Abstract
Tracking olfactory bulb mitral cell development with BrdU labeling, we find that mitral cells are generated from Pax6+ radial glial cells in the ventricular zone of the embryonic olfactory bulb. Unlike cortical projection neurons, postmitotic mitral cell precursors express both Tbr1 and Tbr2. Our tracking experiments revealed that down-regulation of Pax6 preceded up-regulation of Tbrs, and that Tbr1 emerged earlier than Tbr2. Using in utero electroporation, we also show that Pax6 negatively regulates the expression of Tbr1 and Tbr2 in postmitotic mitral cell precursors. Exogenous expression of Pax6 in embryonic olfactory bulb postmitotic precursors decreased the number of cells that progressed to a mitral cell fate. In contrast, exogenous expression of Pax6 resulted in an increase of GABAergic and/or dopaminergic interneurons. These results indicate that Pax6 is a regulator of fate determination of precursor cells.
Keywords: olfactory bulb, mitral cell, transcription factor, fate determination, in utero electroporation
INTRODUCTION
Mitral and tufted cells, the glutamatergic olfactory bulb (OB) projection neurons, receive synaptic input from olfactory sensory neuron axons and transmit information to the olfactory cortex (Mori et al., 1999). Mitral cells are generated from ventricular zone (VZ) progenitors in the anterior telencephalic vesicle (Blanchart et al., 2006; Imamura et al., 2011). Postmitotic mitral cell precursors migrate radially toward the intermediate zone (IZ) where they differentiate into mitral cells. However, the molecular mechanisms regulating mitral cell differentiation remain enigmatic. Here, we studied the mechanisms of differentiation by focusing on transcription factors, Tbr1, Tbr2, and Pax6.
In developing neocortex, Pax6 is expressed by radial glial cells and is an intrinsic fate determinant of their neurogenic potential (Hack et al., 2004; Haubst et al., 2004; Heins et al., 2002). During cortical pyramidal neuron development Pax6 is down-regulated in radial glial-derived intermediate progenitor cells (IPCs). Down-regulation of Pax6 is associated with an up-regulation of Tbr2, while a down-regulation of Tbr2 results in an up-regulation of Tbr1 in postmitotic pyramidal cells. Therefore, there is a transcription factor expression sequence Pax6 → Tbr2 → Tbr1 in the differentiation of radial glia → IPC → postmitotic pyramidal neuron (Englund et al., 2005). Postmitotic Tbr1+ pyramidal cells do not express Pax6 or Tbr2.
Pax6 is also expressed in the anterior tip of telencephalic vesicle (Hebert et al., 2003; Walther and Gruss, 1991). Like developing neocortex, it was recently suggested that OB mitral and tufted cells are generated from Neurog2+ cells derived from Pax6+ cells in the VZ (Winpenny et al., 2011). These data also suggested the existence of cells expressing both Pax6 and Tbr2, but not Tbr1, in the VZ. However, unlike cortical pyramidal neurons, postmitotic mitral cell precursors in the IZ express not only Tbr1, but also Tbr2 (Bulfone et al., 1999; Bulfone et al., 1995; Faedo et al., 2002; Mizuguchi et al., 2012). Absence of either Tbr1 or Tbr2 in postmitotic mitral cell precursors causes comparable defects in mitral cell development, indicating that both molecules are necessary for the cells to progress toward a mitral/tufted cell phenotype (Arnold et al., 2008; Bulfone et al., 1998; Sessa et al., 2008). Thus, there should be a unique mechanism that regulates the expression of Tbr1 and Tbr2 in postmitotic mitral cell precursors in the developing OB.
Here, we used the mouse to determine the temporal and spatial expression patterns of Pax6, Tbr1, and Tbr2 in developing mitral cells. We first establish that Pax6 and Tbrs show diametrical expression patterns during mitral cell development. Using in utero electroporation to control Pax6 expression, we also show that exogenous expression of Pax6 in postmitotic mitral cell precursors impairs both Tbr1 and Tbr2 expression and therefore, mitral cell fate. Interestingly, as a consequence of ectopic Pax6 expression, mitral cell precursors changed their fate and expressed molecular phenotypes characteristic of OB interneurons including dopaminergic and GABAergic periglomerular cells. These data demonstrate the importance of transcription factor expression pathways and that mitral cell fate is critically dependent upon down-regulation of Pax6 and the ensuing up-regulation of Tbr2 and Tbr1.
Materials and Methods
Animals
All the experiments were performed using the CD-1 mouse strain (Charles River Laboratories; Wilmington, MA). The day on which we found a copulation plug was called E0, and the succeeding days of gestation were numbered in order. Prenatal embryos were harvested and fixed in 4% paraformaldehyde (PFA) for overnight after pregnant mothers were euthanized with CO2 inhalation. All animal care and use were approved by the Yale University Animal Care and Use Committee.
BrdU injection
5-bromo-2′-deoxyuridine (BrdU; Sigma; St. Louis, MO) was intraperitoneally injected into pregnant mothers at E11 or E13 (50mg/kg). Injections were performed once in the morning between 10am and noon.
Plasmids
Both pCAGEN (Plasmid #11160), a plasmid vector having CAG enhancer, and pGFP (pCAG-GFP; Plasmid #11150), a plasmid having egfp cDNA in the multiple cloning site of pCAGEN, (Matsuda and Cepko, 2004) were obtained from Addgene. To construct pPax6, cDNAs were first synthesized by reverse-transcription of total RNAs obtained from E17 mouse brain using the SuperScript III First-Strand Synthesis System (Invitrogen). Then, full-length mouse pax6 cDNA was cloned with PCR; the primer sequences used were: AGTCTCGAGCCACCATGCAGAACAGTCACAGCGG; and AGTGCGGCCGCTTACTGTAATCGAGGCCAGT. The obtained PCR fragment was inserted into the pCAGEN vector after cut with restriction enzymes, XhoI and NotI.
In utero electroporation
In utero electroporation was performed according to the procedure previously reported (Saito and Nakatsuji, 2001). Pregnant mothers were anesthetized with an intraperitoneal injection of ketamine (100 mg/kg) and xylazine (10 mg/kg), and the uterine horns were carefully taken out from the abdominal cavity. Approximately 0.5 μl of DNA solution (1.5 – 4 μg/μl in 50% TE) was injected into the lateral cerebral ventricle of embryos by inserting a glass pipette. The DNA solution was mixed with 100 μg/ml of Fast Green to visibly confirm the injection site. Then, electroporation was carried out by applying square electric pulses: 2 pulses of 30 V, 50 ms duration with a 950 msec interval. To efficiently label the mitral cell precursors in the presumptive OB, positive current was given from posterior to anterior. Following electroporation, the uterine horns were repositioned in the abdominal cavity, and following suturing the animals recovered in a warm environment. More than 70% of operated embryos survived and expressed transgene products (Supplementary Table 1).
Immunohistochemistry
The fixed brains were cryopreserved in 30% sucrose (wt/vol) in 0.1 M phosphate buffer (pH 7.4), and embedded in optimal cutting temperature compound (Sakura Finetek). The olfactory tissues were cut on a cryostat into 20 μm slices and stored at −20 °C until use. The slices were pretreated for 30 min in 0.025 M HCl at 65 °C, and rinsed with 0.1 M borate buffer (pH 8.5), PBS and TBS-T (10 mM Tris-HCl (pH 7.4), 100 mM NaCl with 0.3% Triton-X100 (vol/vol)). The slices were then blocked with blocking buffer (5% normal donkey serum (vol/vol) in TBS-T) at 20 – 25 °C for 1 h and incubated with primary antibodies diluted in blocking buffer overnight at 4 °C. Sections were washed with TBS-T, then incubated with secondary antibodies with 4′6-diamino-2-phenylindole dihydrochrolide (DAPI; Invitrogen) or DRAQ5 (Biostatus Ltd.) for nucleus staining for 1 h. The immunoreacted sections were washed and mounted with Gel/Mount mounting medium (Biomeda).
For primary antibodies, we used anti-BrdU (Accurate Chemical & Scientific Corporation, rat, 1:00), anti-GFP (Millipore, chicken, 1:1000), anti-Pax6 (Developmental Studies Hybridoma Bank, mouse, 1:5), anti-Pax6 (Millipore, rabbit, 1:200), anti-Tbr1 (Abcam, rabbit, 1:5000), anti-Tbr2 (Abcam, rabbit, 1:5000), anti-NCAM (Millipore, rat, 1:300), anti-BLBP (Millipore, rabbit, 1:100), anti-vGluT1 (Synaptic Systems, rabbit, 1:1000), anti-NeuN (Millipore, mouse, 1:100), anti-PH3 (Millipore, rabbit, 1:1000), anti-Ki67 (Novus Biologicals, rabbit, 1:500), anti-GAD67 (Millipore, mouse, 1:500), anti-TH (Millipore, rabbit, 1:1000), anti-cleaved caspase-3 (Cell Signaling Technology, rabbit, 1:100), and anti-Tbx21 (kindly provided by Dr. Yoshihiro Yoshihara at RIKEN, rabbit, 1:10000).
