Abstract
Human cutaneous DCs have the ability to prime and bias Th17 lymphocytes. However, the factors that stimulate cutaneous DCs to induce Th17 responses are not well known. Alarmins, such as ATP, likely play a pivotal role in the induction and maintenance of cutaneous immune responses by stimulating DC maturation, chemotaxis, and secretion of IL-1β and IL-6, Th17 biasing cytokines. Here, utilizing a well-established human skin model we have demonstrated that signaling purinergic receptors, predominantly P2X7R, via an ATP analog initiates innate proinflammatory inflammation, DC17 differentiation, and the subsequent induction of Th17 biased immunity. Moreover, our results suggest a potential role for P2X7R signaling in the initiation of psoriasis pathogenesis, a Th17 dependent autoimmune disease. In support of this, we observed the increased presence of P2X7R in non-lesional and lesional psoriatic skin compared to normal healthy tissues. Interestingly, there was also a P2X7R variant (P2X7RB) that was highly expressed in lesional psoriatic skin compared to non-lesional psoriatic and normal healthy skin. Furthermore, we demonstrated that psoriatic responses could be initiated via P2X7R signaling in non-lesional skin following treatment with a P2X7R agonist. Mechanistic studies revealed a P2X7R-dependent mir-21 angiogenesis pathway that leads to the expression of VEGF and IL-6, and which may be involved in the development of psoriatic lesions. In conclusion, we have established that purinergic signaling in the skin induces innate inflammation leading to the differentiation of human Th17 responses, which have implications in the pathogenesis and potential treatment of psoriasis.
INTRODUCTION
Efficient activation and differentiation of naïve CD4+ T cells requires the recognition of antigen-peptide complexes in the context of MHC-II molecules (signal 1) followed by positive co-stimulation (signal 2). T cell polarization is determined by the presence of a specific cytokine profile secreted during antigen presentation (signal 3), which is provided by DCs (1). However, the stimuli that induce DCs to secrete Th polarizing cytokines, particularly Th17-biasing cytokines, are complex and not completely understood.
The skin is a very immunogenically active organ capable of triggering inflammation and potent T cell responses by appropriately responding to antigenic stimuli. The immunogenicity of skin correlates with a substantial number of resident DCs including epidermal Langerhans cells (LCs) and dermal dendritic cells (DDCs), which are both capable of activating naïve T cells and biasing Th1 and Th17 immune responses (2–4).
In addition to cellular elements the epidermis and dermis contain a vast network of regulatory cytokines, neuropeptides, and other endogenous factors with either proinflammatory or tolerogenic activities that contribute to the initiation and control of cutaneous inflammatory immune responses. One particular family of endogenous factors is the damaged associated molecular patterns (DAMPs), which signal cellular damage. Following trauma endogenous DAMPs, termed alarmins, are released from damaged, stressed, or necrotic cells and initiate proinflammatory responses. Characteristics of alarmins are that they 1) are released during non-programmed cell death, 2) are secreted by immune cells, 3) recruit and activate innate immune cells, including DCs, and 4) have regulatory capacities enabling them to promote tissue homeostasis and wound healing (5). However, when regulatory responses fail in autoimmune and inflammatory diseases such as psoriasis, rheumatoid arthritis, and systemic lupus erythematosus alarmins also act as indicators of inflammation and disease severity (6). Thus, it is likely that alarmins also play a pivotal role in the induction and pathogenesis of such diseases by positive-feedback inflammatory mechanisms that break immunological self-tolerance and perpetuate the immune response (7). However, little is known regarding the mechanisms employed by alarmins in perpetuating inflammatory diseases, including the early progression of cutaneous psoriasis, a common accessible model of inflammatory diseases in which T helper (Th) 17 and Th1 cells are effectors.
ATP is a particularly interesting alarmin that via purinergic signaling induces DC maturation, chemotaxis, and secretion of IL-1β and IL-6, cytokines involved in the induction of Th17 immunity (8–14). Furthermore, ATP has been described as an adjuvant that induces an “alternative” DC maturation phenotype characterized by inhibition of IL-12 and stimulation of IL-23 production, which enhances and terminally differentiates Th17 responses (15, 16). In support of these findings, Atarashi et al. (17) demonstrated in mice that ATP secreted by mucosal commensal bacteria bias lamina propria Th17 responses by stimulating CD70highCD11clow colonic DCs. Additionally, several key studies have indicated that ATP plays a role in cutaneous inflammation and wound healing (18–22). For instance, Weber et al. demonstrated that contact hypersensitivity responses dependent on IL-1β were inhibited in P2X7 receptor (P2X7R) −/− mice (22). Furthermore, P2X7R is functionally expressed on cutaneous keratinocytes and LCs (14, 19, 23, 24). Thus, we hypothesize that ATP signaling via the P2X7R has the capacity to stimulate cutaneous innate and adaptive Th17 inflammatory responses in humans.
To explore our hypothesis we began by treating human cutaneous explants and DCs with 2’(3’)-O-(4-Benzoylbenzoyl) adenosine 5’-triphosphate (BzATP), an ATP analog and P2X7R agonist. In this regard, we determined that purinergic signaling provokes innate cutaneous inflammatory responses, DC17 differentiation, and Th17 responses. Moreover, our results further define a potential role for ATP in the induction of psoriasis by demonstrating the substantial presence of P2X7R in psoriatic skin. In conclusion, our results demonstrate that cutaneous inflammatory responses induced via purinergic signaling, predominantly P2X7R, have implications in the pathogenesis and potential treatment of inflammatory diseases, such as psoriasis.
MATERIAL AND METHODS
Human Tissues
Normal human skin was procured from healthy donors undergoing breast reduction or abdominoplasty at the University of Pittsburgh Medical Center (UPMC) or tissues were procured from the National Disease Research Interchange (NDRI) or the Cooperative Human Tissue Network (CHTN). Psoriatic lesional and non-lesional skin samples were obtained with informed consent through the UPMC Dermatology clinic or NDRI. Human peripheral blood samples (leukopacks) from healthy volunteers were acquired from the Central Blood Bank. All human samples were procured in accordance with the Declaration of Helsinki protocols and University of Pittsburgh Institutional Review Board approval.
Isolation and Culture of Skin Migratory Dendritic Cells (smiDCs)
SmiDCs were collected after migration from human skin explants as previously described (3, 25, 26). Briefly, a cutaneous mini-knife (Integra-Padgett; Plainsboro, NJ) was used to prepare explants composed of epidermis and a thin layer of dermis (0.5 mm thick). Explants were rinsed and cultured epidermal side up on sterile stainless steel mesh screens (0.1 mm pore) placed inside 100 mm2 culture dishes. Plates were filled with serum free Aim V medium (Invitrogen; Carlsbad, CA), supplemented with 10 µg/ml gentamicin sulfate (Invitrogen), so that skin explants were cultured in the liquid/air interface for 72 h. Following culture, the non-adherent cell fraction containing smiDCs was collected.
Overnight cultures were established to analyze smiDC activation and maturation. For these experiments, smiDCs were cultured at a concentration of 2.5×105–1×106 cells/ml in 24-well plates in 500 µl of serum-free AIM V media and treated with PBS (control), BzATP [350 µM; P2X7R agonist (Sigma Aldrich, St. Louis, MO or Tocris Biosciences, Bristol, UK)], and/or KN-62 (1 µM; P2X7R antagonist, Sigma Aldrich), unless otherwise indicated. Doses for BzATP and KN-62 were selected based on titration curves and selected dose was utilized throughout this manuscript in vitro and in vivo (Supplemental Figure 1A–1C and data not shown). Following 24 h incubation at 37°C the supernatants were collected for analysis of cytokine production by ELISA. The cells were harvested and stained for flow cytometric analysis or utilized in real time quantitative RT-PCR (qRT-PCR) experiments.
Cutaneous Injections
12 mm punch biopsies prepared from epidermal-dermal explants and psoriatic biopsies were injected intradermally with 100 µl of PBS, BzATP (350 µM), KN-62 (1 µM), mir-21 antagomirs [(Atg) 20 µM; Exiqon; Woburn, MA)], and/or a combination of 10 µg/ml of IL-1β and IL-6Rα antibodies (R&D systems; Minneapolis, MN). Biopsies were then floated on 0.5 ml of AIM V media plus gentamicin (10 µg/ml) and incubated at 37°C. Tissue samples were incubated for 24–72 h then snap-frozen for microscopy or stored in RNAlater solution (Ambion; Austin, TX) for qRT-PCR.
Isolation and Culture of Naïve CD4+ T Cells
Human naïve CD45RA+ CD4+ T cells, utilized as responders in mixed leukocyte cultures (MLCs), were purified from peripheral blood mononuclear cells (PBMCs) by ficoll gradient followed by positive selection using human naïve CD4+ T cell selection beads according to manufacturer’s protocol (Miltenyi Biotec; Auburn, CA). Purity of CD45RA+CD4+ T cells was ≥90%.
MLCs were established by first plating smiDCs at concentration of 1×104 cells/well in 96-well round-bottom plates containing serum free AIM V media supplemented with PBS, BzATP (350 µM), and/or KN-62 (1 µM), for 3 h. Cells were washed thoroughly and resuspended in AIM V media. Naïve CD45RA+CD4+ T cells were then added to the cultures at a concentration of 1×105 cells/well. In some experiments IL-1β or IL-23 antibodies (R&D systems) were added to the cultures. Depending upon the experimental protocol, cultures were incubated for 1–5 d. Cellular proliferation was measured in MLCs using the commercially available Quick Cell Proliferation Assay kit (BioVision; Mountain View, CA) according to manufacturer’s instructions.
Flow Cytometric Analysis
SmiDCs and responder CD4+T cells were stained by blocking with 10% normal donkey serum and then incubating cells with combinations of Abs against HLA-DR (BioLegend, San Diego, CA), Langerin (Dendritics; Lyon, France), P2X7R (internal epitope; LifeSpan Biosciences; Seattle, WA), RAGE (R&D systems), CD86 (BioLegend), IL-23R (R&D systems), CD25 (BD Biosciences; San Diego, CA), and CD4 (BD Biosciences). Internal P2X7R staining was followed by secondary anti-rabbit-PerCP Ab (Jackson Immuno; West Grove, PA) and biotinylated RAGE staining was followed by Streptavidin-Percp-cy5.5 (BioLegend). Cells were fixed in 2% paraformaldehyde (PFA) and analyzed using an LSR II flow cytometer (BD Immunocytometry Systems; San Jose, CA).
WinList 3D (Verity Software House;Topsham, ME) or FlowJo (Tree Star Inc; Ashland,OR) software was utilized for analysis. Populations were initially gated on live cells in the FSC vs. SSC and by eFluor 780 viability dye (eBioscience; San Diego, CA). Gates were then set based on negative controls, single-staining, and fluorescence minus one (FMO) controls.
Immunoflourescent Microscopy
Cross-sections of skin were prepared and immunofluorescently labeled as previously described (3). Briefly, biopsies and explants were embedded in Shandon Cryomatrix medium (Thermo Scientific; Waltham, MA) and snap frozen in pre-chilled methyl-butane (Sigma Aldrich). 8 µm cross-sections were cut using a cryostat, mounted onto slides pre-treated with Vectabond (Vector Laboratories; Burlingame, CA), air-dried, fixed in 96% ethanol, and immunofluorescently labeled with combinations of Abs against P2X7R [internal epitope (LS-A9585); LifeSpan Biosciences], HLA-DR (BioLegend), DC-LAMP (R&D systems), CD206 (BioLegend), iNOS (R&D systems), Langerin (Dendritics), CD163 (BioLegend), and IL-23R (R&D systems). Primary P2X7R Ab was followed by anti-rabbit-Cy3 and DC-LAMP, CD206, and iNOS primary Abs were followed by anti-mouse-Cy3 (secondary Abs were purchased from Jackson Immuno). Biotinylated IL-23R was followed by Streptavidin -Cy3 (Jackson Immuno). Slides were counter-stained with DAPI nuclear stain, fixed with 4% PFA, and coverslips were mounted with gelvatol (10.5% polyvinyl alcohol in Tris buffer). Images were acquired using an Olympus Provis AX-70 microscope system (Olympus; Center Valley, PA) with FluoView 500 software. In some experiments cells were counted during microscopic examination in up to 15 40× high powered fields.
