Abstract
The increasing resistance of uropathogens to antibiotics, and recognition of generally self-limiting nature of uncomplicated urinary tract infection (UTI) suggests that it is time to reconsider empirical treatment of UTI using antibiotics. Identifying new and effective strategies to prevent recurrences and alterative treatment strategies are a high priority. We review the recent literature regarding the effects of functional food products, probiotics, vaccines, and alternative treatments on treating and preventing UTI.
Keywords: functional foods, probiotics, vaccines, anti-inflammatories
Especially among sexually active young women aged 18 to 24, uncomplicated urinary tract infection (UTI) is extremely common, affecting almost one out of five US women in this age group annually. [1]. Even among those experiencing frequent infections, uncomplicated UTI is not associated with loss of renal function or increased mortality [reviewed by[2]. However, the condition causes pain and suffering, and negatively impacts quality of life albeit transiently[3]. Pre-menopausal women invariably present with frequent, urgent and painful urination; suprapubic pressure and hematuria may also be present. By contrast post-menopausal women are more likely to present with generalized symptoms, such as lower abdominal pain[4]. Because uncomplicated UTI is generally self-limiting, and responds rapidly to short courses of antibiotic therapy, it is generally treated empirically.
There are several reasons to reconsider empirical UTI treatment using antibiotics. First, there is increasing resistance of E. coli, the primary causative agent of uncomplicated UTI, to a variety of antibiotics, including fluoroquinolones[5], and Extended Spectrum Beta Lactamase (ESBL) resistance is increasingly observed among community-acquired UTI [6,7,8]. Second, studies of the human microbiota demonstrate there can be significant impact of short courses of antibiotics on the gut microbiota[9]; this probably holds for microbiota in the vaginal cavity and periurethral area where uropathogens also live. If the uropathogen is resistant to the antibiotic, the antibiotic may enhance the pathogenicity of the uropathogen by adversely affecting the inherent pathogen resistance of the normal microbiota. Notably, the most common ESBL-producing E. coli identified to date, denoted by its multilocus sequence type ST131, has fewer virulence factors than other uropathogens [10]. Instead, ST131’s virulence seems a function of inadequate initial antibiotic therapy [11]. Third, antibiotic therapy is not without harm. Of every 100,000 persons treated with trimethoprim sulfamathoxazole, three will develop Erythema multiforme, Stevens Johnson syndrome or toxic epidermal necrolysis requiring hospitalization[12]. Fluoroquinolones cause nausea, diarrhea, vomiting, rash or abnormal liver function tests in up to 2.5%[13]. Rarely, but more serious, fluoroquinolones can cause ventricular arrhythmia (0.3/cases per 10 million [14]), tendinopathy (0.4% [14]) or antibiotic associated diarrhea (4.6/1000 days treated [15]). By far the most common adverse event is development of a vaginal candida infection, which occurs in up to 22% of women treated for uncomplicated UTI [16]. Lastly, bacteria have rapidly become resistant to each introduced antibiotic (Figure 1).
Figure 1.
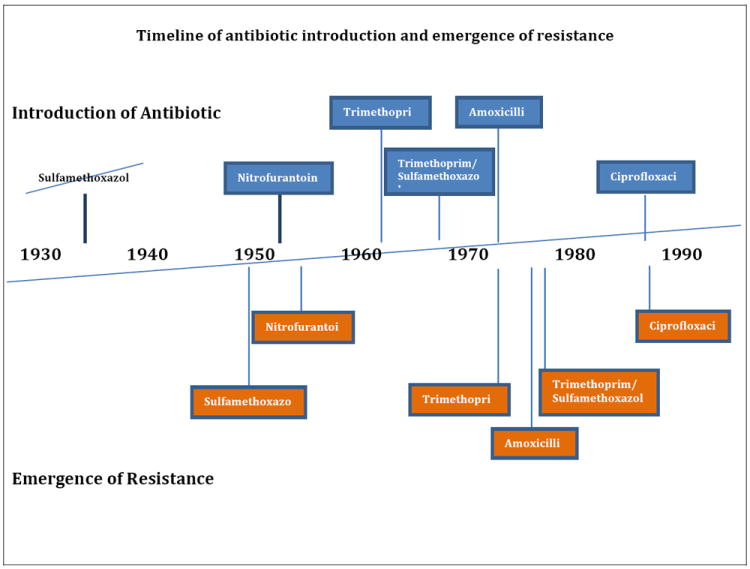
Timeline of the introduction of commonly prescribed antibiotics for urinary tract infections and the emergence of resistance.