Goat or donkey anti-species IgG conjugated with Alexa 488, Alexa 555, Alexa 674 (Invitrogen), or Cy2 (Jackson Immunochemicals) were used as secondary antibodies.
Double-labeling with two rabbit antibodies was done using Zenon Rabbit IgG Labeling Kit (Invitrogen). Anti-Tbr1 and anti-Tbr2 antibodies were directly labeled with Alexa 488 and Alexa 555, respectively, according to the manufacturer instruction before applying to the sections (1:100).
Image acquisition and statistical analysis
Quantification was done using images acquired with a laser scanning confocal microscope (Leica TCS SL). Levels were adjusted in Photoshop software (Adobe), but the images were otherwise unaltered. The numbers of BrdU–labeled or GFP+ cells in the embryonic OB were manually counted to quantify the distribution in the OB and/or percentages expressing molecular markers. Six OBs (3 embryos from a mother for E11, E11.5, E12, E13 and E14; 3 embryos from 3 mothers for E15) were analyzed at each time point to quantify the expression of transcription factors in BrdU-labeled cells. We selected a horizontal section of the OB defined based on NCAM expression in the olfactory nerve layer (Supplementary Fig. 1), and took the images using 40x objective lens. All BrdU-labeled cells within the OB region were analyzed. To quantify the Tbr expressions in GFP+ cells and/or their distributions within the OBs electroporated with pGFP along with pCAGEN or pPax6, we analyzed nine E14 pCAGEN-electroported OBs (7 embryos from 6 mothers), nine E14 pPax6-electroported OBs (9 embryos from 6 mothers), five E17 pCAGEN-electroported OBs (5 embryos from 5 mothers), and four E17 pPax6-electroported OBs (4 embryos from 4 mothers). We selected a sagittal (E14) or a horizontal (E17) OB section, and took images using 40x objective lens. All GFP-labeled cells within the OB region were analyzed. To quantify the densities of Ki67+ cells, four non-electroporated (no IUE) OBs (4 embryos from 4 mothers), five pCAGEN-electroported OBs (4 embryos from 4 mothers), and four pPax6-electroporated OBs (3 embryos from 3 mothers) were analyzed. We selected a sagittal OB section, and took images using 40x objective lens. The VZ regions were defined with DRAQ5 staining and the numbers of cells labeled with anti-Ki67 antibody in the VZ were manually counted to calculate the Ki67 density. No significant difference was observed in Ki67 density among the different conditions (no IUE, pCAGEN, or pPax6). Because Pax6, Tbr1 and Tbr2 are transcription factors and Ki67 is a nuclear protein, they are localized in cell nuclei. Therefore, the sections were always co-labeled with cell nuclei marker, DRAQ5, and we manually defined the cells whose signal within the nuclei was significantly stronger than background as positive cell. All results are presented as mean and standard error of the mean. Statistical analyses were performed using GraphPad Prism 4 software (GraphPad Software).
RESULTS
Expressions of Pax6, Tbr1, and Tbr2 in mitral cell precursors
To begin probing the molecular signaling pathways that regulate mitral cell fate, we first examined the developmental expression of the candidate transcription factors Pax6, Tbr1, and Tbr2 in mitral cell precursors. Because we previously showed that mouse mitral cells were generated predominately between embryonic day (E) 10 and E13, with a peak of genesis at E11, (Blanchart et al., 2006; Imamura et al., 2011), we made a single injection of BrdU into pregnant mothers at E11, and then double labeled for Pax6, Tbr1, and Tbr2 at 2 hours (E11), 1 day (E12), 2 days (E13), 3 days (E14), or 4 days (E15) post-BrdU (Fig. 1). Progenitor cells divide asymmetrically to produce a postmitotic cell as well as a second progenitor cell. Both daughter cells would be labeled with BrdU. Nevertheless, because the general cell cycle duration in the VZ of E11 cerebral cortex is about 8 hours (Takahashi et al., 1995), we can reasonably conclude that almost all BrdU-labeled cells examined at each of the sacrifice times were generated around E11.
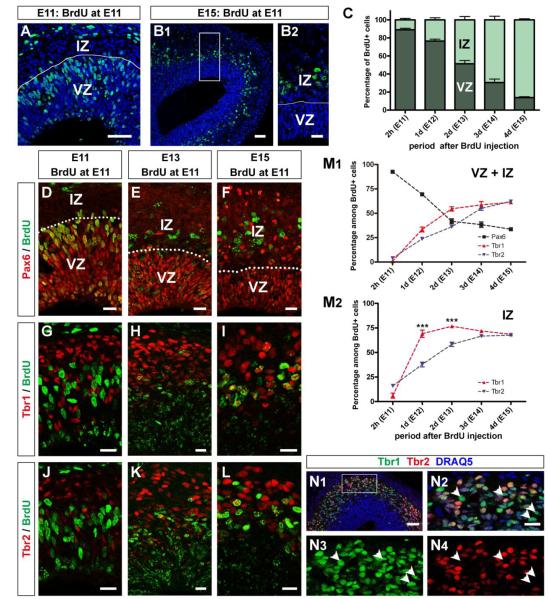
Figure 1. Expression of Pax6, Tbr1, and Tbr2 in mitral cell precursors in embryonic olfactory bulbs.
(A, B) Horizontal sections of E11 (A) and E15 OBs (B). BrdU (Cy2, green) was intraperitoneally injected into pregnant mothers at E11. All nuclei were stained with DRAQ5 (blue). The OBs were divided into VZ and IZ based on the morphology of cell nuclei. (C) Graph showing the distribution of BrdU-labeled cells between VZ and IZ at 2 hours (E11), 1 day (E12), 2 days (E13), 3 days (E14), and 4 days (E15) after BrdU injection. (D – L) Expression of Pax6 (D – F; Alexa 555, red), Tbr1 (G – I; Alexa 555, red), and Tbr2 (J – L; Alexa 555, red) were immunohistochemically examined at 2 hours (E11; D, G, J), 2 days (E13; E, H, K) and 4 days (E15; F, I, L) after injection. Distributions within OBs and co-labeling with BrdU (green) were examined. VZ: ventricular zone; IZ: intermediate zone. (M) Graphs showing percentages of BrdU-labeled cells expressing Pax6 (black), Tbr1 (red), or Tbr2 (blue) at each time point after BrdU injection. The percentages were calculated among BrdU-labeled cells in both VZ and IZ (M1) and only IZ (M2). In total, decrease of percentage of Pax6+ cells occurs in parallel with increases of Tbr1+ and Tbr2+ cells. Percentages of Tbr1+ cells in IZ are significantly higher than those of Tbr2+ cells at E12 and E13 (***p<0.0001; n=6) (unpaired t test). (N) Expression patterns of Tbr1 (Alexa 488, green) and Tbr2 (Alexa 555, red) in E13 OB. There are many cells expressing only Tbr1 (N2 – N4; arrowheads) while all Tbr2+ cells are expressing Tbr1. Error bars, s.e.m. Scale bars, 50 μm in (A) and (B1); 20 μm in (B2), (D – L), and (N2); 100 μm in (N1).
We first examined the distribution of BrdU-labeled cells in developing OB. Evagination of the anterior telencephalon was not evident at the earliest developmental stages including E11 and E12. To determine the location of the presumptive OB at these ages we labeled for NCAM, a pan-marker of olfactory sensory axons (Miller et al., 2010) (Supplementary Fig. 1). The embryonic OB was divided into two prominent layers (Hinds, 1972). The inner layer, the VZ, was densely packed with radially oriented oval-shaped cells. The outer half, the IZ, was sparsely filled with tangentially oriented oval or round-shaped cells. Two hours after injection (E11), BrdU-labeled cells were restricted to the VZ; their localization shifted to the IZ during the next 4 days (Fig. 1A – C). This is consistent with a radial migration of postmitotic mitral cell precursors from the VZ to the IZ and the conclusion that mitral cell precursors were labeled with BrdU.
Next, we examined the expression of Pax6, Tbr1, and Tbr2. The percentages of BrdU-labeled cells expressing these transcription factors were calculated at each time point from E11 to E15. Most of the cells in VZ expressed Pax6, therefore BrdU-labeled cells were mostly Pax6+ at 2 hours after injection (E11) (Fig 1D, M1). At later time points Pax6 was also expressed by some cells in IZ (Fig. 1E, F) although only a few of them were labeled with BrdU. Thus, the percentage of Pax6+ cells among BrdU-labeled cells decreased from 92.6 ± 1.7% (E11) to 33.7 ± 1.5% (E15) during the 4 days following BrdU injection. This suggests that mitral cell precursors lose Pax6 expression as they migrate into the IZ. In contrast to Pax6, both Tbr1+ cells and Tbr2+ cells were found predominantly in the IZ (Fig. 1G – L). Although there were some VZ cells that express Tbr1 or Tbr2 at E11, these were only rarely labeled with BrdU. Thus, few BrdU-labeled cells express Tbr1 or Tbr2 at 2 hours post-BrdU. However, the percentages of BrdU-labeled cells expressing Tbr1 and Tbr2 increased from E11 to E15 (Fig. 1M1); Tbr1: from 0.8 ± 0.2% to 61.2 ± 1.2%; Tbr2: from 3.8 ± 0.7% to 62.0 ± 1.8%. These results suggest the up-regulation of Tbr1 and Tbr2 expression in mitral cell precursors is synchronous with their migration into the IZ.
