Multiple experimental approaches have been used to assess the free-running period, or “tau,” of humans. One approach has been to study circadian rhythms in free-running blind humans. For example, Sack et al. (1992) measured the plasma melatonin rhythm in 11 free-running blind people and reported an average tau of 24.55 h.
In sighted humans, at least 3 different experimental paradigms have been used to derive tau. First, tau has been measured in people free-running in temporal isolation. In the classic bunker experiments, Wever (1979) reported an average tau of 25.00 h in 147 subjects. During spontaneous internal desynchronization in 52 of these subjects, tau from core body temperature recordings shortened to 24.86 h (Wever, 1992). Wever also noted shorter free-running periods in females, and longer free-running periods when subjects could alter light levels in association with their sleep, rather than sleeping in continuous ambient light. A background of constant bright light (3000–5000 lux) also lengthened tau (Wever, 1986, 1992). When subjects in the same isolation units were permitted to nap, tau was shorter in 3 subjects who napped (24.22 h) versus 4 subjects who rarely napped (24.73 h; Campbell et al., 1993). When groups of subjects lived in isolation units in dim light (<8 lux), but with knowledge of clock time, average tau estimated from 6-sulfatoxymelatonin was 24.36 h in 6 male subjects (Middleton et al., 1996) and 24.33 h in 10 male subjects (Middleton et al., 1997).
Human tau has also been estimated during forced desynchrony protocols in which humans live in a light-dark (LD) cycle beyond the range of entrainment. It has been argued that tau derived from the classic free-running protocol is longer than tau derived from forced desynchrony protocols, because in the free-running protocol subjects stay awake during the phase-delay portion and sleep through the phase-advance portion of their light phase response curve (PRC), thus producing phase delays. By contrast in the forced desynchrony protocols light exposure is more equally distributed across each subject's light PRC (Czeisler et al., 1999). When measuring plasma melatonin, temperature, and cortisol rhythms during a 28-h day, Czeisler et al. (1999) reported an average tau in 11 young males and 13 older females and males of 24.18 h. Hiddinga et al. (1997) measured core body temperature in 12 male subjects during a 20-h day and reported an average tau of 24.30 h.
A 3rd approach to estimating tau in sighted humans is through the use of ultradian LD cycles, which are also beyond the range of entrainment. Kripke et al. (2005) measured salivary melatonin and 6-sulfatoxymelatonin during 3 days of a 1.5-h ultradian LD cycle (60 min light, 30 min dark) in a sample of 62 young and 25 elderly humans and reported an average tau of 24.38 h.
There is still considerable interest in measuring tau, for example, in patients with circadian-based disorders such as delayed sleep phase type, and across the lifespan from adolescents to the elderly. Here we report on a method to estimate tau in sighted humans that is shorter and less expensive than the classic free-running protocol and the forced desynchrony protocols with long day lengths. Subjects lived in the laboratory on 2 different occasions, separated by 9 days. Both laboratory sessions contained 3 days of a 4-h ultradian LD cycle (2.5 h light < 100 lux, 1.5 h dark). During 1 session subjects were given melatonin or bright light, and in the other session either a placebo pill or no bright light, counterbalanced as previously described (Burgess et al., 2008; Revell and Eastman, 2005). Here we report tau only from the sessions without bright light or melatonin (Fig. 1). We also calculated tau from the subjects who had the placebo pill or no bright light first (“placebo 1st”) and those who had it second (Table 1), but there was no statistical difference between these groups (t(30) = 0.49, p = 0.63). These results are quite similar to those observed in other forced desynchrony protocols. Our average tau (24.22 h) is similar to the tau of young males reported by Czeisler et al. (1999) from the average of melatonin, temperature, and cortisol rhythms (24.18 h). In our sample, 12.5% (4 of 32) of the subjects had a tau < 24.0 h. This percentage is similar to the 9.1% (1 of 11) of young humans that Czeisler et al. (1999) first reported, but less than their more recent reports of 23.8% (5 of 21; Duffy and Wright, 2005) and 35.7% (5 of 14; Wright et al., 2005).
Figure 1.
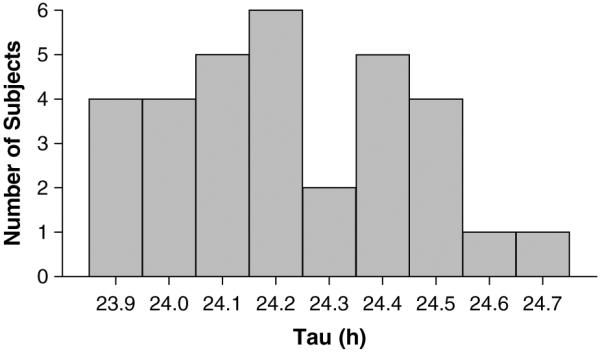
The frequency histogram of tau calculated from 32 young healthy subjects exposed to an ultradian light-dark cycle.
Table 1.
