Abstract
Cell-penetrating peptides such as TAT or R9 labeled with small organic fluorophores can lyse endosomes upon light irradiation. The photo-endosomolytic activity of these compounds can in turn be used to deliver proteins and nucleic acids to the cytosol of live cells with spatial and temporal control. In this report, we examine the mechanisms by which such fluorescent peptides exert a photolytic activity using red blood cells as a membrane model. We show that the peptides TAT and R9 labeled with tetramethylrhodamine photolyse red blood cells by promoting the formation of singlet oxygen in the vicinity of the cells’ membranes. In addition, unlabeled TAT and R9 accelerate the photolytic activity of the membrane-bound photosensitizer Rose Bengal in trans, suggesting that the cell-penetrating peptides participate in the destabilization of photo-oxidized membranes. Peptides and singlet oxygen generators therefore act in synergy to destroy membranes upon irradiation.
INTRODUCTION
Photochemical internalization (PCI) is a strategy used to deliver macromolecules into cells. First described by Berg and co-workers, this methodology is based on two essential steps [1, 2]. First, cells are incubated in the dark with a macromolecule of interest and a PCI agent that can disrupt membranes upon light irradiation. Endocytosis causes the accumulation of both macromolecule and PCI agent within the lumen of endocytic organelles. In a second step, light is used to activate the PCI agent trapped inside endosomes. The irradiated PCI agent promotes endosomal lysis and escape of the macromolecule from endosomes. Upon endosomal escape and entry into the cytosolic space, the macromolecule can then exert its biological function. Overall, this methodology is very attractive because light provides temporal and spatial control over the delivery process [3]. In addition, endosomal release, a typical bottleneck in most delivery strategies, is efficient with this approach. Several investigators have recently shown that fluorescently labeled cell-penetrating peptides (Fl-CPPs) possess a photo-endosomolytic activity and could therefore be used as PCI agents. For instance, the CPPs TAT or R9 labeled with fluorescein, tetramethylrhodamine (TMR), alexa fluors and Cy3 have been used to lyse endosomes and deliver proteins and nucleic acids to the cytosol of live cells successfully [3–6].
The mechanism by which Fl-CPPs lyse the membrane of endosomes upon irradiation remains unclear. The fluorophores used for CPP-mediated photo-endosomolysis are commonly used in a variety of live-cell fluorescence microscopy assays and membrane damage during these assays is usually not observed. CPPs such as TAT therefore appear to confer a photolytic activity to otherwise innocuous fluorophores. A possible explanation for the photo-endosomolytic activity of Fl-CPPs might be that CPPs simply tend to accumulate inside endosomes. Above a certain threshold concentration of endocytosed Fl-CPP, reactive oxygen species (ROS) could for instance be produced upon irradiation of the fluorophores and these ROS could then cause lysis. However, the peptide TMR-K9 does not cause endosomal release upon irradiation as TMR-TAT does, even when both peptides are endocytosed by cells to similar extent [5]. These results therefore suggest that the peptide moiety of a Fl-CPP might directly participate in the endosomolytic activity observed.
In this report, we test the hypothesis that CPPs enhance the photolytic activity of fluorophores by destabilizing membranes photo-oxidized by singlet oxygen. A previous report demonstrated that Fl-CPPs are photolytic towards membranes other than that of endosomes [5, 7]. In particular, the plasma membrane of epithelial cells and that of red blood cells (RBCs) are photolysed by TMR-TAT. Because RBCs represent a simpler system than endosomes, TMR-TAT photohemolysis is investigated as a means to gain possible mechanistic insights into the photo-endosomolytic activity of Fl-CPPs.
MATERIALS AND METHODS
Reagents for solid phase peptide synthesis (SPPS) were purchased from Novabiochem. The fluorophore 5,6-carboxy-tetramethylrhodamine was purchased from Novabiochem. Eosin Y, Rose Bengal, α-tocopheryl acetate and imidazole were obtained from Sigma. Crocetin was purchased from MP Biomedicals. Whole blood was purchased from Gulf Coast Regional Blood Center (Houston, TX).