With regard to diagnosis, the combination of symptoms and results from urinalysis are not very good predictors of a positive urine culture [17]. It is notable that almost one-fourth of women presumed to have a lower UTI based on the presence of dysuria and frequency, and urgency or suprapubic pressure or macroscopic hematuria have a negative midstream urine culture [unpublished data of the authors]. This may reflect sampling error (such as if the woman was well hydrated), or indicate that the infection was spontaneously resolving. Voiding is part of the host defense against UTI, and more frequent voiding in an intervention study was associated in one study with lower UTI risk[18]. Women with a history of 3 or more UTI in the previous year compared to controls with stress incontinence but no UTI history have oversensitive bladders based on filling cystometry data [19] – perhaps a natural preventive strategy.
In the two published placebo controlled trials, bacteriologic cure rates 4 to 7 weeks post treatment were only marginally better among those treated with antibiotics. Although symptoms resolve more quickly with antibiotics, it is notable that antibiotics also have anti-inflammatory effects[20]. Surprisingly, anti-inflammatories are not generally prescribed to relieve UTI symptoms, although analgesics are. Clinical cure rates in a non-inferiority trial comparing ciprofloxacin to ibuprofen were not significantly different [21], however, this trial was small; a larger trial is ongoing.
The concern that lower UTI will progress to pyelonephritis is commonly cited as a reason to treat empirically. While this progression does occur, the published evidence suggests that it is rare. One of the two placebo controlled trials observed one case of pyelonephritis among those in the placebo group; while the point estimate was 2% (1/38) the 95% confidence intervals range from 0.13 to 12.3%[22]. In the placebo controlled trial of Ferry et al.[23], no cases of pyelonephritis were observed among 288 women treated with placebo, and none in the more recent trial among 40 women treated with ibuprofen[21]. In five randomized controlled trials that reported incidence of pyelonephritis, there were 2 cases of pyelonephritis out of 582 participants (3/1000); both cases occurred in women receiving a 3 day versus a longer course of antibiotics (reviewed by Katchman, et al., 2005. [24])
The greatest challenge presented by uncomplicated UTI is the propensity to recur. Following an initial UTI, the risk of a second is 24.5% within 6 months[25], and a up to 5% will have 3 or more episodes per year [26]. While antibiotic therapy either daily or as post-coital prophylaxis is effective at reducing recurrences, the increasing rate of antibiotic resistance among uropathogens, and concerns about the effect of antibiotic prophylaxis on microbiota, makes this strategy less desirable. Thus, identifying new and effective strategies to prevent recurrences and alterative treatment strategies are a high priority. As reviewed herein, several recent publications have explored the effects of functional food products, probiotics, vaccines, and alternative treatments on treating and preventing UTI.
Functional Food Products
The best-studied natural therapeutic and preventative for UTI is the American cranberry. A well known folk remedy, there have been mixed reports of effectiveness in clinical trials. The different clinical trials have used various dosages and formulations and many included only a small number of participants. However, a meta analysis which included recent clinical trials with larger numbers of participants suggests little or no effect of cranberry juice on treatment or prevention in otherwise healthy women[27]. Other natural products that have been suggested, for which there is only limited scientific evidence include garlic for non-E. coli UTI;][27,28], a preparation containing horseradish and nasturtium [29], rice vinegar [30], and a sage herb found in Asia, Salvia Plebeia [31], which is a folk remedy for UTI.
