Abstract
Identification of murine mammary stem cells (MaSCs) has been attempted with various in vitro and in vivo assays. While, the in vivo repopulation assay remains as the most definitive assay for MaSC detection, it is expensive, time-consuming, and technically challenging. The in vitro mammosphere assay was considered unreliable because of major concerns about its clonal origin. In the current study, co-culture experiments with mammary cells from fluorescent protein transgenic mice and time-lapse video microscopy revealed that > 90% mammospheres formed from sorted basal epithelial-enriched cells were of clonal origin in terms of stem cell. These basal-cell derived mammospheres were further distinguished morphologically in a 3-dimensional extracellular matrix culture and functionally in the in vivo repopulation assay. Transplant of single mammospheres or the resultant 3-dimensional solid structures into gland-free mammary fat pads yielded a 70% success rate of multilineage mammary gland reconstitution. Thus, this in vitro sphere formation and differentiation assay is a reliable alternative to the in vivo repopulation assay for the study of MaSCs.
Keywords: Mammary stem cell, Mammosphere, Lineage differentiation, In vivo repopulation
Introduction
The mammary fat pad in vivo transplant (IVT) assay is widely used for demonstrating multilineage differentiation of murine mammary stem cells (MaSCs). However, this assay is costly, time-consuming, and technically challenging (Stingl, 2009). A less expensive and faster assay for qualifying MaSCs is the in vitro mammosphere assay, in which cells with self-renewal properties, such as stem cells, form spherical structures. This assay was established to identify MaSCs, similar to the neurosphere assay (Dontu et al., 2003). Yet, these assays have been unreliable because of concerns about the clonal origin of the resulting spheres (Deleyrolle, Rietze, and Reynolds, 2008; Louis et al., 2008; Reynolds and Rietze, 2005; Singec et al., 2006; Stingl, 2009)
In the current study, we found that murine mammospheres formed from lineage-specific epithelial-enriched fractions, purified by fluorescence-activated cell sorting (FACS), were small and non-aggregated when compared with the spheres formed from primary unfractionated mammary cells. The mammospheres were further differentiated into morphologically distinct basal or luminal lineage-specific structures in a 3-dimensional (3D) extracellular matrix (ECM). IVT analysis indicated that single spheres or 3D solid structures derived from the MaSC-enriched basal cells repopulated cleared fat pads with very high efficiency, demonstrating that these single spheres or 3D structures originated from mammary stem cells. Furthermore, co-culture experiments with purified basal mammary cells from green and red fluorescent protein transgenic mice demonstrated that most of these spheres were derived from single stem cells, indicating clonal origin. This report provides evidence that in vitro mammosphere formation, in combination with subsequent single sphere differentiation in 3D-ECM, provides a good alternative to the commonly used IVT assay for the identification and quantification of mouse basal MaSCs.
Materials and methods
Mice
C57BL/6, BALB/c, UBC-GFP (C57BL/6), and Actb-DsRed.T3 (C57BL/6), originally purchased from the Jackson Laboratory, were bred and maintained in our animal facility according to institutional guidelines. All experiments were approved by the Institutional Animal Care and Use Committee of the University of Texas Health Science Center at San Antonio.
Mammary cell preparation
Inguinal and thoracic mammary glands were dissected from individual female mice and placed in a 50-mL tube containing dissociation medium (1 part 10x gentle collagenase/hyaluronidase [Catalog No. 07919] and 9 parts EpiCult-B complete medium [Catalog No. 05610] supplemented with 5% fetal bovine serum [FBS] and 0.05 mg/mL gentamicin) (StemCell Technologies, Vancouver, Canada). The tissue was digested for 15 h at 37°C in a 5% CO2 incubator. The resultant organoid pellet was resuspended first in 0.64% NH4Cl for 5 min and spun down, followed by 0.25% trypsin-EDTA for 1 to 3 min and spun down, and 5 mg/mL dispase and 0.1 mg/mL DNase I for 1 to 3 min and spun down. The cell suspension was then filtered through a 40-micron mesh, and labeled with various antibodies (see below) for further purification. The detailed protocol can be found in our previous publication (Bandyopadhyay, Dong, and Sun, 2012).
Antibodies
Antibodies included anti-CD24-PE, anti-CD49f-FITC, biotinylated anti-CD31/CD45/Ter119 cocktail (StemCell Technologies), anti-CD16/CD32 (BD Pharmingen), anti-CD24-PE-Cy7 (Biolegend), and anti-CD49f-PE-Cy5 (BD Biosciences). APC-conjugated streptavidin (Invitrogen) was used to visualize the biotinylated antibody cocktail.
Cell labeling and flow cytometry sorting
All incubations and washes were performed in HBSS supplemented with 2% FBS. Cells were first incubated with anti-CD16/CD32 (Fcγ III/II receptor) for 10 min on ice to reduce Fc receptor-mediated binding, followed by a 15-min incubation on ice with the biotinylated CD31/CD45/Ter119 antibody cocktail. After washing, cells were incubated with anti-CD24-PE, anti-CD49f-FITC, and streptavidin-APC on ice for 10 min. After 1 more wash, cells were sorted (FACSAria cell sorter, BD Bioscience) according to the gates illustrated in Fig. S1b. Purity of sorted populations was routinely > 95%.
Mammosphere assay
Sphere formation was performed in ultralow attachment 96-well plates (Corning) with mouse EpiCult-B complete medium (150 μL per well) supplemented with 2% B27 without vitamin A (Invitrogen), 20 ng/mL bFGF, 20 ng/mL EGF, 10 μg/mL heparin, 10 μg/mL insulin, 1 μg/mL hydrocortisone, and 50 μg/mL gentamicin (referred to as mammosphere or MMS medium). Each sorted cell population was plated at 2 different densities with 3 replicates per density: 5000 and 10 000 cells/well for the basal cells; 1000 and 2000 cells/well for luminal cells; and 5000 and 10 000 cells/well for stromal cells. For co-culture experiments, a 1:1 ratio was used for mixing the same lineage cells and resultant final plating density was 20 000 cells/well for basal cells and 10 000 cells/well for stromal cells. Cells were cultured at 37°C, in 5% CO2. After 7 to 10 days, the culture was agitated with a P200 micropipette to segregate individual spheres to facilitate quantification.