Detection of Secreted Cytokines
The release of cytokines by smiDCs or responder CD4+ T cells was analyzed in culture supernatants using commercially available IL-1β (BD Biosciences), IL-6 (BD Biosciences), IL-17A (R&D Systems), high mobility group-box 1 [(HMGB1) Shino-Test Corporation; Japan] and VEGF (R&D Systems) ELISA kits following manufacturer’s instructions. All ELISA plates were analyzed using SpectraMax 340PC, 384 plate reader (Molecular Devices). Assays were performed in duplicates and the results are expressed as mean concentration ± SD.
Real-time qRT-PCR
Real-time qRT-PCR experiments were conducted using either total RNA or microRNA. Total RNA was extracted from cells and tissues using an RNAqueous-4PCR kit (Ambion) according to manufacturer’s instructions and quantified using the Qubit RNA assay kit (Invitrogen). For each RT assay, 10–100 ng of RNA was converted to cDNA using RNA to cDNA High Capacity Master Mix (Applied Biosystems, Carlsbad, CA).
For multiplex PCR reactions, TaqMan Gene Expression Assay mixes specific for RORC, IL-6, P2X7R, IL-23, VEGF (Applied Biosystems), and IL-12 (Integrated DNA Technologies; Coralville, IA) were utilized and set up according to manufacturer’s protocol. Additionally, for experiments where skin samples were injected, mRNA transcript expression was assessed by Custom TaqMan Array Fast Plates (Applied Biosystems) 24 h and 72 h following injections. For this, Custom TaqMan Array Fast Plates were pre-spotted with TaqMan Gene Expression Assays for CXCL10, IL-6, IL-1β, TNFR1, NOS2, TNFα, S100A7, CCL20, and TRAIL. Endogenous controls included 18s, B2M, and/or GAPDH.
MicroRNA was harvested from samples using the MirVana MicroRNA Isolation Kit (Ambion) and quantified using the Qubit RNA Assay Kit. TaqMan microRNA assays RNU6B, hsa-mir-26b, and RNU49 were used as endogenous controls and sequence specific assays were hsa-mir-21, hsa-mir-221, and hsa-mir-326 (Applied Biosystems). For the RT step, the TaqMan MicroRNA Reverse Transcription Kit (Applied Biosystems) was used with a modified protocol. Each 15 µl reaction consisted of 10 ng of microRNA in 5 µl H2O, 1.5 µl reverse transcriptase, 1.5 µl 10× RT buffer, 0.15 µl dNTPs, and 0.2 µl RNase inhibitor. To this reaction mix, 13.3 µl of a 1:1 mix of the seven microRNA-specific primers were added.
All cDNA samples were amplified using Gene Expression Master mix (Applied Biosystems) and analyzed using the real-time Step One Plus sequence detection system (Applied Biosystems). In some instances preamplification of small cDNA quantities was necessary and performed using the TaqMan PreAmp Master Mix (Applied Biosystems). Relative fold-changes of RNA expression were calculated and normalized based on the 2−ΔΔCt method.
Western blot analysis
Psoriasis and normal cutaneous biopsies were minced and placed into pre-chilled RIPA buffer [50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, 1 mM EDTA, PMSF 10ml/ml, and 1× protease inhibitor cocktail (Sigma Aldrich)]. Samples were then homogenized and clarified by centrifugation at 10,000 × g for 10 min. Lysates were mixed with Laemmli buffer (20% glycerol, 10% β-mercaptoethanol, 5% SDS, 0.2 M Tris-HCl, pH 6.8, and 0.4% bromphenol blue) and boiled for 5 min. 40 µg of protein/lane were separated on a 12% SDS-PAGE gel followed by transfer to polyvinylidene difluoride (PVDF) membrane (Bio-Rad), after which membranes were blocked with 5% non-fat dry milk in PBS/0.1% Tween-20 (PBST) solution. Proteins were detected by overnight incubation with anti-P2X7R Ab [extracellular epitope (ab77413); Abcam, Cambridge, MA)] followed by anti-rabbit horseradish peroxidase-conjugated secondary Ab (Sigma Aldrich) for 1 h at room temperature. Antibody specificity was confirmed on a separate gel with a P2X7R peptide (Abcam), blocking was performed according to manufacturer’s instructions. Chemiluminescence reactions were carried out with ECL substrate (GE Healthcare Biosciences, Piscataway, NJ) and exposed to film according to manufacturer’s directions.
RESULTS
Signaling via P2X7R stimulates innate cutaneous inflammation
To test our hypothesis that P2X7R signaling potentiates innate and adaptive Th17 immune responses in the skin, we utilized a well-established and physiological model of human skin epidermal-dermal explants, which allows for culturing human skin explants and for the isolation of a high number of smiDCs mobilizing from the skin to the skin DLNs via lymphatic vessels (25, 27). Our initial ex vivo experiments in which we injected cutaneous explants with BzATP confirmed human in vitro and murine results (8, 9, 12, 13, 17) by demonstrating a significant increase in the innate IL-1β and IL-6 transcripts in the skin 24 h following P2X7R stimulation compared to non-treated controls (Figure 1). By 72 h following injections IL-1β and IL-6 return to baseline levels (data not shown). To confirm that BzATP responses were occurring through P2X7R we treated cutaneous explants with KN-62 (a specific P2X7R antagonist) or KN-62 plus BzATP and did not observe a significant difference compared to non-treated controls (Figure 1). The study of P2X7R-dependent release of IL-1β has heavily focused on the ability of P2X7R signaling to induce the activation and assembly of the NALP3 inflammasome (28, 29). However, here we have also determined that P2X7R signaling induces the increased expression of IL-1β mRNA in human skin.
FIGURE 1.
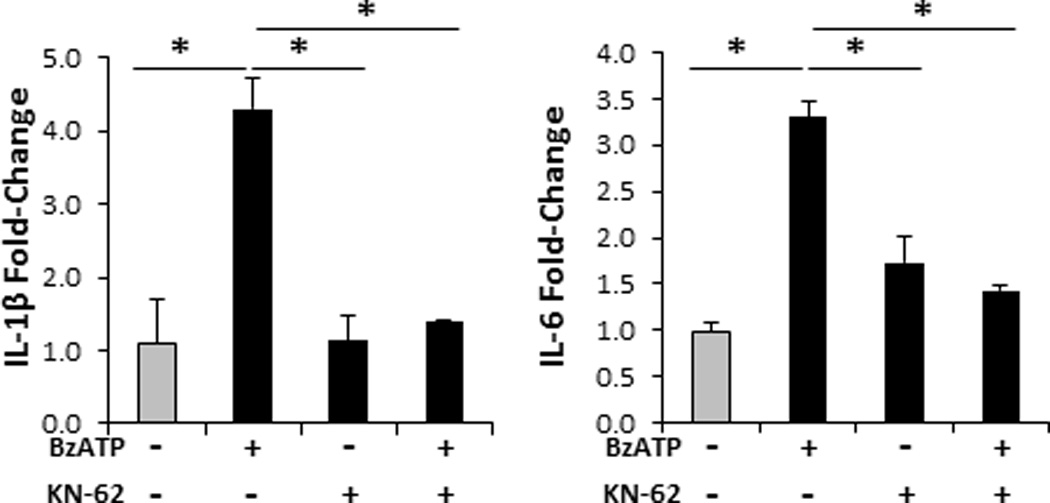
Specific signaling through the P2X7R induces innate Th17-biasing cytokines. Bar graphs demonstrate the relative fold change in mRNA expression of IL-1β and IL-6 24 h following cutaneous injections with 350 µM of BzATP, 1 µM KN-62 (P2X7R antagonist), BzATP + KN-62 (control), or PBS (control; gray bar). Fold-change was determined by relative qRT-PCR using the 2−ΔΔCt method in which samples were normalized to GAPDH followed by PBS controls. Data expressed as mean ± SD of triplicates. Asterisk indicates a significant difference compared to indicated treatment group, p < 0.05. Representative of three independent experiments.
We next expanded our studies to assess the expression levels of innate factors involved in cutaneous inflammation and autoimmune disorders. CXCL10 (Th1 chemokine) and TNFR1 (TNFα receptor) were significantly increased in cutaneous explants 24 h following BzATP injections compared to PBS injected controls (Figure 2A). Furthermore, 72 h following BzATP treatments CXCL10 and TNFR1 mRNA levels remained significantly elevated while S100A7, CCL20 (Th17 chemokine), TNF-α, VEGF, NOS2, and TRAIL were significantly upregulated (Figure 2A). Additionally, the presence of KN-62 demonstrated a significant reduction in the BzATP-dependent increase in all innate factors examined here. To determine if the responses observed at 72 h were a direct result of P2X7R signaling or a secondary effect of early IL-1β and IL-6 secretion we injected cutaneous explants with BzATP ± IL-1β and IL-6Rα neutralizing antibodies. VEGF, S100A7, and NOS2 mRNA expression levels were significantly decreased in the antibody treated group compared to BzATP treatment alone (Figure 2B). Overall these results demonstrate for the first time that specifically signaling through the cutaneous P2X7R induces potent innate inflammatory responses in human skin directly and as a secondary response to IL-1β and IL-6.
FIGURE 2.
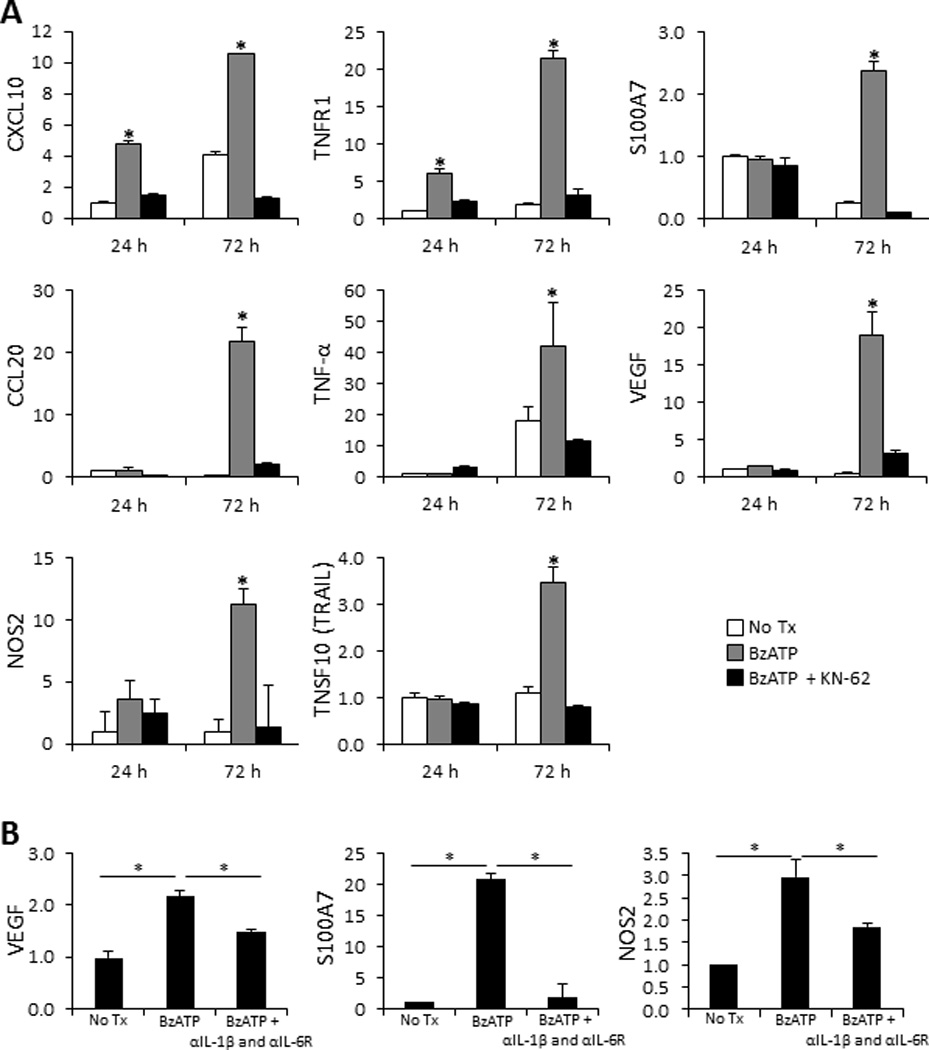
Cutaneous proinflammatory innate immune responses induced ex vivo following signaling through the P2X7R. (A) Bar graphs demonstrate the relative fold change in mRNA expression of CXCL10, TNFR1, S100A7, CCL20, TNF-α, VEGF, NOS2, and TRAIL 24 and 72 h following cutaneous injections with 350 µM of BzATP ± 1 µM KN-62 normalized to 24 h PBS injected controls (No Tx). (B) Examination of VEGF, S100A7, and NOS2 mRNA fold-change, normalized to PBS control (No Tx), 72 h following treatment with BzATP ± IL-1β and IL-6Rα antibodies. Fold-change was determined using the relative qRT-PCR 2−ΔΔCt method. Data expressed as mean ± SD of triplicates. Asterisk indicates a significant difference compared to no Tx for each time point, unless otherwise indicated, p < 0.05. Representative of three independent experiments.
