Abstract
Studies in rodents have shown that psychostimulant drugs such as cocaine and amphetamine cause endorphin release in the brain reward system. There is also evidence for the involvement of the opioid system in human psychostimulant dependence. The acute effects of an i.v. psychostimulant drug on the brain opioid system, however, have not yet been investigated in humans. We hypothesized that an i.v. dose of amphetamine as compared to placebo would cause an opioid release in the human brain reward system, measurable as a reduction of the binding potential of the μ-opioid receptor radioligand [11C]carfentanil. Ten healthy young men were examined using positron emission tomography (PET) and [11C]carfentanil in three sessions: at baseline; after placebo; after an i.v. amphetamine dose of 0.3 mg/kg bodyweight. The order of amphetamine and placebo was double-blinded and randomized. PET examinations were performed with a Siemens high resolution research tomograph. Data were analysed with the simplified reference tissue model, applying manually drawn regions of interest for every subject. Using repeated measures analysis of variance, we found no significant differences in [11C]carfentanil binding potential between amphetamine and placebo conditions in any of the investigated brain regions. In contrast to data from rodent studies and a recent study of oral amphetamine administration in humans, an i.v. dose of amphetamine does not cause any acute opioid release in healthy human subjects. The postulated role of the opioid system in mediating the effects of amphetamine needs to be further investigated in animal models of the disease as well as in patient populations.
Key words: Amphetamine, brain imaging, carfentanil, positron emission tomography
Introduction
While psychostimulants have acute effects on brain dopamine systems, there is also evidence from several lines of research for the involvement of the opioid system in psychostimulant dependence. For instance, positron emission tomography (PET) studies have revealed functionally important changes in the brain opioid systems of cocaine users as compared to controls (Gorelick et al. 2005). There is also pharmacological evidence from animal studies showing that the opioid antagonist naltrexone attenuates amphetamine-induced locomotor sensitization and reinstatement of drug-seeking in rats (Häggkvist et al. 2009, 2011). In human subjects, naltrexone has been shown to significantly attenuate the subjective effects of amphetamine in amphetamine-dependent subjects and healthy controls, as well as reducing craving and risk of relapse to amphetamine use (Jayaram-Lindström et al. 2004, 2008a, b ). This is in line with studies linking the opioid system to the hedonic component of reward (Berridge et al. 2009).
A possible explanation of naltrexone's effect could be that amphetamine causes an activation of the opioid system and that this activation is inhibited by opioid antagonists, which thereby attenuate the rewarding effects of amphetamine. Indeed, several studies have shown evidence of such stimulant-induced opioid release in the ventral striatum of rodents (Olive et al. 2001; Roth-Deri et al. 2003; Soderman & Unterwald, 2009). Recently, a dose of oral amphetamine was shown to induce decreases in [11C]carfentanil binding in healthy control subjects. However, in this study there was no within-subject control for expectation effects and the measurements were performed 3 h after dosing (Colasanti et al. 2012). The acute effects of i.v. administration of amphetamine on the opioid system have not yet been investigated in humans.
In this placebo controlled PET study of healthy, drug-naive subjects, we aimed to examine the hypothesis that i.v. amphetamine induces an acute opioid release in the brain, specifically in the ventral striatum, measurable as a reduction in binding of the μ-opioid receptor radioligand [11C]carfentanil.
Materials and method
The study was approved by the Swedish Medical Products Agency, the regional Ethics Review Board and the Radiation Safety Committee and performed in accordance with ICH guidelines for Good Clinical Practice.
Participants
Ten healthy young men, aged 26.7±2.5 yr (mean±s.d.), were recruited by advertising within the Karolinska Institutet. Screening procedures, including the Alcohol Use Disorders Identification Test, Drug Use Disorders Identification Test and Mini International Neuropsychiatric Interview, were performed by a study physician in order to exclude subjects with any somatic or psychiatric disease or a history of any substance use disorders either themselves or in a first-degree relative. At screening and on the test days, subjects were tested for alcohol in exhaled air and with urine toxicology to exclude use of any illicit drugs.
