Abstract
Previous studies indicate that reproductive condition can alter the stress response and glucocorticoid release. Although the functional significance of hypothalamic-pituitary-adrenal (HPA) axis modulation by breeding condition is not fully understood, one possible explanation is the behavior hypothesis, which states that an animal’s need to express parental behavior may be driving modulation of the HPA axis. This possibility is consistent with findings of blunted activity and reactivity of the HPA axis in lactating female mammals; however, effects of reproductive status on HPA function have not been well characterized in male mammals that express parental behavior. Therefore, we tested this hypothesis in the monogamous and biparental California mouse. Several aspects of HPA activity were compared in males from three reproductive conditions: virgin males (housed with another male), non-breeding males (housed with a tubally ligated female), and first-time fathers (housed with a female and their first litter of pups). In light of the behavior hypothesis we predicted that new fathers would differ from virgin and non-breeding males in several aspects of HPA function and corticosterone (CORT) output: decreased amplitude of the diurnal rhythm in CORT, a blunted CORT increase following predator-odor stress, increased sensitivity to glucocorticoid negative feedback, and/or a blunted CORT response to pharmacological stimulation. In addition, we predicted that first-time fathers would be more resistant to CORT-induced suppression of testosterone secretion, as testosterone is important for paternal behavior in this species. We found that virgin males, non-breeding males and first-time fathers did not display any CORT differences in diurnal rhythm, response to a predator-odor stressor, or response to pharmacological suppression or stimulation. Additionally, there were no differences in circulating testosterone concentrations. Adrenal mass was, however, significantly lower in new fathers than in virgin or non-breeding males. These results suggest that the behavior hypothesis does not explain HPA function across reproductive conditions in male California mice.
Keywords: HPA axis, Corticosterone, glucocorticoid, stress, paternal behavior, California mouse, Peromyscus californicus, behavior hypothesis
1. Introduction
Stress and reproduction are intimately intertwined, and as such the effects of stress on reproduction have been investigated in a variety of taxa. Stress, both acute and chronic, can dampen reproductive physiology as well as suppress reproductive behavior [1-5]. Conversely, reproductive condition can alter the hypothalamic-pituitary-adrenal (HPA) axis stress response and glucocorticoid (GC) release [6-13]. For example, changes in HPA axis function (e.g., response to GC negative feedback) and GC release patterns (e.g., amplitude and range of the diurnal rhythm; response to stressors) occur during the breeding season in several species [see 12 for review; 5,14].
The functional significance of HPA-axis modulation during the breeding season has yet to be fully elucidated. One of several posited explanations is the behavior hypothesis [9, 12], which states that the need to express parental behavior may be driving seasonal modification of the HPA axis [12]. Various types of stress have been shown to disrupt parental behavior, and evidence suggests that GCs are at least partially responsible for this effect [15-18]. The disruption of parental investment (e.g., abandonment or cannibalism of offspring) that can occur in response to major stressors (e.g., prolonged severe weather or food shortage) may increase overall lifetime fitness of parents by increasing the parent’s survival, but triaging reproductive effort in response to every minor perturbation (e.g., attempted predation or a minor storm) could be detrimental to reproductive success. Lowering both baseline and stress-induced GC concentrations during the time of intensive parental behavior would reduce the chances of GC levels increasing to a point that would disrupt parental care, and therefore could be beneficial both for offspring survival and for parental fitness [5,13,19].
Due to the universal expression of maternal behavior in mammals, numerous studies have addressed the interactions between motherhood and HPA activity [20-23]; however, little is known about the relationship between fatherhood and the HPA axis. In 6-10% of mammals, including humans, both parents provide care for their offspring [24]. In these biparental systems, both mothers and fathers make important contributions to the survival and growth of young (e.g., by providing food, warmth, and protection) and can influence behavioral and neuroendocrine development of offspring [25-27]. A reasonable hypothesis, therefore, is that in biparental species, both sexes modulate HPA-axis function during periods of parental care in an effort to ensure offspring survival.