BzATP increases cutaneous DC maturation and inflammation
We next examined the capability of extracellular ATP to activate cutaneous inflammatory DCs ex vivo by injecting explants with BzATP or PBS (No Tx control) and characterizing the DC populations by four-color immunofluorescence. 24 h following BzATP injections there was a significant increase in HLA-DR+ cells that was maintained at 72 h (Figure 3A and 3B). Furthermore, 24 and 72 h following BzATP injections, there was a significant increase in the expression of DC-LAMP, a DC maturation marker highly expressed in inflammatory tissues (30, 31), on both epidermal and dermal DCs (Figure 3A and 3C). CD206, a mannose receptor expressed by cutaneous DCs during severe inflammation (32), is also significantly increased by dermal cells, some of which are HLA-DR+ (Figure 3D and 3F). Characterization of the CD206+ cells identified an abundance of two CD206+ populations, the CD206+CD163+ macrophages and CD206+CD163− DCs (Figure 3E); both of which are increased in cutaneous inflammatory disorders (31–33). iNOS, a mediator of cutaneous inflammation secreted by DCs (34), was found to be significantly increased predominantly on dermal HLA-DR+ DCs following BzATP treatments (Figure 3G and 3H). Further assessment found dermal populations of DC-LAMP+, CD206+, and iNOS+ cells that correspondingly express Langerinand HLA-DR (Figure 3A, 3D, 3G), which are likely inflammatory LCs migrating into the dermis. Of note Langerin has not been described on dermal DCs in human skin as in murine skin. These findings indicate that BzATP signaling stimulates cutaneous DCs to mature and differentiate into highly inflammatory DCs.
FIGURE 3.
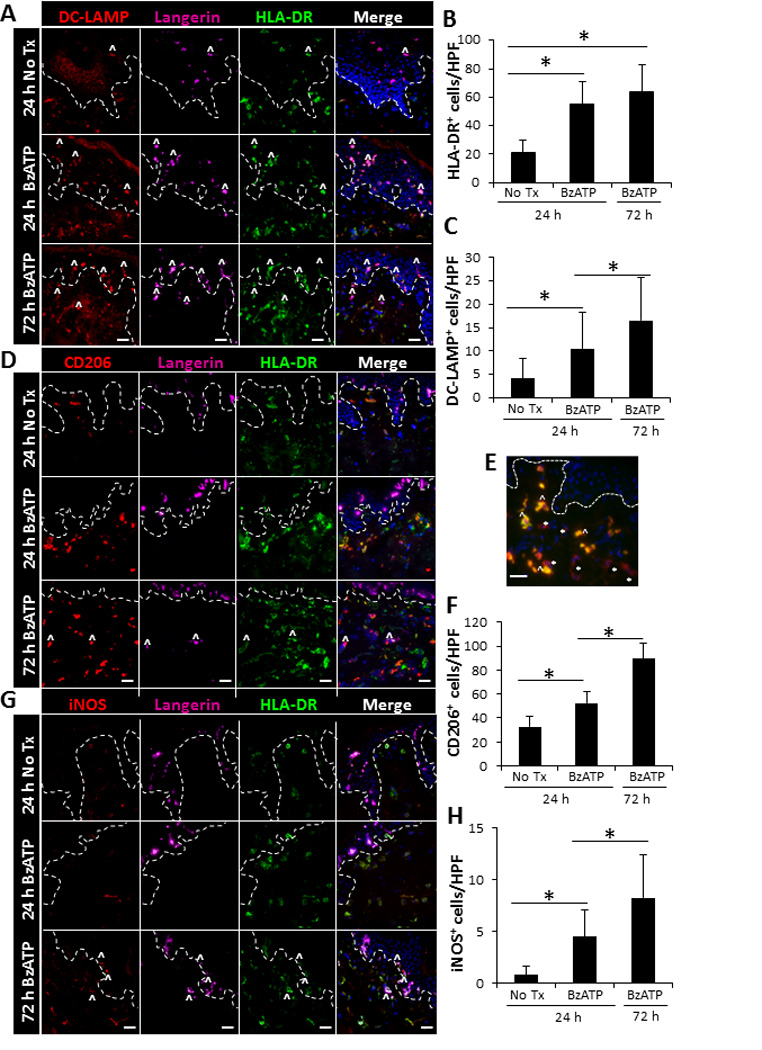
Increased expression of inflammatory markers on cutaneous DCs following ex vivo stimulation with BzATP. Skin explants were injected with 350 µM of BzATP or PBS (No Tx). 24 and 72 h following injections skin sections were immunofluorescently labeled with Langerin, HLA-DR, and (A) DC-LAMP, (D) CD206, or (G) iNOS. Dotted line represents epidermal-dermal junction and ^ indicates representative Langerin+ cells that are expressing DC-LAMP, CD206, or iNOS. Merged panels include all three stains and DAPI nuclear counter-stain. (E) Characterization of CD206+ cells 72 h following BzATP treatment. CD206-red and CD163-green. * indicates representative DCs (CD206+CD163−) and ^ indicates representative macrophages (CD206+CD163+). 40× original magnification, bar= 20µm. (B, C, F, H) Quantitation of cells positive for HLA-DR, DC-LAMP, CD206, or iNOS. Bars indicate the mean ± SD of 11–15 replicates. Asterisk indicates a significant difference, p< 0.05.
Signaling directly through the P2X7R on smiDCs induces DC maturation
Based on the above findings it could not be discerned whether the maturation and differentiation of DCs is through direct DC signaling, stimulation of precursor DCs, or solely through keratinocyte stimulation. Thus, the next set of experiments was performed to address this question. Previous studies have confirmed in mice and humans that cutaneous LCs and keratinocytes express functional P2X7R (23, 24). Additionally, we determined that approximately 20% of total smiDCs and 33% of LCs maintained their P2X7R expression following migration, indicating that activated cutaneous DCs have the capacity to directly respond to P2X7R stimulation and that smiDCs can be utilized in P2X7R mechanistic studies (Supplemental Figure 2).
We next examined the ability of P2X7R agonist to further increase smiDC maturation and terminal differentiation. To determine the optimal in vitro BzATP concentrations we performed a titration experiment and examined IL-1β and IL-6 secretion and DC viability. Following treatment with 350 µM BzATP IL-1β secretion was significantly increased by smiDCs and peaked at 3500 µM. IL-6 levels were also significantly increased by treatment with 350 µM BzATP and began to decrease at 3500 µM. Likewise, cellular viability began decreasing at the highest dose of 3500 µM BzATP (Figure 4A and Supplemental Figure 1). Thus, the 350 µM concentration is used throughout these experiments as the concentration that induces a maximal response in DCs without inducing cellular death. Additionally, smiDCs cultured in the presence of BzATP had significantly increased IL-23 and VEGF mRNA expression levels compared to smiDCs cultured in media alone (Figure 4B). Next we determined that signaling was occurring through the P2X7R by culturing smiDCs in the presence of BzATP and/or KN-62. In the presence of KN-62 there was a significant decrease in the BzATP-dependent IL-1β, IL-6, IL-23, and VEGF levels compared to BzATP treatments alone, confirming that P2X7R was directly stimulated on smiDCs via BzATP (Figure 4A and 4B, and Supplemental Figure 1D). Furthermore, in some cases KN-62 alone was able to suppress IL-1β and IL-6 below control levels indicating that endogenous ATP is present to some extent in DC cultures (Supplemental Figure 1D).
FIGURE 4.
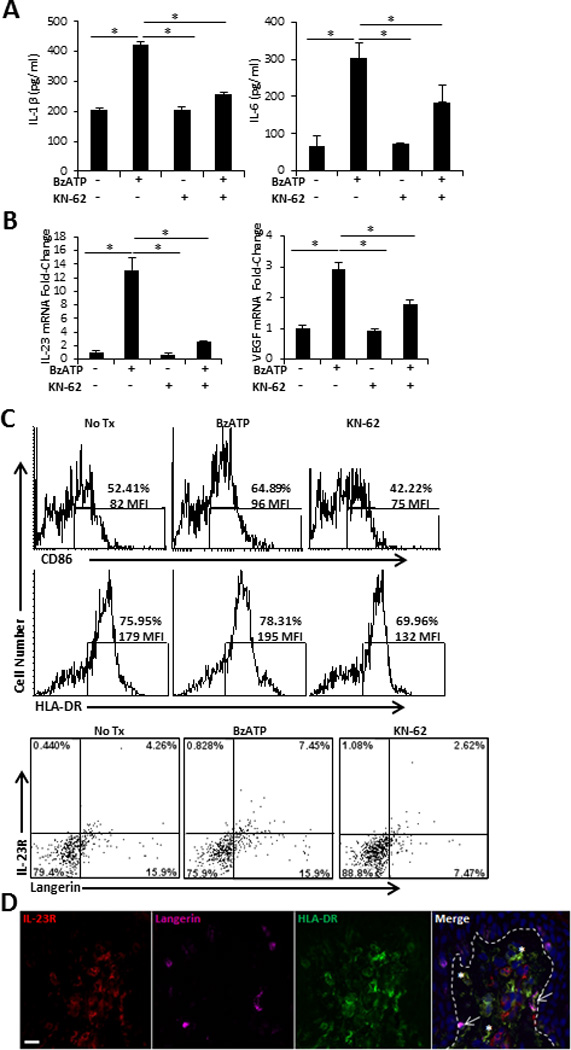
Direct stimulation of human smiDCs with P2X7R agonist. SmiDCs were cultured in the presence of the indicated treatments for 24 h and analyzed by (A) ELISA for the detection of secreted IL-1β and IL-6 in the culture supernatants and (B) qRT-PCR for the fold-change of IL-23 and VEGF expression. Data are representative of three-eight independent experiments; bars represent the means ± SD from triplicates. Asterisks indicate a significant difference between indicated groups, p < 0.05. (C) SmiDCs were stained with HLA-DR-, CD86-, IL-23R-, and Langerin-Abs. Top histograms are CD86 and HLA-DR expression; numbers represent percent positive and MFI within the gated region. Bottom dot-plots signify Langerin and IL-23R expression; numbers represent the percent positive within the respective quadrant. Flow cytometry data is one representative of four independent subjects with similar results. (D) Cutaneous explants were injected with 350 µM of BzATP and immunofluorescently labeled with IL-23R, Langerin, and HLA-DR 72 hr following treatment. Dashed line indicates epidermal-dermal junction. 40× original magnification, bar= 20µm. Data is one representative of two independent experiments.
Utilizing flow cytometry, we determined the maturation status of BzATP stimulated smiDCs by assessing the expression levels of CD86 and HLA-DR. Following treatment with BzATP there was an increase in the mean fluorescent intensity (MFI) of CD86 and HLA-DR expression on the already highly activated migratory DCs (Figure 4C). Furthermore, the percentage and MFI of CD86 and HLA-DR were decreased on smiDCs treated with KN-62 (Figure 4C).