Tbr1+ cells and Tbr2+ cells were localized in the IZ of the developing OB. This is consistent with previous findings that mitral/tufted cells in late developmental stages express both Tbr1 and Tbr2 (Bulfone et al., 1999; Bulfone et al., 1995; Faedo et al., 2002; Mizuguchi et al., 2012). Here, by examining the Tbr1 or Tbr2 expression in BrdU-labeled cells in the IZ, we found that the percentages of BrdU-labeled cells expressing Tbr1 at E12 (69.1 ± 3.7%) and E13 (76.7 ± 0.9%) was significantly higher than those cells expressing Tbr2 (E12: 37.8 ± 2.5%; E13: 58.3 ± 2.2%), while they finally reached similar percentages by E15 (Tbr1: 68.5 ± 1.5%; Tbr2: 67.7 ± 0.9%) (Fig. 1M2). This indicates that expression of Tbr1 in postmitotic mitral cell precursors precedes the expression of Tbr2. Consistent with this interpretation, many Tbr1+ cells did not express Tbr2 in the IZ of E13 OB, while all of the Tbr2+ cells expressed Tbr1. (Fig. 1N).
Complementary expression of Pax6 and Tbrs in embryonic olfactory bulb
In the embryonic neocortex, Pax6 is expressed by radial glial cells which divide throughout neurogenesis and give rise to the majority of IPCs and projection neurons (Götz et al., 1998; Heins et al., 2002). To determine if the Pax6 expressing cells that give rise to mitral cells also have a radial glial phenotype in embryonic OB, we immunohistochemically labeled with antibodies against Pax6 and BLBP, a well-known marker for radial glial cells (Feng et al., 1994). BLBP+ cells in the E11 OB have a soma near the ventricle and neurites that extend radially toward the surface of the brain (Fig. 2A). The BLBP+ cells were largely Pax6+, and the Pax6+ cells were also BLBP+. This strongly suggests that the Pax6+ cells in the E11 OB are radial glial cells and serve as the neuroprogenitors that give rise to mitral cells.
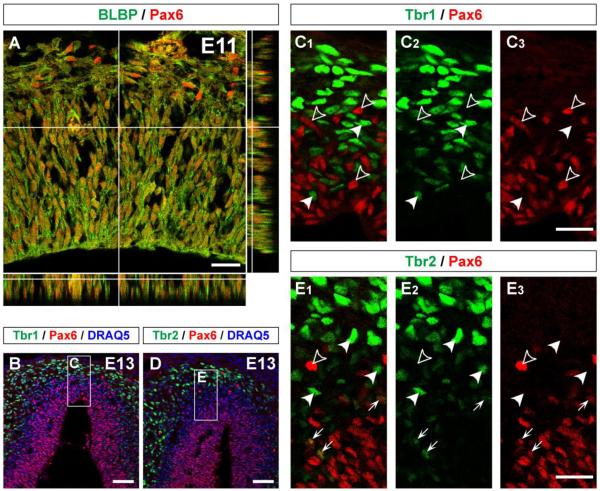
Figure 2. Expression of Pax6 by radial glial cells but absence from postmitotic cells in embryonic olfactory bulb.
(A) Horizontal section of E11 OB immunostained with Pax6 (Alexa 555, red) and BLBP (Alexa 488, green) that is expressed by radial glial cells. BLBP+ radial glial cells are mostly Pax6+. (B – E) Sagittal sections of E13 OB immunostained with Pax6 (Alexa 555, red) and Tbr1 (B; Alexa 488, green) or Tbr2 (D; Alexa 488, green). All nuclei were stained with DRAQ5 (blue). (C, E) High magnification images of boxed regions of (B) and (D). Pax6+ cells are mostly located in VZ and do not express Tbr1 or Tbr2 (open-arrowheads), while Tbr1 and Tbr2 are mostly expressed by IZ cells that are negative for Pax6 (closed-arrowheads). A few Pax6+ cells in VZ express Tbr2 (arrows in E). Scale bars, 20 μm in (A), (C3), and (E3); and 50 μm in (B) and (D).
We next tested whether Tbr1 and Tbr2 are co-expressed with Pax6 in the same cell during development of the OB. In the IZ of E13 OB, both Tbr1 and Tbr2 had complementary expression patterns with regard to Pax6 (Fig. 2B – E). We did not find any Tbr1+ cells or Tbr2+ cells that expressed Pax6 in the IZ. Together with the results from expression time course in the IZ (Fig. 1M2), these data are consistent with the down-regulation of Pax6 preceding the up-regulation of Tbr1 followed by Tbr2 in postmitotic mitral cell precursors.
As we noted above, a few Tbr1+ and Tbr2+ cells could be found in the VZ. When BrdU was injected at E11, the percentage of BrdU-labeled cells expressing Tbr2 at 2 hours after injection (3.8 ± 0.7%) was slightly higher than Tbr1 expressing cells (0.8 ± 0.2%) (Fig. 1M1). At E13, a few of the Tbr2+ cells in the VZ expressed Pax6 (arrows in Fig. 2E), while there were no Tbr1+ cells that were also Pax6+. These data may suggest that Tbr2 is upstream of Tbr1 in the progenitors of mitral cells, but that Tbr2 expression is highly transient. To test this possibility, we examined Tbr2 expression in BrdU-labeled cells at 12 hours after injection (E11.5). We found that percentage of BrdU-labeled cells expressing Tbr2 was 21.4 ± 1.1%, which is intermediate to the value found at 2 hours (E11; 3.8 ± 0.7%) and 1 day after injection (E12; 23.7 ± 1.5%). This indicates that there is no obvious transient increase of Tbr2 expression in E11-generated cells between E11 and E12. To acquire deeper insights, we injected BrdU into pregnant mothers at E13 and examined expression of Tbr1/Tbr2 in BrdU-labeled cell in the OB 2 hours after injection (Supplementary Fig. 2). In this case, 14.3 ± 1.6% of BrdU-labeled cells expressed Tbr2, while the percentage of Tbr1 expressing cells was 2.5 ± 0.6%. Therefore, the fraction of BrdU-labeled cells expressing Tbr2 at 2 hours after injection was significantly higher with E13 injection (14.3 ± 1.6%) than E11 injection (3.8 ± 0.7%) (p<0.001). Because the largest numbers of mitral cells are generated at E11 while only a few are generated at E13, different subpopulations of cells were labeled with BrdU among E11 and E13 injections. These results may suggest that E11-generated BrdU-labeled cells expressing Tbr2 at 2 hours after BrdU injection and Pax6+ cells expressing Tbr2 found in the VZ of E13 OB are precursors of non-mitral glutamatergic cell that follow an alternative transcription cascade of Pax6 → Tbr2 → Tbr1 (Winpenny et al., 2011).
Electroporation of plasmid vectors into mitral cell precursors
Next, we hypothesized that the down-regulation of Pax6 is necessary for the expression of Tbr1 and Tbr2 in postmitotic mitral cell precursors, and their subsequent differentiation into mature mitral cells. We tested this hypothesis using in utero electroporation, which has been used to introduce plasmid vectors into cells in developing brain (Saito and Nakatsuji, 2001), although it has not yet been widely applied to the embryonic OB. Here, we applied negative current from posterior to anterior of the embryonic brain to deliver negatively charged plasmids to the anterior part of the telencephalic vesicle (Fig. 3A). Beginning with our previous analyses of mitral cell genesis (Imamura et al., 2011), we found that we could efficiently introduce the plasmid vectors into mitral cell precursors by performing the in utero electroporation in E11 embryos. Figures 3B – G show the developing OBs in which the plasmid vectors containing egfp cDNA under the CAG enhancer (pGFP) and the control mock plasmid (pCAGEN) were introduced at E11. Expression of GFP in the OB was evident one day after electroporation (E12) (Fig. 3B, C). The GFP+ cells were in both VZ and IZ, and those in the VZ expressed both BLBP and Pax6 (Fig. 3C), suggesting that the radial glial cells were electroporated with plasmid vectors. By E17, GFP+ cells were predominately localized in mitral cell layer (MCL) and double labeled with Tbx21, a mitral/tufted cell specific marker (Fig. 3D). While these GFP+ cells did not express Pax6 (Fig. 3D), they were positive for Tbr1 and Tbr2 (Fig. 3E, F). To further confirm that the electroporated cells differentiated into glutamatergic projection neurons we examined vGluT1, the glutamate transporter expressed by OB mitral cells. We found that GFP+ cells in the MCL expressed vGluT1 (Fig. 3G), consistent with the notion that the majority of electroporated cells had followed a mitral cell fate.