Magnetic resonance scanning
A T1 weighted magnetic resonance (MR) scan (1.5 T) was performed for every subject prior to PET procedures to exclude intracranial pathology and obtain anatomical references for definition of regions of interest (ROIs).
Design and test procedures
Applying a double-blind, cross-over randomized design, three PET examinations were performed for each subject. The test days were approximately 1 wk apart, although three examinations were delayed for technical reasons (performed after an interval of 20–40 d). The first examination served as a baseline measure, while the second and third measurements were done after administration of an i.v. dose of dexamphetamine (0.3 mg/kg bodyweight) or placebo. The order of amphetamine or placebo was randomized and double-blind to avoid confounding with expectation effects.
After the amphetamine and placebo sessions, subjects were asked to subjectively rate, on a scale from 0 to 100, how strongly they felt any effect of the drug and how strongly they experienced any arousal or ‘being high’. They were also asked to rate how strongly they would like to have more of the drug.
PET examinations
We used the PET radioligand [11C]carfentanil, a selective μ-opioid receptor agonist (Frost et al. 1985). [11C]carfentanil has been shown to have excellent test–retest reliability, making it a suitable ligand for cross-over experiments (Hirvonen et al. 2009). [11C]carfentanil is considered sensitive to acute changes in opioid neurotransmission, based on previous findings of reduced [11C]carfentanil binding potential (BPND) in response to behavioural or pharmacological challenges that have been interpreted as increased endogenous opioid release (Hirvonen et al. 2009; Mitchell et al. 2012; Scott et al. 2007; Zubieta et al. 2001). The injected radioactivity was 306.8±15.1 MBq (mean±s.d.) and injected mass 0.300± 0.181 μg (mean± s.d.). There were no significant differences in these variables between the baseline, placebo and amphetamine conditions [repeated measures analysis of variance (ANOVA) for injected dose: F2,18=0.41, p=0.67 and for injected mass: F2,18=0.14, p=0.87].
PET examinations were performed using the high resolution research tomograph (Siemens Molecular Imaging; Germany). For each subject, an individual helmet was made and attached to a holder on the coach to minimize head movement. After a 6 min transmission scan using a single 137Cs source, the study drug (amphetamine/placebo) was injected into an i.v. catheter and flushed with saline. Two minutes later, the radioligand was administered as a rapid bolus and flushed with saline. List-mode data were acquired for 69 min, starting at the time of ligand injection. PET images were reconstructed from a series of 16 time frames (3×1, 4×3 and 9×3 min), including modelling of the point spread function, after correction for attenuation, randoms and scatter. This reconstruction procedure yields a spatial resolution of 1.5 mm (Varrone et al. 2009). PET images were corrected for head movement using frame-by-frame realignment (Montgomery et al. 2006) using the first frame as reference.
Data analysis
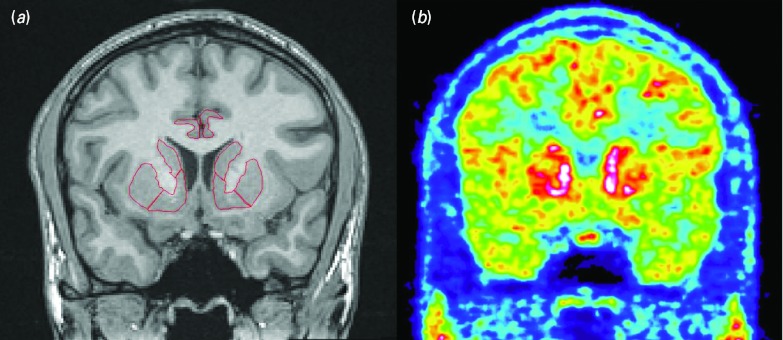
MR images were reoriented into the AC-PC plane. ROIs were delineated on the MR images for each subject individually, using the Human Brain Atlas software (Fig. 1) (Seitz et al. 1990). Ventral striatum was selected as the primary ROI, while a secondary, exploratory analysis included other brain regions involved in drug abuse and reward, i.e. associative and sensorimotor striatum, prefrontal cortex (divided into orbitofrontal, dorsolateral and medial), anterior cingulate cortex, hippocampus and amygdala. The definitions of ROIs were based on previously published guidelines (Abi-Dargham et al. 2002; Ballmaier et al. 2004; Crespo-Facorro et al. 2000; Mawlawi et al. 2001; Pruessner et al. 2000).