DC-secreted IL-23 is a key cytokine associated with the development of cutaneous inflammation and autoimmune diseases, through its terminal differentiation of Th17 responses, induction of IL-22, and perhaps via its adjuvant activity that directly stimulates DCs to present self-peptides (16, 35–38). Furthermore, IL-23R has been described to be up-regulated on DCs present in psoriatic lesions (36). Here we determined that the expression of IL-23R on smiDCs was increased following BzATP treatment compared to PBS treated controls and that the predominant population expressing IL-23R was Langerin+ smiDCs (Figure 4C bottom panels). Importantly, the presence of KN-62 in smiDC cultures led to the down-regulation of IL-23R (Figure 4C bottom panels). Consistent with these results we observed IL-23R expression on LCs that have begun to migrate from the epidermis 72 h following cutaneous BzATP injections (Figure 4D, arrows). However, in the skin several dermal DCs (dDC) were also expressing IL-23R (Figure 4D, asterisks), indicating that IL-23R+ dDCs are maintained in the skin following P2X7R stimulation or IL-23R is down regulated upon dDC migration. Overall, these experiments demonstrate that directly signaling through the P2X7R on cutaneous smiDCs is capable of further inducing maturation and differentiation of smiDCs.
P2X7R signaling induces the release of HMGB1
We next wanted to determine if P2X7R signaling of smiDCs could stimulate the release of other inflammatory alarmins, such as HMGB1. For this, smiDCs were stimulated overnight in the presence or absence of BzATP and/or KN-62. Assessment of smiDC culture supernatants by ELISA demonstrated a significant increase in the amount of HMGB1 released following P2X7R stimulation compared to non-treated controls (Figure 5A). Furthermore, KN-62 had the capacity to significantly inhibit the BzATP induced response (Figure 5A). The HMGB1 receptor of advanced glycation end products (RAGE) is also increased on smiDCs following P2X7R signaling and decreased following KN-62 treatment (Figure 5B and 5C). Moreover, RAGE is increased on Langerin+ LCs in the presence of BzATP and decreased by KN-62 (Figure 5C). Importantly, this data indicates that a potential role of ATP is to further trigger other alarmins, such as HMGB1, in an autocrine/paracrine loop, which induces and perpetuates inflammatory responses.
FIGURE 5.
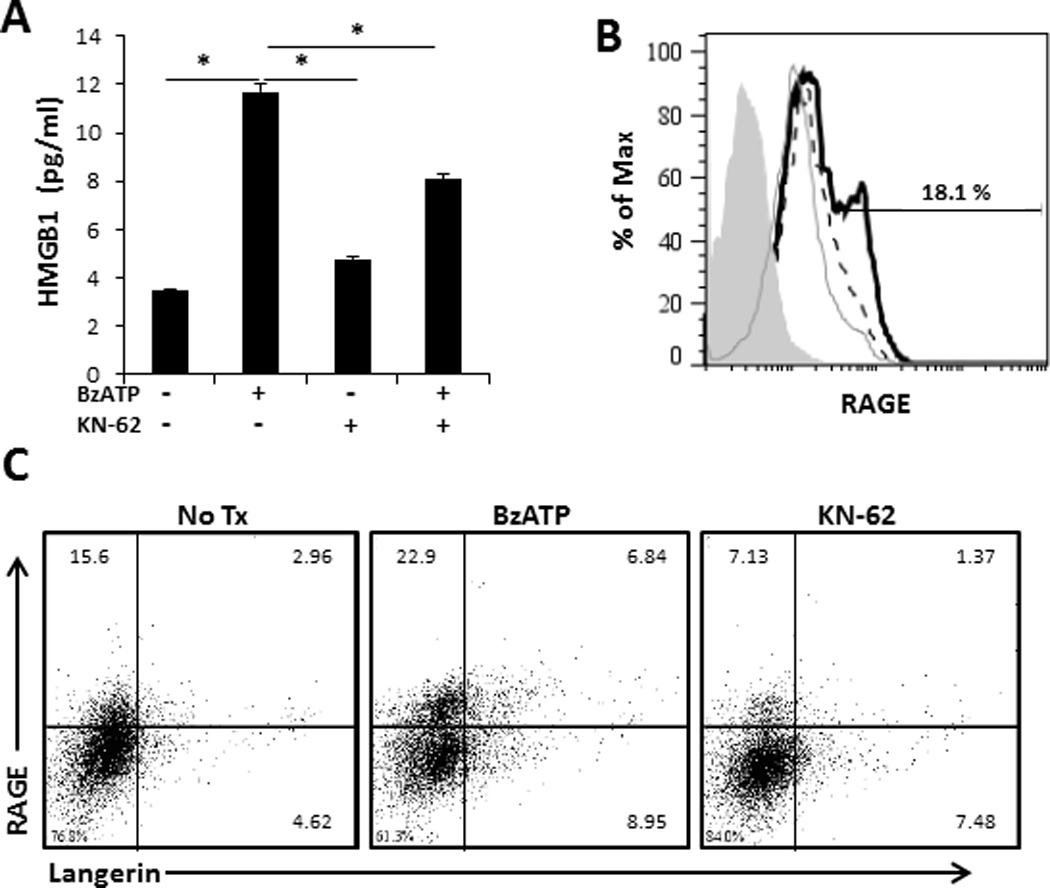
Activation of smiDCs via P2X7R stimulates increased HMGB1 secretion and RAGE expression. SmiDCs were stimulated overnight with BzATP and/or KN-62. (A) Following overnight culture, supernatants were collected and assessed for the presence of HMGB1 by ELISA. Data expressed as mean ± SD of replicates. Asterisk indicates a significant difference between designated groups, p < 0.05. Data are representative of four independent experiments. (B) SmiDCs were collected and examined by flow cytometry for the surface expression of RAGE, an HMGB1 receptor,(filled histogram represents control antibody, dashed lined represents No Tx, grey line represents KN-62, and black line represents BzATP treatments), and (C) RAGE expression on Langerin+ cells, numbers represent the percent positive within the respective quadrant. Flow cytometry data is one representative of four independent subjects with similar results.
BzATP-differentiated smiDCs bias Th17 responses
Alloreactive CD4+ T cells stimulated with smiDCs proliferate and secrete significantly high levels of IFN-γ and IL-17 (3, 4). In the present work we examined the ability of P2X7R signaling of smiDCs to further potentiate Th17 responses. Because CD3/CD28 activated CD4+ T cells secrete IL-17 when directly stimulated by BzATP (Supplemental Figure 3), smiDCs were cultured with treatments for 3 h and washed well prior to being added to MLCs. In data not shown we assessed the residual level of BzATP in smiDC cultures after 3 h of treatment and found that BzATP concentrations had returned to baseline levels. Following a 5 d incubation the MLCs were significantly increased in CD4+ effector T cells expressing an intermediate level of CD25 (CD25int) when cultured with BzATP-stimulated smiDCs (BzDCs) compared to non-treated smiDCs (NtDCs; Figure 6A and 6B). Moreover, BzDCs have the capacity to potentiate Th17 differentiation compared to NtDCs, which was indicated by the increase in IL-17 secretion (Figure 6C). However, due to the high variability inherent to the human model the significant increase in IL-17 expression was not always consistent (Supplemental Table I); however, when we inhibited P2X7R signaling with KN-62 we did observe a more consistent decrease in the Th17 response (Figure 6C and Supplemental Table I), indicating high levels of endogenous ATP present in the DC cultures, which potentially leads to negative feedback. Additionally, to address P2X7R signaling specificity we confirmed that KN-62 had the capacity to inhibit the BzATP-dependent increase in IL-17 expression (Figure 6C and Supplemental Table I). To verify that these findings were not dependent on an increase in T cell number we also assessed the proliferation induced by smiDCs and did not see a significant difference in T cell proliferation amongst the treatment groups when examined by mitochondrial dehydrogenase activity (data not shown).
FIGURE 6.
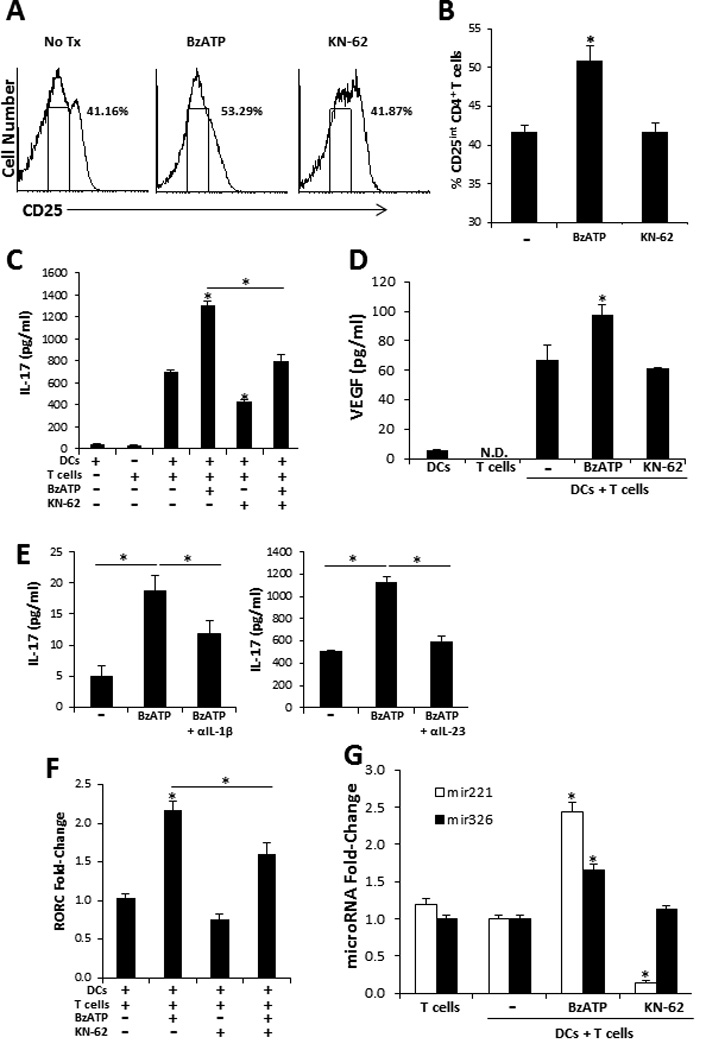
P2X7R-stimulated smiDCs significantly increase effector Th17 biased responses. Responder allogeneic naïve CD4+ T cells were cultured with BzATP-, KN-62-, BzATP + KN-62-, or non-stimulated-smiDCs for 1 or 5 d. (A) Following 5 d culture CD25 expression was examined by flow cytometry. CD25 histograms represent cells that were gated on CD4. Gated region on histogram represents intermediate CD25 expression, characteristic of effector CD4+ T cells. (B) The percentage of cells expressing CD25int graphically illustrated, bars represent the mean ± SD of triplicates. (C) IL-17 and (D) VEGF were detected in supernatants from 5 d MLCs and (E) MLCs stimulated with BzDCs were treated with IL-1β or IL-23 neutralizing Abs for 5 d then IL-17 levels were assessed by ELISA. Bars represent the mean ± SD from replicates. Controls are smiDCs and naïve T cells alone. (F) RORC mRNA transcripts and (G) microRNAs were detected 1 d following initial co-culture. Bars are the mean ± SD from triplicates and are expressed as the fold-increase compared to no treatment control (−) as determined by the 2−ΔΔCt method. Data are representative of 3–11 independent experiments. Asterisk indicates a significant difference compared to non-treated DC + T cell controls (−), unless otherwise indicated, p < 0.05. N.D. non-detected.
In addition to being a potent pro-inflammatory cytokine IL-17 also appears to be an angiogenesis factor involved in tumor progression and autoimmune diseases via the induction of VEGF (39, 40). Thus, in addition to detecting a significant increase of VEGF in ex vivo skin and smiDCs following BzATP treatment (Figure 2A and 4B) we also determined that VEGF was significantly increased in MLCs at both the mRNA (data not shown) and protein levels when naïve T cells were stimulated by BzDCs compared to NtDCs (Figure 6D). Finally, by neutralizing IL-1β or IL-23 in BzDC-stimulated MLCs we determined that the P2X7R-dependent increase in Th17 differentiation was in part induced by IL-1β and IL-23 (Figure 6E). Of note, inhibition of IL-6 signaling did not reduce the P2X7R-dependent Th17 responses in these cultures (data not shown).
