Abstract
TLR9 suppresses TLR7-driven pathogenesis in the MRL.Faslpr murine model of systemic lupus erythematosus, but the mechanisms by which TLR7 promotes and TLR9 prevents disease in this and other lupus models remain unclear. Type I interferons have also been implicated in the pathogenesis of lupus both in patients and in several murine models of disease, but their role in MRL.Faslpr mice is controversial. Using MRL.Faslpr mice genetically deficient in a subunit of the receptor for type I interferon, Ifnar1, we show that type I interferons contribute significantly to renal disease in this model. Ifnar1 had no effect on anti-nucleosome or anti-Sm autoantibody titers, but instead regulated anti-cytoplasmic and anti-RNA specificities. Moreover, Ifnar1 deficiency prevented the exacerbation of clinical disease observed in Tlr9-deficient animals in this lupus model. Thus, type I interferon signaling is an important mediator of lupus pathogenesis and anti-RNA antibody production that is dysregulated in the absence of Tlr9.
Keywords: autoimmunity, systemic lupus erythematosus, TLR9, IFN–I
Introduction
Systemic lupus erythematosus (SLE) is an autoimmune disease characterized by the loss of B cell tolerance to nucleic acid-associated self antigens (1). The clinical diagnostic hallmark of lupus is the presence of serum autoantibodies with affinity for dsDNA or nucleosomes. In addition to anti-nucleosome autoantibodies, autoantibodies reactive to RNA-associated protein antigens including Sm or to RNA itself are present in a fraction of SLE patients, particularly those with more severe disease activity (2). These antibodies form immune complexes that deposit in target organs, including the kidneys, where they contribute to pathological inflammation (3).
Recently we demonstrated that anti-nucleosome autoantibodies in the MRL.Faslpr mouse model of SLE are dependent upon Tlr9, an endosomal innate immune sensor of dsDNA expressed in B cells and myeloid cells among others (4–6). Similarly, anti-Sm and anti-RNA autoantibodies required Tlr7, a sensor of RNA (5, 6). MRL.Faslpr mice lacking both Tlr7 and Tlr9, or their common signaling adaptor Myd88, displayed reduced titers of both anti-nucleosome and anti-RNA antibodies and had little clinical disease (6). Surprisingly, however, mice deficient only in Tlr9 but with intact Tlr7 had more severe clinical manifestations of disease, including renal disease, together with elevated titers of certain anti-RNA specificities, despite lacking anti-nucleosome autoAb (4–6). This apparently paradoxical effect of Tlr9 deficiency on disease severity has since been replicated in at least four other mouse models of SLE (7–10).
Previously we observed an increase in the titer of serum IFNα in MRL.Faslpr mice lacking Tlr9 (5) and speculated that this cytokine could have contributed to the exacerbation of disease in those animals. IFNα, a type I interferon (IFN-I), is elevated in some SLE patients with severe disease (11, 12), and has also been implicated in the pathogenesis of several murine lupus models (13–18). In contrast, a previous report suggested that IFN-I was dispensable for disease or even protective in the MRL.Faslpr model, although only a small number of animals were examined in this study (19). Even if IFN-I were not required for MRL.Faslpr disease, it remained possible that IFN-I could have contributed to the enhancement or acceleration of disease specifically in mice lacking Tlr9. We therefore backcrossed mice deficient in the receptor for IFN-I, Ifnar1, onto the MRL.Faslpr model with or without Tlr9. Here we demonstrate that Ifnar1 indeed contributes to renal disease and production of anti-RNA but not anti-nucleosome autoantibodies in the MRL.Faslpr model, in contrast to the previous report. Moreover, and most importantly, the exacerbation of disease seen in Tlr9−/− MRL.Faslpr mice is substantially mitigated in Ifnar1−/− Tlr9−/− MRL.Faslpr mice, suggesting that the proinflammatory effects of TLR9 deficiency in lupus are in large part mediated via increased IFN-I.
Materials and Methods
Mice
Ifnar1−/− mice were previously described (20) and were backcrossed to the MRL/MpJ-Faslpr/lpr/2J genetic background (Jackson Labs #006825) for eight generations prior to intercross. Tlr9−/− mice on the MRL/MpJ-Faslpr/lpr/J background were previously described, and were backcrossed an additional six generations to the MRL/MpJ-Faslpr/lpr/2J background prior to intercross. There were n=11–32 animals per group in all assays except for 24 wk Ifnar+/+Tlr9+/+ where n=5 in all assays. All animal work was approved by the Yale Institutional Animal Care and Use Committee.
Evaluation of Clinical Disease
For skin disease, mice were scored for dorsal lesions on a scale of 0–5 based on affected area, with up to one additional point for presence of ear dermatitis and facial rash or loss of whiskers as described previously (5). Proteinuria was measured using a colorimetric dipstick assay (Albustix; Siemens, Tarrytown, NY). For kidney disease, formalin-fixed and paraffin-embedded tissue sections stained with hematoxylin and eosin were scored for extent of interstitial and perivascular infiltrates on a 0–3 scale by an independent observer blinded to the genotype of the samples. Glomerulonephritis was scored on the same sections on a 0–6 scale as previously described (21).
Measurement of serum autoantibodies
HEp-2 immunofluorescence assays (Antibodies Inc, Davis, CA) were performed as previously described (5) with serum diluted at 1/200 and were scored for relative fluorescence intensity of cytoplasmic staining on a scale of 0–3 and for the presence or absence of mitotic chromatin by an observer blinded to the genotype of the samples.
Anti-nucleosome and anti-Sm Ab ELISAs were performed as previously described (6). Anti-RNA Ab ELISAs were performed as described (22). Total serum IgG was determined by ELISA as previously described (6). Total serum IgM was determined by ELISA by coating polystyrene plates with goat anti-mouse IgM (clone B7-6). After blocking with 1% BSA in PBS, serial dilutions of serum from 1/50,000 to 1/1,350,000 were added. Specific Abs were detected with alkaline phosphatase-conjugated goat anti-mouse IgM (Southern Biotechnology Associates).