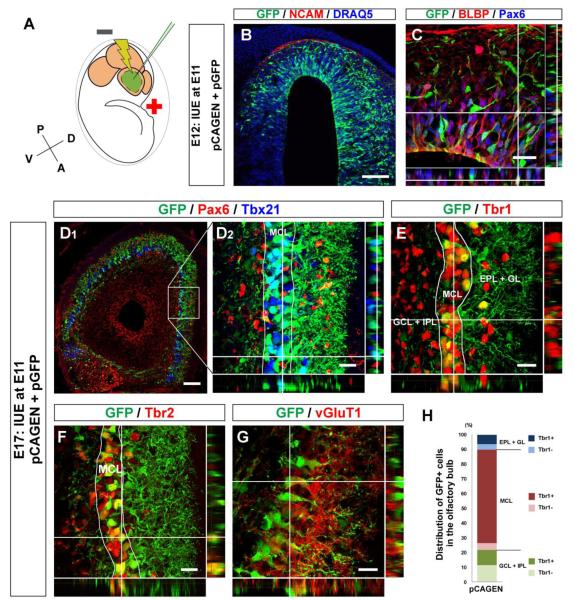
Figure 3. Introduction of plasmid vectors into mitral cell precursors with in utero electroporation.
(A) Schematic diagram of in utero electroporation. Negative current (two 50 ms pulses of 30 V with 950 ms intervals) was applied from posterior to anterior in embryonic mouse brain after injection of plasmid vectors into lateral ventricle to electroporate the embryonic OB cells. All electroporations shown in this study were performed at E11. (B, C) Horizontal sections of E12 OB electroporated with pCAGEN and pGFP at E11. GFP+ cells (Cy2, green) are found in presumptive OB defined with NCAM+ olfactory sensory axons (red). All nuclei were stained with DRAQ5 (blue) (B). The section immunostained with Pax6 (Alexa 647, blue) and BLBP (Alexa 555, red). GFP+ cells (Cy2, green) expressing both Pax6 and BLBP are located in VZ. (D – G) Horizontal sections of E17 OB electroporated with pCAGEN and pGFP. GFP+ cells (Cy2, green) are mostly negative for Pax6 (D; Alexa 555, red), and localize in the mitral cell layer (MCL) that is defined with Tbx21 (D; Alexa 647, blue). These GFP+ cells express Tbr1 (E; Alexa 555, red), Tbr2 (F; Alexa 555, red), and vGluT1 (G; Alexa 555, red). (H) Graph showing distribution and Tbr1 expression of GFP+ cells in E17 OB electroporated with pCAGEN and pGFP. Based on DRAQ5 and Tbr1 staining, layers in the OB were divided into three parts; glomerular and external plexiform layers (GL + EPL), MCL, and internal plexiform and granule cell layers (IPL + GCL) (E). Numbers of GFP+ cells and GFP+ cells expressing Tbr1 were counted, and percentages of Tbr1+ and Tbr1− GFP+ cells among total GFP+ cells were calculated in each part. The graph was made by averaging the data acquired from 4 OBs. Scale bars, 200 μm in (B) and (D1); and 20 μm in (C) and (D2 – G).
We then analyzed the distribution and Tbr1 expression of GFP+ cells in the E17 OB electroporated with pGFP and pCAGEN at E11 (Fig. 3E, H). The majority of the GFP+ cells (68.3 ± 9.3%) were localized in the MCL, and more than 90% of these were Tbr1+, suggesting that ~65% of the electroporated cells differentiated into mitral cells. Smaller numbers of GFP+ cells were also found outside the MCL; 21.7 ± 9.7% and 10.0 ± 1.1% were localized to superficial layers, glomerular layer and external plexiform layer (GL + EPL), and deep layers, internal plexiform layer and granule cell layer (IPL + GCL), respectively. Many of those found outside the MCL also expressed Tbr1. Overall, 80.5 ± 7.2% of GFP+ cells electroporated with pCAGEN at E11 expressed Tbr1 in the E17 OB. These results show that precursor cells that were electroporated with plasmid vectors at E11 differentiate predominantly into mitral cells. Hereafter, we always performed in utero electroporation experiments at E11.
Importance of Pax6 down-regulation for expression of Tbrs in postmitotic mitral cell precursors
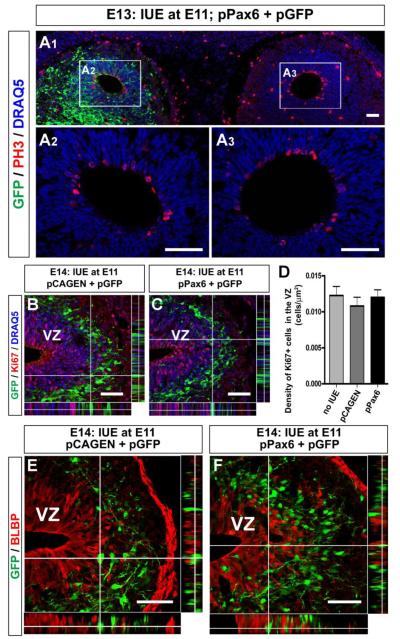
We then tested our hypothesis that Pax6 must be down-regulated in mitral cell precursors for the subsequent up-regulation of Tbr1 and Tbr2 in postmitotic cells. Pax6 was exogenously expressed by electroporating the plasmid vector containing the pax6 cDNA sequence under the CAG enhancer (pPax6) (Fig. 4). We co-electroporated pGFP to visualize the pPax6 electroporated cells, and confirmed that GFP+ cells were mostly Pax6+ at 3 days after electroporation (cf. Fig. 5B, F). The electroporation is effective in labeling the Pax6+ radial glia (Fig. 3C), and because Pax6 is known to regulate the neurogenic function of radial glia to control the number of cortical cells (Quinn et al., 2007), we checked to determine if exogenous expression of Pax6 changed the proliferation rate of electroporated cells and prevented them from further differentiation. We first determined if pPax6 electroporation affected the rate or magnitude of neurogenesis in the embryonic OB. Using an antibody against phospho-histone H3 (PH3), a mitosis-specific marker, we found that there was no difference in the number of PH3+ cells around the ventricles of pPax6 electroporated and non-electroporated OBs at E13 (Fig. 4A). We also compared the expression of a marker of proliferating cells, Ki67, in the E14 OBs electroporated with pCAGEN (Fig. 4B) or pPax6 (Fig. 4C), and found no difference in the distribution of Ki67+ cells. Densities of Ki67+ cells in the VZ were similar among OBs without electroporation or electroporated with pCAGEN or pPax6 (Fig. 4D). Moreover, both pCAGEN electroporated cells and pPax6 electroporated cells no longer expressed BLBP at E14 (Fig. 4E, F), suggesting that they no longer exhibited the molecular phenotype of radial glia. Thus, electroporation of pPax6 performed at E11 did not affect the cell cycle or proliferation of progenitor cells in the developing OB. However, compared to the localization of cells electroporated with pCAGEN in the IZ (Fig. 4E), pPax6 electroporated cells were still found in the VZ (Fig. 4F). Therefore, radial migration of postmitotic cells toward the IZ may be affected.
Figure 4. Exogenous expression of Pax6 had no effect on proliferation of progenitor cells.
(A) Horizontal section of the E13 OBs. Plasmid vectors, pPax6 and pGFP, were electroporated in left hemisphere. GFP expression (Cy2, green) is observed in left OB, while no cells in the right OB expressed GFP (A1). There is no obvious difference in PH3 expression (Alexa 555, red) around the ventricles of pPax6-electroporated (A2) and non-electroporated (A3) OBs. All nuclei were stained with DRAQ5 (blue). (B, C) Sagittal sections of E14 OBs electroporated with pCAGEN and pGFP (B) or pPax6 and pGFP (B). Proliferating cells were detected with anti-Ki67 antibody (Alexa 555, red). (D) Graph showing the densities of Ki67+ cells within the VZ of OBs. No significant difference was found among OBs without electroporation, electroporated with pCAGEN and with pPax6. (E, F) Sagittal section of E14 OB electroporated with pGFP together with pCAGEN (E) or pPax6 (F). In both cases, GFP+ cells (Cy2, green) are mostly negative for BLBP (Alexa 555, red). Scale bars, 50 μm.
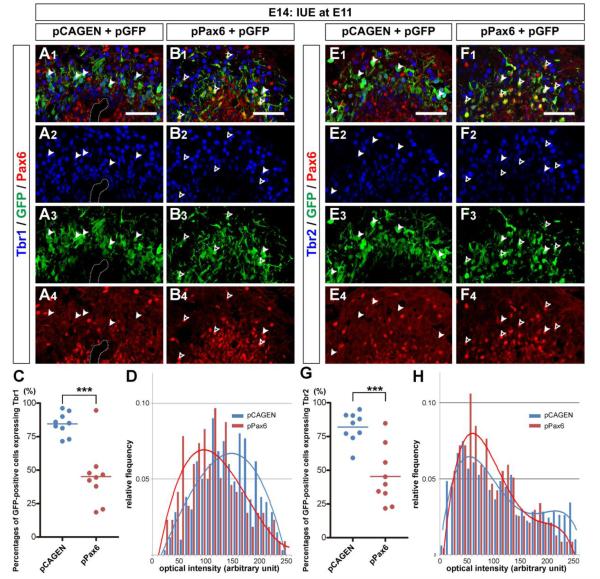
Figure 5. Exogenous expression of Pax6 impairs expressions of Tbr1 and Tbr2 in embryonic olfactory bulb.