To assess whether BzDCs affect Th17 differentiation at the transcriptional level we examined the retinoid-related orphan nuclear receptor (ROR)γt, which is a major transcription factor that drives Th17 differentiation. We clearly demonstrated a significant increase in the RORγt encoding gene, RORC, in cultures stimulated by BzDCs compared to NtDCs (Figure 6F). Additionally, there was a significant decrease in RORC expression when BzDCs were cultured in the presence of KN-62 (Figure 6F). Finally, we assessed the expression levels of two microRNAs involved in T cell activation (mir-221) and Th17 differentiation (mir-326) (41, 42). MLCs stimulated by BzDCs expressed a significant increase in both mir-221 (2.44 ± 0.11 fold increase) and mir-326 (1.66 ± 0.07 fold increase) compared to naïve T cells stimulated with NtDCs (Figure 6G). Additionally, KN-62 significantly suppressed mir-221 but had no effect on mir-326 (Figure 6G). Overall, these results indicate that BzDCs were capable of potentiating Th17 differentiation at both the transcriptional and post-transcriptional levels and thus we can conclude that the direct P2X7R signaling of smiDCs leads to the initiation of a DC17 differentiation profile.
Lesional and non-lesional psoriatic skin expresses P2X7R
In a genetically predisposed environment, it has been suggested that alarmins could lead to the induction of psoriatic lesions by promoting a highly inflammatory positive feedback loop (7); however, the role of alarmins in psoriasis pathogenesis have not been well addressed. In this context, ATP is a particularly appealing alarmin that, via P2X7R signaling, induces NF-κB activation and the IL-23/Th17 axis, both of which have been shown to be psoriasis susceptibility pathways (17, 35, 43). Additionally, aberrant microRNA expression profiles have been observed in psoriasis, which have correlates within the P2X7R signaling pathway, such as mir-21 (44, 45). Therefore, P2X7R signaling links early inflammatory triggers with psoriasis susceptibility factors. We hypothesize that within a genetically susceptible microenvironment cutaneous P2X7R signaling is a mechanism of psoriasis pathogenesis. To determine whether P2X7R has a role in initiating psoriatic lesions we used immunofluorescence microscopy to compare the expression of P2X7R in the skin from healthy donors to that in lesional and non-lesional skin of patients with psoriasis. In figure 7 we observed by immunofluorescence the low expression of P2X7R in the epidermis of normal skin from patients without psoriasis (Figure 7A). Expression is somewhat increased in the epidermis of non-lesional skin from patients with psoriasis and substantially increased in lesional skin from the same donor [Figure 7A and (19)]. To confirm the expression of P2X7R in psoriatic skin western blot analysis was performed on cutaneous lysates. Similar to immunofluorescence data, the full-length canonical receptor represented by the expression of the 74 kDa P2X7R protein was slightly elevated in non-lesional samples and even less so in lesional samples (Figure 7B, upper panel). However, interestingly, we also detected a 45 kDa variant of the P2X7R that was highly expressed in all lesional samples examined, while only minimally expressed in normal tissues and non-detected in most non-lesional samples (Figure 7B, upper panel). We confirmed the specificity of these bands by using a blocking peptide, which inhibited P2X7R antibody binding (Figure 7B, lower panel). Further, analysis of the P2X7R at the mRNA level revealed that non-lesional tissues exhibited a significant increase in P2X7R mRNA compared to lesional samples; while no difference was observed in lesional samples compared to normal controls (Figure 7C).
FIGURE 7.
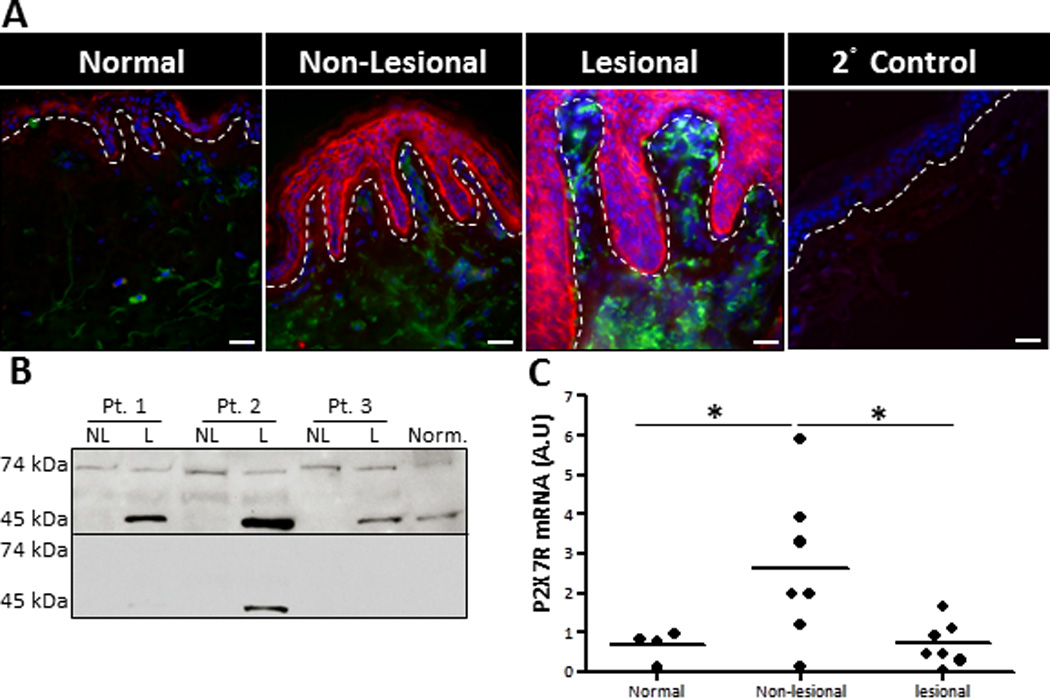
Lesional and non-lesional psoriatic skin exhibit enhanced P2X7R expression. (A) Human healthy donor and psoriatic non-lesional and lesional skin explants were immunofluorescently labeled with markers for P2X7R (red) and HLA-DR (green), plus DAPI (blue) nuclear counter-stain. As a staining control normal skin was stained with the secondary anti-rabbit-Cy3 Abalone (2° Control). Original magnification is 40×, bar = 20µm. Dotted line indicates the epidermal-dermal junction. Representative of four independent experiments. (B) Protein extracts were isolated from normal healthy skin and non-lesional and lesional psoriatic skin. Western blot analysis demonstrated the presence of two specific P2X7R variants, a 74 kDa and 45 kDa protein (upper panel). P2X7R specificity was confirmed with a blocking peptide (lower panel). Three individual patients are presented, which is representative of seven individual donors examined. (C) Scatter plot demonstrates arbitrary units of P2X7R mRNA expression normalized to B2M utilizing the 2−ΔCt method. Each dot represents an individual biopsy from normal, psoriatic non-lesional, or psoriatic lesional skin. Asterisk indicates a significant difference between indicated groups, p < 0.05.
To begin to address the role of P2X7R signaling in the initiation of psoriasis pathogenesis we injected non-lesional psoriatic explants ex vivo with BzATP or PBS (vehicle control) and examined the expression of VEGF, IL-6, and IL-23, factors positively associated with psoriasis pathogenesis (36, 46, 47). An assessment by qRT-PCR revealed a significant increase in VEGF, IL-6, and IL-23 transcript levels in non-lesional skin following injections with BzATP, compared to non-lesional controls (Figure 8). Importantly, there was no difference between non-lesional plus BzATP and lesional skin in the VEGF, IL-23, and IL-6 expression levels (Figure 8). Furthermore, to address whether purinergic signaling is involved in perpetuation of psoriatic lesions we injected lesional tissues with BzATP and determined that IL-23 is significantly increased compared to lesional controls, while VEGF and IL-6 were not increased (Figure 8). Thus, these findings support the hypothesis that P2X7R signaling is involved in the initiation phase of psoriasis pathogenesis in addition to perpetuation of psoriatic lesions. Based on these findings experimental murine models are now being developed to further explore this hypothesis.
FIGURE 8.
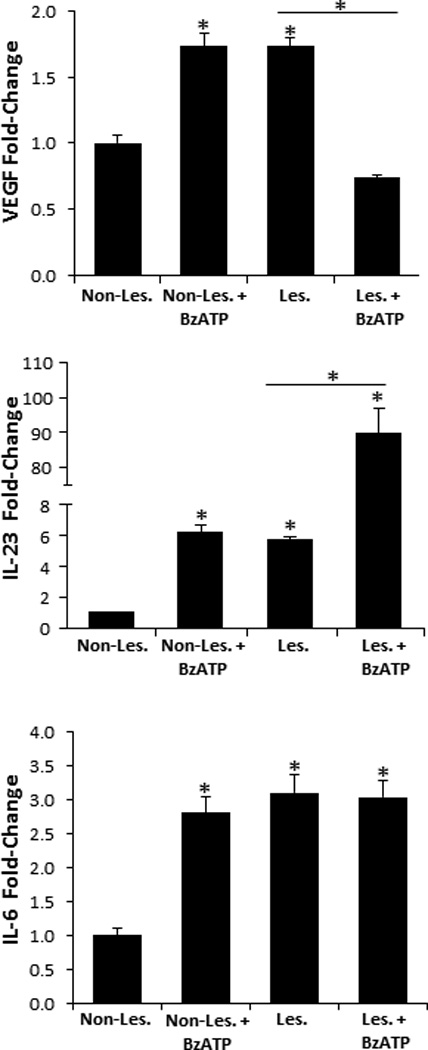
VEGF, IL-23, and IL-6 are significantly increased in non-lesional skin following BzATP treatments. Non-lesional and lesional explants were injected with BzATP or PBS (control). 24 h (VEGF and IL-6) and 72 h (IL-23) following treatment VEGF, IL-6, and IL-23 transcript expression levels were determined by the 2−ΔΔCt method. Bars represent the mean ± SD of triplicates. Asterisk indicates a significant difference compared to non-lesional explants, unless otherwise indicated, p < 0.05. Representative of three independent experiments.
We finally wanted to examine novel P2X7R-depdendent signaling mechanisms involved in the induction of psoriatic cytokines. Interestingly, mir-21 is an angiogenic factor associated with psoriasis and which increases VEGF expression [(44, 45, 48) and (Figure 9A)]. To assess the capacity of P2X7R signaling to initiate the mir-21 pathway we first injected normal human skin with BzATP and demonstrated a significant increase in mir-21 expression compared to PBS injected controls (Figure 9B). Next, we injected human cutaneous explants ex vivo with a mir-21 Atg, to specifically block mir-21 expression, in the presence or absence of BzATP. Consistent with our previous results in Figure 1 and 2, cutaneous treatment with BzATP induced a significant increase of VEGF and IL-6 expression, which was significantly decreased by approximately 80% in the presence of the mir-21 Atg at both the protein and mRNA levels (Figure 9C and data not shown, respectively). IL-6 and VEGF, to a lesser degree, were decreased in the PBS + mir-21 Atg treatment group compared to PBS alone; however, not to the same level as the BzATP treatment group (Figure 9C). Thus, we found that mir-21 stimulates the secretion of VEGF and IL-6 at a low level in the steady state and potently in a P2X7R-dependant manner. For the first time, these results further define a mechanistic role for P2X7R signaling in inflammation and describe a potential mechanism for the induction of psoriasis by stimulating the P2X7R-dependent mir-21 angiogenesis pathway.
FIGURE 9.