3-dimensional extracellular matrix (Matrigel) single sphere differentiation assay
Thirty to 50 individual mammospheres were handpicked using a dissecting microscope and resuspended in 60 μL chilled Matrigel (BD Biosciences). The sphere-Matrigel drop was plated at the center of a well in ultralow attachment 6-well plates, and was allowed to solidify inside a 37°C, 5% CO2 incubator for 15 min before covering with MMS medium supplemented with 5% FBS. After incubation at 37°C for 9 days (with fresh medium added at day 5), the number of 3D structures were counted. For each sample, at least 3 wells (approximately 120 spheres) were analyzed for morphology.
Cleared fat pad transplant and analysis
Single mammospheres or 3D structures (isolated from Matrigel by incubating them for 30 min in medium containing 2.5 mg/mL dispase) were resuspended in HBSS with 0.2% trypan blue (Sigma) and 50% Matrigel. In 5 to 10 μL volumes, they were injected with a Hamilton syringe into the inguinal glands of 21-day-old virgin female mice that had been cleared of endogenous epithelium. The Hamilton syringe was rinsed and checked to ensure the absence of spheres or 3D structures after injection, indicating successful injection. Recipient glands were removed 8 to 10 weeks later for evaluation by GFP imaging and/or whole mount Carnoy staining. An outgrowth was defined as an epithelial structure that included ducts with terminal end buds (virgin) or ducts with lobules (pregnant).
Time-lapse video microscopy
Sorted basal or stromal cells were placed in a 200-μL MMS medium droplet containing 20 000 cells in a 35-mm suspension culture dish (Corning). The drop was covered with 3 mL pre-warmed mineral oil (Sigma) and incubated in a humidified atmosphere at 37°C and 5% CO2 inside the climate chamber of a Nikon BioStation-IM. Images were captured every 30 min for a continuous 192 h.
Calculation of MaSC frequency
In this study, we refer the cells that are capable of forming spheres in suspension culture and subsequently differentiating into solid structures in 3D-ECM culture operationally as sphere formation/differentiation initiating cells (SFD-ICs) to distinguish them from the mammary repopulating units (MRUs) used previously to describe cells that can engraft a cleared mammary fat pad (Stingl et al. 2006). The basal SFD-IC frequency within the basal cell (% SFD-ICb) or total epithelial (% SFD-ICe) population was thus calculated as follows:
| [1] |
| [2] |
| [3] |
%(3D solid) was defined as the percentage of spheres that formed a solid structure in Matrigel culture of the total number of spheres plated; % Basal cell was the percentage of cells gated as Lin−CD24+CD49fhi; % Total epithelial cells was the sum of percentage cells gated as Lin−CD24+CD49fhi (basal) and Lin−CD24hiCD49flow (luminal).
Results
Characterization of in vitro mammosphere formation, proliferation, and differentiation
Primary unfractionated mammary cells (MC) or MC depleted of endothelial (CD31+) and hematopoietic (CD45+, TER119+) cells (Lin−MC) form mammospheres in suspension culture that often are large (> 100 μm) because multiple small spheres form aggregates (Fig. S1a). In contrast, Lin−CD24+CD49fhi basal MaSC-enriched cell populations (hereafter called basal cells) and Lin−CD24hiCD49flo luminal MaSC-enriched cell populations (hereafter called luminal cells)(Fig. S1b) only formed small spheres of 43 ± 9 μm (all stated values are mean ± SD) (n = 210) and 45 ± 9 μm (n = 63) after 7-days of culture (Fig. 1a, Fig. S1c). These spheres did not appear to merge with each other to form larger spheres, even when cultured for > 2 weeks. However, spheres formed from Lin−CD24−CD49f− stromal cells frequently merged into large spheres (Fig. 1a). The sphere formation efficiency (SFE, number of spheres per 1000 cells) was 7 ± 2 (n = 6) for the purified basal cell population and 47 ± 17 (n = 6) for the luminal cell population (Fig. 1b). The former usually did not become evident until 48 h after plating, but spheres formed from the latter were formed overnight.
Figure 1.
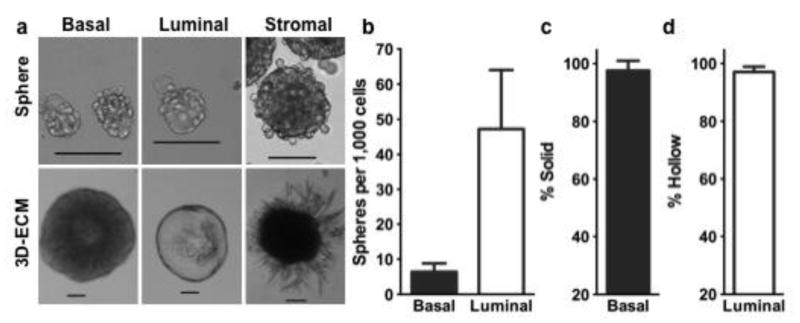
Characteristics of mammospheres derived from different fractions of fluorescence-activated cell sorting (FACS) sorted cells from wild type C57BL/6 mice (a). Single spheres were further plated in Matrigel to form corresponding morphologically unique 3-dimentional extracellular matrix (3D-ECM) structures (a). Bar graphs showing the sphere formation efficiency (b) and percentage of solid (c) or hollow (d) structures in 3D-ECM culture. Scale bars, 100 μm. The mean and standard deviation values, and the number of replicates used for each plotted figures are stated in the Results.