Lactobacillus preparations
E. coli that cause UTI can live in the bowel and vaginal cavities, around the urethral opening and in the urinary tract. During a UTI, the causative uropathogen often is isolated from the vagina, bowel microbiota or periurethrum [32,33]. Bacteria from the bowel, vagina and periurethrum are moved into the bladder during sexual activity; the presence of E. coli in the urinary tract is correlated with time since last sexual activity [34]. Therefore, inhibiting the colonization of these areas by E. coli may decrease UTI risk. This is one of the theories behind using preparations containing Lactobacillus species to prevent UTI. Lactobacillus species also live in the bowel, vagina and periurethrum; some Lactobacillus produce hydrogen peroxide, and modulate the local pH. Others may modulate host immune response, limit adhesion of E. coli to tissue or disrupt E. coli biofilm formation [35]. These characteristics, and perhaps others which reflect their role in the microbial community structure, help the vagina and periurethrum resist invasion by pathogens such as E. coli. In the bowel, Lactobacillus may limit colonization by uropathogens.
A US phase 2 randomized controlled double blind trial using a Lactobacillus crispatus intravaginal suppository probiotic (Lactin-V; Osel) to prevent recurrent UTI in 100 otherwise health premenopausal women with recurrent UTI showed promising results. Fifteen percent of women using the active suppository had a UTI within 8 weeks, compared to 27% of those using placebo at 8 to 10 weeks. However, larger trials are needed [36]. A Dutch double-blind noninferiority trial conducted among 252 postmenopasusal women with recurrent UTI compared the preventive effects of prophylactic oral as opposed to vaginal capsules containing Lactobacillus rhamnonus and Lactobacillus reuteri to prophylactic trimethoprim-sulfamethoxazole [37]. The mean number of clinical recurrences among women taking Lactobacillus was 3.3 (95% CI 2.7, 4.0) compared to 2.9 (95% CI 2.3, 3.6) among women taking trimethoprim-sulfamethoxazole (p=0.42); this did not meet the criteria for noninferiority. There were significantly more microbial recurrences in the Lactobacillus group. However, after one month of trimethoprim-sulfamethoxazole, E. coli resistance to trimethoprim-sulfamethoxazole, trimethoprim and amoxicillin increased fourfold in the feces of asymptomatic women (20% to 80%) and doubled in their urine 50% to 95%). There was no change in antibiotic resistance observed in E. coli from the feces and urine of those using the Lactobacillus capsules. The authors [37] and an accompanying commentary [38] suggest that the slightly lower effectiveness of the probiotic might be worth the benefit in terms of decreased antibiotic resistance. An even more preliminary study tested the effects of vaginally applied lactic acid gel on symptoms and bacteriuria in 20 women with cystitis [39]. Eleven women responded well with symptom resolution, the remaining 9 did not. While further studies are needed, there is increasing evidence suggesting that use of Lactobacillus probiotics may be effective in preventing UTI.
Immunotherapeutics and Vaccines
A sublingual bacterial vaccine (Iromune) and an immunotherapeutic (OM-89 UroVaxom) both based on bacterial extracts are commercially available in Europe. Results of a retrospective record review study comparing 159 women using Iromune to 160 women using trimethoprim/ sulfamethoxazole to prevent recurrent UTI were promising; however since participants were neither randomized nor blinded the results are interesting but need confirmation[40]. By contrast, reports of a multicenter blinded randomized trial of OM-89 among 453 women with acute UTI also found significant reduction in recurrent UTIs in the treated group over a 12 month period (40% versus 55%)[41]. However, 20% of those in the active group, and 16% in the placebo group discontinued therapy early; the regimen of one dose daily for 3 months, followed by treatment for the first 10 days of months 7-9, was long and potentially complicated. Ideally, a vaccine would be given only a few times and be long lasting. This may be challenging for UTI: uropathogenic E. coli are quite diverse and use a variety of mechanisms to cause disease. Several other vaccines have been tested or are under development (Reviewed in [26]), based on surface polysaccharides, adherence factors, toxins, iron acquisition, multiepitope subunit, hypothetical proteins, genetically engineered, or like OM-89 and Iromune, multi-strain whole cell. Most have been tested only in mouse or rat models or primates. The ideal UTI vaccine appears to be far in the future.
Biological Therapies
Although colonization of the urine is required for UTI to occur, treatment of asymptomatic bacteriuria increases risk of symptomatic UTI [[42,43]. This suggests that the presence of an avirulent strain may interfere with colonization by a virulent one. Following this reasoning, a Swedish group conducted a blinded cross-over trial comparing inoculation with a well characterized E. coli strain originally isolated from a girl with asymptomatic bacteriuria (E. coli 83972) to inoculation with saline [44]. The twenty participants had incomplete bladder emptying and a history of recurrent lower UTI. There were significantly fewer UTIs when E. coli 83972 was present. The same group recently reported evidence suggesting that uropathogens may evolve within the individual to be less virulent[45]; if true, this supports less aggressive treatment of uncomplicated UTI (see section on symptomatic relief, below). It seems unlikely that instillation of an asymptomatic bacteriuria strain will become standard therapy anytime soon.