Results
To evaluate the role of IFN-I in the MRL.Faslpr model of SLE, we backcrossed mice genetically deficient in Ifnar1, a subunit of the heterodimeric receptor for IFN-I, onto the MRL.Faslpr/2J genetic background. Although a previous report found little effect of Ifnar1 deficiency on disease in the MRL.Faslpr model, this report evaluated a small number of animals and did not segregate them by gender (19). Due to the variable onset and severity of disease in this model, as well as the gender-dependent difference in disease kinetics (23), we considered that this report may not have had sufficient statistical power to accurately determine effects of Ifnar1 deficiency. In addition, we previously reported that several clinical parameters of disease were exacerbated in Tlr9-deficient MRL.Faslpr mice that also had elevated titers of serum IFNα (5). Therefore, to test the hypothesis that an important mechanism by which TLR9-deficiency paradoxically promotes disease is via stimulation of excessive IFN-I secretion and signaling, we intercrossed Ifnar1−/− MRL.Faslpr animals with MRL.Faslpr genetically deficient in Tlr9. These crosses generated experimental cohorts lacking Ifnar1, Tlr9 or both Ifnar1 and Tlr9. Mice were evaluated at either 16 or 24 weeks of age, and results were further segregated based on gender.
Renal function and glomerular disease are improved in Ifnar1-deficient MRL.Faslpr mice
We first evaluated parameters of renal disease. Proteinuria, a measure of kidney function, was reduced in Ifnar1-deficient female cohorts compared to Ifnar1-intact littermates at both 16 and 24 weeks of age (Fig. 1A). In contrast, males had little proteinuria even by 24 weeks in the Ifnar1-intact or -deficient groups. Importantly, proteinuria was elevated in 16 wk female and male and 24 wk male mice lacking Tlr9 compared to Tlr9-intact animals, in agreement with our previous reports of exacerbated renal disease in the absence of Tlr9 (5, 6). In contrast, mice deficient in both Ifnar1 and Tlr9 had relatively little proteinuria, suggesting that the exacerbation of disease in Tlr9 deficient animals also requires the receptor for IFN-I.
Figure 1. Renal disease in MRL.Faslpr mice requires Ifnar1.
(A) Proteinuria was evaluated in 16 wk old (left graph) or 24 wk old (right graph) mice of indicated genotypes and genders using a dipstick assay. (B) Interstitial and perivascular renal infiltrates were scored by a pathologist on a 0–3 scale. (C) Glomerular renal disease was scored by a pathologist on a 0–6 scale. Data are represented as mean +/− SEM. * p<0.05; ** p<0.01; *** p<0.001, one-tailed Mann-Whitney test.
Renal disease in SLE may be mediated by interstitial and perivascular lymphocyte infiltrates and/or by inflammation of the glomeruli (3). The extent of lymphocytic infiltrates in H&E stained kidney sections was evaluated by a pathologist blinded to the genotypes of the animals, and was not significantly different among any of the experimental groups, suggesting that neither Ifnar1 nor Tlr9 grossly affect recruitment and/or in situ expansion of lymphocytes in the kidneys (Fig. 1B). In contrast, the severity of glomerular disease was significantly diminished in 16wk female and 24 wk female and male mice that lacked both Ifnar1 and Tlr9 compared to mice lacking only Tlr9, as reflected by differences in glomerular scores (Fig. 1C). Incidence of severe disease was also influenced by Ifnar1. The 24 week female Tlr9−/− group had 13/22 severely affected (score >=3) while the doubly-deficient group had 9/32 with severe glomerulonephritis (p=0.0282 by two-sided Fisher’s exact test); among the males the Tlr9−/− group had 15/32 with severe disease while only 3/31 Ifnar1−/−Tlr9−/− mice were severely affected (p=0.0017 by two-sided Fisher’s exact test). Thus, Ifnar1 promotes progression of glomerular but not interstitial renal disease in the MRL.Faslpr model.
Onset of dermatitis is delayed in Ifnar1-deficient mice
We next evaluated other clinical parameters of disease. Aged MRL.Faslpr mice develop dorsal skin lesions, which are more frequently observed and more severe in females than in males, along with a loss of fur and whiskers on the muzzle and inflammation of the ears (24, 25). We did not observe significant skin lesions in Ifnar1-deficient animals at 16 weeks of age regardless of Tlr9 genotype; in contrast, a fraction of female Ifnar1-intact animals had developed modest dermatitis by this age (Fig. 2A–B, left panels). By 24 weeks of age, 30% of female Ifnar1-deficient mice had developed skin lesions that were as large in area as in affected Ifnar1-intact animals (Fig. 2A–B, right panels). Few males in this experimental cohort developed significant skin disease in either age group. Conclusions were fundamentally similar whether we considered skin score (Fig. 2A) or percentage of mice that were affected to a moderate or greater degree (Fig. 2B). Therefore, Ifnar1 deficiency modestly delays the onset of gender-dependent skin disease, but does not have a significant effect on incidence or severity of dermatitis among older affected animals.
Figure 2. Dermatitis onset is delayed and its incidence and severity are reduced in the absence of Ifnar1.
(A) Dorsal skin lesions were scored, with addition of up to 0.5 points for face rash and 0.25 points for inflammation of the ears. Each symbol represents an individual animal. * p<0.05; ** p<0.01, one-tailed Mann-Whitney (B) Incidence of dermatitis was determined as the proportion of animals with a skin score >= 1. * p<0.05, one-sided Fisher’s exact test.