Fig. 1.
T1 weighted magnetic resonance image, coronal slice through the striatum with superimposed regions of interest (a). High-resolution research tomograph positron emission tomography image with [11C]carfentanil, corresponding slice (b).
PET images were co-registered to the MR image using SPM 5 and the parameters derived were used to apply ROIs to the PET images in order to extract time–activity curves. Partial volume effect correction was performed according to Meltzer et al. (1990) . Quantitative analysis was performed using the simplified reference tissue model with the occipital lobe as reference region, as has been validated for [11C]carfentanil (Endres et al. 2003; Hirvonen et al. 2009). The BPND was the parameter of interest, representing the ratio at equilibrium of specifically bound to that of non-displaceable radioligand (Innis et al. 2007).
BPND data from the baseline, placebo and amphetamine conditions were analysed with repeated measures ANOVA for effect of treatment. Statistics were done in SPSS v. 19 and graphs in GraphPad Prism 5.
Results
All 10 subjects participated in the study according to the protocol. Amphetamine immediately caused evident subjective effects in all participants. Ratings of how much the subjects ‘felt’ the drug effect ranged from 50 to 100 (mean 88±16.9), feeling of ‘high’ from 30 to 90 (mean 66±22.2) and how much they wanted more of the drug from 0 to 100 (mean 64±32.6). Placebo ratings were between 0 and 10 for all three questions. No serious adverse events occurred during the study.
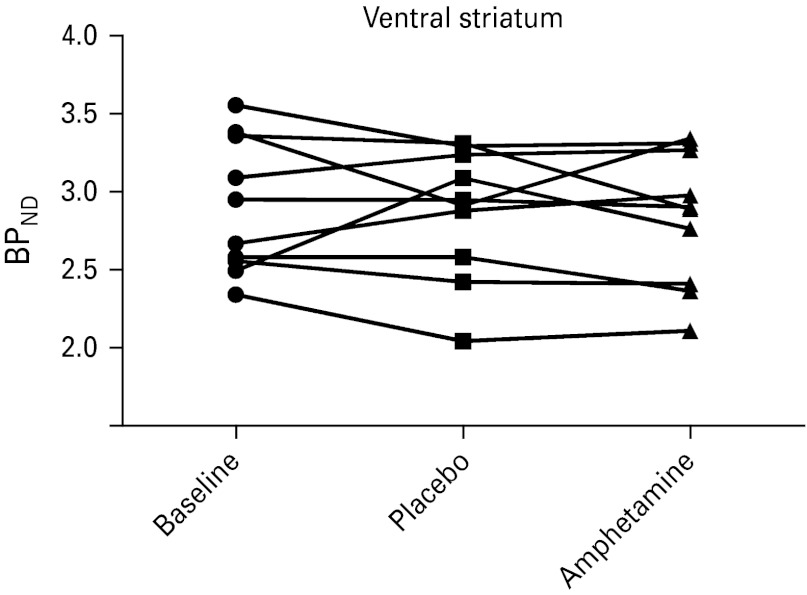
When analysing the PET data with repeated measures ANOVA, we found no significant effects of amphetamine compared to placebo on the [11C]carfentanil BPND for ventral striatum, which was the primary ROI (Fig. 2). Expressed in terms of difference between the placebo and amphetamine conditions, mean ΔBPND was −0.037 in our sample, with s.d.=0.240, giving us a 95% confidence interval of −0.186 to 0.111 (or −6.4 to 3.8%). In other words, we are confident that amphetamine does not reduce BPND in the ventral striatum by >6.4%, as compared to placebo. Neither were there any effects of treatment when comparing to baseline BPND measures. ANOVA revealed no significant effects of order between amphetamine and placebo scans. The same results were found when extending the analysis to other regions of the striatum (Table 1).
Fig. 2.
Individual measures of [11C]carfentanil binding potential (BPND) for the ventral striatum.
Table 1.