(A, B) Sagittal sections of E14 OBs electroporated with pCAGEN (A) or pPax6 (B) together with pGFP. The sections were immunostained with anti-Tbr1 (Alexa 647, blue) and anti-Pax6 (Alexa 555, red) antibodies. GFP+ cells (Cy2, green) electroporated with pCAGEN are mostly localized in IZ and express Tbr1, but not Pax6 (A; closed-arrowheads). In contrast, many GFP+ cells electroporated with pPax6 are found in VZ. While all of them are Pax6+, some are Tbr1+ (B; closed-arrowheads). GFP+ cells that do not express Tbr1 are also found (B; open-arrowheads). Damaged region with a hole in the section shown in (A) is encircled with dotted lines. (C) Scatter plots showing percentages of Tbr1 expressing cells among GFP+ cells in E14 OB electroporated with pCAGEN (n=9) or pPax6 (n=9). The percentage is significantly decreased by pPax6 electroporation (***p<0.0005; unpaired t test). (D) Histogram of optical intensity (arbitrary unit) of Tbr1 in GFP+ cells electroporated with pCAGEN (blue) or pPax6 (red). Only GFP+ cells that were counted as Tbr1+ in (C) were used for the analysis. Trendlines were overlaid in the graph. (E, F) Similar to Tbr1 expression, Tbr2 (Alexa 555, red) is expressed by most of GFP+ cells (Cy2, green) electroporated with pCAGEN (E; closed-arrowheads), while both Tbr2+ (F; closed-arrowheads) and Tbr2– (F; open-arrowheads) cells are found among GFP+ cells electroporated with pPax6). (G) Scatter plots showing percentages of Tbr2 expressing cells among GFP+ cells in E14 OB elctroporated with pCAGEN (n=9) or pPax6 (n=9). The percentage is significantly decreased by pPax6 electroporation (***p<0.0005; unpaired t test). (H) Histogram of optical intensity (arbitrary unit) of Tbr2 in GFP+ cells electroporated with pCAGEN (blue) or pPax6 (red). Only GFP+ cells that were counted as Tbr2+ in (G) were used for the analysis. Trendlines were overlaid in the graph. Scale bars, 50 μm.
Next, we compared the expression of Tbr1 and Tbr2 in cells electroporated with pCAGEN or pPax6 in the E14 OB. When pCAGEN was electroporated, GFP+ cells were negative for Pax6 but positive for Tbr1 (Fig. 5A; closed arrowheads). In sharp contrast, when pPax6 was electroporated with pGFP, GFP+ cells mostly expressed Pax6, suggesting the exogenous Pax6 expression from electroporated pPax6 (Fig. 5B; arrowheads). In this case, there were many GFP+ cells that did not express Tbr1 (Fig. 5B, open arrowheads). Our quantification revealed that 84.5 ± 2.8 % of GFP+ cells expressed Tbr1 in the OB electroporated with pCAGEN, which is in good agreement with the value observed at E17 (Fig. 3F). However, the percentage was significantly decreased to 45.1 ± 7.3 % following pPax6 electroporation (Fig. 5C). Similarly, Tbr2 expression was significantly suppressed by exogenous expression of Pax6 (Fig 5E, F). While 82.0 ± 3.7 % of pCAGEN electroporated cells expressed Tbr2 in the E14 OB, only 45.3 ± 7.1 % of pPax6 electroporated cells were Tbr2+ (Fig. 5G).
Pax6 did not completely inhibit Tbr1 or Tbr2 expression in mitral cell precursors. Since we counted Tbr1+ or Tbr2+ cells regardless of their expression levels, we next examined the expression levels of Tbr1 and Tbr2 in GFP+ cells by analyzing their relative optical intensity. Figures 5D and H show histograms of optical intensities of Tbr1 and Tbr2, respectively, in GFP+ cells that were determined as Tbr1+ or Tbr2+ in previous analyses. Fractions of GFP+ cells exhibiting high optical intensities of Tbr1 and Tbr2 were both decreased by exogenous expression of Pax6, suggesting that Tbr1 and Tbr2 expression were suppressed by Pax6. These results showed that Pax6 negatively regulates the expression of Tbr1 and Tbr2 in postmitotic mitral cell precursors.
Exogenous expression of Pax6 changed cell fate from projection neuron to interneuron
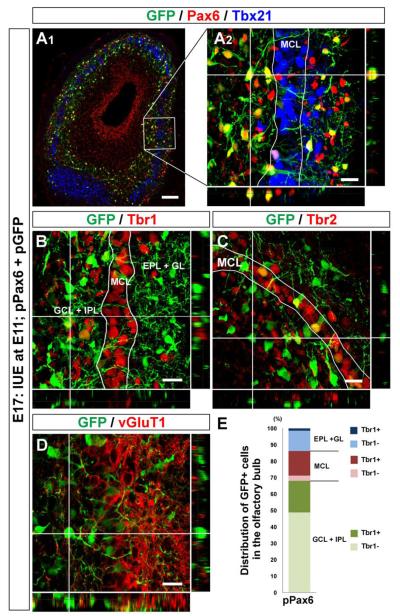
We then examined the fate of cells electroporated with pPax6 at E11 that were negative for Tbr1 and Tbr2 expression. First, we asked whether these cells died without differentiating or progressing to an alternate fate. However, using cleaved-caspase3 as a marker of apoptotic cell death, we found no colocalization with the GFP+ cells at E15 or E17 (data not shown). At E17, a prominent difference between pCAGEN and pPax6 electroporated GFP+ cells was observed in their laminar distributions in the OB. While pCAGEN electroporated cells localized in the MCL (Fig. 3D), there were fewer pPax6 electroporated cells in this layer (Fig. 6A). In contrast, we found that many GFP+ cells that were pPax6 electroporated localized outside of the MCL. These GFP+ cells located outside the MCL did not express the molecular markers characteristic of the excitatory projection neurons found in the OB, such as Tbx21, Tbr1, Tbr2, and vGluT1 (Fig. 6A – D). Analysis of GFP+ cell distribution revealed that less than one fifth (17.9 ± 2.2%) were in the MCL, although most of those were Tbr1+ (Fig. 6E). Compared with pCAGEN electroporated cells (Fig. 3J), percentages of Tbr1-negative cells found outside the MCL were significantly increased. Overall, only one third (35.7 ± 5.6%) of GFP+ cells electroporated with pPax6 expressed Tbr1 in the E17 OB, which is consistent with the value acquired at E14 (Fig. 5C). Considering that there was no evidence of cell death among pPax6 electroporated cells, it is likely that these Tbr1-negative cells were derived from the precursors that were originally fated to differentiate into mitral cells. These results suggest that exogenous expression of Pax6 in embryonic OB cells prohibits differentiation into mitral cells by suppressing the expression of Tbr1 and Tbr2.
Figure 6. Impaired mitral cell differentiation by sustained Pax6 expression in embryonic olfactory bulb.
(A – D) Horizontal sections of E17 OB electroporated with pPax6 and pGFP. GFP+ cells (Cy2, green) are Pax6+ (A; Alexa 555, red), and localize both inside and outside the mitral cell layer that is defined with Tbx21 (A; Alexa 647, blue). GFP+ cells positioned inside the mitral cell layer express Tbr1 (B; Alexa 555, red), Tbr2 (C; Alexa 555, red), and vGluT1 (D; Alexa 555, red). In contrast, GFP+ cells found outside the mitral cell layer are mostly negative for these molecules. (E) Graph showing distribution and Tbr1 expression of GFP+ cells in E17 OB electroporated with pPax6 and pGFP. The graph was made in the same way as described in Figure 3J. Data acquired from 4 OBs were averaged. Scale bars, 50 μm.
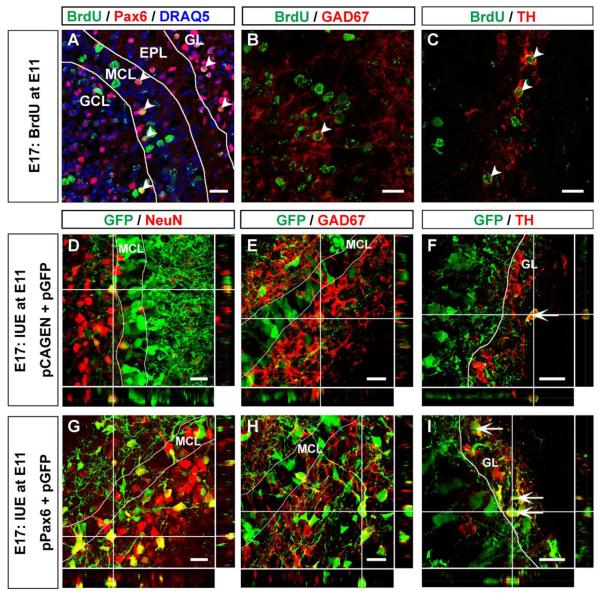
Pax6 regulates the differentiation of specific subtypes of interneurons in the OB (Brill et al., 2008; Ninkovic et al., 2010). Because our BrdU-labeling experiment suggested that some postmitotic cells generated in the E11 OB expressed Pax6 and did not differentiate into mitral cells (Fig. 1), we tested to determine if these Pax6+ cells exhibited interneuronal phenotypes. At E17, many BrdU-labeled cells expressing Pax6 were found in the GL, MCL, and superficial GCL (Fig. 7A). Some BrdU-labeled cells expressed GAD67, a molecular marker of GABAergic neurons (Fig. 7B), and tyrosine hydroxylase (TH), a marker of dopaminergic neurons (Fig. 7C).