Peptide synthesis
TAT (GRKKRRQRRRG-NH2), R9 (GRRRRRRRRR-NH2) and K9 (KKKKKKKKK-NH2) were synthesized by Fmoc solid phase peptide synthesis using rink amide MBHA resin according to previously reported protocols (Novabiochem). Coupling of carboxy-tetramethylrhodamine to the N-terminus of the peptides was performed after Fmoc deprotection of the N-terminal amino group. Peptides and Fl-CPPs were purified using HPLC and their identity was confirmed by mass spectrometry (MALDI-TOF). TAT expected mass: 1451.92 Da, observed mass: 1452.41 Da; R9 expected mass: 1478.96 Da, observed mass: 1479.52 Da; K9 expected mass: 1169.88 Da, observed mass: 1170.96 Da; TMR-TAT expected mass: 1865.07 Da, observed mass: 1866.1 Da; TMR-K9 expected mass: 1583.0 Da, observed mass: 1583.30 Da; TMR-R9 expected mass: 1893.20 Da, observed mass: 1894.4 Da. The pure lyophilized peptides were dissolved in water to make 1 mM stock solutions that were then diluted to desired working concentrations in PBS for photohemolysis experiments.
Photohemolysis Assay
Photo-hemolysis methods have been published previously [5]. Briefly, a concentration of 0.1% by volume of RBCs was used for photohemolysis experiments. Peptides and RBCs were mixed in PBS in a 384-well plate. RBCs were incubated with a given peptide for 15 min during which time RBCs could settle to the bottom of the dish prior to imaging. The photo-hemolytic activity of the photosensitizing compounds was assessed by irradiation of the sample at 560 nm (RFP channel) on the microscope. The number of lysed cells was counted after each irradiation by using bright field images in which intact erythrocytes have a dark contrast while lysed ghosts do not. The data reported represents the averages and the corresponding standard deviations of five experiments in which a minimum of 500 cells were examined.
In parallel experiments, irradiation was performed using light from a 600 W halogen lamp (Utilitech) which was filtered with a 1.5-inch water filter, homogenizing glass and a green optical plastic filter NT46–624 (Edmund optics). The maximum transmission was in the range of 450 nm to 580 nm with a final photon flux output of 3.3×1017 photons×s−1×cm−2. The RBCs incubated with peptides were irradiated for a given amount of time and spun down. The absorbance of the supernatant was measured at 450 nm using a plate reader to establish a percentage photohemolysis (100% hemolysis was set using RBCs lysed with 0.1% Triton X). Each condition was done in triplets to obtain a standard deviation.
For experiments involving annexin V staining of RBCs, 0.1% RBCs were incubated with 2 μM TMR-TAT and 0.25 μg/ml of FITC-annexin V in PBS containing 1.5 mM CaCl2. Photohemolysis was performed as described before with the exception that FITC-annexin V was added to lysed RBCs after irradiation. Images were recorded before irradiation and after irradiation using the RFP, FITC and phase channels. The signal seen in the FITC channel due to RFP cross talk was subtracted from all the FITC images shown (cross talk was determined RFP and FITC images obtained with RBCs and TMR-TAT incubated alone).
Detection of singlet oxygen formation with RNO assay
The RNO spectrophotometric assay was used to detect singlet oxygen formation upon irradiation of TMR-TAT [8]. Briefly, the assay uses p-nitrosodimethylaniline (RNO) and imidazole. Singlet oxygen reacts with imidazole, to form a peroxide intermediate. This intermediate then reacts with RNO to cause bleaching of the chromophore. The rate of loss of RNO absorbance is measured to determine the rate of singlet oxygen production [8]. RNO (50 μM) and imidazole (10 μM) were mixed with the peptide solutions in PBS. Rose Bengal (RB) and eosin Y were used as standards for calculation of the quantum yield since the fluorescence spectra is similar to that of the TMR fluorophore. The singlet oxygen quantum yield of RB and eosin Y in aqueous solution is 0.76 and 0.57 respectively [8]. The absorbance of the samples used in this assay were set to 0.63 at 556 nm in all cases, as described in reported protocols [9]. The decrease in the absorbance of RNO was monitored using the plate reader (450 nm) at periodic intervals. Irradiation was performed using the halogen lamp described in the previous section.
Microscopy
Imaging was performed on an inverted epifluorescence microscope (Model IX81, Olympus, Center Valley, PA). Images were captured with a Rolera-MGI Plus back-illuminated EMCCD camera (Qimaging, Surrey, BC, Canada). Imaging was performed using bright field and fluorescence imaging with the RFP filter set (Ex = 560±20 nm/Em= 630±35 nm). The excitation light was from a 100 W mercury lamp (Leeds Precision Instruments # L202 Osram) passed through the filter cubes and a 100x objective. The bright field and fluorescence images were captured with the SlideBook 4.2 software (Olympus, Center Valley, PA).
