Abstract
Rationale
Sleep disorders and substance abuse are highly comorbid. Although methamphetamine is a very commonly abused drug, to the best of our knowledge, no study has evaluated its effects on sleep during drug use and abstinence under well-controlled conditions in laboratory animals.
Objectives
The objective of this study was to examine the effects of methamphetamine self-administration on sleep-like measures in nonhuman primates.
Methods
Adult male rhesus monkeys (Macaca mulatta; n=4) self-administered methamphetamine (0.01 and 0.03 mg/kg/injection, i.v.) under a fixed-ratio 20 schedule of reinforcement (60-min sessions once a day, 5 days per week) for 5 weeks. Sleep-like measures were evaluated with Actiwatch monitors before, during, and after each period of drug self-administration.
Results
Both doses of methamphetamine reliably maintained self-administration. Methamphetamine (0.03 mg/kg) increased derived measures of latency to sleep onset and sleep fragmentation, and decreased sleep efficiency compared to abstinence, and higher methamphetamine intake predicted worse sleep quality. However, sleep normalized immediately after the discontinuation of methamphetamine self-administration.
Conclusions
Methamphetamine markedly disrupted sleep-like measures; however, methamphetamine self-administration did not disrupt sleep quality during subsequent periods of drug abstinence.
Keywords: Methamphetamine, sleep, self-administration, drug abuse, Actiwatch
Introduction
Methamphetamine use is a serious public health concern (Marshall et al., 2012) as it is one of the most widely abused illicit drugs globally (Cruickshank and Dyer, 2009). Its use is associated with numerous adverse physical, behavioral, and mental health outcomes. As reviewed by Cruickshank and Dyer (2009), disturbed sleep is one of the most prominent symptoms of amphetamines use (Gossop et al., 1982; McGregor et al., 2005; Perez et al., 2008). Relatively little attention has been given to the consequences of methamphetamine abuse on sleep, and mixed results have been reported. For example, Srisurapanont et al. (1999) did not find insomnia to be a marked symptom, while another study reported sleep reductions following a period of hypersomnolence (Gossop et al., 1982).
Studies in humans have revealed substantial comorbidity between sleep disorders and substance abuse. This complex association can be better examined in animal models that allow for the isolation of each process under highly controlled laboratory settings, which may not be entirely feasible or ethical in humans. In this sense, non-human primates provide a translational opportunity to investigate the neurophysiological and behavioral consequences of drug abuse because they are a laboratory animal model with similar responses to humans.
A number of studies have examined methamphetamine self-administration in nonhuman primates; for example, Freeman et al. (2010) reported that methamphetamine functioned as a positive reinforcer in a dose-dependent manner in rhesus monkeys. Methamphetamine self-administration in rhesus monkeys has been examined in comparison to other drugs under a progressive-ratio schedule, and in other contexts. Collectively, these studies demonstrate that methamphetamine has reinforcing effects in monkeys that are sustained over experimental sessions. However, no study has documented nighttime behavioral activity during periods of active methamphetamine self-administration and abstinence in rhesus monkeys. Therefore, the present study evaluated the effects of methamphetamine self-administration and abstinence on sleep-like measures derived from Actiwatch monitors in rhesus monkeys under well-controlled experimental conditions. Disrupted sleep may be considered a risk factor for relapse to substance use (Hasler et al., 2012). Accordingly, studies focusing on methamphetamine’s sleep-related effects will provide a broader understanding of the neurobiology of sleep during stimulant use, help better identify the detrimental effects of methamphetamine on human health, and have important implications for interventions.
Methods
Subjects
Four male adult rhesus monkeys (Macaca mulatta), weighing 15–17kg, served as subjects. Each subject was individually housed in stainless steel home cages and fed Purina monkey chow (Ralston Purina, St. Louis, MO), supplemented with fruit and vegetables. Water was continuously available in the colony. The colony was maintained at an ambient temperature of 22±2°C at 45–50% humidity, and the lights were set to a 12-h light/dark cycle (lights on at hour 07:00; lights off at hour 19:00). Environmental enrichment was provided on a regular, rotating basis. All procedures and studies strictly followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals (Publication No. 85-23, revised 1985), and were approved by the Institutional Animal Care and Use Committee of Emory University. All animals were fitted with collars (Primate Products) prior to the initiation of the studies. All of the animals had a previous history of cocaine self-administration, but had no exposure to any experimental drugs for a minimum of 6 months prior to beginning these studies
Drugs
Methamphetamine HCl was provided by the National Institute on Drug Abuse (Research Technology Branch, Research Triangle Park, N.C., USA), and was dissolved in sterile physiological saline. The drug dose was determined as the salt.
