Abstract
Background
Desmoglein 1 (Dsg1), the pemphigus foliaceus (PF) antigen, is produced as a precursor (preDsg1) and is transported to the cell surface as the mature form (matDsg1). Recent studies show that B cells from North American individuals without pemphigus can potentially produce anti-preDsg1 IgG antibodies, but ELISA screening of large numbers of normal people in North America and Japan hardly ever shows circulating antibodies against preDsg1 or matDsg1. In contrast, in Tunisia, where PF is endemic, anti-Dsg1 IgGs are frequently detected in healthy individuals.
Objective
To characterize these anti-Dsg1 antibodies from normal individuals in Tunisia.
Methods
Sera from 16 healthy individuals and 9 PF patients in the endemic PF area in Tunisia, and sera from Japanese non-endemic PF patients were analyzed by immunoprecipitation-immunoblotting using recombinant proteins of preDsg1, matDsg1, and domain-swapped Dsg1/Dsg2 molecules.
Results
Sera from normal Tunisian individuals reacted to preDsg1 alone (8/16) or more strongly to preDsg1 than to matDsg1 (7/16), while those from all Tunisian PF patients and Japanese non-endemic PF patients reacted similarly to preDsg1 and matDsg1, or preferentially to matDsg1. The epitopes recognized by anti-Dsg1 IgGs from normal Tunisian individuals were more frequently found in the C-terminal extracellular domains (EC3 to EC5), while those in Tunisian endemic PF patients were more widely distributed throughout the extracellular domains, suggesting IgGs against EC1 and EC2 developed during disease progression.
Conclusions
These findings indicate that IgG autoantibodies against Dsg1 are mostly raised against preDsg1 and/or C-terminal domains of Dsg1 in healthy Tunisians in the endemic area of PF.
1. Introduction
Pemphigus foliaceus (PF) is a tissue-specific autoimmune disease characterized by superficial blisters in the epidermis and circulating autoantibodies against the desmosomal cadherin desmoglein 1 (Dsg1), which is involved in cell-cell adhesion [1]. PF has two forms: a sporadic form that occurs throughout the world and an endemic form (fogo selvagem) first reported in Brazil in 1903 [2, 3]. In 1993, the presence of an endemic form of PF in southern Tunisia was reported [4, 5]. Both sporadic and endemic forms are clinically, histologically, and immunologically similar; however, the endemic form has several unique features, such as geographic and familial clustering and high prevalence among young adults [5, 6].
Characteristics of the Tunisian endemic form of PF include a significantly higher incidence in southern regions as compared with northern regions [6, 7]. The observation that the majority of PF family cases in Tunisia were also located in the southern regions implies the role of genetic susceptibility or environmental factors, and DRB1*03 was concluded to be the main susceptibility allele for the Tunisian endemic PF in previous studies [8, 9]. Anti-Dsg1 antibodies (Abs) have been detected in normal subjects living in an area where PF is endemic [3]. Furthermore, Abs against Dsg1 were detected in 7.4% of normal subjects throughout Tunisia. The rate was higher in the south (9.23% vs. 5.71% in the north), where there are more patients with the endemic form of PF [7].
Dsg1 is synthesized in the endoplasmic reticulum as an inactive precursor protein (preDsg1) with a propeptide at its amino-terminus. It is then processed by proteases such as furin during transport to the cell surface, where the mature form of Dsg1 (matDsg1) (without a propeptide) forms desmosomes, which are involved in cell-cell adhesion [10–12]. A recent study from North America isolated monoclonal antibodies (mAbs) specific for preDsg1 from individuals without pemphigus using phage display[13]. This type of study indicates that there may be B cells in individuals without pemphigus capable of producing anti-preDsg1 antibodies, however they probably do not usually do so because most people in North America and Japan without PF are negative in anti-Dsg1 ELISA [14]. This ELISA assay is known to detect both anti-preDsg1 and anti-matDsg1 antibodies [15, 16]. In contrast, anti-Dsg1 antibodies have been detected from normal individuals by ELISA, in 19% of the population without PF in endemic areas for PF in Brazil, and 9% in Tunisia, respectively [3, 7]. We hypothesized that in these endemic areas a subset of people that may be predisposed to developing PF may have activated these preDsg1-specific B cells as the first step toward developing disease, and in doing so actually produce anti-preDsg1 antibodies that are detected by ELISA.
To test this possibility, we characterized autoantibodies against Dsg1 in individuals with and without PF in endemic areas for PF in Tunisia. We found that anti-Dsg1 Abs from healthy Tunisian individuals preferentially reacted with preDsg1, unlike those from individuals with Tunisian PF, which preferably bound to matDsg1. Our findings suggest that Abs against preDsg1, which are usually not detected by ELISA in normal individuals, can be detected in individuals without pemphigus who live in or near areas endemic for pemphigus foliaceus. The production of these anti-preDsg1 Abs, whether due to the environment or the genetics of the population, may be an initial step in developing anti-matDsg1 antibodies and disease.
2. Materials & Methods
2.1. Subjects and sera
We examined sera from nine Tunisian patients with endemic PF (TPF) and 16 healthy subjects found in previous studies by our group to have anti-Dsg1 Abs [6, 9, 13]. Among these 16 normal subjects, seven were relatives of PF patients (THR) and nine were healthy controls (THC). We also included sera from nine Japanese patients with sporadic PF (JPF). All patients showed typical clinical and histological features of PF. All patients and healthy controls gave written consent to participate in the study.