RESULTS
Fl-CPPs lyse red blood cells upon irradiation by generating singlet oxygen
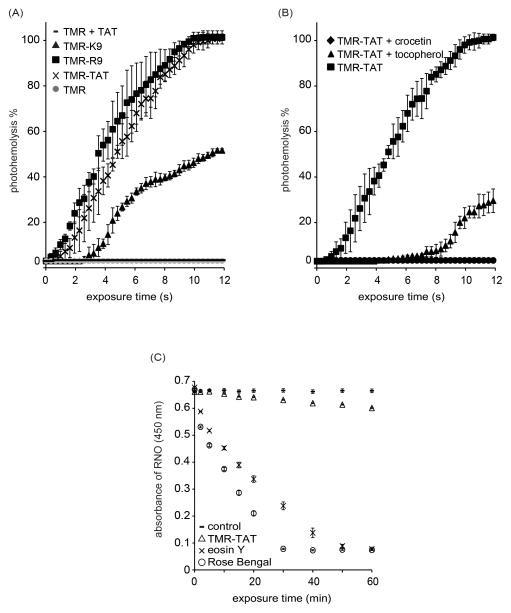
TMR-TAT and RBCs were irradiated with light using the microscope and photohemolysis was monitored as a function of irradiation time. As shown in Figure 1A, TMR-TAT photolyses RBCs after a short lag time. Irradiation of TMR or TAT alone did not reproduce this activity, indicating that conjugation of TMR to TAT is required for photohemolysis. TAT is mainly composed of arginine and lysines residues. To assess the effect of peptide composition, the control peptides TMR-R9 and TMR-K9 were tested. TMR-K9 showed a significantly reduced photohemolytic activity as compared to TMR-TAT. In contrast, TMR-R9 displayed a photohemolysis activity similar to that of TMR-TAT. Together, these results indicate that the arginine residues in TAT account for the photohemolytic activity of this Fl-CPP.
Figure 1.
Fl-CPPs lyse RBCs upon irradiation and the production of singlet oxygen is involved in this process. A) Photohemolysis curves for TMR-TAT, TMR-R9 and TMR-K9. B) Effect of tocopherol and crocetin on TMR-TAT mediated photohemolysis. C) Quenching of RNO absorbance by TMR-TAT as a function of irradiation time. Rose Bengal and eosin Y are used a singlet oxygen generators of known singlet oxygen quantum yields (0.76 and 0.57, respectively). All experiments were performed in triplicates to obtain standard deviation and the data represents the average of 3 sets of readings.
In order to examine the mechanisms by which TMR-TAT promotes membrane lysis upon irradiation, the involvement of singlet oxygen was tested. The lipophilic singlet oxygen quencher tocopherol and the singlet oxygen inhibitor crocetin both led to a significant decrease in photohemolytic activity In order to confirm that singlet oxygen is produced upon irradiation of TMR-TAT, a RNO spectroscopic assay was performed. Rose Bengal and eosin Y, compounds with reported singlet oxygen quantum yields of 0.76 and 0.57 in water were used as standards [8, 10]. As shown in Figure 1C, production of singlet oxygen upon irradiation of RB or eosin Y could be detected at low light doses. In contrast, TMR-TAT caused a decrease in RNO absorbance only after extended irradiation. Together, these results indicate that TMR-TAT produces very low levels of singlet oxygen upon irradiation but that singlet oxygen produced is definitely required for photohemolysis.