Self-administration
All animals were surgically prepared with a chronic indwelling venous catheter under sterile conditions. The apparatus and self-administration procedure were described in detail by Howell and Wilcox (2001). Animals were previously trained to respond under a fixed ratio (FR) schedule of drug delivery. Subjects had the opportunity to self-administer methamphetamine during 60-min sessions once a day, 5 days per week in the morning (starting between 7–9 am). The animals were placed in a primate chair (Primate Products) and placed in a sound-attenuating experimental chamber for the duration of the session. The rest of the day, the animals were maintained in their home-cages. During the test session, the behavioral chamber was illuminated with a red light which served as a discriminative stimulus. Completion of the FR 20 response requirement resulted in a change in the stimulus light from red to white for 15 s and a methamphetamine infusion (0.01 or 0.03 mg/kg in 0.5 ml infused over 3 s). This infusion was followed by a 60-s timeout; during which the stimulus lights were turned off and responding had no programmed consequences. At the end of the timeout, the red light was presented again to signal the availability of drug and the opportunity to complete another FR and earn another infusion. Three weeks of a no-drug condition, in which the subjects remained undisturbed in their home-cages, followed the methamphetamine self-administration.
Protocol design
Before the drug intake, the Actiwatch was attached to the collar of each monkey and spontaneous baseline sleep-like behavior patterns were measured for 3 weeks (pre-drug recording). Each subject underwent the self-administration protocol twice, such that each subject received 2 different doses (0.01 and 0.03 mg/kg/inj, i.v.) of methamphetamine. The first day of SA was considered the onset of the first week of recording under drug-intake (dose of 0.01 mg/kg). SA and recording continued for a total of 5 weeks. The drug at this dose was discontinued and sleep-like measures continued for an additional 3 weeks (post-drug). The sequence was then repeated with a higher unit dose (0.03 mg/kg/inj) of methamphetamine. All animals received the lower dose before proceeding to the higher dose. Doses were separated by a minimum of a 6 week interval during which no self-administration sessions occurred.
Sleep-wake pattern
Actigraphy is one of the most commonly used methods for assessing movements during sleep-wake cycles (Terrill et al., 2010). The Actiwatch technology has been previously shown to be a reliable, non-invasive method for activity monitoring (Mann et al., 2005; Andersen et al., 2010a, 2012). The Actiwatch sensor (Mini Mitter, Bend, OR, USA) was attached to the subject’s collar while the subject was under ketamine (3.0–10mg/kg, i.m.) anesthesia. The following sleep-like behavior parameters were assessed: sleep efficiencies (i.e., the percentage of the dark phase spent sleeping), sleep latencies (i.e., the time between the lights-off time and the first sleep bout) and the fragmentation index (immobile bouts during the dark phase that lasted less than 1 minute during the sleep recording period). The fragmentation index is a measure of how much a rest period is disjointed by physical movements and it is an indicator of restlessness that takes into account the amount of wake episodes during the sleep period. Sleep bouts are the number of sleep episodes events that take place during the entire period of recording. All parameters were calculated using the Actiware software program.
Statistical analysis
The self-administration data, expressed as drug intake, were analyzed using two-way repeated-measures ANOVA with the main factors of unit dose (0.01 vs. 0.03 mg/kg/inf) and time (weeks of self-administration). Post-hoc analyses were conducted with Bonferroni’s post hoc tests to indicate significance. The sleep parameters were analyzed for the cumulative dark phase. The data were analyzed using two-way repeated-measures ANOVA with Bonferroni’s post hoc tests to indicate significance. The data were combined in blocks across 3 (baseline or post-SA) or 5 (active self-administration) weeks of the experiment. Significance was accepted at an alpha of 0.05. Correlational analyses were conducted using linear-regression analysis.