2.2. Anti-Desmoglein1 antibodies titration
Dsg1 ELISA kits (MBL, Nagoya, Japan) were used for quantitative analysis of serum anti-Dsg1 Ab levels as previously described [14]. According to the manufacturer’s instructions, index values >20 were considered positive. In some experiments, to increase the ratio of the mature form of Dsg1 on ELISA plates, we pretreated the plates with 10 U/well furin (New England Biolabs, Ipswich, MA, U.S.A.) in 20 mM Tris, 500mM sodium chloride (pH 7.5; TBS), with 1mM CaCl2 at room temperature overnight.
2.3. Indirect immunofluorescence (IIF)
Each serum sample was subjected to indirect immunofluorescence analysis using normal human skin cryosections. Briefly, human skin cryosections were incubated with serial dilutions (1:10, 1:40, and 1:160) of tested sera for an hour at room temperature or overnight at 4°C. Human IgG Abs were detected with a 1:100 dilution of FITC-labeled polyclonal rabbit anti-human IgG (Dako, Copenhagen, Denmark) [17].
2.4. Recombinant proteins
Recombinant proteins were produced as previously described [18]. Briefly, plasmids were cotransfected with Sapphire Baculovirus DNA (Orbigen, San Diego, CA, U.S.A.) into cultured insect Sf9 cells. Recombinant virus was collected from the supernatant and was amplified by several rounds of passage in Sf9 cells. High Five Cells (Invitrogen, San Diego, CA, U.S.A.) cultured in serum-free EX Cell 405 medium (JRH Biosciences, Lenexa, KS, U.S.A.) were infected with baculovirus and incubated at 27°C for 3–4 days. Baculoproteins were collected as supernatants and stored at −80°C.
In the plasmid pQE-Dsg1-FacX, which carries the entire extracellular domain of Dsg1 fused to an E-tag and a His-tag, the putative endoproteolytic cleavage site of Dsg1 (RQKR) is substituted with the recognition site for blood coagulation factor Xa (IEGR) [16]. The construct was transiently transfected into CHO cells cultured in Ham’s F-12 medium (Sigma, St Louis, MO, U.S.A.) and incubated at 37°C for 7 days. PreDsg1 and matDsg1 proteins were collected as supernatants and stored at −80°C.
2.5. Immunoprecipitation-immunoblotting analysis (IP-IB)
Immunoprecipitation was carried out by mixing a 20 μl bed volume of Protein G Sepharose 4 Fast Flow (GE Healthcare®, Uppsala, Sweden), 200 μl of culture supernatant for each protein, and 2 μl of each serum. In some experiments, the culture supernatant was treated with ethylenediaminetetraacetic acid (EDTA) at 5mM for 1 hour to chelete calcium. The mixture was incubated at 4°C overnight with gentle rotation and then washed five times with Tris-buffered saline containing 1 mM calcium chloride and 0.05% Tween 20. Immunoprecipitated proteins were then re-suspended in sodium dodecyl sulfate (SDS) sample buffer, fractionated by SDS polyacrylamide gel electrophoresis (SDS-PAGE), and labeled using a horseradish peroxidase-conjugated anti-E-tag Ab (GE Healthcare, Buckinghamshire, UK). Proteins were visualized using Western Lightning Chemiluminescence Reagent Plus (PerkinElmer LAS, Shelton, CT, U.S.A.) and by autoradiography.
2. 6. Statistics
All parameters were compared by Fisher’s exact test, as appropriate, with p<0.05 considered significant.
3. Results
3.1. Anti-Dsg1 antibodies from healthy individuals in Tunisia do not show intercellular staining, in contrast to those from PF patients
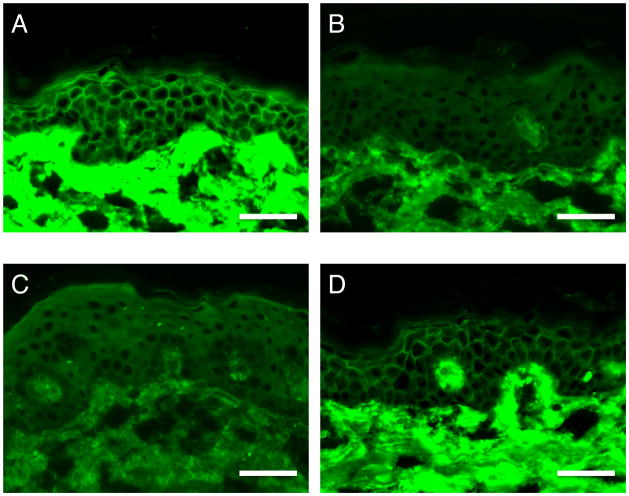
All sera analyzed in this study reacted with Dsg1 in an ELISA, as shown in Supplemental Table 1. We compared the ELISA results with those for indirect immunofluorescence (IIF) using normal human skin as the substrate. Seven of nine TPF sera and all JPF sera showed cell surface staining throughout the epidermis, as is typically seen in PF (Fig. 1A, D). Two TPF sera did not exhibit cell surface staining, presumably because of their lower titers against Dsg1 in the ELISA (their indexes were 36.67 and 37.32). On the other hand, none of the THR or THC sera showed cell surface staining in IIF (Fig. 1B, C). No fluorescence was observed even with sera showing high ELISA reactivity (index values >150 index, e.g., THR2 and THC8), indicating that low antibody titers are not the reason for the lack of fluorescence. We repeated the assay with extended incubation time to overnight with sera of TPF, THR and THC to increase the sensitivity, and no cell surface staining was observed in THR or THC sera as well (Supplemental Fig. 1).