To further investigate the proliferation and differentiation potential of these different types of spheres, we seeded individual spheres into a 3D-ECM culture system (Matrigel). Spheres derived from the basal cells generated predominately solid spherical structures (Fig. 1a, c; Fig. S2a–d), at a plating efficiency of > 90%. Spheres derived from the luminal cells produced mainly hollow spherical structures (Fig. 1a, d), which could be completely hollow (Fig. S2e), partially hollow (Fig. S2f) or irregular (Fig. S2g, h). After 9 days of Matrigel culture, the average diameter of these solid 3D structures was 390 ± 91 μm (n = 23) and hollow 3D structures was 472 ± 219 μm (n = 49) (Fig. S1d, e). Spheres derived from the stromal cells generated branch-like structures (Fig. 1a), which gave rise to oil-filled adipocytes after 2 weeks of culture (data not shown). These findings are consistent with previous observations that MaSCs have an ability to form solid organoids, and luminal progenitors form acinar (hollow) structures in Matrigel cultures (Lim et al., 2009; Shackleton et al., 2006; Stingl et al., 2006). Various structures derived from spheres of luminal origin may represent different progenitor populations within the hierarchy of the luminal lineage. Together, these data showed that only the spheres formed from the basal cells gave rise to solid structures in Matrigel culture, suggesting that these spheres could be initiated from MaSCs (Stingl et al., 2006).
Maintenance of in vivo repopulating potential of basal cell derived spheres and their corresponding 3D solid structures
We used the IVT assay to demonstrate that the solid 3D structures observed in Matrigel culture, initiated from basal cell derived spheres, contain MaSCs that can reconstitute mammary tissue in vivo. Transgenic green fluorescent protein (GFP) C57BL/6 mice were used as donors for mammary cell isolation, FACS sorting, and 2-step in vitro culture of sphere and 3D structures. Upon transplant of a single 3D solid structure per cleared fat pad (CFP), we obtained 8 to 9 positive outgrowths from 12 transplants per trial, in 4 independent trials (Fig. S1f). This represented an overall average engraftment frequency of 71 ± 2%. Single 3D hollow structures did not repopulate CFPs in a similar setting.
The 3D solid structures containing cells with repopulating ability were generated from single mammospheres; therefore, these spheres must also have contained in vivo regenerating cells. To verify this, spheres used to generate the 3D structures from the same donors were tested for presence of MaSCs with in vivo repopulation capacity. Mammospheres from 1 donor demonstrating a plating efficiency of 97% in Matrigel culture had a corresponding IVT engraftment frequency of 75% (9 of 12 total CFP) from single spheres, which is comparable to the IVT of single 3D solid structures (8 of 12 total CFP) (Fig. 2a). Mammospheres from another donor demonstrating a plating efficiency of 36% (or approximately 1 of 3 spheres that yielded solid 3D structures) in Matrigel culture had an IVT engraftment frequency of 25% (3 of 12 total CFP) from single spheres, which is one-third of the engraftment frequency of its corresponding single 3D solid structure (9 of 12 total CFP) (Fig. 2a). This result suggests that the 3D solid structure not only correlates with the basal MaSC repopulating function in vivo, but also is a better predictor than the mammosphere for in vivo repopulation capability.
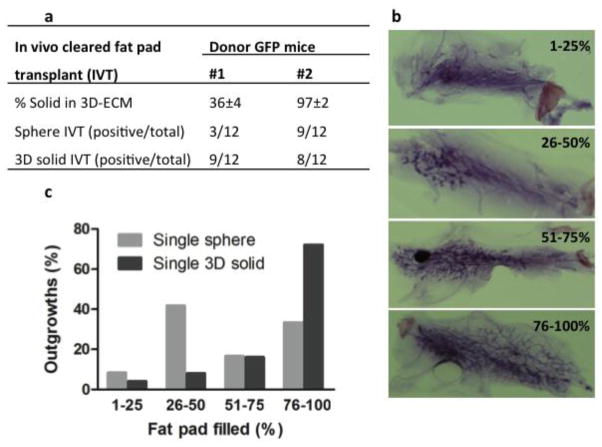
Figure 2.
In vivo cleared mammary fat pad transplant of single mammosphere and single 3D-ECM solid structure derived from FACS sorted basal cell fraction (CD24+CD49fhi) of two transgenic green fluorescent protein (GFP) mice (a). Representative whole mount staining pictures showing different percentage of fill in cleared fat pad with regenerated GFP glands (b). Bar graphs showing percentage of fill in cleared fat pad for all regenerated glands derived from single mammosphere (light gray bars) or single 3D-ECM solid structure (dark gray bars) with in vivo transplant (c).
Subsequent analysis of outgrowths revealed that > 25% CFP area was filled with the regenerated mammary ducts in > 90% regenerated glands (Fig. 2b, c). In addition, mice transplanted with either a single sphere or a 3D solid structure were impregnated and demonstrated full alveolar development (Fig. S1g), indicating that these sphere formation/differentiation initiating cells (SFD-ICs) were capable of multilineage differentiation. To further examine whether these SFD-ICs had retained self-renewal capacity, we prepared single cell suspensions from the primary mammary gland outgrowths formed by a single 3D solid structure and transplanted these cells to 2 CFPs at a cell density of 2 × 105 per CFP. The secondary recipient was impregnated 8 weeks after transplant. Both fat pads displayed full secondary gland reconstitution (75% to 95% outgrowth) with alveolar structures similar to those of primary outgrowth (Fig. S1g); this showed that SFD-ICs also had retained self-renewal ability during the sphere culture and subsequent 3D structure formation.
Time-lapse video microscopy of mammosphere initiation
If, in the above described assays, each mammosphere originated from a single stem cell, then the number of spheres that form 3D solid structures would indicate the number of stem cells within the original cell suspension. To prove the stem cell clonal origin of these spheres, we performed single cell culture in low attachment 384-well plates. However, we found very few spheres formed (3 of 3840 basal cells) and only after an extended 25-day culture, in contrast with approximately 28 spheres from the same number of cells in the 96-well suspension culture for 7 days. All 3 spheres differentiated into solid structures in Matrigel culture, indicating that isolated single SFD-ICs can give rise to single spheres but with much lower frequency and longer culture time.