Results
Self-administration
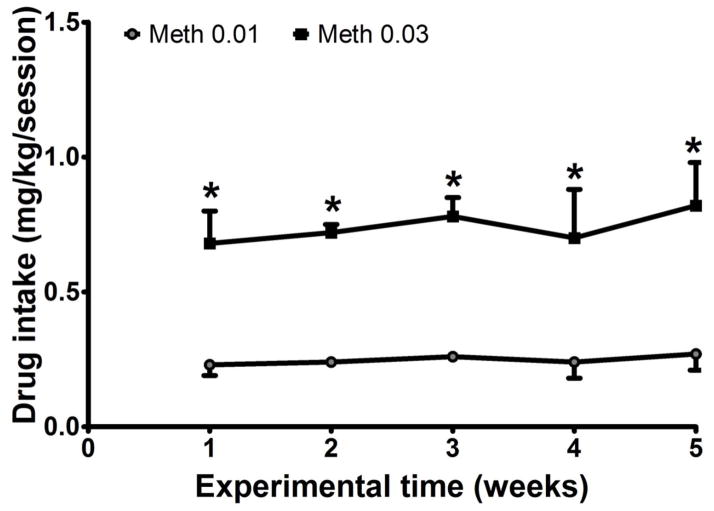
The initial dose of methamphetamine (0.01 mg/kg/infusion) reliably maintained responding in all 4 subjects, with average drug intake ranging from 0.09 to 0.33 mg/kg/session. At 0.03 mg/kg/infusion, methamphetamine intake increased to a range of 0.22 to 0.996 mg/kg/session, with a group (n=4) mean of 0.74±0.05 mg/kg/session (Figure 1). Two-way ANOVA revealed a significant main effect for dose (F(1,39)=79.22; p<0.01). Post-hoc tests revealed that there was significantly higher intake of methamphetamine at 0.03 mg/kg compared to 0.01 mg/kg (p<0.01), with no significant differences across the weeks.
Figure 1.
Methamphetamine intake (mg/kg/session) averaged across the 5-week self-administration period at 2 unit doses (0.01 and 0.03 mg/kg/inj) in a group of 4 rhesus monkeys. Data are expressed as Mean ± SEM. *Significantly different compared to 0.01 mg/kg/inj.
Sleep-wake pattern
Sleep efficiency
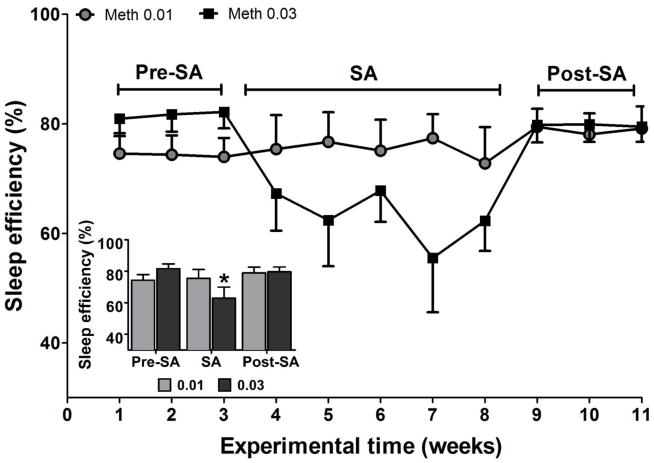
The data were combined across the weeks of each experimental block (the baseline, the period of active SA, and the post-SA period). Two-way ANOVA revealed a significant interaction (F(2,23)=3.31; p<0.05) for sleep efficiency. Post-hoc tests revealed that methamphetamine at 0.03 mg/kg caused a significant decrease in sleep efficiency during SA compared with post-SA weeks (p<0.01 - Figure 2). The 0.01 mg/kg unit dose of methamphetamine did not exhibit any statistical differences across the time-points (pre SA, SA, or post SA). At neither unit dose were the baseline and post-SA values statistically significantly different.
Figure 2.
Sleep efficiency (the percentage of time spent sleeping during the dark period) at baseline (weeks 1–3), during self-administration (weeks 4–8), and during post self-administration periods (weeks 9–11). All of the sleep data were combined across the weeks of each experimental block (baseline, active self-administration, and post-self-administration). The data are expressed as mean ± SEM. *Significantly different from post self-administration (post-SA).