Fig. 1.
Representative results of indirect immunofluorescence using normal human skin. (A) A patient with Tunisian endemic PF (TPF). (B) A healthy relative of a patient in Tunisia with endemic PF (THR). (C) A healthy individual from the area in Tunisia affected by endemic PF (THC). (D) A patient with Japanese sporadic PF (JPF). Scale bars: 50 μm.
Based on their different IIF staining patterns, we assumed that Abs against Dsg1 in THRs and THCs might bind to precursor Dsg1 (preDsg1) and not mature Dsg1 (matDsg1). The majority of preDsg1 is located in the cytoplasm, which is why Abs against preDsg1 show undetectable cell surface staining in IIF and do not cause blister formation in the skin [19].
3.2. Anti-Dsg1 antibodies in healthy Tunisian individuals mainly target preDsg1
To further characterize THR and THC sera with positive Dsg1 ELISAs, immunoprecipitation was performed using recombinant Dsg1 (rDsg1). We produced a mutated form of rDsg1 named precursor-FacX-Dsg1 (preDsg1), in which the original endoproteolytic cleavage site of Dsg1 (RQKR) was replaced with the recognition site for serum coagulation factor Xa (IEGR) (Figure 2A). We also prepared the original construct of rDsg1 (matDsg1). These proteins were expressed in Chinese hamster ovary (CHO) cells, which have more efficient posttranslational modification than insect cells (baculovirus expression system) that have been used for Dsg ELISA [13, 16]. In insect cells, rDsg1 was produced as a mixture of preDsg1 and matDsg1, while in CHO cells pure preDsg1 and matDsg1 were prepared from their respective plasmid constructs (Fig. 2B) [13, 19, 20]. Thus, we could use pure recombinant protein that only contains preDsg1 or matDsg1 for immunoprecipitation.
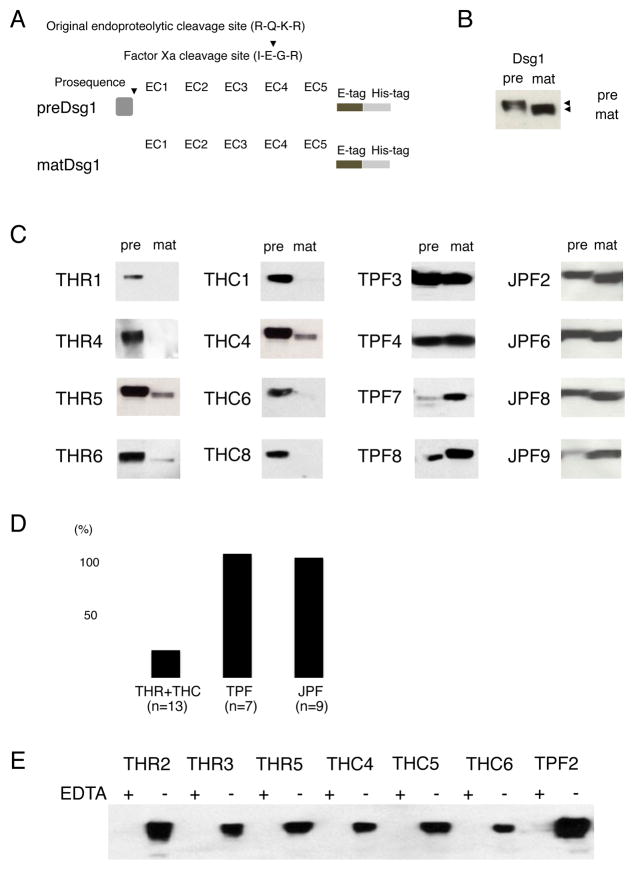
Fig. 2.
Immunoprecipitation analysis using mature and precursor forms of Dsg1.
(A) Recombinant precursor (preDsg1) and mature Dsg1 (matDsg1) proteins were used. (B) Western blotting of preDsg1 and matDsg1. The bands detected by anti-E tag Abs show the molecular weight differences between preDsg1 and matDsg1, the latter of which has a slightly lower molecular weight. Expression levels of preDsg1 and matDsg1 are approximately same in CHO cell culture supernatant. (C) Representative results of immunoprecipitation for sera from each group. THR and THC sera showed preferential binding to preDsg1, while TPF and JPF sera reacted with both preDsg1 and matDsg1, or preferentially with matDsg1. (D) The average of Dsg1 titer after furin-treated ELISA. THR and THC sera decreased to 23.9% after furin treatment. (E) Representative results of immunoprecipitation using preDsg1 before and after EDTA treatment. EDTA treatment abolished the binding activity to preDsg1.