Lymphadenopathy is Ifnar1-dependent
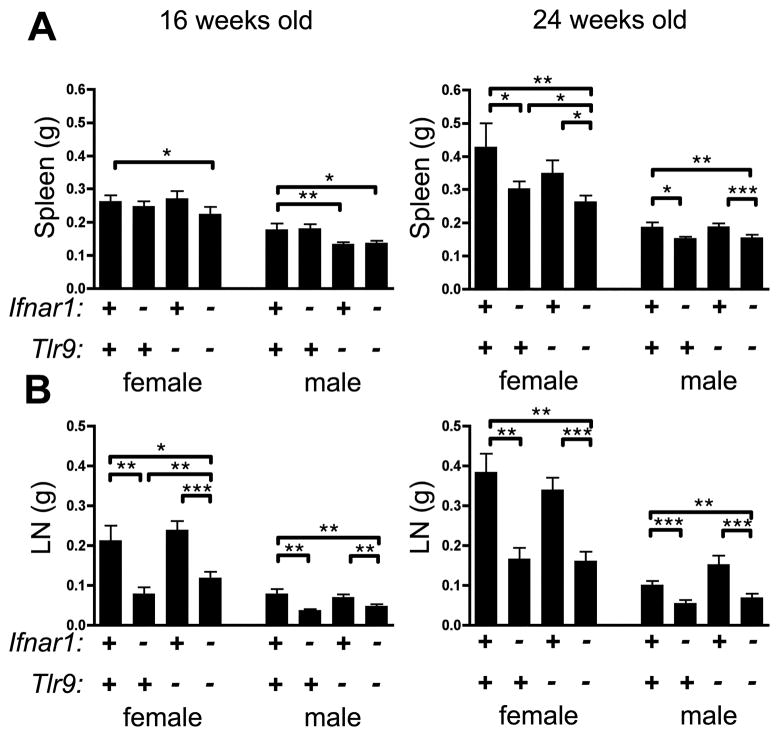
MRL.Faslpr mice develop splenomegaly and lymphadenopathy with age. In contrast, the MRL.Faslpr/2J substrain, which arose via genetic drift in the Jackson Labs colony, has been reported to have less severe splenomegaly and lymphadenopathy than conventional MRL.Faslpr/J, although the genetic alterations responsible have not yet been determined (http://jaxmice.jax.org/strain/006825.html). Consistent with these reports, we found that spleens in 16 week animals in this experimental cohort, which had been backcrossed to the MRL.Faslpr/2J strain, were relatively less expanded in size than in our previous reports (5, 6) (Fig. 3A, left) although moderate splenomegaly was observed in 24 wk animals (Fig. 3A, right). In the 24 wk cohort, splenomegaly was reduced when Ifnar1 was absent regardless of Tlr9 genotype. Similarly, in both 16 and 24 wk animals of either gender, Ifnar1 deficiency consistently led to smaller lymph nodes, independent of Tlr9 genotype (Fig. 3B). Thus, IFN-I contributes to the expansion of spleen and lymph nodes observed in the MRL.Faslpr/2J strain.
Figure 3. Lymphadenopathy is reduced in absence of Ifnar1.
(A) Spleens and (B) axillary LN from indicated animals were weighed. Data are represented as mean +/− SEM. * p<0.05; ** p<0.01; *** p<0.001, one-tailed Mann-Whitney test.
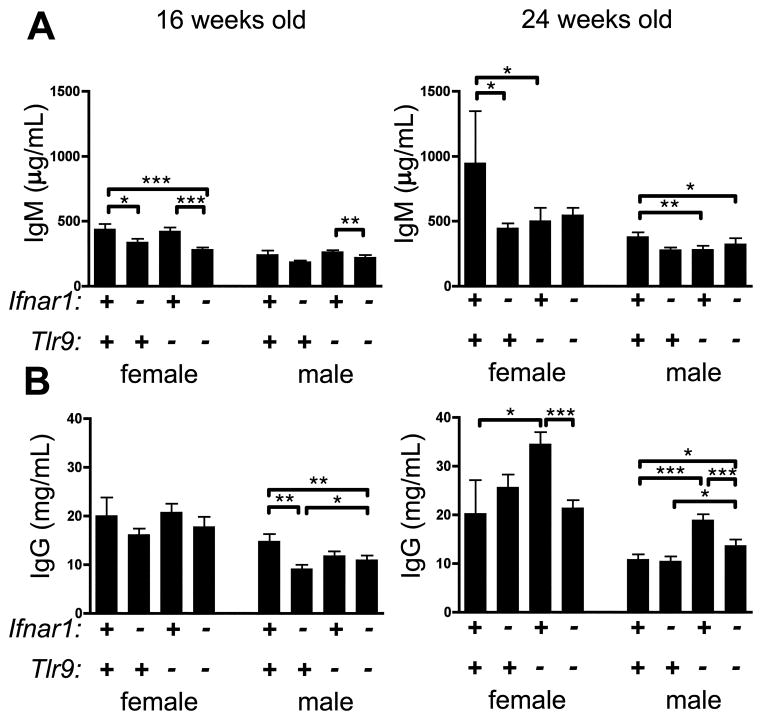
Hypergammaglobulinemia in Tlr9-deficient mice requires Ifnar1
MRL.Faslpr mice have high concentrations of serum immunoglobulins that are even further elevated in Tlr9-deficient animals (5, 6). Therefore we measured total serum IgM and IgG concentrations by ELISA. In 16 week Ifnar1-deficient animals, we observed a modest reduction in serum IgM compared to Ifnar1-intact animals regardless of Tlr9 genotype, although this effect was not observed in older mice (Fig. 4A). In contrast to our previous reports (5, 6), and likely due to differences in disease kinetics between MRL.Faslpr/J and MRL.Faslpr/2J strains, we did not see a significant increase in amounts of serum IgG among 16 wk animals when Tlr9 was absent (Fig. 4B, left). Nonetheless, the absence of Ifnar1 resulted in a reduction in total IgG concentrations among 16 week males (Fig. 4B, left). In contrast, among 24 wk animals, Tlr9 deficiency did lead to the increase in IgG concentrations in both females and males, as expected from our prior studies (5, 6) (Fig. 4B, right). Critically, this increase was not observed in mice also lacking Ifnar1, indicating that increased serum IgG concentrations observed in Tlr9−/− animals depends upon Ifnar1 signaling.