Sleep latency
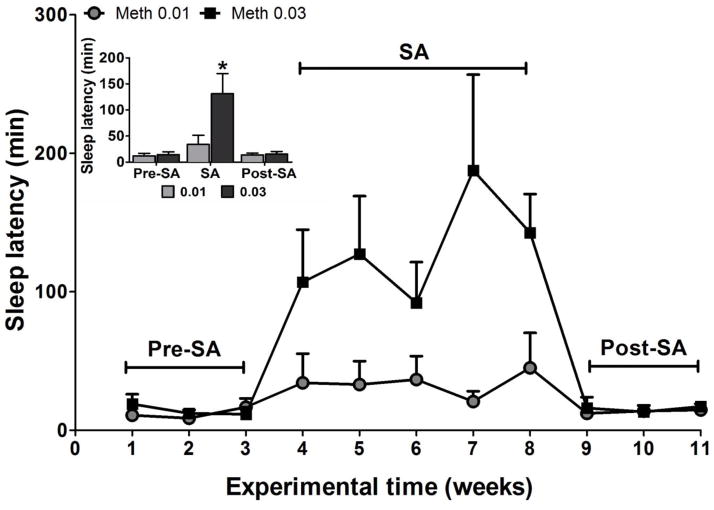
Two-way ANOVA revealed a significant main effect for time (F(2,23)=9.03; p<0.01), dose (F(1,23)=16.39; p<0.05) and a significant interaction (F(2,23)=13.11; p<0.01) for sleep latency. Post-hoc tests indicated that the latency to sleep onset was significantly higher during the period of self-administering the 0.03 mg/kg unit dose compared with before and after this period (ps<0.01). The increase in the time to fall asleep was the greatest during the weeks of SA (0.03 mg/kg) when the average time to sleep for the methamphetamine group increased by a little more than 9-fold. During SA of methamphetamine (0.03mg/kg dose), the group showed more variability in their sleep latency (Figure 3). Moreover, the methamphetamine groups had much higher deviation in sleep latency. No significant differences in sleep latency were found between baseline and post-SA period.
Figure 3.
Sleep latency measured in minutes at baseline (weeks 1–3), self-administration (weeks 4–8) and during post self-administration (weeks 9–11). The data are expressed as mean ± SEM. *Significantly different from baseline (pre-self-administration) and post self-administration.
Sleep fragmentation
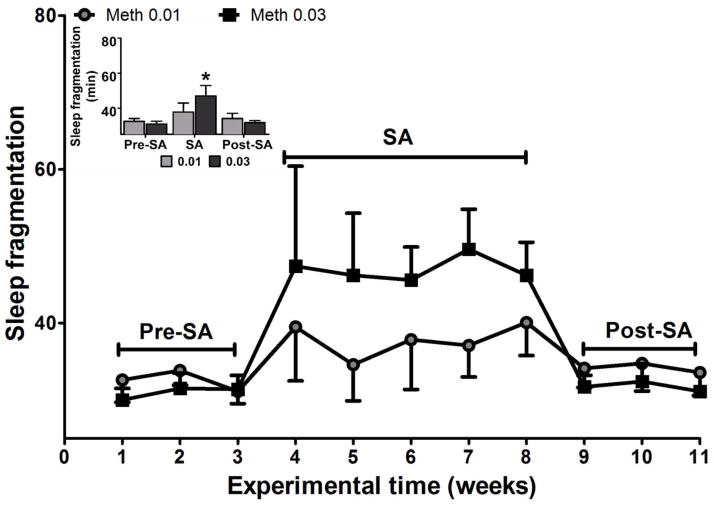
Two-way ANOVA revealed a significant main effect for time (F(2,23)=6.23; p<0.05) and interaction (F(2,23)=7.91; p<0.02) for sleep fragmentation. Post-hoc tests revealed that the sleep fragmentation index was significantly increased during the SA period for the highest dose compared to baseline and post-SA periods (ps<0.01; Figure 4). No significant differences were found between baseline and post-SA time-points.
Figure 4.
Sleep fragmentation (unitless index) at baseline (weeks 1–3), self-administration (weeks 4–8) and during post self-administration (weeks 9–11). The data are expressed as mean ± SEM. Significantly different from baseline (pre-self-administration) and post self-administration.