We suspected that paracrine cues from other cells may have been necessary for efficient sphere formation in suspension culture. Thus, we sought to culture cells at the same density as suspension culture in semisolid methylcellulose, which prevented cell to cell contact yet allowed diffusion of soluble factors. In this semisolid culture, very few spheres were formed. These results indicated that cell-cell contact and/or free movement might be required for efficient sphere formation. Thus, we used time-lapse microscopy to monitor sphere formation from basal cells in suspension culture.
Our findings (n = 2 trials) showed that 49% of 85 total spheres were initiated from single cell proliferation (Supplemental Video 1), 31% from 2 cells (Supplemental Video 2), and 20% from > 2 cells (Supplemental Video 3). The supplemental videos also demonstrated that the growth of the spheres was due to cell proliferation without sphere aggregation despite the fact that the cells were plated at high density. In contrast, spheres derived from stromal cells were formed from random aggregation among single cells with no apparent cell proliferation (Supplemental Video 4). These observations showed that basal cell derived spheres were formed from proliferation rather than cell aggregation and spheres could initiate from single or multiple cells. Yet, the question remained whether the approximately 50% spheres initiated from multiple cells were formed by single or multiple stem cells.
Co-culture of genetically labeled mammosphere-forming cells
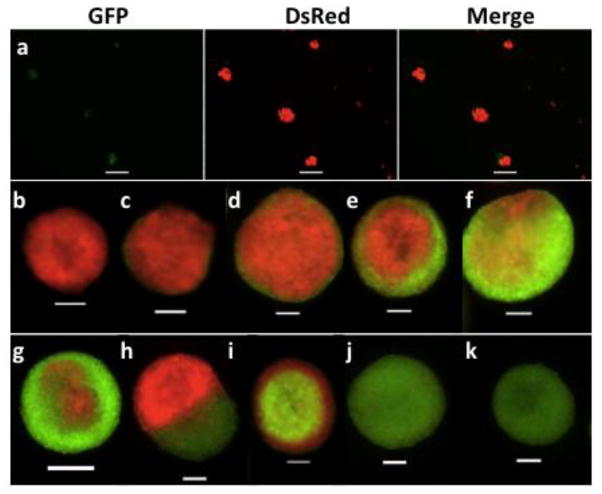
To determine whether single or multiple stem cells were involved in mammosphere initiation, we employed a culture system of mixed basal cell populations isolated from DsRed and GFP mice. Only stem cells have the capability to self-renew, differentiate, and produce a large number of progeny; therefore, we expected that sphere differentiation in Matrigel, using basal cells carrying different labels, would allow us to distinguish spheres formed from single or multiple different stem cells. Assuming equal proliferation potential of individual SFD-ICs, sphere formation involving 2 SFD-ICs should produce 3 random combinations: green with green (GG), green with red (GR), and red with red (RR); sphere formation involving 3 SFD-ICs should produce 4 random combinations: GGG, GGR, GRR, and RRR. Thus, we would expect at least 30% or 50% dual-color structures if the mammospheres were all formed by 2 or 3 SFD-ICs. We found that the resulting spheres of these mixed basal cell co-cultures had single color and mixed color (Fig. 3a). When these spheres were used for 3D structure formation in Matrigel culture in 2 separate experiments (Table 1), we found that the average percentage of single color 3D solid structure (Fig. 3b, k) was 44%. A similar average percentage (51%) of the 3D solid structures showed 2 colors, but these had most cells with 1 color in the center of each structure (Fig. 3c–e, i, and j). Only a small percentage (< 7%) of 3D solid structures showed a more equal mix of dual colors (hereafter termed true chimeras) (Fig. 3f–h). The true chimera shown in Fig. 3h was extremely rare and was observed only once in all experiments. These percentage values were consistent with the above prediction suggesting that a significant fraction of mammospheres were formed by ≥ 2 cells.
Figure 3.
Mammospheres (a) derived from co-culture of FACS sorted basal cells of GFP and DsRed mice, and subsequent 3D-ECM culture derived solid structures (b–k). Scale bars, 100 μm.
Table 1.
The 3-dimensional solid structures formed from single mammospheres derived from co-culture experiments
| Co-culture group | No. of 3D | Color 1
|
Color 2
|
Chimeras | C1/C2 (expected) | ||
|---|---|---|---|---|---|---|---|
| Single | P-single | Single | P-single | ||||
| DsRed/GFP | 222 | 23% | 53% | 11% | 8% | 6% | 3.2 (2.0) |
| DsRed/WT | 112 | 23% | 23% | 32% | 18% | 3% | 1.3 (1.4) |
Mammospheres used for these 3-dimensional (3D) structures were formed from co-culture of basal cells from DsRed mice (color 1) with basal cells from GFP or wild type (WT) C57BL/6 mice (color 2); P-single refers to predominantly single color.
To address whether they were all stem cells, we performed an in vitro Matrigel culture with series passage of cells derived from single 3D solid structures with 2 colors because MaSCs were expected to repeatedly regenerate 3D structures. Cells dissociated from 21 of 30 individual 3D structures were able to grow and form 3D structures upon first passage in Matrigel, translating to a 70% passage rate, which agrees perfectly with our IVT engraftment efficiency using single 3D solid structures. We found that cells dissociated from all 12 of the 3D structures that had a predominant color generated 3D structures with only 1 color, which was the dominant color of the original 3D structure, after 3 consecutive passages (Fig. S3a, b). This observation was evidence against the possibility that the predominantly single color 3D structures were formed by > 1 MaSC and suggested that the other cell(s) that participated in the formation of the sphere and 3D structure was likely a non-stem cell supporting the growth of the sphere and 3D structure. This argument was further supported by the observation that cells derived from 8 of 9 true chimera 3D structures also generated only 1 color 3D structure after 3 consecutive in vitro passages in Matrigel culture, and 1 true chimera 3D structure produced daughter 3D structures with either one or the other color (Fig. S3c). This indicates that although there were approximately 5% true chimera 3D structures from the co-culture experiments (Table 1), only 11% (1 of 9) of these true chimera 3D structures resulted from a mix of SFD-ICs with different color. This translates to 0.6% of all 3D solid structures are formed by two or more SFD-ICs with different color. Assuming a similar frequency for the formation of spheres by 2 SFD-ICs with the same color, we estimated that 1.8% spheres and 3D structures might be initiated from ≥ 2 SFD-ICs.