Previous data on monogamous, biparental male mammals suggest that reproductive condition, as well as pair bonding, can alter HPA function. For example, in male prairie voles (Microtus ochrogaster), loss of a female pairmate, but not a male cagemate, increased circulating GC concentrations and passive stress-coping behavior [28], suggesting that the presence of a pair bond can reduce circulating GC levels in males. Similarly, male California mice (Peromyscus californicus) housed with an ovariectomized female had lower basal and stress-induced GC levels than isolated males, suggesting that social living (and presumably pair bonding) can decrease circulating corticosterone (CORT) concentrations [29]. Pair bonding in male California mice may also buffer the CORT response to a repeated stressor, as virgin males housed with another male, but not males pair-housed with a female (either with or without pups), showed an increased GC response to predator urine following repeated exposure [30]. However, no study to date has systematically investigated baseline, stress-induced, and pharmacologically manipulated HPA activity across reproductive conditions in a biparental male mammal.
The California mouse is a valuable animal model for studying the effects of reproductive condition on the HPA axis, as this rodent is both monogamous and biparental [31-33]. California mouse fathers are highly attracted to pups, engage in all of the same parental behaviors as mothers (with the exception of lactation), and can increase offspring survival under both field and laboratory conditions [25, 34-37]. When presented with a newborn pup, virgin males of this species are more variable in their expression of paternal behavior than are new fathers [38-43]. Consistent with this difference in behavior, circulating concentrations of several hormones thought to be associated with the expression of paternal care differ in male California mice as a function of reproductive condition. For example, new fathers have lower plasma progesterone levels than virgin males [39] and fathers have higher systemic levels of prolactin than virgin males or expectant fathers [44], while expectant fathers have higher systemic levels of oxytocin than do virgin or non-breeding males [45]. Moreover, mated males with pregnant mates have higher testosterone levels than both new fathers and virgins [39] however, fathers have higher levels of aromatase (the enzyme that converts testosterone to estradiol) in the medial preoptic area of the brain [39; a region important for expression of paternal behavior; 46], and testosterone has been shown to increase paternal behavior in California mice via conversion to estradiol [47,48]. Because testosterone is important for paternal care in this species, and because GCs and stress have been shown to decrease circulating testosterone levels [5,49], dampening HPA activity around the time of reproduction may help promote and preserve paternal care. Little is known, however, about how GC (in California mice, CORT) dynamics change with reproductive status in this species.
In this study we evaluated the effects of reproductive status on HPA-axis function in male California mice by characterizing HPA activity and reactivity in virgin males, non-breeding males (pair-housed with tubally ligated females), and first-time fathers. Specifically, we aimed to determine whether fatherhood (or cohabitation with a female) influences 1) baseline HPA activity (diurnal rhythm), 2) HPA response to an acute stressor (predator urine), 3) HPA responsiveness to GC negative feedback (dexamethasone injection), 4) adrenal responsiveness to pharmacological stimulation (corticotropin-releasing hormone [CRH] injection) and, 5) response of the hypothalamic-pituitary-gonadal axis, measured via circulating testosterone, to an acute elevation of CORT. The behavior hypothesis predicts that fathers should show decreased amplitude of the diurnal rhythm in CORT (or an overall reduction in CORT release across the diurnal cycle), a blunted CORT increase following acute stress, increased sensitivity to GC negative feedback, and/or a blunted CORT response to CRH stimulation, as compared to virgins and non-breeding males. In addition, first-time fathers should be more resistant to suppression of testosterone secretion following an increase in CORT, when compared to virgin or non-breeding males, as testosterone is important for paternal behavior in this species.