TAT causes shrinkage of photo-damaged membranes
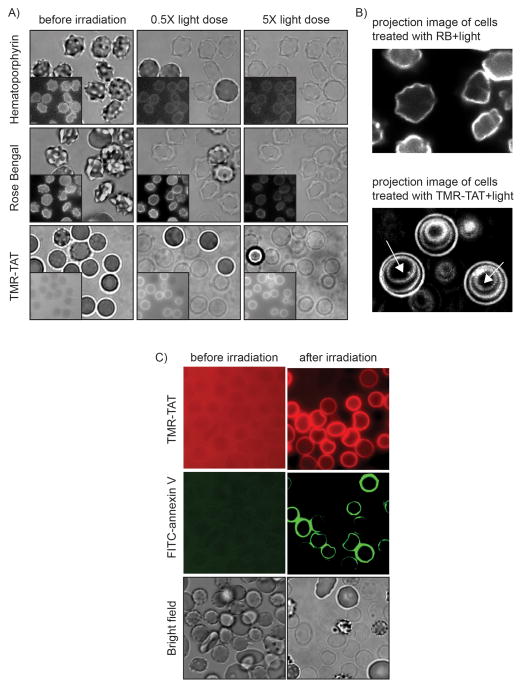
In order to investigate how the peptide moiety of a Fl-CPP might contribute to photohemolysis, TMR-TAT was compared to known photolytic photosensitizers such as RB or hematoporphyrin (HP). In these experiments, RBCs were incubated with TMR-TAT, RB, or HP and the photohemolysis of cells was monitoring by fluorescence and bright field microscopy. Based on the fluorescence distribution of TMR-TAT, the peptide showed little association with RBCs prior to irradiation. In contrast, strong fluorescence signals were present on the surface of the cells when RBCs were incubated with the fluorescent RB and HP. In addition, while the morphology of RBCs incubated with TMR-TAT was that of cells incubated in buffer alone, RBCs incubated with RB or HP formed echinocytes. This morphology is indicative of the insertion of the photosensitizers in the outer leaflet of the lipid bilayer of RBCs [11]. Upon irradiation, cells lysed rapidly and lost their dark contrast in bright field imaging because of the release of hemoglobin into the medium [12]. The lysed membrane of ghost cells remained, however, visible in both bright field and fluorescence imaging. Interestingly, the fluorescence signals of TMR-TAT bound to RBCs significantly increase after lysis (ghosts appear as fluorescent rings in Figure 2A). We have previously reported that TAT displays a stronger affinity for lysed RBCs than for intact cells and that exposure of the negatively charged phosphatidylserine lipids on the surface of lysed cells might be involved in this effect (phosphatidylserine is present in the inner leaflet of the lipid bilayer and not accessible to the peptide prior to lysis)[12]. To test whether phosphatidylserine is also exposed on the membrane of RBCs upon TMR-TAT mediated photohemolysis, FITC-annexin V was added to RBCs before and after irradiation with light. Based on fluorescence imaging, annexin V did not bind to intact RBCs. However, upon irradiation, both TMR-TAT and annexin V stained the membrane of lysed RBCs. These results therefore suggest that phosphatidylserine becomes accessible for binding upon photohemolysis and that TMR-TAT might interact with this negatively charged lipid.
Figure 2.
TMR-TAT-mediated photohemolysis is accompanied by cell shrinkage but photolysis mediated by RB or hematoporphyrin (HP) is not. A) Bright field and fluorescence imaging of RBCs treated with HP, RB, or TMR-TAT. Images were acquired at 0.5x light dose (light dose that yields 50% photohemolysis) and 5x light dose (a light dose 10 times that required for 50% photohemolysis). Insert: fluorescence images. B) Projection images of cells treated with RB or TMR-TAT and light. The fluorescence images of the cells exposed at 0.5x, 2x and 5x light dose were super-imposed in a single overlay image. The fluorescence signal corresponds to either RB or TMR-TAT binding to the surface of lysed cells. In the case of cells treated with RB, the projection image is identical to the 0.5x image, indicating that the morphorlogy of the cells is unchanged during light exposure. In contrast, the projection image of the cells treated with TMR-TAT shows concentric circles that correspond to the cell membrane shrinking with increasing light exposure (white arrows indicate the direction of membrane shrinking with increasing irradiation). C) AnnexinV staining of membrane of RBCs lysed with TMR-TAT upon irradition. FITC-annexinV was added before or after lysis of RBCs with TMR-TAT and light irradiation.
For all the photosensitizers tested, photohemolysis led to the formation of ghosts with an initial morphology similar to that observed for the intact RBCs prior to light exposure (i.e. crenated for RB and HP and round for TMR-TAT, Figure 2). The morphology of ghosts obtained with RB or HP did not change when irradiation was extended beyond what is required for lysis. In contrast, TMR-TAT caused a significant shrinking of the membrane of ghosts. Shrinkage was not observed when ghosts were not irradiated post-photolysis. This indicates that TMR-TAT mediated shrinkage requires continued photolytic degradation of the membrane.