There were uneven percentages of 3D structures formed by red, green, and wild type SFD-ICs when the basal cells with 2 different colors were mixed in equal number (Table 1). To address this, we calculated the ratio of the numbers of 3D structures with red color versus those with green or wild type, and compared it with the expected ratio (based on the ratio of the basal cell number multiplied by SFE of these cells cultured alone). These values were comparable for different co-culture combinations (Table 1), revealing that the red basal cells had higher SFE than the green basal cells. Taken together, these results indicated a clonal origin of a single stem cell for > 90% mammospheres and 3D solid structures.
To test whether the clonal origin of 3D structures in vitro could be validated in vivo, we performed IVT of single 3D structures characterized as having predominantly single color and true chimeras. We also co-injected 1 of the 3D solid structures from DsRed mice together with 1 of the 3D solid structures from GFP mice in 5 CFPs as positive controls, which produced 100% engraftment frequency. All 5 repopulated CFPs were characterized by 2 separate outgrowths without any mosaic branches (Fig. 4a). In contrast, regenerated glands from predominantly single color 3D solid structures (n = 5) showed predominantly 1 color with no branches (Fig. 4b) or 1 to 2 branches showing mosaic appearance (Fig. 4c). Four of 5 regenerated glands from true chimera 3D solid structures also showed a single color dominant pattern like those shown in Fig. 4b, c, and only 1 showed a mosaic appearance throughout the whole gland (Fig. 4d). These results are consistent with our observations from serial in vitro passages of the dual color 3D structures suggesting that the glands regenerated by the dual color 3D structures were mostly from a clonal stem cell. If both DsRed and GFP SFD-ICs were present in the dual color 3D structures, we would have seen either 2 separate outgrowths like that in Fig. 4a or complete mosaic outgrowth like that in Fig. 4d.
Figure 4.
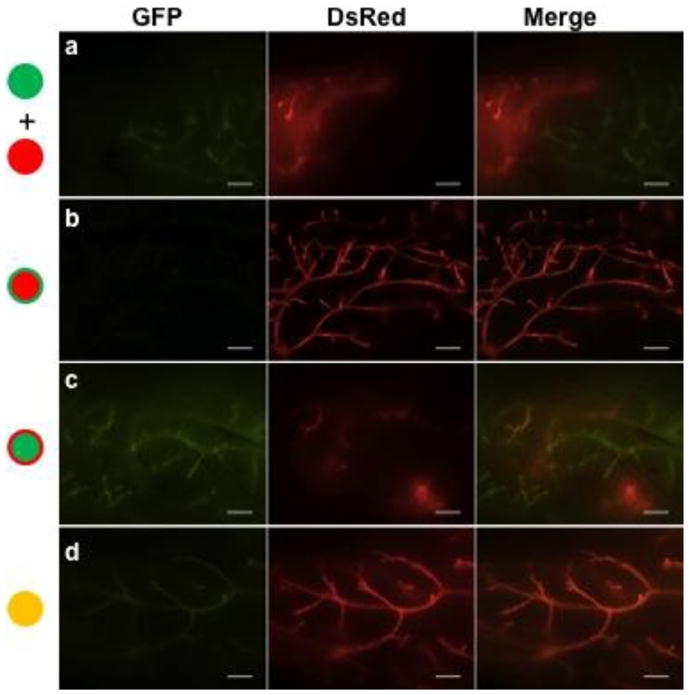
Regenerated glands from in vivo transplant of (a) one 3D solid structure from DsRed mice together with one 3D solid structure from GFP mice or of (b–d) single 3D structures derived from spheres formed from co-culture of FACS sorted basal cells of GFP and DsRed mice that were characterized by predominantly red (b), predominantly green (c), and true chimeras (d). Scale bars, 500 μm.
When stromal cells from DsRed and GFP transgenic mice were co-cultured, all resulting spheres were homogenous chimeras (Fig. S4a), indicating random single cell aggregation as evidenced in time-lapse microscopy. Subsequent Matrigel culture of these chimeric spheres yielded branched structures (Fig. S4b) similar to those formed by stromal cells from wild-type mouse (Fig. 1c), and resulted in no mammary tissue outgrowth upon IVT of these single 3D structures.