Mean [11C]carfentanil binding potential (BPND)±s.d. and mean BPND change from baseline for each condition in different regions of the striatum
| Region | Baseline | Placebo | Change from baseline (%) | Amphetamine | Change from baseline (%) | F 2,18 | p |
|---|---|---|---|---|---|---|---|
| Ventral striatum | 2.90±0.43 | 2.91±0.42 | +0.2 | 2.84±0.43 | −2.2 | 0.292 | 0.750 |
| Associative striatum | 1.84±0.34 | 1.94±0.21 | +5.1 | 1.86±0.29 | +0.6 | 0.298 | 0.746 |
| Sensorimotor striatum | 1.28±0.23 | 1.33±0.13 | +4.5 | 1.25±0.17 | −2.2 | 0.793 | 0.468 |
d.f., F and p values from repeated measures analysis of variance of treatment effect, uncorrected for multiple comparisons.
An exploratory analysis of other brain regions, including the prefrontal cortex, amygdala and hippocampus, did not reveal any effects of amphetamine on [11C]carfentanil BPND (Table 2).
Table 2.
Exploratory analysis of mean [11C]carfentanil binding potential in regions of the prefrontal cortex and medial temporal lobe
| Region | Baseline | Placebo | Amphetamine | F 2,18 | p |
|---|---|---|---|---|---|
| Dorsolateral prefrontal cortex | 1.136 | 1.252 | 1.237 | 1.41 | 0.270 |
| Medial prefrontal cortex | 1.120 | 1.176 | 1.180 | 0.323 | 0.728 |
| Orbitofrontal cortex | 1.185 | 1.236 | 1.212 | 0.405 | 0.673 |
| Anterior cingulate cortex | 1.169 | 1.270 | 1.277 | 0.626 | 0.626 |
| Amygdala | 2.475 | 2.374 | 2.567 | 0.113 | 0.113 |
| Hippocampus | 0.453 | 0.506 | 0.455 | 1.341 | 0.287 |
d.f., F and p values from repeated measures analysis of variance of treatment effect, uncorrected for multiple comparisons.
Measures of baseline [11C]carfentanil BPND were found to be quite similar to the values obtained in a previously published test–retest study (Hirvonen et al. 2009). The inter-individual variation in change between different conditions as indicated by the standard deviation was higher than the corresponding inter-individual variation in test–retest difference shown previously. We therefore proceeded in analysing associations between individual BPND changes and subjective ratings of amphetamine effects. However, we found no evidence of a correlation between changes in [11C]carfentanil BPND and the subjective effects of amphetamine (p>0.1, data not shown).
Discussion
In the present cross-over, randomized, placebo-controlled PET study, we found that an acute i.v. dose of amphetamine did not change [11C]carfentanil BPND in the striatum, nor in the prefrontal cortex, amygdala or hippocampus. This is a surprising finding that gives rise to several important questions.
The dose of amphetamine administered in this study (0.3 mg/kg bodyweight) is the one most often used in human laboratory studies, causing strong, immediate subjective effects in drug-naive individuals. This dose has been shown to induce a significant dopamine release in the ventral striatum, measurable in human PET studies as a decrease of the dopamine D2-receptor radioligand [11C]raclopride BPND by about 15% (Drevets et al. 2001). Importantly, a dose of 0.3 mg/kg bodyweight was also used previously in studies showing that naltrexone blunts the subjective effects of amphetamine (Jayaram-Lindström et al. 2004, 2008b). Amphetamine-dependent patients often inject several hundred mg and such a dose naturally gives rise to stronger pharmacological effects, possibly including an activation of the opioid system. However, due to the risk of adverse reactions, such doses cannot be administered to humans in an experimental setting. Animal studies are therefore needed to investigate the opioid effects of amphetamine across a wider dose range.