2. Methods
Animals were bred and housed at the University of California, Riverside (UCR). The UCR colony was started in 2007 with mice purchased from the Peromyscus Genetic Stock Center (University of South Carolina, Columbia, SC). Mice were housed in polycarbonate cages (44 × 24 × 20 cm) lined with aspen shavings; cotton wool was provided for nesting material. Mice had ad libitum access to food (Purina rodent chow 5001) and water. Lights were maintained on a 14:10 light:dark cycle with lights on at 0500h and lights off at 1900h, and ambient temperature was maintained at approximately 23°C with humidity around 65%. Animals were weaned from their birth cage at 27-32 days of age (prior to the birth of any younger siblings), ear punched for identification and housed in same-sex groups of four until the experiment began.
At the start of the experiment mature male mice were randomly placed into one of three conditions (virgin males, non-breeding males, first-time fathers; n=12 per condition). A power analysis, conducted in G*Power [50] using data from a previous study on diurnal rhythms in CORT [51], indicated that our samples sizes yielded power of >99%.
Virgin males were housed with an unrelated, age-matched male; non-breeding males were housed with a tubally ligated female (see below); and breeding males were housed with an intact female. Non-breeding males were expected to pair-bond (form an emotional attachment) and mate [see 28] with the female, but without conception. After being placed in one of the reproductive conditions, all animals were weighed twice per week in order to monitor body condition and to detect pregnancy in the females from the breeding group. Body mass at the start of the experiment did not differ among the three groups of males (44.48 ± 1.29g, mean ± SEM; range = 30.00-60.02 g). Moreover, male age at the beginning of data collection did not differ significantly among groups (175.0 ± 2.1 days, range = 148-200 days). Data collection on first-time fathers occurred within the first 3 weeks following the birth of the pair’s first litter, and data collection in the other groups was time-matched to that in breeding males. UCR has full AAALAC accreditation, and all procedures were approved by the UCR IACUC and conducted in accordance with the Guide for the Care and Use of Laboratory Animals.
2.1 Experimental design
Data collection spanned 14 days for each mouse. Latency from formation of breeding pairs to birth of each pair’s first litter was 36.3 ± 0.7 days (range: 33-41 days), and litters contained an average of two pups (range: 1-3 pups); testing began when pups were 0-5 days old (2.2 ± 0.5 days). During the first week of data collection two blood samples were collected from each male for baseline CORT analysis, one at 0800h and one at 2000h to capture both the nadir and peak of the daily rhythm in baseline CORT levels, respectively [51]. Consecutive samples from each individual mouse were separated by two days, and the order of sample collection was approximately balanced across animals within each reproductive condition. One week after collection of the first baseline blood sample, mice were exposed to coyote urine (see below) for 5 min, and a blood sample was collected immediately following exposure. One week after predator-urine exposure (14 days after the initial baseline blood sample), each mouse underwent a combined DEX/CRH challenge (see below), after which it was euthanized by CO2 inhalation, and additional blood was collected by cardiac puncture for analysis of testosterone concentration. Right-side adrenal glands and testes were dissected out, placed in physiological saline, blotted three times on a paper towel, and weighed to the nearest 0.0001 g.
2.2 Tubal-ligation surgery
Female mice were tubally ligated using antiseptic techniques and standard surgical procedure. Briefly, mice were anesthetized with isoflurane gas, a ventral midline incision (approximately ½ cm) was made, the uterus was located and the ends of the right and left uterine horns were tied off using absorbable sutures (Monomend MT, Veterinary Products Laboratories, Phoenix, AZ). The oviducts were then located and severed using microscissors. All reproductive structures were repositioned back in the abdominal cavity, the abdominal incision was closed with absorbable sutures and the skin was sealed using tissue glue. Mice were given an injection of Ketoprofen (5 mg/kg, s.c.) to provide analgesia and allowed to recover in isolation for 7 days, after which time they were paired with a male for formation of non-breeding pairs. Upon termination of the experiment, tubally ligated females were sacrificed with CO2 and dissected to check for pregnancy; none of these females had visible embryos or fetuses at the time of sacrifice.