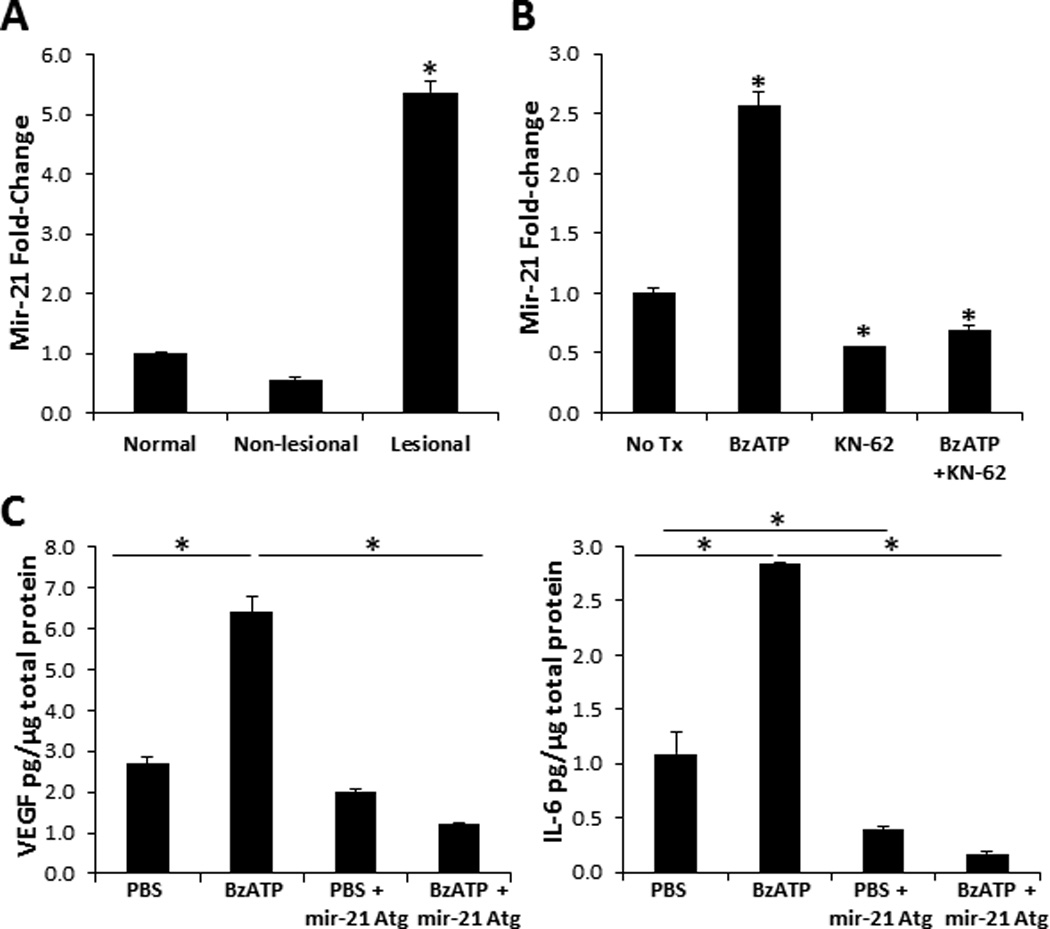
Mir-21 increases VEGF and IL-6 expression following P2X7R signaling. Mir-21 expression levels in (A) normal, non-lesional, and lesional psoriatic skin and in (B) normal human skin 12 h following injections with BzATP, KN-62, BzATP + KN-62, or PBS control (No Tx). Mir-21 expression levels were normalized to non-treated normal human skin. Bars represent the mean ± SD of triplicates. Asterisk indicates a significant difference compared to non-treated normal skin, p < 0.05. (C) VEGF and IL-6 present in cutaneous lysates 72 h following injections with PBS, BzATP, PBS + mir-21 Atg, or BzATP + mir-21 Atg. Bars represent the mean ± SD of replicates. Asterisk indicates significant difference between indicated groups. Representative of three independent experiments.
DISCUSSION
Utilizing human skin explants ex vivo and cutaneous DCs we examined the inflammatory responses initiated by the cutaneous immune system following stimulation through the P2X7R. We demonstrated that P2X7R signaling in human cutaneous tissues induces innate and adaptive immune responses; specifically, responses leading to the differentiation of Th17 cells. Furthermore, we determined that P2X7R signaling is a potential mechanism involved in the initiation of psoriasis pathogenesis, perhaps through the induction of the mir-21 angiogenic network, a critical pathway involved in the pathogenesis of psoriasis (44, 45, 48, 49).
Key innate inflammatory markers, such as IL-6, IL-1β, and TNF-α, characteristic of psoriasis, are increased following cutaneous P2X7R stimulation. IL-6, secreted by endothelial cells and cutaneous DCs, is particularly important in the development of psoriasis for its capacity to block regulatory T cell functions (47). TNF-α, a psoriasis susceptibility gene secreted by TIP-DCs and effector T cells has a critical role in psoriasis by in part initiating the expression of IL-1β and IL-8 (31, 50, 51). Moreover, TNF-α and IL-17 cooperate in the pathogenesis of psoriasis, as is seen by the effect of TNF-α antagonism on IL-17 signaling and in their synergistic effect on the production of inflammatory cytokines by keratinocytes (50, 52). P2X7R stimulation also increases the cutaneous expression of iNOS, VEGF, CXCL10, S100A7, CCL20, and TRAIL, factors highly associated with inflammation and psoriasis compared to atopic dermatitis (31, 49, 51, 53, 54). Thus, the presence of P2X7R agonists, such as ATP, induce innate inflammatory mediators in the skin reminiscent of those observed in psoriatic lesions.
At least two downstream P2X7R signaling mechanisms have been described following P2X7R stimulation, which are inflammasome/IL-1β dependent and independent pathways (55). Barberà-Cremades et al. (55) described the P2X7R-dependent secretion of PGE2 as an inflammasome-independent mechanism mediated through intracellular calcium influx and MAPK signaling. Likewise, CXCL10 and TNFR1 are increased early in the skin following P2X7R activation likely through MAPK signaling (56, 57); whereas, VEGF, iNOS, and S100A7 appear to be downstream of the inflammasome pathway (58). Therefore, we conclude that cutaneous immune responses induced following P2X7R signaling can occur through both inflammasome/IL-1β dependent and independent mechanisms.
DCs are significantly increased in psoriatic lesions and many of the psoriasis pathogenic factors found in lesions, such as IL-23, TNF-α, and iNOS, are secreted by inflammatory DCs (31, 34, 36). The significant increase of HLA-DR+ DCs in the dermis of skin explants treated with the P2X7R agonist is remarkable considering the lack of blood supply to attract circulating inflammatory monocytes; therefore, ATP likely matures and differentiates resident monocytes and/or precursor DCs into inflammatory DCs (59). Cutaneous DC populations are also highly activated expressing DC-LAMP, CD206, and iNOS following P2X7R injections. Moreover, direct P2X7R stimulation is capable of further maturing and differentiating activated migratory DCs into DC17s as determined by the expression of IL-23R, IL-1β, IL-6, IL-23, iNOS, and VEGF. LCs are the cutaneous DC population capable of initiating Th17 responses (2, 3); thus, it is not surprising that inflammatory markers and alarmin receptors increased following BzATP treatment are co-expressed with Langerin.
In murine macrophages ATP is involved in the inflammasome-dependent release of the alarmin, HMGB1, which acts as an inflammatory mediator and adjuvant stimulating DC activation, migration, and T cell-biasing functions (60–62). Specifically signaling through the P2X7R on cutaneous human DCs also induces the active release of HMGB1. Importantly, the downstream effects of HMGB1 signaling on DCs include the release of IL-6, IL-1β, and TNF-α (60, 63). The best characterized receptor for HMGB1 is RAGE, which has been shown to be expressed on immature populations of DCs and which we demonstrate for the first time is upregulated on cutaneous DCs following P2X7R agonist treatment (60, 61, 64). Furthermore, S100A7, which is increased following P2X7R stimulation and induces VEGF secretion, has also been described as an agonist for RAGE (65). Thus, a mechanism of action of P2X7R signaling in human cutaneous DCs is to trigger downstream RAGE inflammatory responses. We hypothesize that ATP is also capable of directly stimulating the release of other alarmins, such as LL-37, IL-33, and heat-shock proteins.
In the presence of P2X7R agonist cutaneous DCs express IL-1β and IL-23 to further potentiate Th17 responses, which are essential for inducing downstream effectors of psoriasis pathogenesis (66, 67). One such effector includes the secretion of VEGF, a potent inflammatory and angiogenesis factor that establishes a positive feedback mechanism by further enhancing Th17 responses and tissue inflammation (39, 40, 46, 68). Overall P2X7R signaling in healthy cutaneous tissues is capable of initiating inflammatory responses reminiscent of those observed in psoriasis lesions and thus warranted further examination of P2X7R signaling as a potential mechanism of the initiation of psoriasis pathogenesis.
This is the first report to demonstrate an increase in P2X7R expression in non-lesional skin and also the expression of P2X7R variants in psoriatic lesions. The increase of P2X7R in non-lesional skin suggests an important primary difference in the skin of patients with psoriasis and not just a secondary effect of inflammation. Interestingly, psoriatic lesions expression both the 74 kDa full-length canonical P2X7R and a unique 45 kDa variant. There has been approximately eleven splice variants identified for the P2X7R, indicated as P2X7RA-K with P2X7RA representing the full-length form (69). Of particular interest is the P2X7RB variant, which 1) is approximately 45 kDA in size, 2) is widely distributed among tissues, and 3) is lacking the intracellular C-terminus that confers P2X7R signaling cytotoxicity (69, 70). Additionally, P2X7RB stimulates increased NFATc1 (NFAT2) activation leading to enhanced cellular proliferation and decreased ATP-induced apoptosis (69, 70). It has been suggested that NFAT2 is involved in keratinocyte hyperproliferation in psoriasis (71, 72). Furthermore, reports have proposed that P2X7R variants are likely involved in human diseases (69, 73). Thus, we hypothesize that the increased expression of P2X7RB in psoriatic lesions is in part responsible for the increased proliferation and reduced apoptosis of lesional keratinocytes, contributing to the perpetuation of the psoriatic lesions.
In addition to increased levels of P2X7R, non-lesional psoriatic tissues also express significantly higher P2X7R transcripts compared to healthy controls and lesional samples. Correspondingly, mir-150, which triggers P2X7R mRNA instability, is decreased in non-lesional samples and returns to baseline levels in lesional psoriatic tissues (74, 75). These findings indicate that non-lesional psoriatic skin is poised to initiate psoriatic lesion formation following the release of danger signals and innate mediators by up-regulating the surface expression of P2X7R. In support of this, P2X7R stimulation in non-lesional skin leads to the significant increase in VEGF, IL-23, and IL-6 expression comparable to levels found in psoriatic biopsies. Moreover, the VEGF and IL-6 pathways are induced by mir-21, which is increased in psoriatic tissues and in healthy skin following P2X7R signaling (44, 45, 48). Thus, it is tempting to speculate that ATP signaling through the P2X7R initiates an inflammatory cascade that is involved in the conversion of non-lesional to lesional psoriatic tissues by initiating the mir-21 pathway.
In summary, the factors that initiate and perpetuate psoriatic lesion from non-lesional skin are not know, here we have demonstrated that a possible trigger is the release of extracellular ATP from trauma or potentially the release of ATP from commensal bacteria (17). Overall, utilizing normal human skin, we determined that signaling through purinergic receptors, particularly P2X7R, plays a role in the development of both innate and adaptive Th17 inflammatory immune responses in human skin. These findings have prompted us to further explore the P2X7R dependent development of psoriasis pathogenesis in experimental murine models of psoriasis.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Adriana Larregina for critical reading of the manuscript. We acknowledge use of tissues procured by the NDRI, CHTN, and University of Pittsburgh Health Sciences Tissue Bank. Tissue sample imaging was performed in the Center for Biological Imaging.
This work was supported by National Institutes of Health Grant 1K22 AI 83882-01 (A.R.M) and a National Psoriasis Foundation Discovery Research Grant (A.R.M). NDRI is supported by NIH grant 5 U42 RR006042 and the CHTN is funded by the National Cancer Institute. The CBI is supported by NIH Grant U54 RR022241.