MaSC quantification and verification
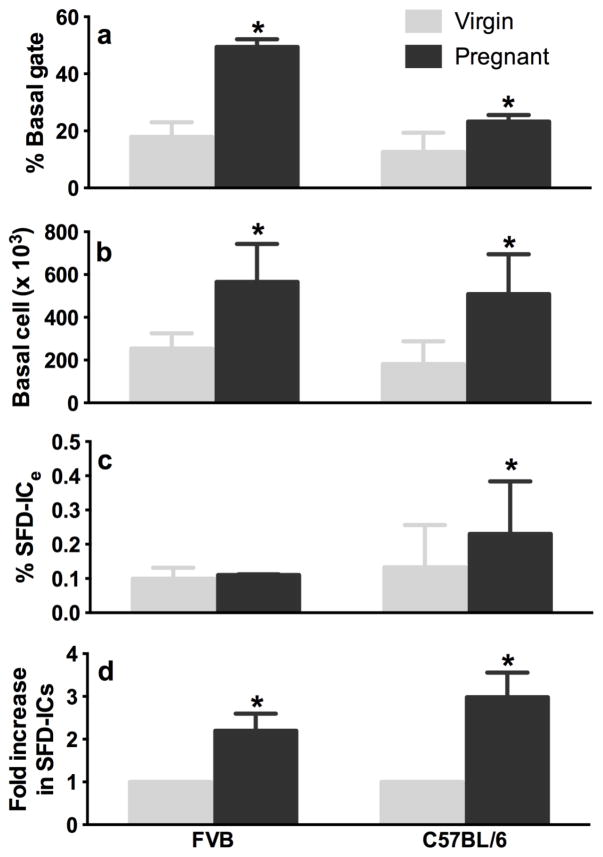
Given the stem cell clonal nature of most spheres derived from basal cells, the in vitro sphere formation and differentiation (SFD) assay described above enables us to estimate the frequency of SFD-IC within the basal cell (% SFD-ICb) or within the total epithelial cell population (% SFD-ICe) (see formula [2] and [3] in Methods), and the absolute SFD-IC number (% SFD-ICb x No. of basal cells) in the murine mammary gland. To verify that our SFD assay quantified MaSCs similarly to that of the IVT assay, we repeated previous experiments that had solely relied on the IVT assay as a measure of MaSC number. A previous study showed that mammary glands of 12.5 day-pregnant 8-week-old FVB/NJ mice contain 11.3-fold more basal MRUs compared with non-pregnant mice (Asselin-Labat et al., 2010). We compared the SFD-IC number of virgin versus age-matched pregnant mice in FVB and C57BL/6 by using our SFD assay. We found an average of 2.16-fold (in FVB) or 2.98-fold (in C57BL/6) increase in the absolute number of SFD-ICs in a mid-pregnancy gland versus virgin gland (Fig. 5). The increase was mainly due to increased total number of basal cells in FVB mice because the %SFD-ICe remained similar in virgin and pregnant glands (Fig. 5). In contrast, the % SFD-ICe also increased upon pregnancy in C57BL/6, which resulted in a greater increase in SFD-IC number than in FVB mice (Fig. 5). Thus, our SFD assay recapitulated the difference in MaSC numbers between virgin and pregnant mice (Asselin-Labat et al., 2010), even though the magnitude of the difference was much lower. However, in the previous study, repopulating cells from mid-pregnant glands showed a 2.5-fold lower self-renewing capacity than MRUs isolated from virgin mice in secondary transplant assays, which translated to a 4.52-fold increase of true repopulating stem cells compared with virgin glands. This difference approximates the 2.16- to 2.98-fold increase in the absolute number of SFC-ICs in a mid-pregnancy gland versus virgin gland.
Figure 5.
Percent basal cell gated within the Lin−CD24+CD49fhi fraction (a), total number of basal cells per mouse (b), %SFD-IC in total epithelial cell (c) and fold increase (d) in basal SFD-IC number from pairs of age-matched virgin (light gray bars) and 12.5-day pregnant (dark gray bars) FVB (n = 4 pairs of same age, approximately 2.5-month old) or C57BL/6 (n = 3 pairs of mice of 2, 3, and 4-month old) mice. Asterisks denote significant difference between virgin and pregnant mice at P < 0.05.
Discussion
The combined findings from time-lapse microscopy and co-culture experiments suggest that most mammospheres formed from basal cells are clonal expansions of stem cells. This does not necessarily indicate a single cell origin because only approximately 50% spheres were initiated from a single cell. This conclusion appears to support the concept that contacts with other cells may provide cues for the stem cells to proliferate and form spheres in suspension culture, in which the natural stem cell niche is absent. This also could be a primary explanation for the low efficiency of sphere formation by single cells. Subsequent sphere differentiation in Matrigel culture showed distinct morphologic differences between 3D structures derived from spheres formed from basal versus luminal cells. These results validate the utility of in vitro sphere differentiation for lineage determination. Furthermore, we directly demonstrated that the single 3D solid structure did contain stem cells that were capable of multilineage differentiation and self-renewal upon transplant in vivo. In concordance with recent findings that MaSCs can be retained by in vitro passage of solid 3D structures in a similar culture system using 5% Matrigel (Guo et al., 2012), our data also suggests that the Matrigel culture system allows MaSC differentiation and retains MaSC self-renewal properties in vitro.
The mammosphere assay was initially designed as a serial passage assay to evaluate self-renewal of human mammary stem cells (Dontu et al., 2003). However, its application in detection of mouse mammary stem cells has been deferred due to concerns on sphere aggregation as well as whether all sphere-initiating cells are truly MaSCs (Booth et al., 2007; Liao et al., 2007; Moraes et al., 2007). Our study for the first time revealed that the basal, luminal and stromal cells within the primary mammary cells are all able to form mammospheres when they were cultured alone under non-adherence condition, and stromal cell formed spheres were from cell aggregation. Our finding also indicated that sphere formation efficiency is approximately 7-fold higher by luminal cells than that by basal cells. Therefore, it is expected that spheres derived from primary mammary cells have a mixed pool of cell origins and were dominated by luminal derived spheres as well as merged spheres united by the stromal cells. This may explain why primary mammosphere formation and stem cell number went in opposite directions and why IVT of individual mammospheres formed from the primary unfractionated cells yielded a low (15%) engraftment frequency in a previous study (Moraes et al., 2007).
The use of MaSCs from GFP mice for in vivo transplant into wild type recipients enabled us to examine the purity of FACS-gated basal and luminal cell populations from the regenerated GFP glands. All epithelial cells (GFP positive) were converged in the CD24+ population, while the combination of CD24 and CD49f enabled the separation of luminal cells from basal cells efficiently (Fig. S5a). However, we also found 22% GFP negative cells within the basal gate and 5% GFP negative cells within the luminal gate (Fig. S5a). This indicates that a substantial portion of non-epithelial cells also share the same cell surface marker phenotype as the basal epithelial cells. Subsequent analysis showed that these non-epithelial cells alone cannot form spheres (Fig. S5b). The higher percentage of non-epithelial cells in the basal cell fraction may explain, in part, why basal cells had much lower SFE than luminal cells. Furthermore, differentiation in Matrigel culture showed predominantly solid or hollow structures for spheres derived from basal or luminal cells from the regenerated glands, similar to those of natural glands (Fig. S5c–5f). We noticed uniform appearance of the complete hollow structure (Fig. S5f) in 3D culture of luminal derived spheres, suggesting a reduced complexity of luminal lineage from regenerated glands in comparison with the natural glands. This reduced complexity concept is consistent with the fact that unipotent basal MaSCs in adult glands (used in this study) are different from the multipotent MaSCs present in the fetus (Van Keymeulen A. et al., 2011). Future studies using multipotent MaSCs derived from fetal mammary glands for transplant may unravel the difference between basal MaSCs at different developmental stages.