2.3 Blood-sample collection
Mice were anesthetized with isoflurane, and blood samples (70-140 μl) were collected from the retro-orbital sinus using heparinized glass microhematocrit tubes. Time from disturbance of the cage or end of the test to collection of the blood sample was less than 3 minutes, with one exception (67.44 ± 1.69 sec; range: 36-210). Blood samples were centrifuged for 12 min (13,300 rpm, 4°C), and plasma was collected and stored at -80°C until assay.
2.4 Corticosterone radioimmunoassay
Plasma was assayed in duplicate for corticosterone using an 125I double-antibody radioimmunoassay kit (#07-120102, MP Biomedicals, Costa Mesa, CA) that has been validated for this species [30]. The standard curve ranged from 12.5 ng/ml (91% bound) to 1000 ng/ml (20% bound), and inter- and intra-assay coefficients of variation (CVs) were 10.7% and 4.11%, respectively (N = 45 assays). Samples from each mouse were analyzed in the same assay run, and treatment conditions were balanced across assays to minimize assay-induced variation.
2.5 Testosterone enzyme immunoassay
Plasma concentrations of testosterone were measured at the Assay Services Laboratories at the Wisconsin National Primate Research Center (University of Wisconsin – Madison, WI, USA) using procedures validated for P. californicus [47]. Briefly, samples were extracted with ethyl ether, and steroids were separated using celite chromatography. Total testosterone was analyzed in duplicate using an enzyme immunoassay (T antibody R156, University of California, Davis diluted to 1:35,000). Assay sensitivity at 90% binding was 0.9 pg, and inter- and intra-assay coefficients of variation (CVs) were 15.5% and 3.9%, respectively (N = 54 assays).
2.6 Predator-odor exposure
Males were stressed alone without their adult cagemate or pups present. We chose to isolate males during predator-urine exposure for two reasons: 1) not all males had pups, and 2) the presence of pups has been shown to increase the response to a psychological stressor in rat dams [52]. Between 0800 and 0930h, males were removed from their home cage, placed into a new cage that contained clean bedding and no food or water, and taken to a testing chamber. A cotton ball soaked with 1ml of coyote urine (Maine Outdoor Solutions, Hermon, ME) was then placed in the corner of the cage for 5 min. Immediately after exposure, mice were blood sampled and then returned to their home cage. Predator-odor stress has been used in our lab previously [see 30 for details] and produces a robust CORT response in California mice at this time of day [51].
2.7 Dexamethasone and corticotropin-releasing hormone injections
On the day prior to the DEX/CRH challenge mice were weighed to permit calculation of accurate, body-mass-corrected hormone doses. On the last day of data collection (day 14) males were injected i.p. with 10 mg/kg dexamethasone sodium phosphate (DEX; 4mg/ml, American Regent, Inc., Shirley, NY) at 0730-0830h and then placed back into their home cage. This dose of DEX has previously been shown to suppress plasma CORT levels of male California mice at 8 h following injection [51].
On day 14, 8 h following DEX injection, each male was blood sampled, injected with CRH (C3042, Sigma Aldrich, St. Louis, MO; 4 ug/kg, i.p.) at 1530-1630h and then placed back into its home cage. Forty-five min after CRH injection each mouse was blood sampled and the animal was again returned to its home cage. Finally, 90 min after CRH injection, another blood sample was collected from the retro-orbital sinus, mice were euthanized by CO2 inhalation, and additional blood was collected by cardiac puncture for analysis of testosterone concentration. CRH was diluted in sterile water to a 1 ug/ml solution, and injection doses were based on male body mass. Dose was determined via pilot studies in our laboratory (data not shown), which indicated that this dose could successfully elevate CORT 8 h after DEX suppression.