Figure 7. Differentiation of pPax6 electroporated cells into interneurons in embryonic olfactory bulb.
(A – C) Horizontal sections of E17 OB. BrdU was injected into pregnant mothers at E11. BrdU+ cells (Cy2, green) expressing Pax6 (A; Alexa 555, red) are found in GL, MCL, and GCL (A; arrowheads). All nuclei were stained with DRAQ5 (blue). There are BrdU+ cells expressing GAD67 (B; Alexa 555, red) or TH (C; Alexa 555, red) (B, C; arrowheads). (D, – F) Horizontal sections of E17 OB electroporated with pCAGEN and pGFP. A few GFP+ cells (green) positioned outside the MCL express NeuN (D; Alexa 555, red), GAD67 (E; Alexa 555, red) or TH (F; Alexa 555, red). (G – I) Horizontal sections of E17 OB electroporated with pPax6 and pGFP. Many GFP+ cells positioned outside the MCL express NeuN (G; Alexa 555, red), GAD67 (H; Alexa 555, red), or TH (I; Alexa 555, red). Scale bars, 20μm.
With in utero electroporation experiments, when pGFP was electroporated with pCAGEN into the embryonic OB at E11, there were only a few GFP+ cells that expressed NeuN, an interneuronal marker in the OB that is not found in mitral cells (Imamura et al., 2006; Mullen et al., 1992), as well as GAD67+ and TH+ (Fig. 7D – F). These observations demonstrate that there are progenitor cells that can generate interneurons, TH+ and/or GABAergic, in E11 OB. In contrast, we observed that larger numbers of pPax6 electroporated cells expressed NeuN (Fig. 7G). Moreover, the numbers of GFP+ cells expressing GAD67 and TH in the superficial GCL and GL of E17 OB also increased (Fig. 7H, I). These data further support our conclusion that the progeny of cells that have Pax6 expression have differentiated into GABAergic and/or dopaminergic interneurons. The pPax6 electroporated cells localized in the superficial GCL did not express TH protein and are most likely granule cells or immature TH periglomerular cells (Baker et al., 2001). Our results suggest that sustained expression of Pax6 in postmitotic cells generated in the E11 OB produces fewer mitral cells but more interneurons, and that the fates of postmitotic cells are regulated by the levels and duration of Pax6 expression. Since pPax6 was introduced into a limited number of cells in the developing OB, the determination of cell fate toward either mitral cell or interneuron is likely to be cell-autonomous.
DISCUSSION
Expression sequence of transcription factors in developing mitral cell
We examined the expression of Pax6, Tbr1, and Tbr2 during the course of differentiation of mitral cells generated at E11. First, we established that mitral cells are generated by Pax6+ radial glial cells in the VZ of the presumptive embryonic OB at the anterior pole of the telencephalic vesicle, and then radially migrate into the IZ prior to differentiation. Both Tbr1 and Tbr2 are expressed by IZ cells of the developing OB as early as E11. Unlike developing pyramidal neurons in the neocortex, postmitotic mitral cell precursors in the IZ can simultaneously express Tbr1 and Tbr2. Here, we showed that Tbr1 expression in the postmitotic mitral cell precursors preceded Tbr2, and that Pax6 regulates their expression. However, not all mitral cells may follow the same pathway of differentiation. Mitral cells display considerable diversity in electrophysiological properties and in ion channel expression (Angelo and Margrie, 2011; Angelo et al., 2012; Padmanabhan and Urban, 2010). Moreover, we previously demonstrated that mitral cells with different birthdates are differentially localized in the OB and project their axons to different regions of the olfactory cortex (Imamura et al., 2011). Late-generated mitral cells (>E12) tangentially migrate in the OB toward the postero-ventro-lateral region after their radial migration to the IZ, suggesting that a longer period would be needed for late-generated mitral cells to mature. Since the focus here has been on E11-generated mitral cells, further analyses of mitral cells generated at additional time points will be necessary to fully understand the temporal and molecular interactions regulating mitral cell differentiation.
Furthermore, although we did not observe a clear subventricular zone that is predominantly occupied by IPCs expressing Tbr2 between the VZ and IZ (Englund et al., 2005), there were a few cells expressing both Pax6 and Tbr2 in the VZ of the developing OB. Because no cell expressed Tbr1 and Pax6 simultaneously, these Tbr2+ cells may be putative IPCs that follow the canonical expression sequence as seen in cortical pyramidal neurons of Pax6 → Tbr2 → Tbr1 during differentiation (Winpenny et al., 2011). Interestingly, a recent study showed that Tbr2 is also involved in dendritic specification of mitral cells during postnatal development (Mizuguchi et al., 2012). These data suggest that Tbr2 may subserve multiple functions, both during early embryonic differentiation and during later postnatal development and refinement of neuronal phenotype.
Electroporation of plasmid vectors into mitral cell precursors
In utero electroporation is widely used to regulate gene expression and study the role(s) of candidate molecules in the nervous system (Chen et al., 2005; Dixit et al., 2011; Saito and Nakatsuji, 2001). However, only a few have applied electroporation to study the development of olfactory tissues (Bai et al., 2008), and none in the embryonic or nascent OB. We searched for a condition to efficiently introduce plasmid vectors into mitral cell precursors, and found that about 65% of control pGFP electroporated cells differentiate into mitral cells when the electroporation was performed at E11. We also found a small number of pGFP (control) electroporated cells differentiated into interneurons having GABAergic and/or dopaminergic phenotypes. The plasmid vectors are predominantly electroporated into the cells that are in S- through M-phases of the cell cycle (Stancik et al., 2010). Thus, the majority of the cells electroporated at E11 can be expected to have been generated at E11. This is consistent with our previous data showing that the largest number of mitral cells is generated at E11 (Imamura et al., 2011).
We showed that this technique can also be used to modulate molecular expression in mitral cell precursors, which affect the differentiation and final fate of mitral cells. We electroporated plasmid vectors encoding pax6 cDNA with a GFP plasmid at E11 and observed GFP expression in radial glial cells by E12. While Pax6 was suggested to regulate the cell cycle of progenitor cells (Quinn et al., 2007), exogenous expression of Pax6 with in utero electroporation did not significantly affect progenitor cell proliferation in the developing OB. Because it takes about a day before the translation of electroporated cDNAs, the expression level of Pax6 may be too low to affect the properties of progenitor cells. Thus, we can conclude that ectopically expressed Pax6 had its primary influence on postmitotic cells. We propose that in utero electroporation is a powerful and adaptable technique for studying the molecular mechanisms regulating olfactory system development, including the fate of postmitotic cells in the OB.
Cell fate determination regulated by Pax6
Here, we showed that exogenous expression of Pax6 in embryonic OB cells with in utero electroporation performed at E11 impaired later expression of Tbr1 and Tbr2 in postmitotic mitral cell precursors. Moreover, we showed that pPax6-electroporated cells failed to differentiate into mitral cells. Unexpectedly, there were significantly more pPax6-electroporated cells that now expressed interneuronal markers. Mutation of Pax6 causes abnormal development of an OB-like structure (OBLS) on the lateral aspect of the telencephalon (Jiménez et al., 2000; López-Mascaraque et al., 1998; Nomura and Osumi, 2004). Mitral-like cells are found in the OBLS and our findings may predict an increase in the number of these ectopic cells. However, Pax6 is also implicated in controlling neuron numbers in the cerebral cortex (Quinn et al., 2007) and in the migratory behavior of mitral cells (Nomura and Osumi, 2004). Therefore, it was not possible to qualitatively determine the Pax6 function in controlling mitral cell numbers by comparing the phenotype of a mutant that has global deletion of Pax6 from the beginning of embryonic development with our results in which Pax6 was exogenously expressed only in postmitotic cells.
Most embryonically generated OB interneurons emerge from the lateral ganglionic eminence (LGE) (Wichterle et al., 2001). While it is possible that an increase in interneurons labeled with pPax6 were derived from LGE progenitor cells that were unintentionally electroporated, we think this is unlikely. First, we found a significant percentage of pPax6 electroporated cells that did not express Tbr1 or Tbr2 in E14 OB, and a similar percentage of pPax6 electroporated cells expressing interneuronal markers at E17. Since we found no evidence of cell death in the OB at E15 or E17, it seems most plausible that the cells negative for Tbr1 and Tbr2 at E14 differentiated into interneurons. Second, the ventricle is still patent at the core of the E14 OB, and the migratory pathway of interneuronal precursors from LGE to the OB is not yet established. The most plausible interpretation is that GFP labeled interneurons were derived from progenitor cells in the embryonic OB. Precursor cells that give rise to interneurons within the embryonic OB were previously demonstrated (Vergaño-Vera et al., 2006). GAD+ and TH+ neurons were generated from cells taken from E13.5 OB, and about half of the GAD+ neurons were Pax6+. It was also previously reported that TH+ periglomerular cells are generated as early as E12.5 (Batista-Brito et al., 2008). Consistent with these reports, we found small numbers of GAD67+ and TH+ interneurons around E11, when the largest number of mitral cells are generated. These data collectively suggest the presence of progenitor cells that can produce GAD67+ and TH+ cells in E11 OB.