All THR and THC sera immunoprecipitated preDsg1, while three THR sera and five THC sera reacted with preDsg1 alone, indicating that 50% (8/16) of Tunisian non-pemphigus sera in this study targeted only preDsg1. Four THR sera and four THC sera immunoprecipitated both preDsg1 and matDsg1; however, all of the sera except one serum that reacted to both equally, reacted more strongly with preDsg1 than with matDsg1 (Fig. 2C). In contrast, all TPF sera showed binding to both preDsg1 and matDsg1, and three TPF sera (33.3%) showed preferential binding to matDsg1. The other six TPF sera (66.7%) bound equally to matDsg1 and preDsg1. Similarly, six out of nine JPF sera (66.7%) showed preferential binding to matDsg1, including one case of exclusive binding to matDsg1, while three JPF sera (33.3%) bound to both preDsg1 and matDsg1 at equal levels (Table 1, Fig. 2C). The major finding is that anti-Dsg1 IgG autoantibodies in the THR and THC groups bound more strongly to preDsg1 than to matDsg1, while this preferential binding to preDsg1 was not found after disease onset (i.e., in the TPF and JPF groups) (P<0.0001).
Table 1.
Reactivity with precursor and mature proteins of Dsg1 and results of epitope mapping
| Number | preDsg1/matDsg1 | Epitopes | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pre | mat | pre>mat | pre=mat | pre<mat | EC1 | EC2 | EC3 | EC4 | EC5 | |||
| Tunisia | Healthy relatives (THR) | 7 | 7 | 4 | 6 | 1 | 0 | 2 | 3 | 5 | 5 | 6 |
| Healthy non-relatives (THC) | 9 | 9 | 4 | 9 | 0 | 0 | 1 | 5 | 8 | 8 | 8 | |
| Endemic PF (TPF) | 9 | 9 | 9 | 0 | 6 | 3 | 9 | 7 | 9 | 5 | 6 | |
|
| ||||||||||||
| Japan | Sporadic PF (JPF) | 9 | 8 | 9 | 0 | 3 | 6 | 9 | 5 | 2 | 1 | 0 |
We also performed ELISA with furin-treated plates to determine whether THR and THC sera were binding to preDsg1 specifically. The proprotein convertase furin cleaves the precursor forms of Dsg1 on the ELISA plate and converts them into the mature form [15]. The average of titer against Dsg1 after furin treatment decreased to 23.9% in THR and THC (n=13), while they were 107.7% in TPF (n=7) and 104.3% in JPF (n=9), respectively (Fig. 2D, supplement Table 1). These results suggest that THR and THC sera bind specifically to preDsg1.
The immunoreactivity of THR and THC sera to preDsg1 was calcium-dependent, as indicated by the fact that EDTA treatment abolished binding in all THR and THC sera (Fig. 2E). This result showed that anti-Dsg1 antibodies from healthy Tunisians bind conformational epitopes on preDsg1.
We speculate that the conformational epitopes recognized by these THR and THC anti-Dsg1 antibodies are only expressed intracellularly and are lost with processing to the mature form. Therefore, these Abs do not detect cell surface matDsg1 by IIF, but only bind intracellular preDsg1, similar to mAbs that have been cloned from other individuals without pemphigus [13]. We assume that Abs from these normal Tunisian individuals that bind both preDsg1 and less to matDsg1 are not high enough titer or avidity against the latter to be positive for cell surface staining by IIF.
3.3 Autoantibodies from healthy Tunisian individuals react with the C-terminal extracellular domains of Dsg1, while PF patients have antibodies against the N-terminal extracellular domains
We examined the epitopes of anti-Dsg1 Abs from each group to investigate whether there is any difference in Ab-binding sites between the THR, THC, TPF, and JPF groups. To analyze autoantibody epitopes in each group, we used the previously constructed swapping molecules containing extracellular (EC) domains 1 to 5 of Dsg1 on the backbone of Dsg2 (Fig. 3A) [18].
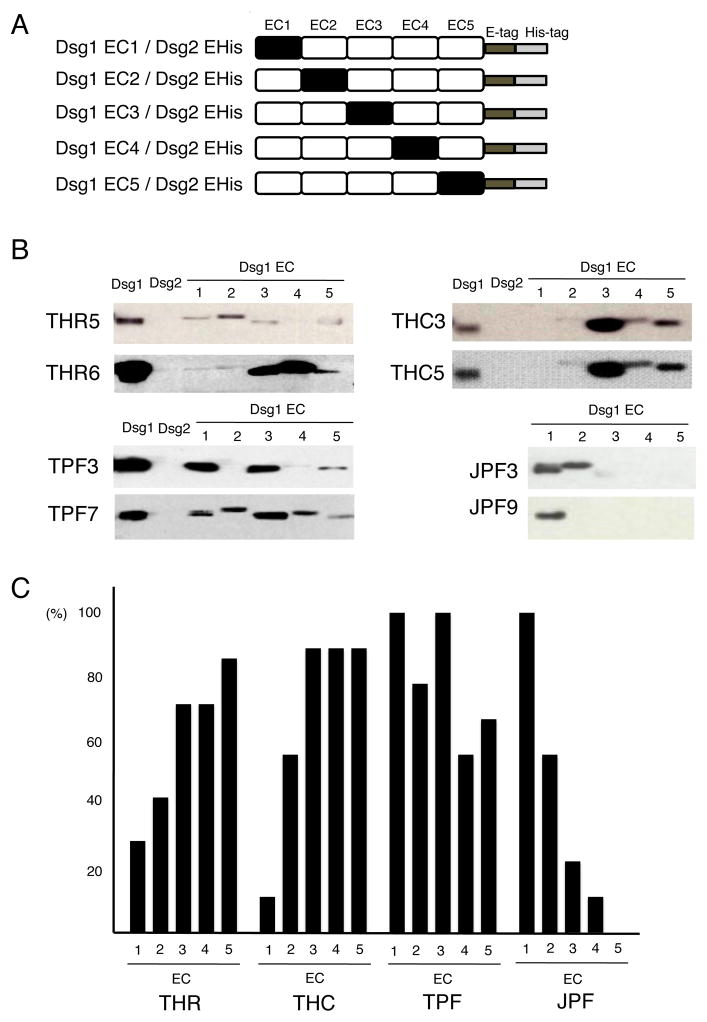
Fig. 3.