2.8 Analysis
Data were checked for normality using the Shapiro-Wilk test and transformed if necessary. All CORT and testosterone values were log10-transformed prior to analysis, but non-transformed values are presented for ease of interpretation. CORT data were analyzed via ANOVA, and area under the curve was calculated in two ways to quantify total CORT release over time following DEX/CRH injection. The first, AUCg, represents the total amount of hormone produced over time with respect to a starting value of zero, thus not accounting for baseline (post-DEX) levels of circulating hormone. The second, AUCi, characterizes the sensitivity of the HPA axis to CRH by evaluating the amount of hormone produced above the starting baseline level (thus taking post-DEX CORT values into consideration) [53]. Associations between CORT and testosterone concentrations were evaluated using Pearson’s correlation. Body masses at the start of data collection and on the day prior to dissection were analyzed via repeated-measures ANOVA; age was analyzed via one-way ANOVA. Testis and adrenal masses were analyzed using ANCOVAs with day-13 body mass as a covariate, following the methods of Tomkins and Simmons [54], except that organ mass was not subtracted from body mass due to differences in the number of significant figures (mass was measured to the nearest 0.01 g and organs to the 0.0001 g).
3. Results
3.1 Basal CORT concentrations
All mice had higher circulating CORT concentrations at 2000h when compared to 0800h (1500.4 ± 81.8 vs. 35.6 ± 4.00 ng/ml, respectively; F1,33=1043.02, P<0.001; Fig. 1), but CORT levels did not differ significantly among the three groups (F2,33=0.07, P=0.993), nor was there a time*group interaction (F2,33=2.37, P=0.108). Additionally, a planned comparison between virgin males and fathers did not reveal an effect of reproductive condition on CORT concentrations at either 2000h (t22=1.36, P=0.094) or 0800h (t22=0.89, P=0.253; 1-tailed, independent-samples t-tests). For all animals analyzed together, baseline CORT levels at the two time points were not correlated (r= 0.133, n=36, P=0.439); thus, higher 0800h CORT values were not associated with higher 2000h CORT concentrations.
Figure 1.
Baseline levels of plasma CORT in adult, male California mice at 2000h and 0800h (14:10 L:D cycle; lights on at 0500h). Plasma CORT concentrations did not differ among virgin males, non-breeding males, and first-time fathers (n=12 per group) at either time point, but CORT was higher at 2000h than at 0800h regardless of reproductive condition (A vs. B; P<0.001).
3.2 CORT response to predator urine
Five-minute exposure to coyote urine elicited a significant increase in plasma CORT above time-matched baseline levels (425.80 ± 66.73 vs. 36.60 ± 4.00 ng/ml, respectively; F1,33=208.33, P<0.001; Fig. 2). However, reproductive status did not affect the CORT response to predator urine (F2,33=0.54, P=0.586), nor was there a status*time interaction (F2,33=0.59, P=0.560). Baseline CORT values at 0800 h were not correlated with post-stress values (r=0.284, n=36, P=0.093).
Figure 2.
Plasma CORT concentrations in adult, male California mice following 5-min exposure to 1 ml coyote urine between 0800 and 0930h. Virgin males, non-breeding males and first-time fathers (n=12 per group) did not differ in their response to predator urine, but predator-urine exposure did elevate CORT in all animals (main effect of time, A vs. B; P<0.001).