Abbreviations used in this article
- P2X7R
P2X7 receptor
- DDC
dermal dendritic cells
- BzATP
2’(3’)-O-(4-Benzoylbenzoyl) adenosine 5’-triphosphate
- BzDCs
DCs stimulated with BzATP
- NtDCs
non-treated DCs
REFERENCES
- 1.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 2.Igyártó Botond Z, Haley K, Ortner D, Bobr A, Gerami-Nejad M, Edelson Brian T, Zurawski Sandra M, Malissen B, Zurawski G, Berman J, Kaplan Daniel H. Skin-resident murine dendritic cell subsets promote distinct and opposing antigen-specific T helper cell responses. Immunity. 2011;35:260–272. doi: 10.1016/j.immuni.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mathers AR, Janelsins BM, Rubin JP, Tkacheva OA, Shufesky WJ, Watkins SC, Morelli AE, Larregina AT. Differential capability of human cutaneous dendritic cell subsets to initiate Th17 responses. J. Immunol. 2009;182:921–933. doi: 10.4049/jimmunol.182.2.921. [DOI] [PubMed] [Google Scholar]
- 4.Morelli AE, Rubin JP, Erdos G, Tkacheva OA, Mathers AR, Zahorchak AF, Thomson AW, Falo LD, Jr, Larregina AT. CD4+ T cell responses elicited by different subsets of human skin migratory dendritic cells. J. Immunol. 2005;175:7905–7915. doi: 10.4049/jimmunol.175.12.7905. [DOI] [PubMed] [Google Scholar]
- 5.Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J. Leukoc. Biol. 2007;81:1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- 6.Ehrchen JM, Sunderkötter C, Foell D, Vogl T, Roth J. The endogenous Toll-like receptor 4 agonist S100A8/S100A9 (calprotectin) as innate amplifier of infection, autoimmunity, and cancer. J. Leukoc. Biol. 2009;86:557–566. doi: 10.1189/jlb.1008647. [DOI] [PubMed] [Google Scholar]
- 7.Gallucci S, Lolkema M, Matzinger P. Natural adjuvants: Endogenous activators of dendritic cells. Nat. Med. 1999;5:1249–1255. doi: 10.1038/15200. [DOI] [PubMed] [Google Scholar]
- 8.Mizumoto N, Kumamoto T, Robson SC, Sevigny J, Matsue H, Enjyoji K, Takashima A. CD39 is the dominant Langerhans cell-associated ecto-NTPDase: modulatory roles in inflammation and immune responsiveness. Nat. Med. 2002;8:358–365. doi: 10.1038/nm0402-358. [DOI] [PubMed] [Google Scholar]
- 9.la Sala A, Ferrari D, Di Virgilio F, Idzko M, Norgauer J, Girolomoni G. Alerting and tuning the immune response by extracellular nucleotides. J. Leukoc. Biol. 2003;73:339–343. doi: 10.1189/jlb.0802418. [DOI] [PubMed] [Google Scholar]
- 10.Idzko M, Dichmann S, Ferrari D, Di Virgilio F, la Sala A, Girolomoni G, Panther E, Norgauer J. Nucleotides induce chemotaxis and actin polymerization in immature but not mature human dendritic cells via activation of pertussis toxin-sensitive P2y receptors. Blood. 2002;100:925–932. doi: 10.1182/blood.v100.3.925. [DOI] [PubMed] [Google Scholar]
- 11.Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat. Immunol. 2007;8:942–949. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- 12.Pizzirani C, Ferrari D, Chiozzi P, Adinolfi E, Sandona D, Savaglio E, Di Virgilio F. Stimulation of P2 receptors causes release of IL-1beta-loaded microvesicles from human dendritic cells. Blood. 2007;109:3856–3864. doi: 10.1182/blood-2005-06-031377. [DOI] [PubMed] [Google Scholar]
- 13.Wilhelm K, Ganesan J, Muller T, Durr C, Grimm M, Beilhack A, Krempl CD, Sorichter S, Gerlach UV, Juttner E, Zerweck A, Gartner F, Pellegatti P, Di Virgilio F, Ferrari D, Kambham N, Fisch P, Finke J, Idzko M, Zeiser R. Graft-versus-host disease is enhanced by extracellular ATP activating P2X7R. Nat. Med. 2010;16:1434–1438. doi: 10.1038/nm.2242. [DOI] [PubMed] [Google Scholar]
- 14.Granstein RD, Ding W, Huang J, Holzer A, Gallo RL, Di Nardo A, Wagner JA. Augmentation of Cutaneous Immune Responses by ATPγS: Purinergic Agonists Define a Novel Class of Immunologic Adjuvants. J. Immunol. 2005;174:7725–7731. doi: 10.4049/jimmunol.174.12.7725. [DOI] [PubMed] [Google Scholar]
- 15.la Sala A, Ferrari D, Corinti S, Cavani A, Di Virgilio F, Girolomoni G. Extracellular ATP induces a distorted maturation of dendritic cells and inhibits their capacity to initiate Th1 responses. J. Immunol. 2001;166:1611–1617. doi: 10.4049/jimmunol.166.3.1611. [DOI] [PubMed] [Google Scholar]
- 16.McGeachy MJ, Chen Y, Tato CM, Laurence A, Joyce-Shaikh B, Blumenschein WM, McClanahan TK, O'Shea JJ, Cua DJ. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat. Immunol. 2009;10:314–324. doi: 10.1038/ni.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Atarashi K, Nishimura J, Shima T, Umesaki Y, Yamamoto M, Onoue M, Yagita H, Ishii N, Evans R, Honda K, Takeda K. ATP drives lamina propria TH17 cell differentiation. Nature. 2008;455:808–812. doi: 10.1038/nature07240. [DOI] [PubMed] [Google Scholar]
- 18.Inoue K, Hosoi J, Denda M. Extracellular ATP has stimulatory effects on the expression and release of IL-6 via purinergic receptors in normal human epidermal keratinocytes. J. Invest. Dermatol. 2006;127:362–371. doi: 10.1038/sj.jid.5700526. [DOI] [PubMed] [Google Scholar]
- 19.Pastore S, Mascia F, Gulinelli S, Forchap S, Dattilo C, Adinolfi E, Girolomoni G, Di Virgilio F, Ferrari D. Stimulation of purinergic receptors modulates chemokine expression in human keratinocytes. J. Invest. Dermatol. 2007;127:660–667. doi: 10.1038/sj.jid.5700591. [DOI] [PubMed] [Google Scholar]
- 20.Holzer AM, Granstein RD. Role of extracellular adenosine triphosphate in human skin. J. Cutan. Med. Surg. 2004;8:90–96. doi: 10.1007/s10227-004-0125-5. [DOI] [PubMed] [Google Scholar]
- 21.Dixon CJ, Bowler WB, Littlewood-Evans A, Dillon JP, Bilbe G, Sharpe GR, Gallagher JA. Regulation of epidermal homeostasis through P2Y2 receptors. Br. J. Pharmacol. 1999;127:1680–1686. doi: 10.1038/sj.bjp.0702653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weber FC, Esser PR, Muller T, Ganesan J, Pellegatti P, Simon MM, Zeiser R, Idzko M, Jakob T, Martin SF. Lack of the purinergic receptor P2X(7) results in resistance to contact hypersensitivity. J. Exp. Med. 2010;207:2609–2619. doi: 10.1084/jem.20092489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Georgiou JG, Skarratt KK, Fuller SJ, Martin CJ, Christopherson RI, Wiley JS, Sluyter R. Human epidermal and monocyte-derived langerhans cells express functional P2X receptors. J. Invest. Dermatol. 2005;125:482–490. doi: 10.1111/j.0022-202X.2005.23835.x. [DOI] [PubMed] [Google Scholar]
- 24.Tran JNSN, Pupovac A, Taylor RM, Wiley JS, Byrne SN, Sluyter R. Murine epidermal Langerhans cells and keratinocytes express functional P2X7 receptors. Exp. Dermatol. 2010;19:e151–e157. doi: 10.1111/j.1600-0625.2009.01029.x. [DOI] [PubMed] [Google Scholar]
- 25.Larregina AT, Morelli AE, Spencer LA, Logar AJ, Watkins SC, Thomson AW, Falo LD. Dermal-resident CD14+ cells differentiate into Langerhans cells. Nat. Immunol. 2001;2:1151–1158. doi: 10.1038/ni731. [DOI] [PubMed] [Google Scholar]
- 26.Pope M, Betjes MG, Hirmand H, Hoffman L, Steinman RM. Both dendritic cells and memory T lymphocytes emigrate from organ cultures of human skin and form distinctive dendritic-T-cell conjugates. J. Invest. Dermatol. 1995;104:11–17. doi: 10.1111/1523-1747.ep12613452. [DOI] [PubMed] [Google Scholar]
- 27.Nestle FO, Zheng XG, Thompson CB, Turka LA, Nickoloff BJ. Characterization of dermal dendritic cells obtained from normal human skin reveals phenotypic and functionally distinctive subsets. J. Immunol. 1993;151:6535–6545. [published erratum appears in J. Immunol. 1994 Jan 1;152(1):376]. [PubMed] [Google Scholar]
- 28.Mariathasan S, Weiss DS, Newton K, McBride J, O'Rourke K, Roose-Girma M, Lee WP, Weinrauch Y, Monack DM, Dixit VM. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 29.Wiley JS, Sluyter R, Gu BJ, Stokes L, Fuller SJ. The human P2X7 receptor and its role in innate immunity. Tissue Antigens. 2011;78:321–332. doi: 10.1111/j.1399-0039.2011.01780.x. [DOI] [PubMed] [Google Scholar]
- 30.Wei-yuan M, Wen-ting L, Chen Z, Qing S. Significance of DC-LAMP and DC-SIGN expression in psoriasis vulgaris lesions. Exp. Molec. Path. 2011;91:461–465. doi: 10.1016/j.yexmp.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 31.Guttman-Yassky E, Lowes MA, Fuentes-Duculan J, Whynot J, Novitskaya I, Cardinale I, Haider A, Khatcherian A, Carucci JA, Bergman R, Krueger JG. Major differences in inflammatory dendritic cells and their products distinguish atopic dermatitis from psoriasis. J. Allergy Clin. Immunol. 2007;119:1210–1217. doi: 10.1016/j.jaci.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 32.Wollenberg A, Mommaas M, Oppel T, Schottdorf E-M, Gunther S, Moderer M. Expression and function of the mannose receptor CD206 on epidermal dendritic cells in inflammatory skin diseases. J. Invest. Dermatol. 2002;118:327–334. doi: 10.1046/j.0022-202x.2001.01665.x. [DOI] [PubMed] [Google Scholar]
- 33.Fuentes-Duculan J, Suarez-Farinas M, Zaba LC, Nograles KE, Pierson KC, Mitsui H, Pensabene CA, Kzhyshkowska J, Krueger JG, Lowes MA. A subpopulation of CD163-positive macrophages is classically activated in psoriasis. J. Invest. Dermatol. 2010;130:2412–2422. doi: 10.1038/jid.2010.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lowes MA, Chamian F, Abello MV, Fuentes-Duculan J, Lin SL, Nussbaum R, Novitskaya I, Carbonaro H, Cardinale I, Kikuchi T, Gilleaudeau P, Sullivan-Whalen M, Wittkowski KM, Papp K, Garovoy M, Dummer W, Steinman RM, Krueger JG. Increase in TNF-alpha and inducible nitric oxide synthase-expressing dendritic cells in psoriasis and reduction with efalizumab (anti-CD11a) Proc. Natl. Acad. Sci. U.S.A. 2005;102:19057–19062. doi: 10.1073/pnas.0509736102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nair RP, Duffin KC, Helms C, Ding J, Stuart PE, Goldgar D, Gudjonsson JE, Li Y, Tejasvi T, Feng B-J, Ruether A, Schreiber S, Weichenthal M, Gladman D, Rahman P, Schrodi SJ, Prahalad S, Guthery SL, Fischer J, Liao W, Kwok P-Y, Menter A, Lathrop GM, Wise CA, Begovich AB, Voorhees JJ, Elder JT, Krueger GG, Bowcock AM, Abecasis GR. Genome-wide scan reveals association of psoriasis with IL-23 and NF-[kappa]B pathways. Nat. Genet. 2009;41:199–204. doi: 10.1038/ng.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tonel G, Conrad C, Laggner U, Di Meglio P, Grys K, McClanahan TK, Blumenschein WM, Qin JZ, Xin H, Oldham E, Kastelein R, Nickoloff BJ, Nestle FO. Cutting edge: A critical functional role for IL-23 in psoriasis. J. Immunol. 2010;185:5688–5691. doi: 10.4049/jimmunol.1001538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Belladonna ML, Renauld JC, Bianchi R, Vacca C, Fallarino F, Orabona C, Fioretti MC, Grohmann U, Puccetti P. IL-23 and IL-12 have overlapping, but distinct, effects on murine dendritic cells. J. Immunol. 2002;168:5448–5454. doi: 10.4049/jimmunol.168.11.5448. [DOI] [PubMed] [Google Scholar]