Figure 4. TLR9-regulated hypergammaglobulinemia requires Ifnar1.
Total serum IgM (A) or IgG (B) was measured by ELISA. Data are represented as mean +/− SEM. * p<0.05; ** p<0.01; *** p<0.001, one-tailed Mann-Whitney test.
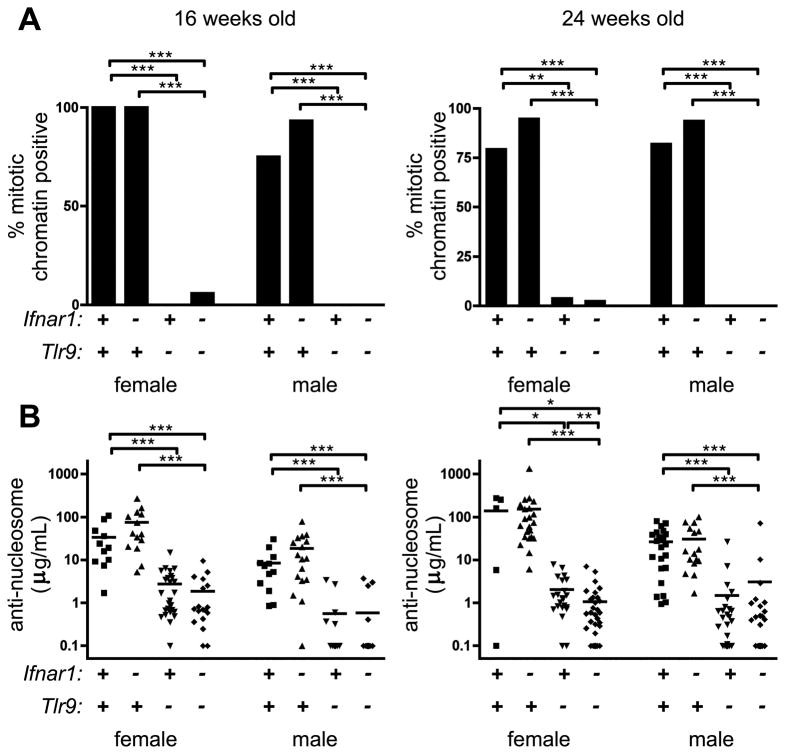
Anti-nucleosome autoantibodies are Ifnar1-independent
We next asked whether Ifnar1 regulated autoantibody titers or specificities. Sera from 16 or 24 week old mice were tested in the HEp-2 ANA (antinuclear antibody) assay for the ability to stain mitotic bodies, a pattern which indicates the presence of anti-nucleosome autoantibodies. The majority of samples from Tlr9-intact mice were indeed positive for mitotic chromatin staining in both 16 and 24 week animals, and this was unaffected by absence of Ifnar1 (Fig. 5A). As previously reported (4–6), Tlr9-deficient animals did not make anti-mitotic chromatin autoantibodies; this was also unaffected by Ifnar1 genotype. Nor did Ifnar1 deficiency affect the titers of anti-nucleosome autoantibodies in 16 or 24 week old MRL.Faslpr/2J mice of either gender (Fig. 5B). In agreement with our previous reports and the HEp-2 ANA, Tlr9-deficient animals had approximately a two-log reduction in anti-nucleosome titer; this was not affected by Ifnar1 genotype. Therefore, Ifnar1 does not affect production of Tlr9-dependent anti-nucleosome autoantibodies.
Figure 5. Antinucleosome autoantibodies require Tlr9 but are Ifnar1-independent.
(A) The proportion of sera positive for mitotic chromatin staining in a HEp-2 ANA is indicated. ** p<0.01; *** p<0.001, one-sided Fisher’s exact test. (B) Serum anti-nucleosome IgG autoantibodies were measured by ELISA. Each symbol represents serum autoantibody concentration in terms of PL2-3 equivalents from an individual animal. * p<0.05; ** p<0.01; *** p<0.001, one-tailed Mann-Whitney test.