Bacteria themselves can be invaded and killed by virus. Reasoning that uropathogens adhering to tissue could be removed by deliberately infecting with a bacteriophage (a virus), a Portuguese group is developing a bacteriophage as a UTI therapy. Preliminary studies suggest that bacteriophage can kill clinical E. coli strains when grown in tissue culture using artificial urine [46]. Phage therapy for UTI and other infections may become standard sometime in the future [47], but for now, any implementation for treatment UTI in humans is premature.
Initial Symptomatic Relief followed by Antibiotics if Needed
Empirical treatment of uncomplicated UTI presumes that the benefits of being able to rapidly treat those with UTI outweigh the risks of erroneously treating those without UTI. Empirical treatment is less costly, and likely increases patient satisfaction. Nonetheless, there are hidden costs, particularly the increased risk of antibiotic resistance bacteria within the individual, risk of adverse reaction to the antibiotic, and applying positive selection for resistance on the population level. An alternative strategy is to treat the symptoms, and if they do not resolve, treat using an antibiotic. This strategy was applied in a pilot study conducted in Germany[21]; a larger trial is ongoing [48]. The pilot study was a double blind, randomly controlled trial conducted among otherwise healthy women presenting with frequency or dysuria and no complicating factors. Women were randomly assigned to either ibuprofen or ciprofloxacin for 3 days. If the symptoms worsened during the 3 days the drug trial was stopped and (another) antibiotic treatment prescribed. While the study was small (40 in the ibuprofen group and 39 in the ciprofloxacin group) there was no significant difference in the proportion symptom free at day 4, or in total symptom score by treatment group. Further, the proportion of women who were given secondary antibiotic treatment due to worsening treatment was not statistically different between the two groups.
Conclusions
Treatment and prevention of uncomplicated UTI is reaching a turning point. The benefits to the individual of empirical antibiotic therapy may no longer outweigh the individual – and societal – risks [38,49,]. We are rapidly approaching a time where there is increasing support for using alternatives to antibiotic therapy – particularly for prevention of recurring infection. This change cannot come soon enough. Urinary isolates are the most common source of ESBL-producing E. coli[6] -- which are often also resistant to fluoroquinolones. Uropathogens rapidly acquire antibiotic resistance, so whenever a new antibiotic is used for treatment resistance rapidly follows. If found on a mobile genetic element, (as both ESBL and fluroquinolone resistance now are), the resistance can rapidly spread to other bacterial species. Uncomplicated UTI in otherwise healthy women are not life threatening (although adverse reactions to antibiotic therapy can be). Advising strategies that enhance the host’s ability to fight infection (encouraging good hydration, frequent voiding, and potentially Lactobacillus probiotics) for prevention, and minimizing inflammatory symptoms (prescribing analgesics), and using antibiotics only for UTIs that do not resolve within three days may be an important strategy for preserving antibiotics for pyelonephritis and other more serious infections.
Table.
| Antibiotic Type | Introduction of Antibiotic | Emergence of Resistance* |
|---|---|---|
| Amoxicillin | 19721 | 19777 |
| Ciprofloxacin | 19871 | 19876,7 |
| Trimethoprim | 19622 | 19723 |
| Sulfamethoxazole | 19354 | 19507 |
| Trimethoprim -Sulfamethoxazole | 19683 | 19788 |
| Nitrofurantoin | 19535 | 19559 |
Some dates are estimaters
eMedExpert. http://www.emedexpert.com. Accessed on November 3, 2012.
Huovinen P, Sundström L, Swedberg G, Sköld O. Trimethoprim and sulfonamide resistance. Antimicrob Agents Chemother 1995;39:279-89.
Eliopoulos G, Huovinen P. Resistance to trimethoprim sulfamethoxazole. Clin Infect Dis. 2001; 32:1608-14.