TAT and R9 promote the photolysis mediated by the membrane-associated photosensitizer Rose Bengal
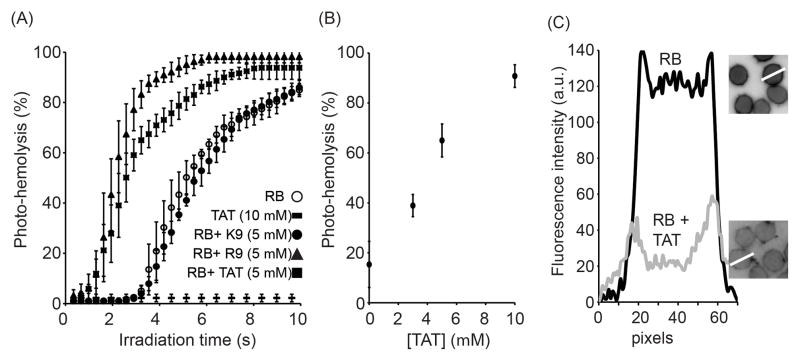
The shrinkage of ghosts by TMR-TAT but not by RB suggested that the Fl-CPP might damage photo-oxidized membranes in a way that cannot be simply accounted for by the generation of singlet oxygen. While shrinkage was only observable after photohemolysis was finished, this phenomenon nonetheless raised the possibility that TAT might destabilize the membrane of intact RBCs before or during the photolytic event. To test this idea, the effect of TAT on the membrane of RBCs photo-damaged with RB was investigated. In this assay, RBCs were incubated with RB, exposed to light and the extent of photohemolysis after each irradiation dose was determined to obtain a photohemolysis curve. In parallel experiments, the unlabeled CPPs TAT and R9 were co-incubated with RB and RBCs and the samples were then irradiated. The non-CPP control K9 was also tested under the same conditions. As shown in Figure 3, photohemolysis was accelerated by addition of TAT in a concentration-dependent manner. This effect was also reproduced with R9 but not with K9. This indicates that arginine residues mediate the enhancement in lysis observed but that positive charges are not sufficient to account for this effect. To examine whether TAT or R9 might cause an increase in lysis by simply causing an increase in binding of the photosensitizer to the membrane of RBCs, the fluorescence signal of RB associated with the cells was quantified. The amount of RB bound to the RBCs decreases by approximately 3-fold in the presence of TAT (10 μM) (similar results were obtained with R9, not shown). These results were further confirmed by measuring the concentration of RB present in solution after incubation with RBCs and with or without TAT. Samples were centrifuged to separate RB associated with RBCs (pellet) from soluble (RB). In this assay, addition of TAT caused an increase in the amount of RB present in solution, indicating again that the association of RB to RBCs decreases in the presence of TAT (results not shown). While the reasons for this decrease remain unclear, these results indicate that the propensity of TAT or R9 to promote photolysis is not caused by an increase in RB binding that would lead to an increase in the singlet oxygen produced in the membrane environment. Instead, the data obtained support the notion that TAT and R9 might act directly on photo-oxidized membranes.
Figure 3.
TAT and R9 enhance the photolytic activity of Rose Bengal (RB). A) Photohemolysis monitored as a function of irradiation time. RB (200 nM) was incubated with RBCs and photohemolysis was assessed by bright field microscopy. B) Photohemolysis mediated by RB as a function of the concentration of TAT present. Photohemolysis was measured after exposing the cells with 560 nm light for 4 s. C) Fluorescence intensity profiles of RBCs incubated with RB alone or with RB and TAT (10 μM). Fluorescence images of the cells are represented as inverted monochrome. White lines highlight the regions of interest from which the intensities were measured. Membrane intensities were calculated for 10 different RBCs and the data was consistent for each cell.