In this study, we started the PET measurement 2 min after the amphetamine/placebo injection and continued for 69 min. A possibility is that amphetamine does cause an endogenous opioid release that either evaporates too fast or commences too late for us to capture it in this time frame. The 69 min time frame was chosen based on our previous studies, where naltrexone's effects were already evident after a couple of minutes and stayed significant over several hours (Jayaram-Lindström et al. 2008b). In a rat microdialysis study, the peak opioid activation following i.p. amphetamine administration came somewhat later, but was clearly evident during the first hour (Olive et al. 2001). Evidence from a recent animal study suggests that opioid activation following cocaine administration i.p. peaks at 20 min (Soderman & Unterwald, 2009). To test for signs of such an early opioid activation, we did a post-hoc kinetic analysis using only the first 39 min of the PET scan, a time frame that has previously been shown to have good reliability with [11C]carfentanil (Hirvonen et al. 2009). This analysis did not reveal any trend of early opioid release, suggesting that our original time frame was adequate.
Several rodent studies have shown evidence of psychostimulant-induced opioid activation, a finding that could not be translated to humans in this study. This might point to physiological differences between species. Experiments using microdialysis in rats have shown that an i.p. dose of 2 mg amphetamine/kg bodyweight gives rise to an increase of dialysate endorphin levels in the accumbens, peaking at 300% (Olive et al. 2001), and a dopamine increase peaking at 350% (Butcher et al. 1988). While i.p. doses in rats are hard to translate directly to i.v. doses in humans, the dose used in this study is probably less potent, even though it did cause strong behavioural effects. Other species differences, particularly in the anatomy of the dopamine system, might therefore be more important in explaining these results (Berger et al. 1991).
In human subjects, as opposed to other animals, it is important to acknowledge expectation effects since they may involve opioid mechanisms (Scott et al. 2008). In this study, the subjects were aware that they would not be administered any study drug during the first, baseline PET examination, but in the second and third they would get either amphetamine or placebo intravenously. By randomizing the order of placebo and amphetamine, we could control for expectation effects and ANOVA showed no effect of order on [11C]carfentanil BPND. Neither was there any significant difference between the baseline (i.e. without study drug) and medication conditions, which means that the expectation of an amphetamine injection did not give rise to any measurable opioid activation.
This study was performed with healthy, drug-naive subjects, but the effects of amphetamine may be quite different in amphetamine-dependent patients. For example, after three doses of the same strength as in this study, a significant sensitization to amphetamine occurs with an increased dopamine release in the striatum (Boileau et al. 2006). Such mechanisms might also cause a downstream recruitment of other neurotransmitter systems with repeated drug intake. Although we saw no evidence of opioid activation in healthy subjects from this first dose of amphetamine, it is possible that the opioid system becomes involved later in the development of stimulant dependence, forming part of the neuro-adaptations in brain reward systems that create a state of hedonic allostasis in the addicted brain (Koob & Le Moal, 2001). Indeed, recent PET studies have shown significant alterations of [11C]carfentanil BPND in chronic cocaine patients as compared to healthy controls, changes that predict treatment outcome and are correlated with drug craving and risk of relapse to cocaine use (Ghitza et al. 2010; Gorelick et al. 2005). Another recent study of alcohol-dependent patients found that opiate-induced dopamine release in the ventral striatum was correlated with the severity of alcohol dependence (Spreckelmeyer et al. 2011), providing further evidence of the important interactions between these two neurotransmitter systems in substance use disorders.
We have previously shown that naltrexone pretreatment attenuates the subjective response to an acute dose of amphetamine to a greater extent in amphetamine-dependent patients than in healthy subjects (Jayaram-Lindström et al. 2004, 2008b). It is therefore possible that amphetamine does cause a significant opioid release in the amphetamine-dependent, but not in the healthy, human brain. Genetic variability between samples, particularly in the prevalence of the Asp40 single nucleotide polymorphism in the μ opioid receptor gene that has been linked to naltrexone response in alcohol dependence, might also have contributed to differences between studies; although we do not have genetic data to prove this (Dlugos et al. 2011).
Very recently, Colasanti et al. (2012) published a study reporting a decrease in [11C]carfentanil BPND 3 h after an oral amphetamine dose of 0.5 mg/kg bodyweight. There are several differences between our studies that may help explain the differences in results.
First, oral dosing differs substantially from i.v. administration of amphetamine in terms of pharmacokinetics and also in the resulting subjective effects. Indeed, in Colasanti's study, the high-dose group did not report any significant euphoria, while the subjects in our study consistently reported strong effects of amphetamine as compared to placebo.