Skold O. Sulfonamide resistance: mechanisms and trends. Drug Resist Updat. 2000;3:155 60.
Thomson CJ. The global epidemiology of resistance to ciprofloxacin and the changing nature of antibiotic resistance: a 10 year perspective. Journal of Antimicrobial Chemotherapy 1999; 43:31-40
http://www.euro.who.int/__data/assets/pdf_file/0011/120143/E94241.pdf. Accessed on November 3, 2012.
Bannatyne RM, Toma S, Cheung R, Hu G, Taylor D, Keystone JS, Devlin HR. Resistance to trimethoprim and other antibiotics in Ontario Shigellæ. The Lancet. 1980; 315:425-26.
Kass EH. Chemotherapeutic and antibiotic drugs in the management of infections of the urinary tract. Am. J. Med. 1955; 18:764-81.
Footnotes
DISCLOSURE
Dr. B. Foxman has been a consultant for Vifor Pharma; Dr. M. Buxton reported no potential conflicts of interest relevant to this article.
References
- 1.Foxman B, Brown P. Epidemiology of urinary tract infections: transmission and risk factors, incidence, and costs. Infect Dis Clin North Am. 2003;17:227–41. doi: 10.1016/s0891-5520(03)00005-9. [DOI] [PubMed] [Google Scholar]
- 2.Naber KG, Wullt B, Wagenlehner FM. Antibiotic treatment of uncomplicated urinary tract infection in premenopausal women. Int J Antimicrob Agents. 2011;38:21–35. doi: 10.1016/j.ijantimicag.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 3.Ellis AK, Verma S. Quality of life in women with urinary tract infections: is benign disease a misnomer. J Am Board Fam Pract. 2002;13:392–397. doi: 10.3122/15572625-13-6-392. [DOI] [PubMed] [Google Scholar]
- 4.Arinzon Z, Shabat S, Peisakh A, Berner Y. Clinical presentation of urinary tract infection (UTI) differs with aging in women. Arch Gerontol Geriatr. 2012;55:145–7. doi: 10.1016/j.archger.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 5•.Hooton TM. Uncomplicated urinary tract infection. N Engl J Med. 2012;366:1028–37. doi: 10.1056/NEJMcp1104429. Case vignette and review of treatment of uncomplicated urinary tract infection. [DOI] [PubMed] [Google Scholar]
- 6.Coque TM, Baquero F, Cantón R. Increasing prevalence of ESBL-producing Enterobacteriaceae in Europe. Euro Surveill. 2008;1319044 [PubMed] [Google Scholar]
- 7•.Meier S, Weber R, Zbinden R, Ruef C, Hasse B. Extended-spectrum β-lactamase-producing Gram-negative pathogens in community-acquired urinary tract infections: an increasing challenge for antimicrobial therapy. Infection. 2011;39:333–340. doi: 10.1007/s15010-011-0132-6. Report of retrospective study comparing community acquired to healthcare associated urinary tract infections due to ESBL-producing Escherichia coli. [DOI] [PubMed] [Google Scholar]
- 8.Fennell J, Vellinga A, Hanahoe B, Morris D, Boyle F, Higgins F, Lyons M, O’Connell K, Keady D, Cormican M. Increasing prevalence of ESBL production among Irish clinical Enterobacteriaceae from 2004 to 2008: an observational study. BMC Infect Dis. 2012;12:116. doi: 10.1186/1471-2334-12-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dethlefsen L, Huse S, Sogin ML, Relman DA. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biol. 2008;6:e280. doi: 10.1371/journal.pbio.0060280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10•.Gibreel TM, Dodgson AR, Cheesbrough J, Fox AJ, Bolton FJ, Upton M. Population structure, virulence potential and antibiotic susceptibility of uropathogenic Escherichia coli from northwest England. J Antimicrob Chemother. 2012;67:346–56. doi: 10.1093/jac/dkr451. Phylogenetic analysis and virulence gene characterization of 300 uropathogenic E. coli. [DOI] [PubMed] [Google Scholar]
- 11.Oteo J, Perez-Vazquez M, Campos J. Extended-spectrum [beta]-lactamase producing Escherichia coli: changing epidemiology and clinical impact. Curr Opin Infect Dis. 2010;23:320–326. doi: 10.1097/qco.0b013e3283398dc1. [DOI] [PubMed] [Google Scholar]
- 12.Chan HL, Stern RS, Arndt KA, Langlois J, Jick SS, Jick H, Walker AM. The incidence of erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis. A population-based study with particular reference to reactions caused by drugs among outpatients. Arch Dermatol. 1990;126(1):43–7. [PubMed] [Google Scholar]
- 13.CIPRO® (ciprofloxacin hydrochloride) TABLETS, CIPRO® (ciprofloxacin*) ORAL SUSPENSION [Internet] Available at: http://www.fda.gov/downloads/Drugs/EmergencyPreparedness/BioterrorismandDrugPreparedness/UCM130802.pdf.
