus macaques. Immunogenetics. 2008;60(1):37–46. doi: 10.1007/s00251-007-0267-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wiseman RW, Karl JA, Bimber BN, et al. Major histocompatibility complex genotyping with massively parallel pyrosequencing. Nat Med. 2009;15(11):1322–1326. doi: 10.1038/nm.2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Solomon C, Southwood S, Hoof I, et al. The most common Chinese rhesus macaque MHC class I molecule shares peptide binding repertoire with the HLA-B7 supertype. Immunogenetics. 2010;62(7):451–464. doi: 10.1007/s00251-010-0450-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wambua D, Henderson R, Solomon C, et al. SIV-infected Chinese-origin rhesus macaques express specific MHC class I alleles in either elite controllers or normal progressors. Journal of Medical Primatology. 2011;40(4):244–247. doi: 10.1111/j.1600-0684.2011.00487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ma X, Tang LH, Qu LB, Ma J, Chen L. Identification of 17 novel major histocompatibility complex-A alleles in a population of Chinese-origin rhesus macaques. Tissue Antigens. 2009;73(2):184–187. doi: 10.1111/j.1399-0039.2008.01168.x. [DOI] [PubMed] [Google Scholar]
- 46.Benson J, Chougnet C, Robert-Guroff M, et al. Recombinant vaccine-induced protection against the highly pathogenic simian immunodeficiency virus SIV(mac251): dependence on route of challenge exposure. J Virol. 1998 May;72(5):4170–4182. doi: 10.1128/jvi.72.5.4170-4182.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chenine AsL, Siddappa NB, Kramer VG, et al. Relative Transmissibility of an R5 Clade C Simian- Human Immunodeficiency Virus Across Different Mucosae in Macaques Parallels the Relative Risks of Sexual HIV-1 Transmission in Humans via Different Routes. Journal of Infectious Diseases. 2010 Apr 15;201(8):1155–1163. doi: 10.1086/651274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Greenier JL, Miller CJ, Lu D, et al. Route of simian immunodeficiency virus inoculation determines the complexity but not the identity of viral variant populations that infect rhesus macaques. J Virol. 2001 Apr;75(8):3753–3765. doi: 10.1128/JVI.75.8.3753-3765.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miller CJ, Marthas M, Greenier J, Lu D, Dailey PJ, Lu Y. In vivo replication capacity rather than in vitro macrophage tropism predicts efficiency of vaginal transmission of simian immunodeficiency virus or simian/human immunodeficiency virus in rhesus macaques. J Virol. 1998 Apr;72(4):3248–3258. doi: 10.1128/jvi.72.4.3248-3258.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.ten Haaft P, Almond N, Biberfeld G, et al. Comparison of early plasma RNA loads in different macaque species and the impact of different routes of exposure on SIV/SHIV infection. Journal of Medical Primatology. 2001;30(4):207–214. doi: 10.1034/j.1600-0684.2001.d01-54.x. [DOI] [PubMed] [Google Scholar]
- 51.Miller CJ, Marthas M, Torten J, et al. Intravaginal inoculation of rhesus macaques with cell-free simian immunodeficiency virus results in persistent or transient viremia. J Virol. 1994 Oct;68(10):6391–6400. doi: 10.1128/jvi.68.10.6391-6400.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Polacino P, Larsen K, Galmin L, et al. Differential pathogenicity of SHIVSF162 P4 infection in pig-tailed and rhesus macaques. Journal of Medical Primatology. 2008;37:13–23. doi: 10.1111/j.1600-0684.2008.00325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]