- 38.Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham-Anderson J, Wu J, Ouyang W. Interleukin-22, a TH17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–651. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- 39.Liu J, Duan Y, Cheng X, Chen X, Xie W, Long H, Lin Z, Zhu B. IL-17 is associated with poor prognosis and promotes angiogenesis via stimulating VEGF production of cancer cells in colorectal carcinoma. Biochem. Bioph. Res. Co. 2011;407:348–354. doi: 10.1016/j.bbrc.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 40.Pickens SR, Volin MV, Mandelin AM, Kolls JK, Pope RM, Shahrara S. IL-17 Contributes to Angiogenesis in Rheumatoid Arthritis. J. Immunol. 2010;184:3233–3241. doi: 10.4049/jimmunol.0903271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grigoryev YA, Kurian SM, Hart T, Nakorchevsky AA, Chen C, Campbell D, Head SR, Yates JR, Salomon DR. MicroRNA regulation of molecular networks mapped by global microRNA, mRNA, and protein expression in activated T lymphocytes. J. Immunol. 2011;187:2233–2243. doi: 10.4049/jimmunol.1101233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Du C, Liu C, Kang J, Zhao G, Ye Z, Huang S, Li Z, Wu Z, Pei G. MicroRNA miR-326 regulates TH-17 differentiation and is associated with the pathogenesis of multiple sclerosis. Nat. Immunol. 2009;10:1252–1259. doi: 10.1038/ni.1798. [DOI] [PubMed] [Google Scholar]
- 43.Liu Y, Xiao Y, Li Z. P2X7 receptor positively regulates MyD88-dependent NF-κB activation. Cytokine. 2011;55:229–236. doi: 10.1016/j.cyto.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 44.Joyce CE, Zhou X, Xia J, Ryan C, Thrash B, Menter A, Zhang W, Bowcock AM. Deep sequencing of small RNAs from human skin reveals major alterations in the psoriasis miRNAome. Hum. Mol. Gen. 2011;20:4025–4040. doi: 10.1093/hmg/ddr331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zibert JR, Løvendorf MB, Litman T, Olsen J, Kaczkowski B, Skov L. MicroRNAs and potential target interactions in psoriasis. J. Dermatol. Sci. 2010;58:177–185. doi: 10.1016/j.jdermsci.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 46.Xia YP, Li B, Hylton D, Detmar M, Yancopoulos GD, Rudge JS. Transgenic delivery of VEGF to mouse skin leads to an inflammatory condition resembling human psoriasis. Blood. 2003;102:161–168. doi: 10.1182/blood-2002-12-3793. [DOI] [PubMed] [Google Scholar]
- 47.Goodman WA, Levine AD, Massari JV, Sugiyama H, McCormick TS, Cooper KD. IL-6 signaling in psoriasis prevents immune suppression by regulatory T cells. J. Immunol. 2009;183:3170–3176. doi: 10.4049/jimmunol.0803721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu L-Z, Li C, Chen Q, Jing Y, Carpenter R, Jiang Y, Kung H-F, Lai L, Jiang B-H. MiR-21 Induced Angiogenesis through AKT and ERK Activation and HIF-1α Expression. PLoS One. 2011;6:e19139. doi: 10.1371/journal.pone.0019139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schonthaler HB, Huggenberger R, Wculek SK, Detmar M, Wagner EF. Systemic anti-VEGF treatment strongly reduces skin inflammation in a mouse model of psoriasis. Proc. Natl. Acad. Sci. U.S.A. 2009;106:21264–21269. doi: 10.1073/pnas.0907550106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zaba LC, Suarez-Farinas M, Fuentes-Duculan J, Nograles KE, Guttman-Yassky E, Cardinale I, Lowes MA, Krueger JG. Effective treatment of psoriasis with etanercept is linked to suppression of IL-17 signaling, not immediate response TNF genes. J. Allergy Clin. Immunol. 2009;124 doi: 10.1016/j.jaci.2009.08.046. 1022-1010.e1021-1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lowes MA, Bowcock AM, Krueger JG. Pathogenesis and therapy of psoriasis. Nature. 2007;445:866–873. doi: 10.1038/nature05663. [DOI] [PubMed] [Google Scholar]
- 52.Chiricozzi A, Guttman-Yassky E, Suarez-Farinas M, Nograles KE, Tian S, Cardinale I, Chimenti S, Krueger JG. Integrative responses to IL-17 and TNF-alpha in human keratinocytes account for key inflammatory pathogenic circuits in psoriasis. J. Invest. Dermatol. 2011;131:677–687. doi: 10.1038/jid.2010.340. [DOI] [PubMed] [Google Scholar]
- 53.Jung K, Lee D, Lim HS, Lee SI, Kim YJ, Lee GM, Kim SC, Koh GY. Double anti-angiogenic and anti-inflammatory protein Valpha targeting VEGF-A and TNF-alpha in retinopathy and psoriasis. J. Biol. Chem. 2011;286:14410–14418. doi: 10.1074/jbc.M111.228130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zaba LC, Fuentes-Duculan J, Eungdamrong NJ, Johnson-Huang LM, Nograles KE, White TR, Pierson KC, Lentini T, Suarez-Farinas M, Lowes MA, Krueger JG. Identification of TNF-related apoptosis-inducing ligand and other molecules that distinguish inflammatory from resident dendritic cells in patients with psoriasis. J. Allergy Clin. Immunol. 2010;125:1261.e1269–1268.e1269. doi: 10.1016/j.jaci.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barberà-Cremades M, Baroja-Mazo A, Gomez AI, Machado F, Di Virgilio F, Pelegrín P. P2X7 receptor-stimulation causes fever via PGE2 and IL-1β release. FASEB J. 2012;26:2951–2962. doi: 10.1096/fj.12-205765. [DOI] [PubMed] [Google Scholar]
- 56.Veglianese P, Lo Coco D, Bao Cutrona M, Magnoni R, Pennacchini D, Pozzi B, Gowing G, Julien JP, Tortarolo M, Bendotti C. Activation of the p38MAPK cascade is associated with upregulation of TNF alpha receptors in the spinal motor neurons of mouse models of familial ALS. Mol. Cell. Neurosci. 2006;31:218–231. doi: 10.1016/j.mcn.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 57.Shen Q, Zhang R, Bhat NR. MAP kinase regulation of IP10/CXCL10 chemokine gene expression in microglial cells. Brain Res. 2006;1086:9–16. doi: 10.1016/j.brainres.2006.02.116. [DOI] [PubMed] [Google Scholar]
- 58.Shimada K, Crother TR, Karlin J, Chen S, Chiba N, Ramanujan VK, Vergnes L, Ojcius DM, Arditi M. Caspase-1 dependent IL-1beta secretion is critical for host defense in a mouse model of Chlamydia pneumoniae lung infection. PLoS One. 2011;6:e21477. doi: 10.1371/journal.pone.0021477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Leon B, Ardavin C. Monocyte-derived dendritic cells in innate and adaptive immunity. Immunol. Cell Bio. 2008;86:320–324. doi: 10.1038/icb.2008.14. [DOI] [PubMed] [Google Scholar]
- 60.Yang D, Chen Q, Yang H, Tracey KJ, Bustin M, Oppenheim JJ. High mobility group box-1 protein induces the migration and activation of human dendritic cells and acts as an alarmin. J. Leukoc. Biol. 2007;81:59–66. doi: 10.1189/jlb.0306180. [DOI] [PubMed] [Google Scholar]
- 61.Dumitriu IE, Bianchi ME, Bacci M, Manfredi AA, Rovere-Querini P. The secretion of HMGB1 is required for the migration of maturing dendritic cells. J. Leukoc. Biol. 2007;81:84–91. doi: 10.1189/jlb.0306171. [DOI] [PubMed] [Google Scholar]
- 62.Lamkanfi M, Sarkar A, Vande Walle L, Vitari AC, Amer AO, Wewers MD, Tracey KJ, Kanneganti T-D, Dixit VM. Inflammasome-Dependent Release of the Alarmin HMGB1 in Endotoxemia. J. Immunol. 2010;185:4385–4392. doi: 10.4049/jimmunol.1000803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Telusma G, Datta S, Mihajlov I, Ma W, Li J, Yang H, Newman W, Messmer BT, Minev B, Schmidt-Wolf IG, Tracey KJ, Chiorazzi N, Messmer D. Dendritic cell activating peptides induce distinct cytokine profiles. Int. Immunol. 2006;18:1563–1573. doi: 10.1093/intimm/dxl089. [DOI] [PubMed] [Google Scholar]
- 64.Lohwasser C, Neureiter D, Weigle B, Kirchner T, Schuppan D. The receptor for advanced glycation end products is highly expressed in the skin and upregulated by advanced glycation end products and tumor necrosis factor-alpha. J. Invest. Dermatol. 2006;126:291–299. doi: 10.1038/sj.jid.5700070. [DOI] [PubMed] [Google Scholar]
- 65.Shubbar E, Vegfors J, Carlstrom M, Petersson S, Enerback C. Psoriasin (S100A7) increases the expression of ROS and VEGF and acts through RAGE to promote endothelial cell proliferation. Breast Cancer Res. Treat. 2011;134:71–80. doi: 10.1007/s10549-011-1920-5. [DOI] [PubMed] [Google Scholar]
- 66.Krueger JG, Fretzin S, Suarez-Farinas M, Haslett PA, Phipps KM, Cameron GS, McColm J, Katcherian A, Cueto I, White T, Banerjee S, Hoffman RW. IL-17A is essential for cell activation and inflammatory gene circuits in subjects with psoriasis. J. Allergy Clin. Immunol. 2012;130:145.e149–154.e149. doi: 10.1016/j.jaci.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li J, Chen X, Liu Z, Yue Q, Liu H. Expression of Th17 cytokines in skin lesions of patients with psoriasis. J. Huazhong Univ. Sci. Technolog. Med. Sci. 2007;27:330–332. doi: 10.1007/s11596-007-0329-1. [DOI] [PubMed] [Google Scholar]
- 68.Choi JP, Kim YS, Tae YM, Choi EJ, Hong BS, Jeon SG, Gho YS, Zhu Z, Kim YK. A viral PAMP double-stranded RNA induces allergen-specific Th17 cell response in the airways which is dependent on VEGF and IL-6. Allergy. 2010;65:1322–1330. doi: 10.1111/j.1398-9995.2010.02369.x. [DOI] [PubMed] [Google Scholar]
- 69.Sluyter R, Stokes L. Significance of P2X7 receptor variants to human health and disease. Recent Pat. DNA Gene Seq. 2011;5:41–54. doi: 10.2174/187221511794839219. [DOI] [PubMed] [Google Scholar]
- 70.Adinolfi E, Cirillo M, Woltersdorf R, Falzoni S, Chiozzi P, Pellegatti P, Callegari MG, Sandona D, Markwardt F, Schmalzing G, Di Virgilio F. Trophic activity of a naturally occurring truncated isoform of the P2X7 receptor. Faseb J. 2010;24:3393–3404. doi: 10.1096/fj.09-153601. [DOI] [PubMed] [Google Scholar]
- 71.Aizu T, Tamai K, Nakano H, Rokunohe D, Toyomaki Y, Uitto J, Sawamura D. Calcineurin/NFAT-dependent regulation of 230-kDa bullous pemphigoid antigen (BPAG1) gene expression in normal human epidermal keratinocytes. J. Dermatol. Sci. 2008;51:45–51. doi: 10.1016/j.jdermsci.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 72.Hampton PJ, Jans R, Flockhart RJ, Parker G, Reynolds NJ. Lithium regulates keratinocyte proliferation via glycogen synthase kinase 3 and NFAT2 (nuclear factor of activated T cells 2) J. Cell. Physiol. 2012;227:1529–1537. doi: 10.1002/jcp.22872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mankus C, Rich C, Minns M, Trinkaus-Randall V. Corneal epithelium expresses a variant of P2X(7) receptor in health and disease. PLoS One. 2011;6:e28541. doi: 10.1371/journal.pone.0028541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhou L, Qi X, Potashkin JA, Abdul-Karim FW, Gorodeski GI. MicroRNAs miR-186 and miR-150 down-regulate expression of the pro-apoptotic purinergic P2X7 receptor by activation of instability sites at the 3'-untranslated region of the gene that decrease steady-state levels of the transcript. J Biol Chem. 2008;283:28274–28286. doi: 10.1074/jbc.M802663200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lerman G, Avivi C, Mardoukh C, Barzilai A, Tessone A, Gradus B, Pavlotsky F, Barshack I, Polak-Charcon S, Orenstein A, Hornstein E, Sidi Y, Avni D. MiRNA expression in psoriatic skin: reciprocal regulation of hsa-miR-99a and IGF-1R. PLoS One. 2011;6:e20916. doi: 10.1371/journal.pone.0020916. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