Correlation
To further explore the relationships between self-administration of methamphetamine and sleep disruption, we performed a statistical correlation between the intake of methamphetamine (total amount average intake/session) and the sleep parameters mentioned above. Significant correlations were found between all of the sleep measures (efficiency: R2=0.20, latency: R2=0.27, and fragmentation: R2=0.19) and intake of methamphetamine (ps<0.01), as shown in Figure 5.
Figure 5.
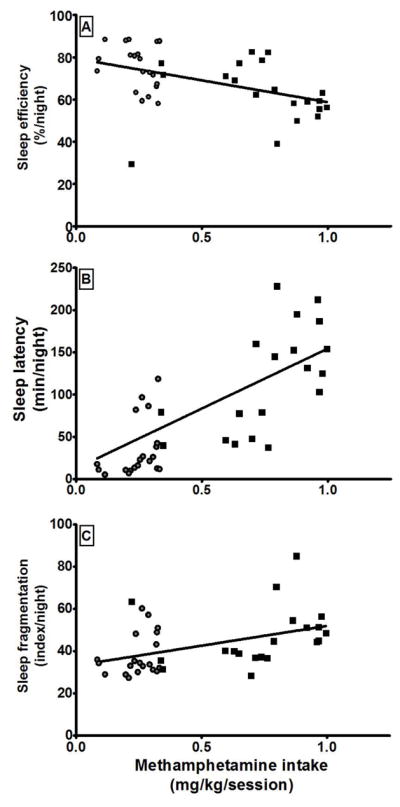
Correlations between the intake of methamphetamine and sleep efficiency (A), sleep latency (B), and sleep fragmentation (C). Each data point represents the mean value over one of the 5 weeks of self-administration testing in each animal at a given unit dose. Circles represent mean values over 1 week of access to methamphetamine at a unit dose of 0.01 mg/kg/inj. Squares represent mean values over 1 week of access to methamphetamine at a unit dose of 0.03 mg/kg/inj.
Discussion
Since methamphetamine is often used repeatedly followed by periods of abstinence, the current study examined the effects of chronic self-administration of methamphetamine upon sleep-like behavior derived from Actiwatch monitors. We also evaluated whether drug-self administration would have an enduring effect on sleep-like behavior during the subsequent periods of drug abstinence. The results showed that sleep-like pattern was disrupted during self-administration of the higher dose, reflected mainly by longer latency to sleep onset, reduced sleep time, and more fragmented sleep. However, the data indicated that sleep-like behavior returned to baseline rapidly following the cessation of self-administration; sleep measures during the 3-week period of no drug access did not reveal any residual effects of the drug self-administration.
In well-controlled laboratory studies, methamphetamine has been self-administered by rats (Roth and Carroll, 2004; Yokel and Pickens, 1973), cats (Balster et al., 1976), nonhuman primates (Balster and Schuster, 1973; Woolverton et al., 1984), and humans (Hart et al., 2001). Subsequent studies in rhesus monkeys showed that methamphetamine is a more potent reinforcer that pseudoephedrine (Freeman et al., 2010) and more effective than MDMA (Wang and Woolverton, 2007). In the current study, rhesus monkeys reliably self-administered methamphetamine at both unit doses during the 5-week blocks of drug availability. To the best of our knowledge, this is the first study to evaluate the consequences of methamphetamine self-administration on nighttime behavioral activity in nonhuman primates. In particular, it should be highlighted that the subjects had a history of stimulant self-administration and were drug-abstinent for at least 6 months prior to the initiation of the study. This unique experimental history may have important relevance for polydrug abusing humans in abstinence, an important clinical population.
As hypothesized, methamphetamine produced marked impairments in sleep-like behavior. Because the Actiwatches used in the current study can only measure activity, it is assumed that during periods of low activity, the animals exhibited behavior that could be inferred as “sleep”. However, only an objective measure (e.g. polysomnography) could indeed provide this definitive information. These impairments began during the first week of self-administration and continued throughout the 5-week period, without the development of any apparent sensitization or tolerance to the reinforcing or sleep-disrupting effects of methamphetamine. The lower dose of methamphetamine did not induce significant changes in sleep-like behavior. However, at the higher maintenance dose of methamphetamine, there were significant increases in sleep latencies and sleep fragmentation, and significant decreases in sleep efficiencies compared to pre-drug baseline. Of note, higher amounts of methamphetamine intake correlated with poorer sleep quality reflected by higher latencies to sleep onset and lower sleep efficiency and continuity. In a recent study, experimenter-administered amphetamine significantly increased sleep latencies and decreased sleep duration, through an apparent dopamine-mediated mechanism (Murnane et al., submitted). Although it is well documented that methamphetamine has dopaminergic effects (Karila et al., 2010 for review), the neurochemical mechanisms underlying the effects of methamphetamine on sleep remain to be determined.