- 14.Mehlhorn AJ, Brown DA. Safety concerns with fluoroquinolones. Ann Pharmacother. 2007;41:1859–66. doi: 10.1345/aph.1K347. [DOI] [PubMed] [Google Scholar]
- 15.Walbrown MA, Aspinall SL, Bayliss NK, Stone RA, Cunningham F, Squier CL, Good CB. Evaluation of Clostridium difficile-associated diarrhea with a drug formulary change in preferred fluoroquinolones. J Manag Care Pharm. 2008;14:34–40. doi: 10.18553/jmcp.2008.14.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu J, Schwartz K, Bartoces M, Monsur J, Severson RK, Sobel JD. Effect of antibiotics on vulvovaginal candidiasis: a MetroNet study. J Am Board Fam Med. 2008;21:261–8. doi: 10.3122/jabfm.2008.04.070169. [DOI] [PubMed] [Google Scholar]
- 17.Schmiemann G, Kniehl E, Gebhardt K, Matejczyk MM, Hummers-Pradier E. The diagnosis of urinary tract infection: a systematic review. Dtsch Arztebl Int. 2010;107:361–7. doi: 10.3238/arztebl.2010.0361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Su SB, Wang JN, Lu CW, Guo HR. Reducing urinary tract infections among female clean room workers. J Womens Health (Larchmt) 2006;15:870–876. doi: 10.1089/jwh.2006.15.870. [DOI] [PubMed] [Google Scholar]
- 19.Arya LA, Northington GM, Asfaw T, Harvie H, Malykhina A. Evidence of bladder oversensitivity in the absence of an infection in premenopausal women with a history of recurrent urinary tract infections. BJU Int. 2012;110:247–51. doi: 10.1111/j.1464-410X.2011.10766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rozin A, Schapira D, Braun-Moscovici Y, Nahir A. Cotrimoxazole treatment for rheumatoid arthritis. Semin Arthritis Rheum. 2001;31:133–41. doi: 10.1053/sarh.2001.27734. [DOI] [PubMed] [Google Scholar]
- 21•.Bleidorn J, Gagyor I, Kochen MM, Wegscheidner K, Hummers-Pradier E. Symptomatic treatment (ibuprofen) or antibiotics (ciprofloxacin) for uncomplicated urinary tract infection? -Results of a randomized controlled pilot trial. BMC Med. 2010;8:30. doi: 10.1186/1741-7015-8-30. Pilot trial comparing ciprofloxacin to ibuprofen suggesting non-inferiority. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Christiaens TC, De Meyere M, Verschraegen G, Peersman W, Heytens S, De Maeseneer JM. Randomised controlled trial of nitrofurantoin versus placebo in the treatment of uncomplicated urinary tract infection in adult women. Br J Gen Pract. 2002;52:729–34. [PMC free article] [PubMed] [Google Scholar]
- 23.Ferry SA, Holm SE, Stenlund H, Lundholm R, Monsen TJ. The natural course of uncomplicated lower urinary tract infection in women illustrated by a randomized placebo controlled study. Scand J Infect Dis. 2004;36:296–301. doi: 10.1080/00365540410019642. [DOI] [PubMed] [Google Scholar]
- 24.Katchman EA, Milo G, Paul M, Christiaaens T, Baerheim A, Leibovici L. Three-day vs longer duration of antibiotic treatment for cystitis in women: systematic review and meta-analysis. Am J Med. 2005;118:1196–207. doi: 10.1016/j.amjmed.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 25.Foxman B, Gillespie B, Koopman J, Zhang L, Palin K, Tallman P, Marsh J, Spear S, Sobel J, Marty MJ, Marrs C. Risk factors for second urinary tract infection among college women. Am J Epidemiol. 2000;151:1194–1205. doi: 10.1093/oxfordjournals.aje.a010170. [DOI] [PubMed] [Google Scholar]