Another important difference is the timing of the PET measurements. We started within minutes of the amphetamine injection, whereas Colasanti et al. waited 3 h before injecting the radioligand. While a part of this interval is needed due to the slower absorption of an oral amphetamine dose, it remains a significant difference between our studies. Our choice of an immediate measurement was based on several lines of evidence, not least previous studies showing a very fast reduction of (3)H-DAMGO binding after cocaine administration in rodents (Soderman & Unterwald, 2009). Also, the euphoric effects of an i.v. amphetamine dose are immediate and start to wear off gradually after 1–2 h. Of course, if opioid release plays any important role in these acute rewarding effects, it has to be present within this time interval rather than 3 or 4 h later.
There are also some technical differences between the studies that might have influenced the results. The increased resolution of the high-resolution research tomograph system provides a higher signal recovery, which should provide additional sensitivity to changes in binding (Schain et al. 2012). Also, we had no bias in terms of differences in injected mass or radioactivity between the different conditions. Finally, a particular strength of our study is the cross-over randomization, which allowed us to do within-subject comparisons while still controlling for expectation effects.
The results of these two PET studies suggest another possible explanation for the fact that naltrexone attenuates the subjective effects of amphetamine, namely, that weak unspecific adverse effects of naltrexone (e.g. nausea) might somewhat blunt the immediate effects of amphetamine, while its specific action as an opioid antagonist blocks the prolonged euphoric effects possibly caused by opioid release 2 or 3 h later.
To conclude, in this placebo-controlled PET study, an acute i.v. dose of amphetamine did not cause any opioid release in the healthy human brain reward system. Furthermore, possible expectation effects were not mediated by opioid activation, since order of placebo/amphetamine did not change [11C]carfentanil BPND. These results are in contrast with earlier findings of stimulant-induced opioid activation in rodents as well as recent data showing later effects of oral amphetamine using a slightly different study design. Further studies are needed to more fully investigate this issue from a translational perspective. With the recent development of small animal PET systems, the effects of amphetamine on the opioid system could be studied across a wider dose range and timescale than what is possible in humans. Studies of stimulant-dependent patients are also needed to investigate the pathophysiological importance of the opioid system and its potential as a therapeutic pharmacological target.
Acknowledgements
The authors thank Per Stenkrona and Andrea Varrone, as well as other members of the Karolinska PET group, for assistance in planning and execution of the study. This study was supported by the Swedish Research Council (Grant 30207123), the Swedish Brain Fund and the Stockholm County Council.
Statement of Interest
Lars Farde also holds a position as Chief Scientist, iMed CNS/Pain, AstraZeneca, Sweden.
References
- Abi-Dargham A, Mawlawi O, Lombardo I, Gil R, et al. (2002). Prefrontal dopamine D1 receptors and working memory in schizophrenia. Journal of Neuroscience 22, 3708–3719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballmaier M, Toga AW, Blanton RE, Sowell ER, et al. (2004). Anterior cingulate, gyrus rectus, and orbitofrontal abnormalities in elderly depressed patients: an MRI-based parcellation of the prefrontal cortex. American Journal of Psychiatry 161, 99–108 [DOI] [PubMed] [Google Scholar]