The average tau and correlation between tau and the DLMO to bedtime interval.
| Tau (h) (mean ± SD) | Correlation between Tau and Phase Angle | |
|---|---|---|
| Entire sample (n = 32) | 24.22 ± 0.22 | r = 0.36* |
| Placebo 1st (n = 18) | 24.20 ± 0.22 | r = 0.53* |
| Placebo 2nd (n = 14) | 24.24 ± 0.22 | r = 0.17 |
NOTE: DLMO = dim light melatonin onset.
p < 0.05.
As in other species (Pittendrigh and Daan, 1976), we found a significant association between tau and the phase angle of entrainment. Table 1 shows that there were significant correlations between tau and the baseline dim light melatonin onset (DLMO) to bedtime or lights-out interval. The correlation in the placebo 1st group of r = 0.53, shown in Figure 2, is remarkably similar in magnitude to that reported by Wright et al. (2005), which was r = 0.56. It shows that people with a shorter tau have a longer DLMO to bedtime interval, and begin their nighttime sleep episode at a later biological time. We believe the correlation for the placebo 1st group is stronger than the placebo 2nd group because the phase angle after treatment with melatonin or bright light may not have returned to baseline by the start of the 2nd laboratory session. There were very small and nonsignificant correlations between tau and bedtime or wake time in the 3 samples, also consistent with the findings of Wright et al. (2005). Thus, the relationship between phase angle of entrainment and tau was stronger when DLMO was used as the circadian phase marker and bedtime (lights-out) was used to mark the time of the zeitgeber than when bed or wake time was used as the circadian phase marker and clock time was used to mark the time of the zeitgeber.
Figure 2.
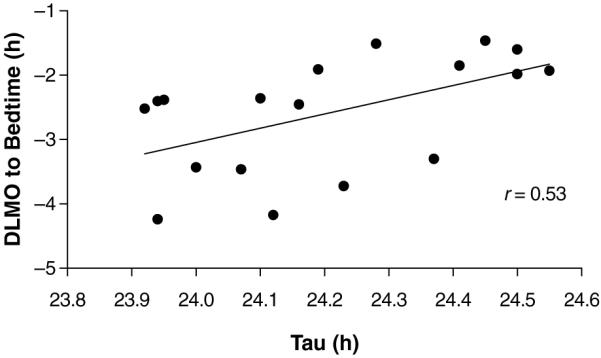
The association between the phase angle of entrainment (baseline dim light melatonin onset [DLMO] relative to bedtime or lights-out) and tau observed during an ultradian light-dark cycle in 14 young healthy subjects (placebo 1st group).
Despite the similarity between our results and the other forced desynchrony protocols, questions remain regarding the tau observed in our ultradian LD cycle. Average light intensity during the ultradian LD cycle was higher than the <20 lux used in the forced desynchrony studies mentioned above. It remains to be determined if the estimate of tau will change if the light levels in the ultradian LD cycle are lowered. It also remains to be determined if the estimate of tau will differ if humans experience the ultradian LD cycle for more than 3 days, because aftereffects from being released from entrainment could produce gradual changes in tau. Such aftereffects could also occur during other forced desynchrony protocols. Further research is required to address these issues. It could also be of interest to compare in an individual subject the estimate of tau derived from this ultradian protocol with an estimate derived from a forced desynchrony protocol, or observed in a free-running blind subject. Finally, if several individual subjects participated in the protocol at least twice, it would also be possible to assess the reliability of the tau estimates. Nevertheless, as it stands, our method can be used to compare tau among different groups, and is a less expensive and shorter experimental paradigm than forced desynchrony protocols with longer day lengths. This may enable more research laboratories to measure tau in humans.
ACKNOWLEDGMENTS
Supported by R01 NR007677 and R01 HL086934.
APPENDIX
MATERIALS AND METHODS
Thirty-two healthy young subjects (14 men, 18 women; mean age ± SD, 27.4 ± 6.5 years) slept at home according to a fixed sleep schedule tailored to their self-reported habitual sleep schedule for a week before entering the laboratory. Across subjects, bedtime varied between 2200 and 0200 h and wake time varied between 0600 and 1000 h. Naps were not permitted. Each 5-day laboratory session consisted of a baseline phase assessment, then 3 days of a 4-h ultradian LD cycle, followed by a final phase assessment. The phase assessments were 21.5–24 h in duration, during which saliva samples were collected half-hourly in dim light (<5 lux) for later determination of the dim light melatonin onset (DLMO) as previously reported (Burgess et al., 2008). After a 9-day return to sleeping on their fixed sleep schedule, the subjects repeated the 5-day laboratory session. During 1 of these laboratory sessions subjects were exposed to bright light or given exogenous melatonin pills, and during the other subjects received either placebo pills or were not exposed to bright light (“placebo session”). Here we report data only from placebo sessions.