Based on our SFD assay, we estimated that 1 SFD-IC in 252 total basal cells or 1 SFD-IC in 1154 total epithelial cells (the sum of basal and luminal cells) was obtained from adult mammary glands from 2-month-old wild type virgin C57BL/6 mice at estrus phase. Our SFD-IC values are very similar to the MRU values (1 MRU in 51 to 160 basal cells) reported previously on the same mouse strain based on limited dilution IVT assay (Stingl et al., 2006). However, MRU frequency has been reported ranging from 1 MRU in 100 total cells to < 1 MRU in 4900 cells in literature (Kordon and Smith, 1998; Moraes et al., 2007; Shackleton et al., 2006; Stingl et al., 2006). The large variation has been attributed to different methods used for mammary tissue dissociation and IVT (Stingl, 2009).
To conclude, the present study revealed that mammospheres formed by purified basal cells can be differentiated into solid structures in Matrigel culture, and these solid 3D structures had high efficiency in reconstituting cleared mammary fat pads. Most importantly, multiple lines of evidence including sphere size, behavior in suspension culture, and analyses of the possibility of multiple MaSCs present in spheres and 3D structures, all suggest that these spheres were most likely derived from single stem cell proliferation. Thus, these findings demonstrated that our SFD assay can serve as a reasonable alternative to the commonly used IVT assay for the identification and quantification of mouse basal MaSCs.
Supplementary Material
In vitro/vivo assays for mouse mammary stem cell (MaSC) identification and quantification. (a): mammospheres formed from primary cells or lineage negative primary cell; (b): FACS profile of primary cells; (c): diameter of mammospheres; (d): growth curves of solid and hollow 3D-ECM structures (Mean ± SD, n = 30); (e): diameter of 3D-ECM structures after 9 days of Matrigel culture; (f): GFP positive outgrowth derived from injecting single mammospheres or 3D solid structures into cleared mammary fat pad of a 21-day-old recipient mouse; (g): full alveolar development of the repopulated gland during the pregnancy.
Representative morphology of 3D-ECM structures derived from single spheres formed by FACS sorted basal (CD24+CD49fhi, upper panel a–d) or luminal (CD24hiCD49flo, bottom panel e–h) cells from C57BL6 mice aged 3 to 4 months. Scale bars, 100 μm.
Pictures showing 1st and 3rd generation 3D-ECM cultures derived from in vitro series passage of single 3D solid structures that were characterized by predominantly red (a), predominantly green (b), and true chimeras (c). The chimeras in Penal c was formed by co-culture of basal cells from DsRed and wild-type mice. Scale bars, 500 μm.
Mammospheres (a) derived from co-culture of FACS sorted stromal cells of GFP and DsRed mice, and representative 3D-ECM structures (b) derived from these spheres. Scale bars, 100 μm.
Regenerated GFP glands from virgin mice (a) showing non-epithelial cells (black) in the luminal (CD24hiCD49f+) or basal (CD24+CD49fhi) gates together with epithelial cells (green). Right panels showing the histograms of %GFP negative (stromal) and positive (epithelial) cells in each gate. FACS sorted basal (GFP+ and GFP−) and luminal cells were further subjected to in vitro mammosphere formation assay (b) and single spheres were plated in Matrigel for 3D-ECM assay where solid (c, e) and hollow structures (d, f) were formed. The data in the plotted figures represent mean ± SD of 6 (b) or 3 (c–d) replicate measurements of pooled glands from 6–8 individual GFP positive mammary fat pads.
Time-lapse video of mammosphere formation from single basal cell within the CD24+CD49fhi gate.
Time-lapse video of mammosphere formation from 2 basal cells within the CD24+CD49fhi gate.
Time-lapse video of mammosphere formation from > 2 basal cells within the CD24+CD49fhi gate.
Time-lapse video of mammosphere formation from stromal cells within the CD24−CD49f− gate.
Highlights.
We report a novel in vitro quantification assay for mouse mammary stem cells.
Stromal cells are responsible for cell merging during mammosphere formation.
Spheres formed from sorted basal epithelial-enriched cells are clonal in stem cell origin.
This assay provides rapid stem cell identification and quantification in vitro.
Our results are in good concordance with the in vivo repopulation assay.