- Berger B, Gaspar P, Verney C (1991). Dopaminergic innervation of the cerebral cortex: unexpected differences between rodents and primates. Trends in Neurosciences 14, 21–27 [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE, Aldridge JW (2009). Dissecting components of reward: ‘liking’, ‘wanting’, and learning. Current Opinion in Pharmacology 9, 65–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boileau I, Dagher A, Leyton M, Gunn RN, et al. (2006). Modeling sensitization to stimulants in humans: an [11C]raclopride/positron emission tomography study in healthy men. Archives of General Psychiatry 63, 1386–1395 [DOI] [PubMed] [Google Scholar]
- Butcher SP, Fairbrother IS, Kelly JS, Arbuthnott GW (1988). Amphetamine-induced dopamine release in the rat striatum: an in vivo microdialysis study. Journal of Neurochemistry 50, 346–355 [DOI] [PubMed] [Google Scholar]
- Colasanti A, Searle GE, Long CJ, Hill SP, et al. (2012). Endogenous opioid release in the human brain reward system induced by acute amphetamine administration. Biological Psychiatry. Published online: 5March2012. . doi: 10.1016/j.biopsych.2012.01.027 [DOI] [PubMed] [Google Scholar]
- Crespo-Facorro B, Kim J, Andreasen NC, Spinks R, et al. (2000). Cerebral cortex: a topographic segmentation method using magnetic resonance imaging. Psychiatry Research 100, 97–126 [DOI] [PubMed] [Google Scholar]
- Dlugos AM, Hamidovic A, Hodgkinson C, Shen PH, et al. (2011). OPRM1 gene variants modulate amphetamine-induced euphoria in humans. Genes Brain and Behaviour 10, 199–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Gautier C, Price JC, Kupfer DJ, et al. (2001). Amphetamine-induced dopamine release in human ventral striatum correlates with euphoria. Biological Psychiatry 49, 81–96 [DOI] [PubMed] [Google Scholar]
- Endres CJ, Bencherif B, Hilton J, Madar I, et al. (2003). Quantification of brain mu-opioid receptors with [11C]carfentanil: reference-tissue methods. Nuclear Medicine and Biology 30, 177–186 [DOI] [PubMed] [Google Scholar]
- Frost JJ, Wagner HN Jr., Dannals RF, Ravert HT, et al. (1985). Imaging opiate receptors in the human brain by positron tomography. Journal of Computer Assisted Tomography 9, 231–236 [DOI] [PubMed] [Google Scholar]
- Ghitza UE, Preston KL, Epstein DH, Kuwabara H, et al. (2010). Brain mu-opioid receptor binding predicts treatment outcome in cocaine-abusing outpatients. Biological Psychiatry 68, 697–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelick DA, Kim YK, Bencherif B, Boyd SJ, et al. (2005). Imaging brain mu-opioid receptors in abstinent cocaine users: time course and relation to cocaine craving. Biological Psychiatry 57, 1573–1582 [DOI] [PubMed] [Google Scholar]
- Hirvonen J, Aalto S, Hagelberg N, Maksimow A, et al. (2009). Measurement of central mu-opioid receptor binding in vivo with PET and [11C]carfentanil: a test-retest study in healthy subjects. European Journal of Nuclear Medicine and Molecular Imaging 36, 275–286 [DOI] [PubMed] [Google Scholar]
- Häggkvist J, Björkholm C, Steensland P, Lindholm S, et al. (2011). Naltrexone attenuates amphetamine-induced locomotor sensitization in the rat. Addiction Biology 16, 20–29 [DOI] [PubMed] [Google Scholar]
- Häggkvist J, Lindholm S, Franck J (2009). The opioid receptor antagonist naltrexone attenuates reinstatement of amphetamine drug-seeking in the rat. Behavioural Brain Research 197, 219–224 [DOI] [PubMed] [Google Scholar]
- Innis RB, Cunningham VJ, Delforge J, Fujita M, et al. (2007). Consensus nomenclature for in vivo imaging of reversibly binding radioligands. Journal of Cerebral Blood Flow and Metabolism 27, 1533–1539 [DOI] [PubMed] [Google Scholar]
- Jayaram-Lindström N, Hammarberg A, Beck O, Franck J (2008a). Naltrexone for the treatment of amphetamine dependence: a randomized, placebo-controlled trial. American Journal of Psychiatry 165, 1442–1448 [DOI] [PubMed] [Google Scholar]
- Jayaram-Lindström N, Konstenius M, Eksborg S, Beck O, et al. (2008b). Naltrexone attenuates the subjective effects of amphetamine in patients with amphetamine dependence. Neuropsychopharmacology 33, 1856–1863 [DOI] [PubMed] [Google Scholar]