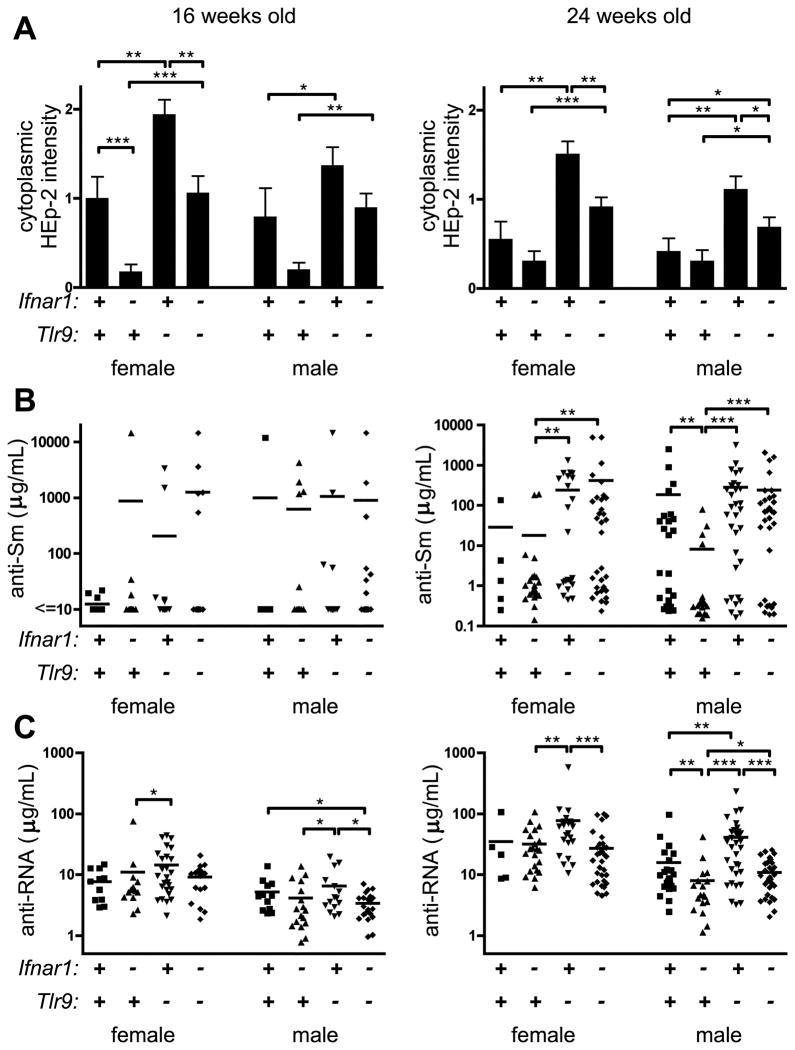
Ifnar1 promotes production of anti-RNA autoantibodies
We previously showed that the exacerbation of disease in Tlr9-deficient mice required intact Tlr7 and was associated with an increase in titers of some autoantibodies to RNA-containing autoantigens (6). To evaluate whether Ifnar1 contributed to Tlr7-dependent anti-RNA autoantibodies either in the presence or absence of Tlr9, we first scored HEp-2 slides for the intensity of staining of cytoplasmic components. Such cytoplasmic staining is Tlr7-dependent and increased in Tlr9-deficient animals (6). In agreement with our previous findings, Tlr9-deficient animals had greater cytoplasmic HEp-2 staining than Tlr9-intact mice (Fig. 6A). However, cytoplasmic HEp-2 staining was reduced when Ifnar1 was also absent, suggesting that this staining pattern includes IFN-I-dependent specificities.
Figure 6. Anti-RNA autoantibodies are reduced in absence of Ifnar1.
(A) Intensity of cytoplasmic staining in the HEp-2 ANA assay was scored. Data are represented as mean +/− SEM. (B) Serum anti-Sm IgG autoantibodies were measured by ELISA. Each symbol represents an individual animal and concentrations are expressed as Y12 equivalents. (C) Serum anti-RNA IgG autoantibodies were measured by ELISA. Each symbol represents an individual animal and concentrations are expressed as arbitrary units. * p<0.05; ** p<0.01; *** p<0.001, one-tailed Mann-Whitney.
To further define this effect we examined the Tlr7-dependent autoantibodies anti-Sm and anti-RNA by ELISA. Few mice were making anti-Sm at 16 weeks of age (Fig. 6B, left). By 24 weeks of age, a greater proportion of female and male animals were making anti-Sm (Fig. 6B, right). Among 24 week old males, Ifnar1 deficiency led to reduced titer and incidence of anti-Sm; however, Ifnar1 did not affect anti-Sm production in Tlr9-deficient mice, with a substantial fraction of Ifnar1−/−Tlr9−/− animals being anti-Sm producers. Thus, we conclude that Ifnar1 is not necessary for the production of anti-Sm autoantibodies. In contrast to anti-Sm, we observed a significant decrease in anti-RNA titers when Ifnar1 was absent, with a 2-fold reduction in titer between 24 wk Ifnar-intact and -deficient males (Fig. 6C). Moreover, while anti-RNA was elevated by 2.2-fold among 24 wk females and 2.6-fold among 24 wk males when Tlr9 only was absent, this elevation was not observed in mice lacking both Ifnar1 and Tlr9. Thus, Ifnar1 contributes to the production of anti-RNA autoantibodies and cytoplasmic HEp-2 staining, but not anti-nucleosome or anti-Sm autoantibodies.
Discussion
The importance of the TLR7 and TLR9 pathways in systemic lupus erythematosus has been clearly demonstrated in several in vivo murine models of lupus pathogenesis (4–10). Autoreactive B cells in lupus break tolerance due to the ability of their nucleic-acid containing ligands to co-ligate both the BCR and either TLR7 or TLR9 (26–28). Mice lacking TLR7 or the adaptor MyD88 have reduced anti-RNA titers as well as reduced renal disease (5, 6). Paradoxically, however, mice lacking TLR9 instead have exacerbated disease, despite lacking anti-nucleosome autoantibodies (6–10). The reasons for the divergent effects of TLR7 and TLR9 deficiency on disease remain incompletely understood, and resolving these issues will have important implications for our understanding of lupus pathogenesis.