Epitope mapping using domain-swapped molecules.
(A) Schematic representation of domain-swapped molecules comprising EC domains of Dsg1 and the backbone of Dsg2. Domains EC1 to EC5 of human Dsg1 were domain-swapped with the corresponding domains of human Dsg2 to generate five recombinant molecules. (B) Representative results of epitope mapping of THR, THC, TPF, and JPF sera. Each serum sample was immunoprecipitated with rDsg1, rDsg2, and five recombinant domain-swapped molecules (labeled at the top of each panel) and subjected to immunoblotting using anti-E tag Abs. (C) Immunoreactivity of sera from each group (seven, nine, nine and nine from the THR, THC, TPF, and JPF groups, respectively) to each extracellular domain of Dsg1 (EC1 to EC5).
Epitope mapping revealed that all sera from clinically healthy subjects from Tunisia (THR and THC) showed immunoreactivity to more than one EC domain and tended to react with the C-terminal domains of Dsg1, such as EC4 and EC5 (Fig. 3B). The proportions of THR and THC sera that reacted with each EC domain were 28.6% and 11.1% (average 18.8%) for EC1, 42.2% and 55.6% (50.0%) for EC2, 71.4% and 88.9% (81.3%) for EC3, 71.4% and 88.9% (81.3%) for EC4, and 85.7% and 88.9% (87.5%) for EC5, respectively (Fig. 3C). We could not completely exclude a possibility that the swapping molecules used in this study may contain the precursor forms because they were produced by baculovirus expression system. However, the results of the epitope mapping will not be obscured because the domain swapping molecules for EC2 to EC5 have the propeptide of Dsg2 and the sera did not show any binding to Dsg2 (Fig. 3C).
All TPF sera were immunoreactive to more than one EC domain of Dsg1 (Supplemental Table 1), and reacted to sites throughout the entire extracellular portion of Dsg1, from EC1 to EC5 (Fig. 3B). The proportions of TPF sera that recognized each Dsg1 EC domain were 100% for EC1, 77.8% for EC2, 100% for EC3, 55.6% for EC4, and 66.7% for EC5 (Fig. 3C). Comparing the results for endemic TPF with those for sporadic JPF, JPF sera mainly bound to EC1 and showed much less frequent reactivity to C-terminal extracellular domains of Dsg1 (Fig. 3B). The proportions of JPF sera that recognized each Dsg1 EC domain were 100% for EC1, 55.6% for EC2, 22.2% for EC3, 11.1% for EC4, and 0% for EC5 (Fig. 3C).
These findings imply the existence of differences in disease development between endemic PF in Tunisia and sporadic PF in Japan. The THR and THC results suggest that individuals in this particular region initially have Abs against preDsg1, which mainly react with C-terminal extracellular domains such as EC4 and EC5. Individuals who develop the endemic form of PF (TPF) produce Abs that recognize epitopes in the N-terminal EC1 domain. This is why Tunisian patients with endemic PF have Abs against EC1 in addition to the other C-terminal domains of Dsg1. In Japanese patients with sporadic PF, however, anti-matDsg1 Abs targeting N-terminal domains of Dsg1 are apparently produced at disease onset because patients with sporadic PF rarely have Abs against the C-terminal domains.
4. Discussion
Our findings provide the first evidence that individuals without pemphigus produced Abs against the precursor form of Dsg1 in the peripheral blood. Previous reports that monoclonal Abs against preDsg1 were isolated from individuals without PF using a phage display technique, suggested that preDsg1-specific B cells could survive in healthy people [13, 19]. In other words, B cell tolerance is specific for matDsg1 on the cell surface and not for preDsg1 inside the cells, which is not exposed to the immune system. However, retaining preDsg1-specific B cells does not mean that PF will develop. In fact, Abs against preDsg1 are usually not detected in people without pemphigus because preDsg1-specific B cells are never activated to produce Abs even if they survive by escaping tolerance. Our results support the existence of preDsg1-specific B cells in individuals without PF and Abs against preDsg1 could be detected in certain situations, such as the preclinical stage of endemic PF.