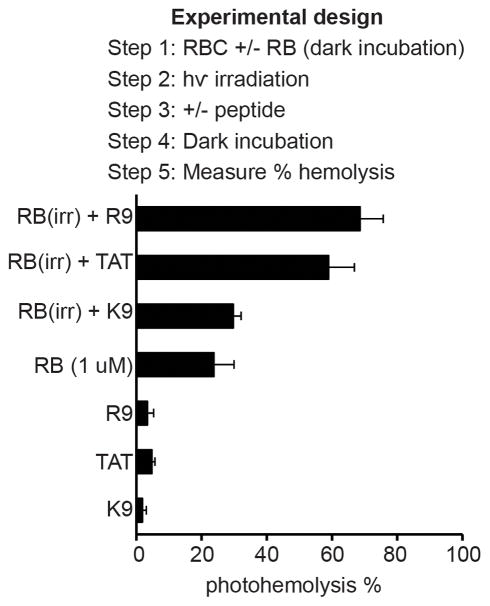
To further characterize how TAT or R9 might accelerate photolysis, RBCs were treated with RB and the peptide was added post-irradiation as opposed to during illumination. RBCs were treated with RB and irradiated with a single dose of light. After completing the irradiation, the peptides TAT, R9, or K9 were added (10 μM) to the cells. A control experiment where PBS was added instead of TAT or R9 was also performed. Hemolysis was measured before and after addition of peptides to the samples. As shown in Figure 4, addition of TAT and R9 post-irradiation caused an increase in hemolysis. In particular, while the photohemolysis obtained for RB alone was only 24±6% under the conditions tested, addition of R9 led to 69±7% (for comparison, the experiment performed with RB and R9 co-incubated leads to 93±3% lysis, data not shown). In contrast, the addition of K9 did not have a significant effect on lysis. The peptides alone had no effect in the dark or when cells were irradiated without RB present. These data therefore suggest that TAT or R9 promote the lysis of membranes that have been photo-oxidized by a photosensitizer.
Figure 4.
TAT and R9 lyse the membrane of RBCs photoxidized by Rose Bengal. Irradiation of RBCs incubated with RB followed by addition of K9, TAT and R9. Addition of TAT and R9 (10μM) post-irradiation increases hemolysis but addition of K9 does not have a significant effect. Conditions of 100% hemolysis were determined by addition of Triton X to RBCs. The control sample consists of untreated RBCs. The data represent the average of triplicate experiments and error bars represent the corresponding standard deviations
DISCUSSION
Our results indicate that Fl-CPPs such as TMR-TAT are photolytic by promoting the formation of singlet oxygen within biological membranes upon irradiation. Singlet oxygen is a reactive oxygen species that is generated when a molecule absorbing light forms an excited triplet state and transfers its energy to molecular oxygen. Singlet oxygen can in turn oxidize both lipid and membrane proteins [13]. Singlet oxygen quencher tocopherol and inhibitor crocetin abolished the photolytic activity of TMR-TAT [5]. Moreover, the production of singlet oxygen upon irradiation of TMR-TAT could be detected using an RNO spectrometric assay. Yet, under conditions of irradiation comparable to those used for photohemolysis, the production of singlet oxygen detected from TMR-TAT was very poor. On one hand, this result is consistent with the low triplet state quantum yield (0.001–0.003) reported for TMR [13, 14]. On the other hand, it is surprising that an inefficient singlet oxygen generator such as TMR might cause lysis of membranes.
Looking for clues on how TMR-TAT might lyse RBCs, the activity of the fluorescent peptide was compared to that of the singlet oxygen generator Rose Bengal (RB). A striking difference between cells photolysed by RB and those photolysed by TMR-TAT was the significant shrinking that accompanied the photolysis mediated by TMR-TAT. The shrinkage observed suggests that TMR-TAT might dissolve certain membrane components, thereby leading to a reduction in the membrane surface area. Similar phenomena have been observed with antimicrobial peptides [15]. In this case, however, our results indicate that the “detergent-like” effect of the peptide is only expressed upon photo-oxidation of the membrane. Based on these results, we hypothesized that TAT might contribute to photolysis by destabilizing photo-oxidized membrane components. To test this hypothesis, RBCs were photo-oxidized with the photosensitizer RB, a photosensitizer that produces singlet oxygen within the membrane environment upon irradiation. Unlabeled TAT and R9 were added in trans to assess whether the peptides might promote the lysis of the photo-damaged membranes. When present during irradiation, both TAT and R9 significantly enhanced the photolytic activity of RB. This effect was observed at a concentration range at which TMR-TAT or TMR-R9 can cause photolysis of RBC (e.g. 2 μM), suggesting that what is observed when RB and CPPs are co-incubated might also take place in the context of Fl-CPPs. Moreover, TAT or R9 did not increase the binding of RB to RBCs. This therefore excludes the possibility that photohemolysis was enhanced because more singlet-oxygen generating RB was bound to the membrane of RBCs in the presence of the peptides. These results instead suggested that TAT or R9 might participate directly in the lytic event. In particular, while TAT and R9 are not lytic by themselves, these peptides could aggravate the oxidative damage initiated by the photosensitizers or fluorophores. In this model, TAT and R9 would therefore have a latent membrane-disrupting activity that is only expressed on partially oxidized membranes. To test this possibility, TAT was added to RBCs treated with RB after rather than during irradiation. In these experiments, both TAT and R9 caused an increase in hemolysis while the control peptide K9 did not. These results therefore support the notion that arginine-rich CPPs such as TAT and R9 can interact with oxidized membranes and promote their lysis. Interestingly, it has been shown that TAT and R9 have membrane translocation properties [16, 17]. Whether photolysis and translocation share some mechanistic features remains, however, to be investigated.