- Jayaram-Lindström N, Wennberg P, Hurd YL, Franck J (2004). Effects of naltrexone on the subjective response to amphetamine in healthy volunteers. Journal of Clinical Psychopharmacology 24, 665–669 [DOI] [PubMed] [Google Scholar]
- Koob GF, Moal M Le (2001). Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology 24, 97–129 [DOI] [PubMed] [Google Scholar]
- Mawlawi O, Martinez D, Slifstein M, Broft A, et al. (2001). Imaging human mesolimbic dopamine transmission with positron emission tomography: I. Accuracy and precision of D(2) receptor parameter measurements in ventral striatum. Journal of Cerebral Blood Flow and Metabolism 21, 1034–1057 [DOI] [PubMed] [Google Scholar]
- Meltzer CC, Leal JP, Mayberg HS, Wagner HN Jr., et al. (1990). Correction of PET data for partial volume effects in human cerebral cortex by MR imaging. Journal of Computer Assisted Tomography 14, 561–570 [DOI] [PubMed] [Google Scholar]
- Mitchell JM, O'Neil JP, Janabi M, Marks SM, et al. (2012). Alcohol consumption induces endogenous opioid release in the human orbitofrontal cortex and nucleus accumbens. Science Translational Medicine 4, 116ra6. [DOI] [PubMed] [Google Scholar]
- Montgomery AJ, Thielemans K, Mehta MA, Turkheimer F, et al. (2006). Correction of head movement on PET studies: comparison of methods. Journal of Nuclear Medicine 47, 1936–1944 [PubMed] [Google Scholar]
- Olive MF, Koenig HN, Nannini MA, Hodge CW (2001). Stimulation of endorphin neurotransmission in the nucleus accumbens by ethanol, cocaine, and amphetamine. Journal of Neuroscience 21, RC184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner JC, Li LM, Serles W, Pruessner M, et al. (2000). Volumetry of hippocampus and amygdala with high-resolution MRI and three-dimensional analysis software: minimizing the discrepancies between laboratories. Cerebral Cortex 10, 433–442 [DOI] [PubMed] [Google Scholar]
- Roth-Deri I, Zangen A, Aleli M, Goelman RG, et al. (2003). Effect of experimenter-delivered and self-administered cocaine on extracellular beta-endorphin levels in the nucleus accumbens. Journal of Neurochemistry 84, 930–938 [DOI] [PubMed] [Google Scholar]
- Schain M, Tóth M, Cselényi Z, Stenkrona P, et al. (2012) Quantification of serotonin transporter availability with [11C]MADAM–a comparison between the ECAT HRRT and HR systems. Neuroimage 60, 800–807 [DOI] [PubMed] [Google Scholar]
- Scott DJ, Domino EF, Heitzeg MM, Koeppe RA, et al. (2007). Smoking modulation of mu-opioid and dopamine D2 receptor-mediated neurotransmission in humans. Neuropsychopharmacology 32, 450–457 [DOI] [PubMed] [Google Scholar]
- Scott DJ, Stohler CS, Egnatuk CM, Wang H, et al. (2008). Placebo and nocebo effects are defined by opposite opioid and dopaminergic responses. Archives of General Psychiatry 65, 220–231 [DOI] [PubMed] [Google Scholar]
- Seitz RJ, Bohm C, Greitz T, Roland PE, et al. (1990). Accuracy and precision of the computerized brain atlas programme for localization and quantification in positron emission tomography. Journal of Cerebral Blood Flow and Metabolism 10, 443–457 [DOI] [PubMed] [Google Scholar]
- Soderman AR, Unterwald EM (2009). Cocaine-induced mu opioid receptor occupancy within the striatum is mediated by dopamine D2 receptors. Brain Research 1296, 63–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreckelmeyer KN, Paulzen M, Raptis M, Baltus T, et al. (2011). Opiate-induced dopamine release is modulated by severity of alcohol dependence: an [(18)F]fallypride positron emission tomography study. Biological Psychiatry 70, 770–776 [DOI] [PubMed] [Google Scholar]
- Varrone A, Sjöholm N, Eriksson L, Gulyás B, et al. (2009). Advancement in PET quantification using 3D-OP-OSEM point spread function reconstruction with the HRRT. European Journal of Nuclear Medicine and Molecular Imaging 36, 1639–1650 [DOI] [PubMed] [Google Scholar]
- Zubieta JK, Smith YR, Bueller JA, Xu Y, et al. (2001). Regional mu opioid receptor regulation of sensory and affective dimensions of pain. Science 293, 311–315 [DOI] [PubMed] [Google Scholar]