The role of anti-preDsg1 Abs in healthy people remains unclear. Ab production against preDsg1 might precede disease development because some individuals without PF, in whose sera we detected anti-preDsg1 Abs in the current study, may develop endemic PF in the future. Anti-preDsg1 monoclonal Abs cloned from individuals without PF in a previous study showed many characteristics of so-called natural autoantibodies (NAAs), which have long been recognized as self-reactive Abs in healthy people [13, 21, 22]. NAAs are often directed against nuclear or cytoplasmic antigens, and their specificity is mostly carried by the heavy chain, which has few somatic mutations [13, 23]. One theory of autoimmunity is that B cells with NAA receptors become stimulated by continuous exposure to antigen through molecular mimicry or tissue injury, with resulting somatic mutations causing pathologic autoantibody production [13, 24, 25]. This could not be the case for anti-preDsg1 mAbs in previous studies because anti-preDsg1 and anti-matDsg1 mAbs from sporadic PF patients were derived from different B cell clones [13, 19]. However, it would be interesting to analyze the situation in the area where PF is endemic because the disease onset mechanism might be different. Of course, pemphigus does not develop immediately after breakdown of B cell tolerance, because activation of Dsg-specific autoreactive T cells is also presumably necessary for autoantibody production [13, 26, 27].
From our epitope mapping experiments, the epitopes recognized by anti-Dsg1 Abs expanded to include the N-terminal domains (EC1 and EC2) when TPF developed. In autoimmune blistering diseases, this epitope shift phenomenon is an important pathogenic mechanism in which autoimmunity extends to new epitopes in the same or different molecules [28, 29]. In fact, a previous study using sera from patients in Brazil with fogo selvagem showed that shifting of epitopes from C-terminal domains (EC5) to N-terminal domains was associated with the development of endemic PF [13, 30]. Our results suggest that the endemic forms of PF in Tunisia and Brazil may share similar epitope shift mechanisms of disease onset. Interestingly, the proportion of Abs against N-terminal domains of Dsg increased as the proportion of Abs against C-terminal domains decreased during disease development in a previous study performed using a mouse model of pemphigus vulgaris [31]. Based on these studies, it appears that, in certain circumstances, specific recognition of Dsg most likely occurs through Abs against C-terminal extracellular domains, which contain more isoform-specific residues, and then spreads to N-terminal domains, which are more conserved among the Dsg isoforms. On the contrary, Abs in patients with sporadic pemphigus mainly target N-terminal domains of Dsg without binding to C-terminal domains, suggesting there must be at least two ways of developing pemphigus, i.e., through epitope spreading from C-terminal domains (as occurs in endemic PF) and by the direct emergence of antibodies specific for N-terminal domains (sporadic pemphigus).
Even though we assume that anti-Dsg1 antibodies in THR and THC are mostly against the precursor form from our results, it is still unclear which part of preDsg1 they are binding to. It is speculated that anti-preDsg1 antibodies reacted with the propeptide themselves or conformation-dependent epitopes generated by combination of propeptide and some parts of matDsg1. Unfortunately, we were unable to address this question because we failed to produce a recombinant protein for the propeptide alone. In addition, some THR and THC sera (e.g. THR6, THC7) with no or weak reactivity with matDsg1 showed stronger reactivity with EC3 or EC5 of Dsg1 on the swapping molecules (Supplemental table 1). Although we could not fully explain the exact reason of this discrepancy, we presume that the swapping molecules may have higher sensitivity to detect Abs reacting with EC3-5 domains of matDsg1 which may be too low to be detected by IIF. Our results raise questions regarding the dynamic state of preDsg1 in living keratinocytes and further studies are needed to clarify the precise conditions of preDsg1 in the epidermis.
Our detection of autoantibodies against a precursor form of Dsg1 in the circulation in individuals without PF may help to elucidate the pathogenesis of pemphigus. Investigating the pathophysiological significance of these Abs may lead to a novel approach of treating the pre-development stage of pemphigus and the prevention of pemphigus development.
Supplementary Material
Results of indirect immunofluorescence using normal human skin with overnight incubation with the sera. A serum from a patient with Tunisian endemic PF showed cell surface staining (A), while none of the sera from healthy relatives of patients in Tunisia with endemic PF (THR) or healthy individuals from the area in Tunisia affected by endemic PF (THC) showed cell surface staining. (B–F). Scale bars: 50 μm.
Acknowledgments
Funding sources
This work was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan, Research on Measures for Intractable Diseases” Project: matching fund subsidy (H23-028) from Ministry of Health, Labour and Welfare, and the Keio Gijuku Academic Development Fund.
We thank Ms. Minae Suzuki for preparing the cryosections. This work was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan, Research on Measures for Intractable Diseases” Project: matching fund subsidy (H23-028) from Ministry of Health, Labour and Welfare, and the Keio Gijuku Academic Development Fund.