Here we have connected the IFN-I signaling pathway to exacerbated disease in the Tlr9-deficient MRL.Faslpr model. As was the case in mice lacking both Tlr7 and Tlr9 (6), mice lacking both Ifnar1 and Tlr9 had reduced anti-RNA autoantibodies and reduced renal disease compared to mice lacking Tlr9 only. Thus, disease that is normally suppressed by Tlr9 requires both Tlr7 and Ifnar1. This represents an important advance in understanding the puzzle of why Tlr9 deficiency paradoxically exacerbates lupus disease. In fact, most but not all of the Tlr7-dependent disease exacerbation phenotypes are also mitigated by the loss of Ifnar1. Hence we propose that TLR7 and IFN-I work in series or alternatively in a positive feedback loop: either TLR7 signals are enhanced in the absence of TLR9, leading to increased production of IFN-I that ultimately promotes disease; or lack of TLR9 directly leads to increased IFN-I secretion, which upregulates expression of TLR7 in one or more target cell types. If both effects were in play then they would be self-reinforcing; genetic inactivation of either Tlr7 or Ifnar1 breaks this proposed feedback cycle and ameliorates disease. Nonetheless, Ifnar1 deficiency did not absolutely prevent disease in all Tlr9-deficient animals; a fraction of older females in the Ifnar1−/−Tlr9−/− group did progress to develop glomerulonephritis and/or dermatitis. It is likely that the delay in disease progression in Ifnar1−/− mice can be overcome by alternative pathways, including for example pathways downstream of IFN-γ or IL-6 (29, 30)
In keeping with the effects of Ifnar1 in modulating disease exacerbation in the absence of Tlr9, but in contrast to a previous report (19), we found that Ifnar1 by itself did indeed contribute to full expression of renal disease in the MRL.Faslpr model. In our experience, and in agreement with other reports (23), the age of spontaneous disease onset and the kinetics of disease progression can vary significantly from animal to animal even among genetically identical mice. As a result, the previous report may have examined too few animals to reveal the effects of Ifnar1 deficiency upon disease. Moreover, lupus disease expression and autoantibody titers are highly gender-dependent in the MRL.Faslpr model (23), as is also the case in human patients (31); the previous study did not report the gender of the animals examined. Our analysis, which was robust with respect to numbers of animals and considered gender, indeed did show that Ifnar1 is important for mediating renal disease progression in MRL.Faslpr, consistent with reports of the role of Ifnar1 in other mouse models of SLE (13–16). This is of significance because heretofore the MRL.Faslpr model has been frequently cited as differing from other murine lupus models with respect to a protective rather than pathogenic role for IFN-I (32, 33); we show here that this is not the case.
An unfortunate confounding factor in the present study is the existence of two commercially available substrains of MRL.Faslpr with significantly different disease kinetics. Over time, the Jackson Laboratories MRL.Faslpr colony experienced genetic drift, acquiring as yet unidentified mutation(s) which reduced the severity and increased the age of onset of clinical disease, most likely due to selective pressures in a breeding colony toward animals with reduced disease and hence increased fertility. All of the genetic alleles examined in the present study were backcrossed to the MRL.Faslpr substrain with slower disease kinetics, MRL.Faslpr/2J, for at least six generations prior to intercross. As a result, the kinetics of disease differed substantially from our previous reports on Tlr9-deficient MRL.Faslpr (5, 6). Most strikingly, while we previously found that Tlr9−/− MRL.Faslpr/J mice reached 50% morbidity or mortality by 16.4 weeks of age (5), in the experimental cohorts described here we observed few deaths before 24 weeks of age in any experimental group. Total antibody and autoantibody titers, spleen and lymph node weights, and extent of renal disease were all reduced in the Ifnar1+/+Tlr9+/+ and Ifnar1+/+Tlr9−/− groups compared to similar animals in previous experimental cohorts at the same age. Nonetheless, Tlr9−/− MRL.Faslpr/2J did exhibit more severe renal disease and elevated IgG titers than their Tlr9+/+ littermates, in broad agreement with our previous reports. It is therefore important to emphasize that all comparisons in this paper were generated via a “littermate-control” design from progeny of a similar genetic background.
An interesting implication of our findings is that they further emphasize that anti-nucleosome autoantibodies, despite being the clinical diagnostic hallmark of SLE, are actually entirely dispensable for renal disease. Tlr9−/− animals lack anti-nucleosome autoantibodies, but have more severe renal disease, likely due to the presence of other pathogenic specificities including anti-RNA, as well as an increase in class switch to complement-fixing IgG2a and IgG3 isotypes (6). Conversely, Ifnar1−/− mice have comparable titers of anti-nucleosome autoantibodies to the Ifnar1+/+ group, but have relatively little clinical disease. This could be due to the reduction in anti-RNA titers, and/or Ifnar1 may mediate inflammation in situ downstream of or independent of antibody deposition, for example by promoting T cell activation.
One of the most notable effects of Ifnar1-deficiency was the shift in autoantibody specificities. Whether Ifnar1 acts directly in autoreactive B cells to exert its effects on repertoire or indirectly through other cell types has not yet been determined. One group described a model in which IFN-I affected reactivity to TLR7-dependent autoantigens by upregulating TLR7 itself in B cells, thereby increasing the likelihood that RNA-containing self antigens that crosslink the autoreactive BCR would also deliver a TLR7-mediated “second signal” to break tolerance (34). Such an effect could explain the differences in anti-RNA titer and cytoplasmic HEp-2 staining that we observed between Ifnar1+/+ and Ifnar1−/− animals, particularly when Tlr9 was absent. However, anti-Sm, which is also a Tlr7-dependent specificity (5, 6), was not affected by the absence of Ifnar1. One explanation for this discrepancy is that these specificities may differ in their ability to receive T cell help; Sm is an RNA-associated protein antigen, while anti-RNA may lack a specific protein component and therefore could be more strongly dependent on Ifnar1 and Tlr7-dependent signals. Nonetheless, with regard to the interaction between Tlr7 and Ifnar1, these data indicate that not all effects are strictly in series.
In conclusion, in addition to clarifying that in fact some components of MRL.Faslpr lupus disease do have a dependency on IFN-I, our studies add a significant piece to the puzzle in understanding how TLR9 exacerbates lupus in murine models. Lack of TLR9 causes increased IFN-I secretion (5) and here we demonstrate its relevance, as loss of IFNAR-I signaling negates many of the disease-enhancing effects of TLR9-deficiency. In this regard the phenotype of Tlr9−/−Ifnar1−/− MRL.Faslpr mice resembles that of Tlr9−/−Tlr7−/− MRL.Faslpr mice (6), although (as discussed above with regard to autoantibody repertoire) they are not complete phenocopies. The connections between TLR7 and IFNAR1 signaling in vivo remain to be worked out—issues that will require a combination of tissue-specific deletion as well as defined signaling experiments. Furthermore, the effects of Tlr7 deletion on exacerbated disease in Tlr9−/− MRL.Faslpr mice that are non-overlapping with and therefore independent of Ifnar1 also are still unresolved. Thus, the ongoing work to unravel these highly interrelated pathways—of which this report is but one significant component—continues to be of great interest, with clear implications for understanding disease and therapeutic targeting.