The interrelationship between sleep and acute methamphetamine administration has been investigated in humans. Among these studies, Comer et al. (2001) reported that 10 mg methamphetamine given orally significantly decreased sleep efficiency compared to placebo-baseline recordings in healthy humans. More recently, Perez et al. (2008), under a double-blind, in-patient, within-participant, study, evaluated the residual effects of different doses of intranasal methamphetamine administration on several dependent variables, including sleep. The findings showed that drug effects on objective sleep (i.e., total number of hours slept and number of awakenings) were statistically significant only at the highest dose of methamphetamine (50 mg), whereas subjective measures (e.g., ratings of “Slept Well”) were markedly decreased by both the 25 mg and 50 mg doses. These data are in concordance with the current results, where the higher dose promoted marked activity-sustaining effects and a significant impact on sleep, whereas the lower lose was ineffective.
There is a growing awareness that long-term disruption of sleep can encompass a range of comorbidities. Major outcomes include cardiovascular mortality (Drager et al., 2007), delayed task learning (Zanini et al., 2012), propensity to diabetes (Van Cauter et al., 2008), and erectile dysfunction (Andersen et al., 2010b; Szymanski et al., 2011). For instance, methamphetamine abuse is associated with several negative health consequences, such as cardiovascular disease (Naidoo and Smith, 2011). Chronic disruption of sleep due to long-term methamphetamine abuse could exacerbate these negative effects. Indeed, sleep deprivation can potentiate convulsions induced by cocaine in rats (Andersen et al., 2010c), illustrating the potential risks of sleep disruption on the adverse effects of illicit drug use. Moreover, there is also the potential concern that exposure to high doses of methamphetamine or different patterns of methamphetamine intake may lead to even more pronounced effects on sleep. This could cause ongoing methamphetamine abusers to take even more drug or abstinent methamphetamine abusers to be more likely to relapse, further disrupting sleep and creating a vicious cycle.
Rhesus monkeys have been used with marked validity and reliability to investigate drug abuse disorders. These animals have been used to study many aspects of drug abuse, including binge intake, chronic self-administration, and relapse, among others. While the present findings clearly document that methamphetamine self-administration can lead to non-consolidated sleep (represented by a decrease in sleep efficiency and more awakenings, i.e., a process that reduces sleep quality after its onset), the disruption of sleep did not extend into the no-drug period under the conditions employed. Thus, future studies should examine the long-term effects of methamphetamine self-administration on sleep using different regimens and durations of exposure. As the failure to manage methamphetamine withdrawal symptoms during treatment may contribute to the high rates of relapse in the first days or weeks post-cessation (Brecht et al., 2000; McGregor et al., 2005), the success of these future studies would allow for a better understanding of the role of sleep disruptions in relapse, and for the development of treatment options.
In summary, we provide evidence that methamphetamine has a marked impact on sleep-related behavior, especially sleep consolidation. Rhesus monkeys exhibited poorer sleep efficiency, spent more time awake, and had higher fragmented sleep indexes. These data indicate that during the normal sleep period, methamphetamine has a profound impact on sleep quality in nonhuman primates. Additional preclinical and clinical studies are needed to evaluate the impact of disrupted sleep on measures of drug relapse. These results will have important implications given that methamphetamine is a very accessible drug and its use has increased in several countries including the United States. Its impact on sleep should be considered as a risk factor in relapse prevention strategies.
Acknowledgments
The authors express their gratitude to Lisa Neidert, Juliet Brown, and Mi Zhou for their excellent assistance with the experiments; and Eileen Kessler Sawyer who gave helpful comments on the manuscript. This research was supported by USPHS Grants DA10344 (LLH), DA31246 (LLH), P51OD11132 (Yerkes National Primate Research Center), and by AFIP and CNPq (MLA).