Overall, our data establish how the fluorophore and CPP moieties of a Fl-CPP might both contribute to photolysis. Upon irradiation, the fluorophore generates singlet oxygen and this singlet oxygen damages membrane components. The peptide is then able to aggravate this damage and lysis takes place more readily. Fluorophore and peptide might therefore act in synergy and this in turn might explain how a poor singlet oxygen generator such as TMR-TAT might be able to achieve lysis relatively efficiently. The molecular basis for the lytic activity of the peptide remains to be explored. Understanding what membrane components interact with CPPs after initial photo-damage should be especially interesting. CPPs are well known to interact with lipid bilayers [18]. One can therefore speculate that CPPs might interact with oxidized lipids and that these interactions are involved in destabilizing membranes.
RBCs provide a membrane environment different than that found inside endosomes. It is therefore unclear whether photohemolysis closely resembles the photolysis activity of Fl-CPPs observed inside endocytic organelles. Both protein composition and membrane curvature are for instance very different between RBCs and endosomes [19]. Yet, RBCs and endosomes have similar lipid composition and the lipid asymmetry between outer and inner leaflets is conserved in these membrane systems [20]. It should also be noted that the difference in photo-endosomolytic activity observed between TMR-K9 and TMR-TAT can be reproduced with photohemolysis [5]. The photolytic effects of TMR-TAT on RBCs presented in this report might therefore be relevant to those observed with endocytic organelles. We therefore propose that, just as with RBCs, Fl-CPPs cause the photolysis of endosomes by the combined action of singlet oxygen and of a peptide moiety that destabilizes oxidized membranes. Understanding how the oxidized membranes are destabilized could lead to the development of efficient light-inducible drug delivery tools. Moreover, it is interesting to note that the enhancement that TAT or R9 provides towards the photolytic activity of photosensitizers such as Rose Bengal could potentially be exploited in other applications. In particular, photosensitizers are commonly used in photodynamic therapy (PDT), a treatment modality that exploits light to kill cells locally. As photolysis is one of the modes of action by which photosensitizers kill cells, it is therefore possible that CPPs might sensitize cells to the photo-killing of photodynamic therapy drugs and thereby enhance their potency.
Acknowledgments
This work was supported by Award Number R01GM087227 and R01GM087981 from the National Institute of General Medical Sciences, the Norman Ackerman Advanced Research Program, and the Robert A. Welch foundation (Grant A-1769).