Footnotes
Conflict of interest
Dr. Kuroda and Dr. Hachiya are employed by Medical and Biological Laboratories Co. Ltd., who supply the Dsg1 and Dsg3 ELISA kits.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stanley JR, Amagai M. Pemphigus, bullous impetigo, and the staphylococcal scalded-skin syndrome. The New England journal of medicine. 2006;355:1800–1810. doi: 10.1056/NEJMra061111. [DOI] [PubMed] [Google Scholar]
- 2.Diaz LA, Sampaio SA, Rivitti EA, Martins CR, Cunha PR, Lombardi C, et al. Endemic pemphigus foliaceus (fogo selvagem). I. Clinical features and immunopathology. Journal of the American Academy of Dermatology. 1989;20:657–669. doi: 10.1016/s0190-9622(89)70079-7. [DOI] [PubMed] [Google Scholar]
- 3.Warren SJ, Lin MS, Giudice GJ, Hoffmann RG, Hans-Filho G, Aoki V, et al. The prevalence of antibodies against desmoglein 1 in endemic pemphigus foliaceus in Brazil. Cooperative Group on Fogo Selvagem Research. The New England journal of medicine. 2000;343:23–30. doi: 10.1056/NEJM200007063430104. [DOI] [PubMed] [Google Scholar]
- 4.Morini JP, Jomaa B, Gorgi Y, Saguem MH, Nouira R, Roujeau JC, et al. Pemphigus foliaceus in young women. An endemic focus in the Sousse area of Tunisia. Archives of dermatology. 1993;129:69–73. doi: 10.1001/archderm.129.1.69. [DOI] [PubMed] [Google Scholar]
- 5.Bastuji-Garin S, Souissi R, Blum L, Turki H, Nouira R, Jomaa B, et al. Comparative epidemiology of pemphigus in Tunisia and France: unusual incidence of pemphigus foliaceus in young Tunisian women. J Invest Dermatol. 1995;104:302–305. doi: 10.1111/1523-1747.ep12612836. [DOI] [PubMed] [Google Scholar]
- 6.Kallel Sellami M, Ben Ayed M, Mouquet H, Drouot L, Zitouni M, Mokni M, et al. Anti-desmoglein 1 antibodies in Tunisian healthy subjects: arguments for the role of environmental factors in the occurrence of Tunisian pemphigus foliaceus. Clinical and experimental immunology. 2004;137:195–200. doi: 10.1111/j.1365-2249.2004.02493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abida O, Kallel-Sellami M, Joly P, Ben Ayed M, Zitouni M, Masmoudi A, et al. Anti-desmoglein 1 antibodies in healthy related and unrelated subjects and patients with pemphigus foliaceus in endemic and non-endemic areas from Tunisia. Journal of the European Academy of Dermatology and Venereology : JEADV. 2009;23:1073–1078. doi: 10.1111/j.1468-3083.2009.03265.x. [DOI] [PubMed] [Google Scholar]
- 8.Loiseau P, Lecleach L, Prost C, Lepage V, Busson M, Bastuji-Garin S, et al. HLA class II polymorphism contributes to specify desmoglein derived peptides in pemphigus vulgaris and pemphigus foliaceus. Journal of autoimmunity. 2000;15:67–73. doi: 10.1006/jaut.2000.0388. [DOI] [PubMed] [Google Scholar]
- 9.Abida O, Zitouni M, Kallel-Sellami M, Mahfoudh N, Kammoun A, Ben Ayed M, et al. Tunisian endemic pemphigus foliaceus is associated with the HLA-DR3 gene: anti-desmoglein 1 antibody-positive healthy subjects bear protective alleles. The British journal of dermatology. 2009;161:522–527. doi: 10.1111/j.1365-2133.2009.09207.x. [DOI] [PubMed] [Google Scholar]
- 10.Posthaus H, Dubois CM, Muller E. Novel insights into cadherin processing by subtilisin-like convertases. FEBS letters. 2003;536:203–208. doi: 10.1016/s0014-5793(02)03897-8. [DOI] [PubMed] [Google Scholar]
- 11.Wahl JK, 3rd, Kim YJ, Cullen JM, Johnson KR, Wheelock MJ. N-cadherin-catenin complexes form prior to cleavage of the proregion and transport to the plasma membrane. The Journal of biological chemistry. 2003;278:17269–17276. doi: 10.1074/jbc.M211452200. [DOI] [PubMed] [Google Scholar]
- 12.Futei Y, Amagai M, Ishii K, Kuroda-Kinoshita K, Ohya K, Nishikawa T. Predominant IgG4 subclass in autoantibodies of pemphigus vulgaris and foliaceus. Journal of dermatological science. 2001;26:55–61. doi: 10.1016/s0923-1811(00)00158-4. [DOI] [PubMed] [Google Scholar]
- 13.Yamagami J, Kacir S, Ishii K, Payne AS, Siegel DL, Stanley JR. Antibodies to the desmoglein 1 precursor proprotein but not to the mature cell surface protein cloned from individuals without pemphigus. Journal of immunology. 2009;183:5615–5621. doi: 10.4049/jimmunol.0901691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ishii K, Amagai M, Hall RP, Hashimoto T, Takayanagi A, Gamou S, et al. Characterization of autoantibodies in pemphigus using antigen-specific enzyme-linked immunosorbent assays with baculovirus-expressed recombinant desmogleins. Journal of immunology. 1997;159:2010–2017. [PubMed] [Google Scholar]