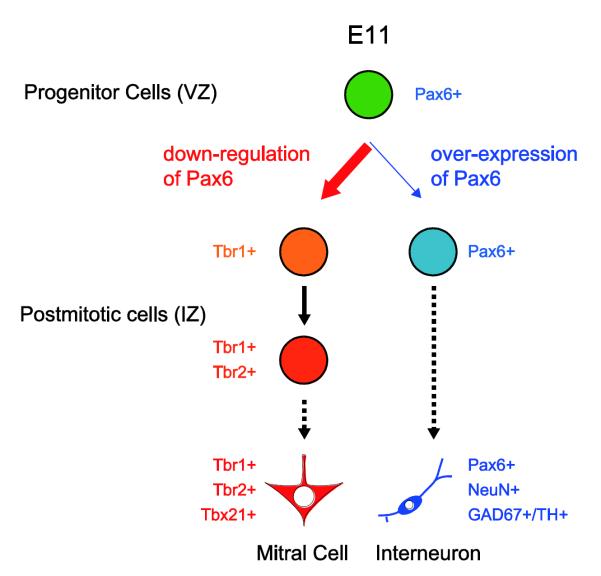
Whether different types of cells in the brain are produced from small subsets of multi-potential progenitor cells or larger populations of heterogenous progenitors remains a central question. Because our exogenous expression of Pax6 resulted in: (1) a decrease in mitral cells; (2) an increase in the number of interneurons; and (3) no change in cell proliferation or death, we are led to suggest that a multi-potential progenitor cell in E11 OBs can give rise to both mitral cells and interneurons, and that Pax6 is a regulator of the differentiation fate. After the final division of progenitors in the embryonic OB, Pax6 is down-regulated in postmitotic cells to produce mitral cells (Fig. 8). Whether intrinsic or extrinsic, Pax6 expression in postmitotic cells of the developing OB should be precisely regulated by yet unidentified mechanisms in a time-dependent manner. However, because our in utero electroporation experiments could cause sustained exogenous Pax6 expression, it is still not clear whether sustained Pax6 expression or failure of Pax6 down-regulation at the right time led the E11-generated postmitotic cells to an interneuronal phenotype. This should be clarified in future studies.
Figure 8. Model for differentiations of mitral cell and interneurons in embryonic olfactory bulb.
Both mitral cell and specific subsets of interneurons are generated from multi-potential Pax6+ progenitor cells in embryonic OB (green) around E11. The differentiation fates of postmitotic cells are regulated by Pax6 expression. Pax6 is down-regulated in majority of E11 generated postmitotic cells to produce mitral cells that are Tbr1+, Tbr2+, and Tbx21+ (thick red arrow). Tbr1 expression precedes Tbr2. If Pax6 is not down-regulated at right time point, the E11-generated postmitotic cells differentiate into interneurons expressing NeuN, GAD67, and/or TH (thin blue arrow).
Conclusions
In conclusion, we showed novel functions of Pax6 in developing OB mitral cells. Pax6 regulates the fate of mitral cells and interneurons. Pax6 down-regulation in precursors is a prerequisite for a mitral cell fate. In contrast, with sustained Pax6, the precursors can follow an interneuron fate. Interestingly, the functions of Pax6 are region specific. Pax6 down-regulates in the cells in developing cerebral cortex as they differentiate into pyramidal neurons, not mitral cells (Englund et al., 2005). Therefore, down-regulation of Pax6 is necessary, but not sufficient, to produce mitral cells. Conditional loss of a transcription factor Gsx2 changes the fate of progenitor cells in dorsal LGE from Sp8+ interneurons to Tbr1+ projection neurons, suggesting that the differentiation pathway toward projection neurons is, in part, suppressed by Gsx2 in dorsal LGE (Waclaw et al., 2009). Our next goal is to identify the upstream regulators of Pax6 expression precursor cells in the developing OB and the downstream targets mediating the differing cellular phenotypes. Uncovering the cascades of molecules and signaling pathways regulating mitral cell differentiation is central to unraveling olfactory circuits and will also provide new insights into the mechanisms generating neuronal diversity in the developing brain.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Drs. Kenneth Kwan, Mandy Lam, and Nenad Šestan for guidance in the use of in utero electroporation and Dr. Yoshihiro Yoshihara for the antibody to Tbx21. We also thank Drs. Arie Mobley and Diego Rodriguez-Gil for the critical reading of the manuscript and all the members of Greer laboratory for technical assistance and discussion. The antibody to Pax6 developed by Atsushi Kawakami was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the US National Institute of Child Health and Human Development and maintained by the Department of Biology, University of Iowa. This work was supported by NIH grants DC000210 (C.A.G.) and DC011134 (F.I.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Angelo K, Margrie TW. Population diversity and function of hyperpolarization-activated current in olfactory bulb mitral cells. Sci Rep. 2011;1:50. doi: 10.1038/srep00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelo K, Rancz EA, Pimentel D, Hundahl C, Hannibal J, Fleischmann A, Pichler B, Margrie TW. A biophysical signature of network affiliation and sensory processing in mitral cells. Nature. 2012 doi: 10.1038/nature11291. (in publish) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold SJ, Huang GJ, Cheung AF, Era T, Nishikawa S, Bikoff EK, Molnar Z, Robertson EJ, Groszer M. The T-box transcription factor Eomes/Tbr2 regulates neurogenesis in the cortical subventricular zone. Genes Dev. 2008;22:2479–2484. doi: 10.1101/gad.475408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai J, Ramos RL, Paramasivam M, Siddiqi F, Ackman JB, LoTurco JJ. The role of DCX and LIS1 in migration through the lateral cortical stream of developing forebrain. Dev Neurosci. 2008;30:144–156. doi: 10.1159/000109859. [DOI] [PubMed] [Google Scholar]
- Baker H, Liu N, Chun HS, Saino S, Berlin R, Volpe B, Son JH. Phenotypic differentiation during migration of dopaminergic progenitor cells to the olfactory bulb. J Neurosci. 2001;21:8505–8513. doi: 10.1523/JNEUROSCI.21-21-08505.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista-Brito R, Close J, Machold R, Fishell G. The distinct temporal origins of olfactory bulb interneuron subtypes. J Neurosci. 2008;28:3966–3975. doi: 10.1523/JNEUROSCI.5625-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchart A, De Carlos JA, López-Mascaraque L. Time frame of mitral cell development in the mice olfactory bulb. J Comp Neurol. 2006;496:529–543. doi: 10.1002/cne.20941. [DOI] [PubMed] [Google Scholar]
- Brill MS, Snapyan M, Wohlfrom H, Ninkovic J, Jawerka M, Mastick GS, Ashery-Padan R, Saghatelyan A, Berninger B, Götz M. A dlx2- and pax6-dependent transcriptional code for periglomerular neuron specification in the adult olfactory bulb. J Neurosci. 2008;28:6439–6452. doi: 10.1523/JNEUROSCI.0700-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulfone A, Martinez S, Marigo V, Campanella M, Basile A, Quaderi N, Gattuso C, Rubenstein JL, Ballabio A. Expression pattern of the Tbr2 (Eomesodermin) gene during mouse and chick brain development. Mech Dev. 1999;84:133–138. doi: 10.1016/s0925-4773(99)00053-2. [DOI] [PubMed] [Google Scholar]
- Bulfone A, Smiga SM, Shimamura K, Peterson A, Puelles L, Rubenstein JL. T-brain-1: a homolog of Brachyury whose expression defines molecularly distinct domains within the cerebral cortex. Neuron. 1995;15:63–78. doi: 10.1016/0896-6273(95)90065-9. [DOI] [PubMed] [Google Scholar]
- Bulfone A, Wang F, Hevner R, Anderson S, Cutforth T, Chen S, Meneses J, Pedersen R, Axel R, Rubenstein JL. An olfactory sensory map develops in the absence of normal projection neurons or GABAergic interneurons. Neuron. 1998;21:1273–1282. doi: 10.1016/s0896-6273(00)80647-9. [DOI] [PubMed] [Google Scholar]
- Chen JG, Ras̆in MR, Kwan KY, S̆estan N. Zfp312 is required for subcortical axonal projections and dendritic morphology of deep-layer pyramidal neurons of the cerebral cortex. Proc Natl Acad Sci U S A. 2005;102:17792–17797. doi: 10.1073/pnas.0509032102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixit R, Lu F, Cantrup R, Gruenig N, Langevin LM, Kurrasch DM, Schuurmans C. Efficient gene delivery into multiple CNS territories using in utero electroporation. J Vis Exp. 2011;52:e2957. doi: 10.3791/2957. DOI: 2910.3791/2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englund C, Fink A, Lau C, Pham D, Daza RA, Bulfone A, Kowalczyk T, Hevner RF. Pax6, Tbr2, and Tbr1 are expressed sequentially by radial glia, intermediate progenitor cells, and postmitotic neurons in developing neocortex. J Neurosci. 2005;25:247–251. doi: 10.1523/JNEUROSCI.2899-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faedo A, Ficara F, Ghiani M, Aiuti A, Rubenstein JL, Bulfone A. Developmental expression of the T-box transcription factor T-bet/Tbx21 during mouse embryogenesis. Mech Dev. 2002;116:157–160. doi: 10.1016/s0925-4773(02)00114-4. [DOI] [PubMed] [Google Scholar]