- 26•.Brumbaugh AR, Mobley HL. Preventing urinary tract infection: progress toward an effective Escherichia coli vaccine. Expert Rev Vaccines. 2012;11:663–76. doi: 10.1586/erv.12.36. Recent comprehensive review of UTI vaccines. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27•.Jepson RG, Williams G, Craig JC. Cranberries for preventing urinary tract infections. Cochrane Database of Systematic Reviews 2012. (10) doi: 10.1002/14651858.CD001321.pub5. Art No.: CD001321. Metaanalysis of randomized controlled trials assessing cranberry effectiveness in UTI treatment. [DOI] [Google Scholar]
- 28.Harjai K, Kumar R, Singh S. Garlic blocks quorum sensing and attenuates the virulence of Pseudomonas aeruginosa. FEMS Immunol Med Microbiol. 58:161–168. doi: 10.1111/j.1574-695X.2009.00614.x. [DOI] [PubMed] [Google Scholar]
- 29.Albrecht U, Goos KH, Schneider B. A randomised, double-blind, placebo-controlled trial of a herbal medicinal product containing Tropaeoli majoris her-ba (Nasturtium) and Armoraciae rusticanae radix (Horseradish) for the prophylactic treatment of patients with chronically recurrent lower urinary tract infections. Curr Med Res Opin. 2007;23:2415–22. doi: 10.1185/030079907X233089. [DOI] [PubMed] [Google Scholar]
- 30.Chung YC, Chen HH, Yeh ML. Vinegar for decreasing catheter-associated bacteriuria in long-term catheterized pateints: a randomized controlled trial. Biol Res Nurs. 2012;14:294–301. doi: 10.1177/1099800411412767. [DOI] [PubMed] [Google Scholar]
- 31.Peng MM, Fang Y, Hu W, Huang Q. The pharmacological activities of Compound Salvia Plebeia Granules on treating urinary tract infection. J Ethnopharmacol. 2010;129:59–63. doi: 10.1016/j.jep.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 32.Foxman B, Manning S, Tallman P, Bauer R, Zhang L, Koopman J, Gillespie B, Sobel J, Marrs C. Uropathogenic Escherichia coli are more likely than commensal E. coli to be shared between heterosexual sex partners. Am J Epidemiol. 2002;158:1133–40. doi: 10.1093/aje/kwf159. [DOI] [PubMed] [Google Scholar]
- 33.Schlager TA, Ashe KM, Hendley JO. The ability of periurethral Escherichia coli to grow in a voiding system is a key for the dominance of E. coli cystitis. Microb Pathog. 1997;22:235–40. doi: 10.1006/mpat.1996.0109. [DOI] [PubMed] [Google Scholar]
- 34.Xie J, Foxman B, Zhang L, Marrs CF. Molecular epidemiologic identification of Escherichia coli genes that are potentially involved in movement of organisms from intestinal tract to the vagina and bladder. J Clin Microbiol. 2006;44:2434–2441. doi: 10.1128/JCM.00397-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karlsson M, Scherbak N, Khalaf H, Olsson PE, Jass J. Substances released from probiotic lactobacillus rhamnosus GR-1 potentiate NF-κB activity in Escherichia coli-simulated urinary bladder cells. FEMS Immunol Med Microbiol. 2012;66:147–56. doi: 10.1111/j.1574-695X.2012.00994.x. [DOI] [PubMed] [Google Scholar]
- 36.Stapleton AE, Au-Yeung M, Hooton TM, Fredricks DN, Roberts PL, Czaja CA, Yarova-Yarovaya Y, Fiedler T, Cox M, Stamm WE. Randomized, placebo-controlled phase 2 trial of a lactobacillus crispatus probiotic given intravaginally for prevention of recurrent urinary tract infection. Clin Infect Dis. 2011;52:1212–1217. doi: 10.1093/cid/cir183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beereport MA, ter Riet G, Nys S, van der Wal WM, de Borgie CA, de Reijke TM, Prins JM, Koeijers J, Verbon A, Stobberingh E, Geerlings SE. Lactobacilli vs antibiotics to prevent urinary tract infections: a randomized, double blind, noninferiority trial in postmenopausal women. Arch Intern Med. 2012;172:704–12. doi: 10.1001/archinternmed.2012.777. [DOI] [PubMed] [Google Scholar]