The ultradian LD cycle consisted of 1.5-h dark/sleep episodes alternating with 2.5-h wake episodes in average light levels of 35.1 lux (<98 lux; light was measured at the eyes of subjects at their angle of gaze twice during each wake/light episode). Further methodological details have been reported elsewhere (Burgess et al., 2008). The protocol was approved by the Rush University Medical Center Institutional Review Board and all subjects gave written informed consent prior to participation.
The DLMOs were calculated as previously described (Burgess et al., 2008). Each melatonin profile was smoothed with a locally weighted least-squares curve. A threshold for each melatonin profile was calculated as the mean of 5 low consecutive daytime values (raw data points) plus twice the standard deviation of these points (Voultsios et al., 1997). The highest threshold from the 2 profiles was then applied to both melatonin profiles for that individual subject. The DLMO for each profile was the point in time (as determined with linear interpolation) when the smoothed melatonin curve exceeded the threshold. As there were 4 days between the baseline and final phase assessments, the tau for each subject was estimated by dividing the shift in the DLMO by 4 and adding 24 h.
REFERENCES
- Burgess HJ, Revell VL, Eastman CI. A three pulse phase response curve to three milligrams of melatonin in humans. J Physiol. 2008;586.2:639–647. doi: 10.1113/jphysiol.2007.143180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell SS, Dawson D, Zulley J. When the human circadian system is caught napping: Evidence for endogenous rhythms close to 24 hours. Sleep. 1993;16:638–640. [PubMed] [Google Scholar]
- Czeisler CA, Duffy JF, Shanahan TL, Brown EN, Mitchell JF, Rimmer DW, Ronda JM, Silva EJ, Allan JS, Emens JS, Dijk DJ, Kronauer RE. Stability, precision, and near-24-hour period of the human circadian pacemaker. Science. 1999;284:2177–2181. doi: 10.1126/science.284.5423.2177. [DOI] [PubMed] [Google Scholar]
- Duffy JF, Wright KP. Entrainment of the human circadian system by light. J Biol Rhythms. 2005;20:326–338. doi: 10.1177/0748730405277983. [DOI] [PubMed] [Google Scholar]
- Hiddinga AE, Beersma DGM, Van Den Hoofdakker RH. Endogenous and exogenous components in the circadian variation of core body temperature in humans. J Sleep Res. 1997;6:156–163. doi: 10.1046/j.1365-2869.1997.00047.x. [DOI] [PubMed] [Google Scholar]
- Kripke DF, Youngstedt SD, Elliott JA, Tuunainen A, Rex KM, Hauger RL, Marler MR. Circadian phase in adults of contrasting ages. Chronobiol Int. 2005;22:695–709. doi: 10.1080/07420520500180439. [DOI] [PubMed] [Google Scholar]
- Middleton B, Arendt J, Stone BM. Human circadian rhythms in constant dim light (8 lux) with knowledge of clock time. J Sleep Res. 1996;5:69–76. doi: 10.1046/j.1365-2869.1996.d01-67.x. [DOI] [PubMed] [Google Scholar]
- Middleton B, Arendt J, Stone BM. Complex effects of melatonin on human circadian rhythms in constant dim light. J Biol Rhythms. 1997;12:467–477. doi: 10.1177/074873049701200508. [DOI] [PubMed] [Google Scholar]
- Pittendrigh CS, Daan S. A functional analysis of circadian pacemakers in nocturnal rodents. IV. Entrainment: Pacemaker as clock. J Comp Physiol. 1976;106:291–331. [Google Scholar]
- Revell VL, Eastman CI. How to trick mother nature into letting you fly around or stay up all night. J Biol Rhythms. 2005;20:353–365. doi: 10.1177/0748730405277233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack RL, Lewy AJ, Blood ML, Keith LD, Nakagawa H. Circadian rhythm abnormalities in totally blind people: Incidence and clinical significance. J Clin Endocrinol Metab. 1992;75:127–134. doi: 10.1210/jcem.75.1.1619000. [DOI] [PubMed] [Google Scholar]
- Voultsios A, Kennaway DJ, Dawson D. Salivary melatonin as a circadian phase marker: Validation and comparison to plasma melatonin. J Biol Rhythms. 1997;12:457–466. doi: 10.1177/074873049701200507. [DOI] [PubMed] [Google Scholar]
- Wever RA. The Circadian System of Man. Springer-Verlag; New York-Heidelberg-Berlin: 1979. [Google Scholar]
- Wever RA. Characteristics of circadian rhythms in human functions. J Neural Transm. 1986;21:323–373. [PubMed] [Google Scholar]
- Wever RA. Basic principles of human circadian rhythms. In: Schmidt T, Engel B, Blumchen T, editors. Temporal Variations of the Cardiovascular System. Springer-Verlag; Berlin: 1992. pp. 15–84. [Google Scholar]
- Wright KP, Gronfier C, Duffy JF, Czeisler CA. Intrinsic period and light intensity determine the phase relationship between melatonin and sleep in humans. J Biol Rhythms. 2005;20:168–177. doi: 10.1177/0748730404274265. [DOI] [PMC free article] [PubMed] [Google Scholar]