- 15.Sharma PM, Choi EJ, Kuroda K, Hachiya T, Ishii K, Payne AS. Pathogenic anti-desmoglein MAbs show variable ELISA activity because of preferential binding of mature versus proprotein isoforms of desmoglein 3. J Invest Dermatol. 2009;129:2309–2312. doi: 10.1038/jid.2009.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yokouchi M, Saleh MA, Kuroda K, Hachiya T, Stanley JR, Amagai M, et al. Pathogenic epitopes of autoantibodies in pemphigus reside in the amino-terminal adhesive region of desmogleins which are unmasked by proteolytic processing of prosequence. J Invest Dermatol. 2009;129:2156–2166. doi: 10.1038/jid.2009.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kwon EJ, Yamagami J, Nishikawa T, Amagai M. Anti-desmoglein IgG autoantibodies in patients with pemphigus in remission. J Eur Acad Dermatol Venereol. 2008;22:1070–1075. doi: 10.1111/j.1468-3083.2008.02715.x. [DOI] [PubMed] [Google Scholar]
- 18.Chan PT, Ohyama B, Nishifuji K, Yoshida K, Ishii K, Hashimoto T, et al. Immune response towards the amino-terminus of desmoglein 1 prevails across different activity stages in nonendemic pemphigus foliaceus. The British journal of dermatology. 2010;162:1242–1250. doi: 10.1111/j.1365-2133.2010.09696.x. [DOI] [PubMed] [Google Scholar]
- 19.Ishii K, Lin C, Siegel DL, Stanley JR. Isolation of pathogenic monoclonal anti-desmoglein 1 human antibodies by phage display of pemphigus foliaceus autoantibodies. J Invest Dermatol. 2008;128:939–948. doi: 10.1038/sj.jid.5701132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hanakawa Y, Schechter NM, Lin C, Garza L, Li H, Yamaguchi T, et al. Molecular mechanisms of blister formation in bullous impetigo and staphylococcal scalded skin syndrome. The Journal of clinical investigation. 2002;110:53–60. doi: 10.1172/JCI15766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lacroix-Desmazes S, Kaveri SV, Mouthon L, Ayouba A, Malanchere E, Coutinho A, et al. Self-reactive antibodies (natural autoantibodies) in healthy individuals. Journal of immunological methods. 1998;216:117–137. doi: 10.1016/s0022-1759(98)00074-x. [DOI] [PubMed] [Google Scholar]
- 22.Mouthon L, Nobrega A, Nicolas N, Kaveri SV, Barreau C, Coutinho A, et al. Invariance and restriction toward a limited set of self-antigens characterize neonatal IgM antibody repertoires and prevail in autoreactive repertoires of healthy adults. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:3839–3843. doi: 10.1073/pnas.92.9.3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Avrameas S, Ternynck T, Tsonis IA, Lymberi P. Naturally occurring B-cell autoreactivity: a critical overview. Journal of autoimmunity. 2007;29:213–218. doi: 10.1016/j.jaut.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 24.Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC. Predominant autoantibody production by early human B cell precursors. Science. 2003;301:1374–1377. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- 25.Goodnow CC, Sprent J, Fazekas de St Groth B, Vinuesa CG. Cellular and genetic mechanisms of self tolerance and autoimmunity. Nature. 2005;435:590–597. doi: 10.1038/nature03724. [DOI] [PubMed] [Google Scholar]
- 26.Soulas P, Woods A, Jaulhac B, Knapp AM, Pasquali JL, Martin T, et al. Autoantigen, innate immunity, and T cells cooperate to break B cell tolerance during bacterial infection. The Journal of clinical investigation. 2005;115:2257–2267. doi: 10.1172/JCI24646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takahashi H, Amagai M, Nishikawa T, Fujii Y, Kawakami Y, Kuwana M. Novel system evaluating in vivo pathogenicity of desmoglein 3-reactive T cell clones using murine pemphigus vulgaris. Journal of immunology. 2008;181:1526–1535. doi: 10.4049/jimmunol.181.2.1526. [DOI] [PubMed] [Google Scholar]
- 28.Tchernev G, Orfanos CE. Antigen mimicry, epitope spreading and the pathogenesis of pemphigus. Tissue antigens. 2006;68:280–286. doi: 10.1111/j.1399-0039.2006.00677.x. [DOI] [PubMed] [Google Scholar]
- 29.Di Zenzo G, Thoma-Uszynski S, Calabresi V, Fontao L, Hofmann SC, Lacour JP, et al. Demonstration of Epitope-Spreading Phenomena in Bullous Pemphigoid: Results of a Prospective Multicenter Study. J Invest Dermatol. 2011 doi: 10.1038/jid.2011.180. [DOI] [PubMed] [Google Scholar]
- 30.Li N, Aoki V, Hans-Filho G, Rivitti EA, Diaz LA. The role of intramolecular epitope spreading in the pathogenesis of endemic pemphigus foliaceus (fogo selvagem) The Journal of experimental medicine. 2003;197:1501–1510. doi: 10.1084/jem.20022031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anzai H, Fujii Y, Nishifuji K, Aoki-Ota M, Ota T, Amagai M, et al. Conformational epitope mapping of antibodies against desmoglein 3 in experimental murine pemphigus vulgaris. Journal of dermatological science. 2004;35:133–142. doi: 10.1016/j.jdermsci.2004.03.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Results of indirect immunofluorescence using normal human skin with overnight incubation with the sera. A serum from a patient with Tunisian endemic PF showed cell surface staining (A), while none of the sera from healthy relatives of patients in Tunisia with endemic PF (THR) or healthy individuals from the area in Tunisia affected by endemic PF (THC) showed cell surface staining. (B–F). Scale bars: 50 μm.