- 38.Trautner BW, Gupta K. The advantages of second best: comment on “Lactobacilli vs antibiotics to prevent urinary tract infections”. Arch Intern Med. 2012;172:712–4. doi: 10.1001/archinternmed.2012.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Swidsinski A, Loening-Baucke V, Mendling W, Swidsinski S. Positive effects of local therapy with a vaginal lactic acid gel on dyuria and E.coli bacteriuria question our current views on recurrent cystitis. Arch Gynecol Obstet. 2012;285:1619–25. doi: 10.1007/s00404-011-2196-z. [DOI] [PubMed] [Google Scholar]
- 40.Lorenzo-Gomez MF, Padilla-Fernandez B, Garcia-Criado FJ, Miron-Canelo JA, Gil-Vicente A, Nieto-Huertos A, Silva-Abuin JM. Evaluation of a therapeutic vaccine for the prevention of recurrent urinary tract infections versus prophylactic treatment with antibiotics. Int Urogynecol J. 2012 doi: 10.1007/s00192-012-1853-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bauer HW, Alloussi S, Egger G, Blumlein HM, Cozma G, Schulman CC. A long-term, multicenter, double-blind study of an Escherichia coli extract (OM-89) in female patients with recurrent urinary tract infections. Eur Urol. 2005;47(4):542–8. doi: 10.1016/j.eururo.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 42•.Cai T, Mazzoli S, Mondaini N, et al. The Role of Asymptomatic Bacteriuria in Young Women with Recurrent Urinary Tract Infections: To Treat or Not to Treat? Clin Infect Dis. 2012;55:771–7. doi: 10.1093/cid/cis534. Trial demonstrating increased risk of symptomatic UTI among women with asymptomatic bacteriuria treated with antibiotics. [DOI] [PubMed] [Google Scholar]
- 43.Hansson S, Jodal U, Lincoln K, Svanborg-Eden C. Untreated asymptomatic bacteriuria in girls: II–Effect of phenoxymethylpenicillin and erythromycin given for intercurrent infections. BMJ. 1989;298:856–9. doi: 10.1136/bmj.298.6677.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sundén F, Håkansson L, Ljunggren E, Wullt B. Escherichia coli 83972 bacteriuria protects against recurrent lower urinary tract infections in patients with incomplete bladder emptying. J Urol. 2010;184:179–85. doi: 10.1016/j.juro.2010.03.024. [DOI] [PubMed] [Google Scholar]
- 45.Hernandez JG, Sunden F, Connolly J, Svanborg C, Wullt B. Genetic control of the variable innate Immune response to asymptomatic bacteriuria. PloS ONE. 2011;6:e28289. doi: 10.1371/journal.pone.0028289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chibeu AA, Lingohr EJE, Masson LL, Manges AA, Harel JJ, Ackermann H-WH, Kropinski AMA, Boerlin PP. Bacteriophages with the ability to degrade uropathogenic Escherichia coli biofilms. Viruses. 2012;4:471–487. doi: 10.3390/v4040471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Housby JN, Mann NH. Phage therapy. Drug Discovery Today. 2009;14:536–540. doi: 10.1016/j.drudis.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 48.Gagyor I, Hummers-Pradier E, Kochen MM, Schmiemann G, Wegscheider K, Bleidorn J. Immediate versus conditional treatment of uncomplicated urinary tract infection - a randomized-controlled comparative effectiveness study in general practices. BMC Infect Dis. 2012;12:146. doi: 10.1186/1471-2334-12-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baerheim A. Empirical treatment of uncomplicated cystitis. Scand J Prim Health Care. 2012;30:1–2. doi: 10.3109/02813432.2012.649629. [DOI] [PMC free article] [PubMed] [Google Scholar]