References
- 1.Berg K, Selbo PK, Prasmickaite L, Tjelle TE, Sandvig K, Moan J, Gaudernack G, Fodstad O, Kjolsrud S, Anholt H, Rodal GH, Rodal SK, Hogset A. Photochemical internalization: a novel technology for delivery of macromolecules into cytosol. Cancer Res. 1999;59:1180–1183. [PubMed] [Google Scholar]
- 2.Berg K, Prasmickaite L, Selbo PK, Hellum M, Bonsted A, Hogset A. Photochemical internalization (PCI)--a novel technology for release of macromolecules from endocytic vesicles. Oftalmologia. 2003;56:67–71. [PubMed] [Google Scholar]
- 3.Endoh T, Sisido M, Ohtsuki T. Spatial regulation of specific gene expression through photoactivation of RNAi. J Control Release. 2009;137:241–245. doi: 10.1016/j.jconrel.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 4.Maiolo JR, 3rd, Ottinger EA, Ferrer M. Specific redistribution of cell-penetrating peptides from endosomes to the cytoplasm and nucleus upon laser illumination. J Am Chem Soc. 2004;126:15376–15377. doi: 10.1021/ja044867z. [DOI] [PubMed] [Google Scholar]
- 5.Srinivasan D, Muthukrishnan N, Johnson GA, Erazo-Oliveras A, Lim J, Simanek EE, Pellois JP. Conjugation to the Cell-Penetrating Peptide TAT Potentiates the Photodynamic Effect of Carboxytetramethylrhodamine. PLoS ONE. 2011;6:e17732. doi: 10.1371/journal.pone.0017732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsushita M, Noguchi H, Lu YF, Tomizawa K, Michiue H, Li ST, Hirose K, Bonner-Weir S, Matsui H. Photo-acceleration of protein release from endosome in the protein transduction system. FEBS Lett. 2004;572:221–226. doi: 10.1016/j.febslet.2004.07.033. [DOI] [PubMed] [Google Scholar]
- 7.Muthukrishnan N, Johnson GA, Lim J, Simanek EE, Pellois JP. TAT-mediated photochemical internalization results in cell killing by causing the release of calcium into the cytosol of cells. Biochim Biophys. 2012;1820:1734–43. doi: 10.1016/j.bbagen.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kraljić I, Mohsni SE. A new method for the detection of singlet oxygen in aqueous solutions. Photochem Photobiol. 1978;28:577–581. [Google Scholar]
- 9.Kochevar IE, Redmond RW. Photosensitized production of singlet oxygen. Methods Enzymol. 2000;319:20–28. doi: 10.1016/s0076-6879(00)19004-4. [DOI] [PubMed] [Google Scholar]
- 10.Redmond RW, Gamlin JN. A compilation of singlet oxygen yields from biologically relevant molecules. Photochem Photobiol. 1999;70:391–475. [PubMed] [Google Scholar]
- 11.Sheetz MP, Singer SJ. Biological membranes as bilayer couples. A molecular mechanism of drug-erythrocyte interactions. Proc Natl Acad Sci U S A. 1974;71:4457–4461. doi: 10.1073/pnas.71.11.4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee YJ, Johnson G, Pellois JP. Modeling of the Endosomolytic Activity of HA2-TAT Peptides with Red Blood Cells and Ghosts. Biochemistry. 2010;49:7854–7866. doi: 10.1021/bi1008408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Redmond RW, Kochevar IE. Spatially resolved cellular responses to singlet oxygen, Photochem. Photobiol. 2006;82:1178–1186. doi: 10.1562/2006-04-14-IR-874. [DOI] [PubMed] [Google Scholar]
- 14.Eggeling C, Widengren J, Rigler R, Seidel CA. Photobleaching of Fluorescent Dyes under Conditions Used for Single-Molecule Detection: Evidence of Two-Step Photolysis. Anal Chem. 1998;70:2651–2659. doi: 10.1021/ac980027p. [DOI] [PubMed] [Google Scholar]
- 15.Bechinger B. Structure and Function of Membrane-Lytic Peptides. Crit Rev Plant Sci. 2004;23:271–292. [Google Scholar]
- 16.Jiao CY, Delaroche D, Burlina F, Alves ID, Chassaing G, Sagan S. Translocation and endocytosis for cell-penetrating peptide internalization. J Biol Chem. 2009;284:33957–65. doi: 10.1074/jbc.M109.056309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Futaki S, Goto S, Suzuki T, Nakase I, Sugiura Y. Structural variety of membrane permeable peptides. Curr Prot Pept Sci. 2003;4:87–96. doi: 10.2174/1389203033487261. [DOI] [PubMed] [Google Scholar]
- 18.Ziegler A. Thermodynamic studies and binding mechanisms of cell-penetrating peptides with lipids and glycosaminoglycans. Adv Drug Deliv Rev. 2008;60(4–5):580–597. doi: 10.1016/j.addr.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 19.Steck TL. The organization of proteins in the human red blood cell membrane. A review. J Cell Biol. 1974;62:1–19. doi: 10.1083/jcb.62.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vogt AM, Winter G, Wahlgren M, Spillmann D. Heparan sulphate identified on human erythrocytes: a Plasmodium falciparum receptor. Biochem J. 2004;381:593–597. doi: 10.1042/BJ20040762. [DOI] [PMC free article] [PubMed] [Google Scholar]