Footnotes
Conflict of interest: The authors declared no conflict of interest.
References
- Andersen ML, Kessler E, Murnane KS, Mcclung JC, Tufik S, Howell LL. Dopamine transporter-related effects of modafinil in Rhesus monkeys. Psychopharmacol. 2010a;210:439–448. doi: 10.1007/s00213-010-1839-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen ML, Santos-Silva R, Bittencourt LR, Tufik S. Prevalence of erectile dysfunction complaints associated with sleep disturbances in Sao Paulo, Brazil: a population-based survey. Sleep Med. 2010b;11:1019–1024. doi: 10.1016/j.sleep.2009.08.016. [DOI] [PubMed] [Google Scholar]
- Andersen ML, Martins RCS, Matos GP, Tufik S. Effect of sleep deprivation on cocaine-induced convulsions in rats. Sleep Science. 2010c;3:69–73. [Google Scholar]
- Andersen ML, Sawyer EK, Howell LL. Influence of chronic RTI-336 treatment on behavior and hormonal levels in rhesus monkeys. Exp Clinical Psychopharm. 2012;20:77–83. doi: 10.1037/a0026034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balster RL, Kilbey MM, Ellinwood EH., Jr Methamphetamine self-administration in the cat. Psychopharmacologia. 1976;46:229–33. doi: 10.1007/BF00421107. [DOI] [PubMed] [Google Scholar]
- Balster RL, Schuster CR. A comparison of d-amphetamine, l-amphetamine, and methamphetamine self-administration in rhesus monkeys. Pharm Biochem Behav. 1973;1:67–71. doi: 10.1016/0091-3057(73)90057-9. [DOI] [PubMed] [Google Scholar]
- Brecht ML, von Mayrhauser C, Anglin MD. Predictors of relapse after treatment for methamphetamine use. J Psychoactive Drugs. 2000;32:211–220. doi: 10.1080/02791072.2000.10400231. [DOI] [PubMed] [Google Scholar]
- Comer SD, Hart CL, Ward AS, Haney M, Foltin RW, Fischman MW. Effects of repeated oral methamphetamine administration in humans. Psychopharmacology. 2001;155:397–404. doi: 10.1007/s002130100727. [DOI] [PubMed] [Google Scholar]
- Cruickshank CC, Dyer KR. A review of the clinical pharmacology of methamphetamine. Addiction. 2009;104:1085–99. doi: 10.1111/j.1360-0443.2009.02564.x. [DOI] [PubMed] [Google Scholar]
- Drager LF, Krieger EM, Lorenzi-Filho G. Sympathetic activity, heart failure, obesity, and metabolic syndrome: is there any role for obstructive sleep apnea? Hypertension. 2007;49:e38. doi: 10.1161/HYPERTENSIONAHA.107.090274. [DOI] [PubMed] [Google Scholar]
- Freeman KB, Wang Z, Woolverton WL. Self-administration of (+)-methamphetamine and (+)-pseudoephedrine, alone and combined, by rhesus monkeys. Pharmacol Biochem Behav. 2010;95:198–202. doi: 10.1016/j.pbb.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossop MR, Bradley BP, Brewis RK. Amphetamine withdrawal and sleep disturbance. Drug Alcohol Depend. 1982;10:177–83. doi: 10.1016/0376-8716(82)90010-2. [DOI] [PubMed] [Google Scholar]
- Hart CL, Ward AS, Haney M, Foltin RW, Fischman MW. Methamphetamine self-administration by humans. Psychopharmacology (Berl) 2001;157:75–81. doi: 10.1007/s002130100738. [DOI] [PubMed] [Google Scholar]
- Hasler BP, Smith LJ, Cousins JC, Bootzin RR. Circadian rhythms, sleep, and substance abuse. Sleep Med Rev. 2012;16:67–81. doi: 10.1016/j.smrv.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell LL, Wilcox KM. Intravenous drug self-administration in nonhuman primates. In: Buccafusco JJ, editor. Methods of Behavior Analysis in Neuroscience. CRC Press; Boca Raton, FL: 2001. pp. 91–110. [Google Scholar]
- Karila L, Weinstein A, Aubin HJ, Benyamina A, Reynaud M, Batki SL. Pharmacological approaches to methamphetamine dependence: a focused review. Br J Clin Pharmacol. 2010;69:578–92. doi: 10.1111/j.1365-2125.2010.03639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann TM, Williams KE, Pearce PC, Scott EA. A novel method for activity monitoring in small non-human primates. Lab Anim. 2005;39:69–77. doi: 10.1258/0023677053739783. [DOI] [PubMed] [Google Scholar]