Acknowledgments
We thank Warren Shlomchik for providing Ifnar1−/− mice on the C57BL/6 background. We thank the technicians of the Yale Animal Resources Center for superb work in animal husbandry.
Abbreviations used in this work
- ANA
antinuclear antibody
- IFN-I
type I interferon
- SLE
systemic lupus erythematosus
Footnotes
This work was supported by the National Institutes of Health Grant P01-AR050256. K.M.N. was supported by the National Research Service Award training grant 5T32-HL007974 and a grant from the S.L.E. Lupus Foundation. The authors have no conflicting financial interests.
References
- 1.Fairhurst AM, Wandstrat AE, Wakeland EK. Systemic lupus erythematosus: multiple immunological phenotypes in a complex genetic disease. Adv Immunol. 2006;92:1–69. doi: 10.1016/S0065-2776(06)92001-X. [DOI] [PubMed] [Google Scholar]
- 2.Kirou KA, Lee C, George S, Louca K, Peterson MGE, Crow MK. Activation of the interferon-alpha pathway identifies a subgroup of systemic lupus erythematosus patients with distinct serologic features and active disease. Arthritis Rheum. 2005;52:1491–1503. doi: 10.1002/art.21031. [DOI] [PubMed] [Google Scholar]
- 3.Nowling TK, Gilkeson GS. Mechanisms of tissue injury in lupus nephritis. Arthritis research & therapy. 2011;13:250. doi: 10.1186/ar3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Christensen SR, Kashgarian M, Alexopoulou L, Flavell RA, Akira S, Shlomchik MJ. Toll-like receptor 9 controls anti-DNA autoantibody production in murine lupus. J Exp Med. 2005;202:321–331. doi: 10.1084/jem.20050338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christensen SR, Shupe J, Nickerson K, Kashgarian M, Flavell RA, Shlomchik MJ. Toll-like receptor 7 and TLR9 dictate autoantibody specificity and have opposing inflammatory and regulatory roles in a murine model of lupus. Immunity. 2006;25:417–428. doi: 10.1016/j.immuni.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 6.Nickerson KM, Christensen SR, Shupe J, Kashgarian M, Kim D, Elkon K, Shlomchik MJ. TLR9 Regulates TLR7- and MyD88-Dependent Autoantibody Production and Disease in a Murine Model of Lupus. J Immunol. 2010;184:1840–1848. doi: 10.4049/jimmunol.0902592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lartigue A, Courville P, Auquit I, François A, Arnoult C, Tron F, Gilbert D, Musette P. Role of TLR9 in anti-nucleosome and anti-DNA antibody production in lpr mutation-induced murine lupus. J Immunol. 2006;177:1349–1354. doi: 10.4049/jimmunol.177.2.1349. [DOI] [PubMed] [Google Scholar]
- 8.Santiago-Raber M-L, Dunand-Sauthier I, Wu T, Li Q-Z, Uematsu S, Akira S, Reith W, Mohan C, Kotzin BL, Izui S. Critical role of TLR7 in the acceleration of systemic lupus erythematosus in TLR9-deficient mice. J Autoimmun. 2009 doi: 10.1016/j.jaut.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 9.Stoehr AD, Schoen CT, Mertes MMM, Eiglmeier S, Holecska V, Lorenz AK, Schommartz T, Schoen A-L, Hess C, Winkler A, Wardemann H, Ehlers M. TLR9 in Peritoneal B-1b Cells Is Essential for Production of Protective Self-Reactive IgM To Control Th17 Cells and Severe Autoimmunity. J Immunol. 2011 doi: 10.4049/jimmunol.1003340. [DOI] [PubMed] [Google Scholar]
- 10.Yu P, Wellmann U, Kunder S, Quintanilla-Martinez L, Jennen L, Dear N, Amann K, Bauer S, Winkler TH, Wagner H. Toll-like receptor 9-independent aggravation of glomerulonephritis in a novel model of SLE. Int Immunol. 2006;18:1211–1219. doi: 10.1093/intimm/dxl067. [DOI] [PubMed] [Google Scholar]
- 11.Lichtman EI, Helfgott SM, Kriegel MA. Emerging therapies for systemic lupus erythematosus--focus on targeting interferon-alpha. Clinical immunology. 2012;143:210–221. doi: 10.1016/j.clim.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Niewold TB. Interferon alpha as a primary pathogenic factor in human lupus. Journal of interferon & cytokine research: the official journal of the International Society for Interferon and Cytokine Research. 2011;31:887–892. doi: 10.1089/jir.2011.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agrawal H, Jacob N, Carreras E, Bajana S, Putterman C, Turner S, Neas B, Mathian A, Koss MN, Stohl W, Kovats S, Jacob CO. Deficiency of type I IFN receptor in lupus-prone new zealand mixed 2328 mice decreases dendritic cell numbers and activation and protects from disease. J Immunol. 2009;183:6021–6029. doi: 10.4049/jimmunol.0803872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nacionales DC, Kelly-Scumpia KM, Lee PY, Weinstein JS, Lyons R, Sobel E, Satoh M, Reeves WH. Deficiency of the type I interferon receptor protects mice from experimental lupus. Arthritis Rheum. 2007;56:3770–3783. doi: 10.1002/art.23023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Braun D, Geraldes P, Demengeot J. Type I Interferon controls the onset and severity of autoimmune manifestations in lpr mice. J Autoimmun. 2003;20:15–25. doi: 10.1016/s0896-8411(02)00109-9. [DOI] [PubMed] [Google Scholar]