Acknowledgments
This work was supported in part by funding from NIH Grants R01CA75253 and R01ES022057, the Bank of America Shelby Rae Tengg Foundation, the Mary Kay Foundation (#082-12), and the Cancer Therapy and Research Center at University of Texas Health Science Center at San Antonio through the NCI Cancer Center Support Grant 2P30CA054174-17 to the flow cytometry core facility. We thank Dr. John Stingl for his kind instruction in establishing the in vitro and in vivo assays in our laboratory. We also thank Dr. Rong Li for the use of the Nikon BioStation-IM.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Asselin-Labat ML, Vaillant F, Sheridan JM, Pal B, Wu D, Simpson ER, et al. Control of mammary stem cell function by steroid hormone signalling. Nature. 2010;465:798–802. doi: 10.1038/nature09027. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay A, Dong Q, Sun LZ. Stem/progenitor cells in murine mammary gland: isolation and functional characterization. Methods Mol Biol. 2012;879:179–193. doi: 10.1007/978-1-61779-815-3_12. [DOI] [PubMed] [Google Scholar]
- Booth BW, Boulanger CA, Smith GH. Alveolar progenitor cells develop in mouse mammary glands independent of pregnancy and lactation. J Cell Physiol. 2007;212:729–736. doi: 10.1002/jcp.21071. [DOI] [PubMed] [Google Scholar]
- Deleyrolle LP, Rietze RL, Reynolds BA. The neurosphere assay, a method under scrutiny. Acta Neuropsychiatrica. 2008;20:2–8. doi: 10.1111/j.1601-5215.2007.00251.x. [DOI] [PubMed] [Google Scholar]
- Dontu G, Abdallah WM, Foley JM, Jackson KW, Clarke MF, Kawamura MJ, et al. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes and Development. 2003;17:1253–1270. doi: 10.1101/gad.1061803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo WJ, Keckesova Z, Donaher JL, Shibue T, Tischler V, Reinhardt F, et al. Slug and Sox9 Cooperatively Determine the Mammary Stem Cell State. Cell. 2012;148:1015–1028. doi: 10.1016/j.cell.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordon EC, Smith GH. An entire functional mammary gland may comprise the progeny from a single cell. Development. 1998;125:1921–1930. doi: 10.1242/dev.125.10.1921. [DOI] [PubMed] [Google Scholar]
- Liao MJ, Zhang CC, Zhou B, Zimonjic DB, Mani SA, Kaba M, et al. Enrichment of a population of mammary gland cells that form mammospheres and have in vivo repopulating activity. Cancer Res. 2007;67:8131–8138. doi: 10.1158/0008-5472.CAN-06-4493. [DOI] [PubMed] [Google Scholar]
- Lim E, Vaillant F, Wu D, Forrest NC, Pal B, Hart AH, et al. Aberrant luminal progenitors as the candidate target population for basal tumor development in BRCA1 mutation carriers. Nat Med. 2009;15:907–913. doi: 10.1038/nm.2000. [DOI] [PubMed] [Google Scholar]
- Louis SA, Rietze RL, Deleyrolle L, Wagey RE, Thomas TE, Eaves AC, et al. Enumeration of neural stem and progenitor cells in the neural colony-forming cell assay. Stem Cells. 2008;26:988–996. doi: 10.1634/stemcells.2007-0867. [DOI] [PubMed] [Google Scholar]
- Moraes RC, Zhang XM, Harrington N, Fung JY, Wu MF, Hilsenbeck SG, et al. Constitutive activation of smoothened (SMO) in mammary glands of transgenic mice leads to increased proliferation, altered differentiation and ductal dysplasia. Development. 2007;134:1231–1242. doi: 10.1242/dev.02797. [DOI] [PubMed] [Google Scholar]
- Reynolds BA, Rietze RL. Neural stem cells and neurospheres - re-evaluating the relationship. Nature Methods. 2005;2:333–336. doi: 10.1038/nmeth758. [DOI] [PubMed] [Google Scholar]
- Shackleton M, Vaillant F, Simpson KJ, Stingl J, Smyth GK, Asselin-Labat ML, et al. Generation of a functional mammary gland from a single stem cell. Nature. 2006;439:84–88. doi: 10.1038/nature04372. [DOI] [PubMed] [Google Scholar]
- Singec I, Knoth R, Meyer RP, Maciaczyk J, Volk B, Nikkhah G, et al. Defining the actual sensitivity and specificity of the neurosphere assay in stem cell biology. Nat Methods. 2006;3:801–806. doi: 10.1038/nmeth926. [DOI] [PubMed] [Google Scholar]
- Stingl J. Detection and analysis of mammary gland stem cells. Journal of Pathology. 2009;217:229–241. doi: 10.1002/path.2457. [DOI] [PubMed] [Google Scholar]
- Stingl J, Eirew P, Ricketson I, Shackleton M, Vaillant F, Choi D, et al. Purification and unique properties of mammary epithelial stem cells. Nature. 2006;439:993–997. doi: 10.1038/nature04496. [DOI] [PubMed] [Google Scholar]
- Van Keymeulen A, Rocha AS, Ousset M, Beck B, Bouvencourt G, Rock J, et al. Distinct stem cells contribute to mammary gland development and maintenance. Nature. 2011;479:189–193. doi: 10.1038/nature10573. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
In vitro/vivo assays for mouse mammary stem cell (MaSC) identification and quantification. (a): mammospheres formed from primary cells or lineage negative primary cell; (b): FACS profile of primary cells; (c): diameter of mammospheres; (d): growth curves of solid and hollow 3D-ECM structures (Mean ± SD, n = 30); (e): diameter of 3D-ECM structures after 9 days of Matrigel culture; (f): GFP positive outgrowth derived from injecting single mammospheres or 3D solid structures into cleared mammary fat pad of a 21-day-old recipient mouse; (g): full alveolar development of the repopulated gland during the pregnancy.
Representative morphology of 3D-ECM structures derived from single spheres formed by FACS sorted basal (CD24+CD49fhi, upper panel a–d) or luminal (CD24hiCD49flo, bottom panel e–h) cells from C57BL6 mice aged 3 to 4 months. Scale bars, 100 μm.
Pictures showing 1st and 3rd generation 3D-ECM cultures derived from in vitro series passage of single 3D solid structures that were characterized by predominantly red (a), predominantly green (b), and true chimeras (c). The chimeras in Penal c was formed by co-culture of basal cells from DsRed and wild-type mice. Scale bars, 500 μm.
Mammospheres (a) derived from co-culture of FACS sorted stromal cells of GFP and DsRed mice, and representative 3D-ECM structures (b) derived from these spheres. Scale bars, 100 μm.
Regenerated GFP glands from virgin mice (a) showing non-epithelial cells (black) in the luminal (CD24hiCD49f+) or basal (CD24+CD49fhi) gates together with epithelial cells (green). Right panels showing the histograms of %GFP negative (stromal) and positive (epithelial) cells in each gate. FACS sorted basal (GFP+ and GFP−) and luminal cells were further subjected to in vitro mammosphere formation assay (b) and single spheres were plated in Matrigel for 3D-ECM assay where solid (c, e) and hollow structures (d, f) were formed. The data in the plotted figures represent mean ± SD of 6 (b) or 3 (c–d) replicate measurements of pooled glands from 6–8 individual GFP positive mammary fat pads.
Time-lapse video of mammosphere formation from single basal cell within the CD24+CD49fhi gate.
Time-lapse video of mammosphere formation from 2 basal cells within the CD24+CD49fhi gate.
Time-lapse video of mammosphere formation from > 2 basal cells within the CD24+CD49fhi gate.
Time-lapse video of mammosphere formation from stromal cells within the CD24−CD49f− gate.