- Marshall BD, Grafstein E, Buxton JA, Qi J, Wood E, Shoveller JA, Kerr T. Frequent methamphetamine injection predicts emergency department utilization among street-involved youth. Public Heath. 2012;126:47–53. doi: 10.1016/j.puhe.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor C, Srisurapanont M, Jittiwutikarn J, Laobhripatr S, et al. The nature, time course and severity of methamphetamine withdrawal. Addiction. 2005;100:1320–1329. doi: 10.1111/j.1360-0443.2005.01160.x. [DOI] [PubMed] [Google Scholar]
- Murnane KS, Andersen ML, Rice KC, Howell LL. Selective serotonin 2A receptor antagonism attenuates the wake-promoting and dopamine releasing effects of amphetamine. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidoo S, Smith D. Methamphetamine abuse: a review of the literature and case report in a young male. DJ. 2011;66:124–7. [PubMed] [Google Scholar]
- Perez AY, Kirkpatrick MG, Gunderson EW, Marrone G, Silver R, Foltin RW, Hart CL. Residual effects of intranasal methamphetamine on sleep, mood, and performance. Drug Alcohol Depend. 2008;94:258–262. doi: 10.1016/j.drugalcdep.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth ME, Carroll ME. Sex differences in the acquisition of IV methamphetamine self-administration and subsequent maintenance under a progressive ratio schedule in rats. 2004;172:443–9. doi: 10.1007/s00213-003-1670-0. [DOI] [PubMed] [Google Scholar]
- Srisurapanont M, Jarusuraisin N, Jittiwutikan J. Amphetamine withdrawal: I. reliability, validity and factor structure of a measure. Aust NZ J Psychiatry. 1999;33:89–93. doi: 10.1046/j.1440-1614.1999.00517.x. [DOI] [PubMed] [Google Scholar]
- Szymanski FM, Filipiak KJ, Hrynkiewicz-Szymanska A, Grabowski M, Dabrowska-Kugacka A, Opolski G. The high risk of obstructive sleep apnea-an independent risk factor of erectile dysfunction in ST-segment elevation myocardial infarction patients. J Sex Med. 2011;8:1434–8. doi: 10.1111/j.1743-6109.2010.02075.x. [DOI] [PubMed] [Google Scholar]
- Terrill PI, Mason DG, Wilson SJ. Development of a continuous multisite accelerometry system for studying movements during sleep. Conf Proc IEEE Eng Med Biol Soc. 2010:6150–3. doi: 10.1109/IEMBS.2010.5627780. [DOI] [PubMed] [Google Scholar]
- Van Cauter E, Spiegel K, Tasali E, Leproult R. Metabolic consequences of sleep and sleep loss. Sleep Med. 2008;9S:S23–8. doi: 10.1016/S1389-9457(08)70013-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Woolverton WL. Estimating the relative reinforcing strength of (+/−)-3,4-methylenedioxymethamphetamine (MDMA) and its isomers in rhesus monkeys: comparison to (+)-methamphetamine. Psychopharmacology (Berl) 2007;189:483–8. doi: 10.1007/s00213-006-0599-5. [DOI] [PubMed] [Google Scholar]
- Woolverton WL, Cervo L, Johanson CE. Effects of repeated methamphetamine administration on methamphetamine self-administration in rhesus monkeys. Pharmacol Biochem Behav. 1984;21:737–41. doi: 10.1016/s0091-3057(84)80012-x. [DOI] [PubMed] [Google Scholar]
- Yokel RA, Pickens R. Self-administration of optical isomers of amphetamine and methylamphetamine by rats. J Pharmacol Exp Ther. 1973;187:27–33. [PubMed] [Google Scholar]
- Zanini GAV, Tufik S, Andersen ML, Martins RCS, Bueno OFA, Rodrigues CC, Pompeia S. Free recall of word lists under total sleep deprivation and after recovery sleep. Sleep. 2012;35:223–30. doi: 10.5665/sleep.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]