- 16.Santiago-Raber ML, Baccala R, Haraldsson KM, Choubey D, Stewart TA, Kono DH, Theofilopoulos AN. Type-I interferon receptor deficiency reduces lupus-like disease in NZB mice. J Exp Med. 2003;197:777–788. doi: 10.1084/jem.20021996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thibault DL, Graham KL, Lee LY, Balboni I, Hertzog PJ, Utz PJ. Type I interferon receptor controls B-cell expression of nucleic acid-sensing Toll-like receptors and autoantibody production in a murine model of lupus. Arthritis Res Ther. 2009;11:R112. doi: 10.1186/ar2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jørgensen TN, Roper E, Thurman JM, Marrack P, Kotzin BL. Type I interferon signaling is involved in the spontaneous development of lupus-like disease in B6.Nba2 and (B6.Nba2 x NZW)F(1) mice. Genes Immun. 2007;8:653–662. doi: 10.1038/sj.gene.6364430. [DOI] [PubMed] [Google Scholar]
- 19.Hron JD, Peng SL. Type I IFN protects against murine lupus. J Immunol. 2004;173:2134–2142. doi: 10.4049/jimmunol.173.3.2134. [DOI] [PubMed] [Google Scholar]
- 20.Müller U, Steinhoff U, Reis LF, Hemmi S, Pavlovic J, Zinkernagel RM, Aguet M. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264:1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- 21.Campbell AM, Kashgarian M, Shlomchik MJ. NADPH oxidase inhibits the pathogenesis of systemic lupus erythematosus. Science translational medicine. 2012;4:157ra141. doi: 10.1126/scitranslmed.3004801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blanco F, Kalsi J, Isenberg DA. Analysis of antibodies to RNA in patients with systemic lupus erythematosus and other autoimmune rheumatic diseases. Clin Exp Immunol. 1991;86:66–70. doi: 10.1111/j.1365-2249.1991.tb05775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andrews BS, Eisenberg RA, Theofilopoulos AN, Izui S, Wilson CB, McConahey PJ, Murphy ED, Roths JB, Dixon FJ. Spontaneous murine lupus-like syndromes. Clinical and immunopathological manifestations in several strains. The Journal of experimental medicine. 1978;148:1198–1215. doi: 10.1084/jem.148.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kanauchi H, Furukawa F, Imamura S. Characterization of cutaneous infiltrates in MRL/lpr mice monitored from onset to the full development of lupus erythematosus-like skin lesions. J Invest Dermatol. 1991;96:478–483. doi: 10.1111/1523-1747.ep12470176. [DOI] [PubMed] [Google Scholar]
- 25.Furukawa F, Tanaka H, Sekita K, Nakamura T, Horiguchi Y, Hamashima Y. Dermatopathological studies on skin lesions of MRL mice. Archives of dermatological research. 1984;276:186–194. doi: 10.1007/BF00414018. [DOI] [PubMed] [Google Scholar]
- 26.Rawlings DJ, Schwartz MA, Jackson SW, Meyer-Bahlburg A. Integration of B cell responses through Toll-like receptors and antigen receptors. Nature reviews Immunology. 2012;12:282–294. doi: 10.1038/nri3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lau CM, Broughton C, Tabor AS, Akira S, Flavell RA, Mamula MJ, Christensen SR, Shlomchik MJ, Viglianti GA, Rifkin IR, Marshak-Rothstein A. RNA-associated autoantigens activate B cells by combined B cell antigen receptor/Toll-like receptor 7 engagement. J Exp Med. 2005;202:1171–1177. doi: 10.1084/jem.20050630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leadbetter EA, I, Rifkin R, Hohlbaum AM, Beaudette BC, Shlomchik MJ, Marshak-Rothstein A. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature. 2002;416:603–607. doi: 10.1038/416603a. [DOI] [PubMed] [Google Scholar]
- 29.Cash H, Relle M, Menke J, Brochhausen C, Jones SA, Topley N, Galle PR, Schwarting A. Interleukin 6 (IL-6) deficiency delays lupus nephritis in MRL-Faslpr mice: the IL-6 pathway as a new therapeutic target in treatment of autoimmune kidney disease in systemic lupus erythematosus. J Rheumatol. 2010;37:60–70. doi: 10.3899/jrheum.090194. [DOI] [PubMed] [Google Scholar]
- 30.Schwarting A, Wada T, Kinoshita K, Tesch G, Kelley VR. IFN-gamma receptor signaling is essential for the initiation, acceleration, and destruction of autoimmune kidney disease in MRL-Fas(lpr) mice. Journal of immunology. 1998;161:494–503. [PubMed] [Google Scholar]
- 31.Rubtsov AV, Rubtsova K, Kappler JW, Marrack P. Genetic and hormonal factors in female-biased autoimmunity. Autoimmunity Reviews. 2010;9:494–498. doi: 10.1016/j.autrev.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kiefer K, Oropallo MA, Cancro MP, Marshak-Rothstein A. Role of type I interferons in the activation of autoreactive B cells. Immunology and cell biology. 2012;90:498–504. doi: 10.1038/icb.2012.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barrat FJ, Coffman RL. Development of TLR inhibitors for the treatment of autoimmune diseases. Immunol Rev. 2008;223:271–283. doi: 10.1111/j.1600-065X.2008.00630.x. [DOI] [PubMed] [Google Scholar]
- 34.Green NM, Laws A, Kiefer K, Busconi L, Kim YM, Brinkmann MM, Trail EH, Yasuda K, Christensen SR, Shlomchik MJ, Vogel S, Connor JH, Ploegh H, Eilat D, Rifkin IR, van Seventer JM, Marshak-Rothstein A. Murine B cell response to TLR7 ligands depends on an IFN-beta feedback loop. J Immunol. 2009;183:1569–1576. doi: 10.4049/jimmunol.0803899. [DOI] [PMC free article] [PubMed] [Google Scholar]