- Feng L, Hatten ME, Heintz N. Brain lipid-binding protein (BLBP): a novel signaling system in the developing mammalian CNS. Neuron. 1994;12:895–908. doi: 10.1016/0896-6273(94)90341-7. [DOI] [PubMed] [Google Scholar]
- Götz M, Stoykova A, Gruss P. Pax6 controls radial glia differentiation in the cerebral cortex. Neuron. 1998;21:1031–1044. doi: 10.1016/s0896-6273(00)80621-2. [DOI] [PubMed] [Google Scholar]
- Hack MA, Sugimori M, Lundberg C, Nakafuku M, Götz M. Regionalization and fate specification in neurospheres: the role of Olig2 and Pax6. Mol Cell Neurosci. 2004;25:664–678. doi: 10.1016/j.mcn.2003.12.012. [DOI] [PubMed] [Google Scholar]
- Haubst N, Berger J, Radjendirane V, Graw J, Favor J, Saunders GF, Stoykova A, Götz M. Molecular dissection of Pax6 function: the specific roles of the paired domain and homeodomain in brain development. Development. 2004;131:6131–6140. doi: 10.1242/dev.01524. [DOI] [PubMed] [Google Scholar]
- Hebert JM, Lin M, Partanen J, Rossant J, McConnell SK. FGF signaling through FGFR1 is required for olfactory bulb morphogenesis. Development. 2003;130:1101–1111. doi: 10.1242/dev.00334. [DOI] [PubMed] [Google Scholar]
- Heins N, Malatesta P, Cecconi F, Nakafuku M, Tucker KL, Hack MA, Chapouton P, Barde YA, Götz M. Glial cells generate neurons: the role of the transcription factor Pax6. Nat Neurosci. 2002;5:308–315. doi: 10.1038/nn828. [DOI] [PubMed] [Google Scholar]
- Hinds JW. Early neuron differentiation in the mouse of olfactory bulb. I. Light microscopy. J Comp Neurol. 1972;146:233–252. doi: 10.1002/cne.901460207. [DOI] [PubMed] [Google Scholar]
- Imamura F, Ayoub AE, Rakic P, Greer CA. Timing of neurogenesis is a determinant of olfactory circuitry. Nat Neurosci. 2011;14:331–337. doi: 10.1038/nn.2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura F, Nagao H, Naritsuka H, Murata Y, Taniguchi H, Mori K. A leucine-rich repeat membrane protein, 5T4, is expressed by a subtype of granule cells with dendritic arbors in specific strata of the mouse olfactory bulb. J Comp Neurol. 2006;495:754–768. doi: 10.1002/cne.20896. [DOI] [PubMed] [Google Scholar]
- Jiménez D, García C, de Castro F, Chédotal A, Sotelo C, de Carlos JA, Valverde F, López-Mascaraque L. Evidence for intrinsic development of olfactory structures in Pax-6 mutant mice. J Comp Neurol. 2000;428:511–526. [PubMed] [Google Scholar]
- López-Mascaraque L, García C, Valverde F, de Carlos JA. Central olfactory structures in Pax-6 mutant mice. Ann N Y Acad Sci. 1998;855:83–94. doi: 10.1111/j.1749-6632.1998.tb10549.x. [DOI] [PubMed] [Google Scholar]
- Matsuda T, Cepko CL. Electroporation and RNA interference in the rodent retina in vivo and in vitro. Proc Natl Acad Sci U S A. 2004;101:16–22. doi: 10.1073/pnas.2235688100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AM, Maurer LR, Zou DJ, Firestein S, Greer CA. Axon fasciculation in the developing olfactory nerve. Neural Dev. 2010;5:20. doi: 10.1186/1749-8104-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuguchi R, Naritsuka H, Mori K, Yoshihara Y. Tbr2 deficiency in mitral and tufted cells disrupts excitatory-inhibitory balance of neural circuitry in the mouse olfactory bulb. J Neurosci. 2012;32:8831–8844. doi: 10.1523/JNEUROSCI.5746-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori K, Nagao H, Yoshihara Y. The olfactory bulb: coding and processing of odor molecule information. Science. 1999;286:711–715. doi: 10.1126/science.286.5440.711. [DOI] [PubMed] [Google Scholar]
- Mullen RJ, Buck CR, Smith AM. NeuN, a neuronal specific nuclear protein in vertebrates. Development. 1992;116:201–211. doi: 10.1242/dev.116.1.201. [DOI] [PubMed] [Google Scholar]
- Ninkovic J, Pinto L, Petricca S, Lepier A, Sun J, Rieger MA, Schroeder T, Cvekl A, Favor J, Götz M. The Transcription Factor Pax6 Regulates Survival of Dopaminergic Olfactory Bulb Neurons via Crystallin alphaA. Neuron. 2010;68:682–694. doi: 10.1016/j.neuron.2010.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura T, Osumi N. Misrouting of mitral cell progenitors in the Pax6/small eye rat telencephalon. Development. 2004;131:787–796. doi: 10.1242/dev.00984. [DOI] [PubMed] [Google Scholar]
- Padmanabhan K, Urban NN. Intrinsic biophysical diversity decorrelates neuronal firing while increasing information content. Nat Neurosci. 2010;13:1276–1282. doi: 10.1038/nn.2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn JC, Molinek M, Martynoga BS, Zaki PA, Faedo A, Bulfone A, Hevner RF, West JD, Price DJ. Pax6 controls cerebral cortical cell number by regulating exit from the cell cycle and specifies cortical cell identity by a cell autonomous mechanism. Dev Biol. 2007;302:50–65. doi: 10.1016/j.ydbio.2006.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T, Nakatsuji N. Efficient gene transfer into the embryonic mouse brain using in vivo electroporation. Dev Biol. 2001;240:237–246. doi: 10.1006/dbio.2001.0439. [DOI] [PubMed] [Google Scholar]
- Sessa A, Mao CA, Hadjantonakis AK, Klein WH, Broccoli V. Tbr2 directs conversion of radial glia into basal precursors and guides neuronal amplification by indirect neurogenesis in the developing neocortex. Neuron. 2008;60:56–69. doi: 10.1016/j.neuron.2008.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stancik EK, Navarro-Quiroga I, Sellke R, Haydar TF. Heterogeneity in ventricular zone neural precursors contributes to neuronal fate diversity in the postnatal neocortex. J Neurosci. 2010;30:7028–7036. doi: 10.1523/JNEUROSCI.6131-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Nowakowski RS, Caviness VS., Jr. The cell cycle of the pseudostratified ventricular epithelium of the embryonic murine cerebral wall. J Neurosci. 1995;15:6046–6057. doi: 10.1523/JNEUROSCI.15-09-06046.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergaño-Vera E, Yusta-Boyo MJ, de Castro F, Bernad A, de Pablo F, Vicario-Abejón C. Generation of GABAergic and dopaminergic interneurons from endogenous embryonic olfactory bulb precursor cells. Development. 2006;133:4367–4379. doi: 10.1242/dev.02601. [DOI] [PubMed] [Google Scholar]
- Waclaw RR, Wang B, Pei Z, Ehrman LA, Campbell K. Distinct temporal requirements for the homeobox gene gsx2 in specifying striatal and olfactory bulb neuronal fates. Neuron. 2009;63:451–465. doi: 10.1016/j.neuron.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther C, Gruss P. Pax-6, a murine paired box gene, is expressed in the developing CNS. Development. 1991;113:1435–1449. doi: 10.1242/dev.113.4.1435. [DOI] [PubMed] [Google Scholar]
- Wichterle H, Turnbull DH, Nery S, Fishell G, Alvarez-Buylla A. In utero fate mapping reveals distinct migratory pathways and fates of neurons born in the mammalian basal forebrain. Development. 2001;128:3759–3771. doi: 10.1242/dev.128.19.3759. [DOI] [PubMed] [Google Scholar]
- Winpenny E, Lebel-Potter M, Fernandez ME, Brill MS, Götz M, Guillemot F, Raineteau O. Sequential generation of olfactory bulb glutamatergic neurons by Neurog2-expressing precursor cells. Neural Dev. 2011;6:12. doi: 10.1186/1749-8104-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.