3.3 CORT response to DEX and CRH
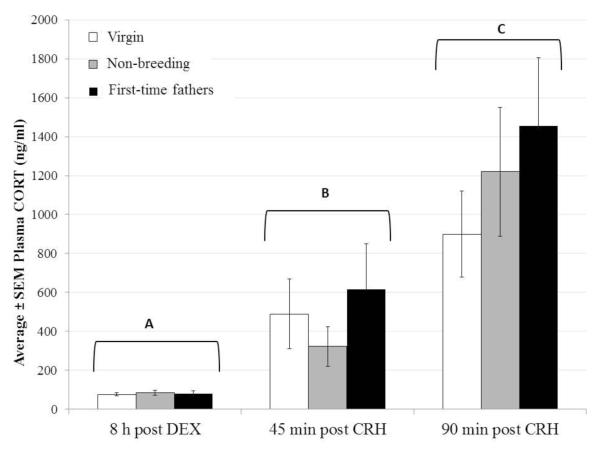
One post-DEX plasma sample was lost during processing. Therefore, data are available from 12 virgin males, 12 non-breeding males, and 11 first-time fathers. CORT levels at 1530-1630h, 8 h following DEX injection, did not differ among reproductive conditions (F2,34=0.05, P=0.948; Fig. 3). Subsequent CRH injection caused an increase in plasma CORT, as reflected in a significant main effect of time (F2,64=67.57, P<0.001), but CORT levels were not influenced by reproductive condition (F2,32=0.29, P=0.744), nor was there a time*reproductive condition interaction (F4,64=0.35, P=0.842). Sidak-corrected post-hoc tests revealed that CORT concentrations at both 45 and 90 min post-CRH were higher than those 8 h following DEX injection (t=5.79, P<0.001; t=10.47, P<0.001, respectively; Fig. 3). Additionally, plasma CORT was higher at 90 min than at 45 min post-CRH injection (t=6.73, P<0.001). Post-DEX CORT concentrations correlated positively and significantly with both post-CRH measures (45 min: r=0.478, n=35, P=0.004; 90 min: r=0.338, n=35, P=0.047); thus, mice that had higher CORT levels before CRH injection also had higher CORT levels after CRH injection. The two post-CRH CORT values were also positively correlated (r=0.749, n=36, P<0.001).
Figure 3.
Effects of dexamethasone (DEX; injected at 0730-0830 h) and corticotropin-releasing hormone (CRH; injected at 1530-1630 h, 8 h after DEX injection) on plasma CORT concentrations in adult, male California mice. Virgin males (n=12), non-breeding males (n=12) and first-time fathers (n =11) did not differ in circulating CORT concentration within any time point. Irrespective of group, plasma CORT levels changed over time; results for the main effect of time (P<0.001) are displayed (time points with different letters differed significantly from one another following Sidak-corrected post-hoc tests, P<0.001).
In addition to repeated-measures ANOVA, we analyzed time-integrated CORT responses to CRH using two calculations for area under the curve (AUC), as described above. Reproductive condition did not influence AUCg (F2,32=0.42, P=0.664) or AUCi (F2,31=0.23, P=0.795).
3.4 Testosterone
Data from five animals were omitted due to assay problems, leaving data from 31 males in the analysis (10 virgin males, 10 non-breeding males, 11 first-time fathers). Reproductive condition did not affect plasma testosterone concentrations measured 90 minutes after CRH injection (F2,28=0.18, P=0.838). Testosterone concentration averaged 409.06 ± 56.31 pg/ml with a range of 88.21-1203 pg/ml, consistent with testosterone concentrations previously reported for virgin male California mice around the same time of day [55]. Since neither testosterone nor CORT levels differed with reproductive condition, the post-injection log10 hormone concentrations were analyzed for all three conditions together. Testosterone concentrations at 90 min post-CRH were positively correlated with CORT concentrations at both the 45- and 90-min post-CRH time points (r=0.489, n=31, P=0.005; r=0.396, n=31, P=0.028; respectively), but not with post-DEX injection CORT levels prior to CRH injection (r=0.200, n=30, P=0.289).
3.5 Body mass
Body mass did not change significantly the day prior to the start of data collection to the day before dissection, 13 days after the first baseline blood sample (44.48 ± 1.29 vs. 44.76 ±1.43 g; F1,32=2.01, P=0.166). Additionally, reproductive condition did not influence body mass (F3,32=1.47, P=0.245), and there was no reproductive condition*time interaction (F2,32=1.58, P=0.221).
3.6 Testis and adrenal mass
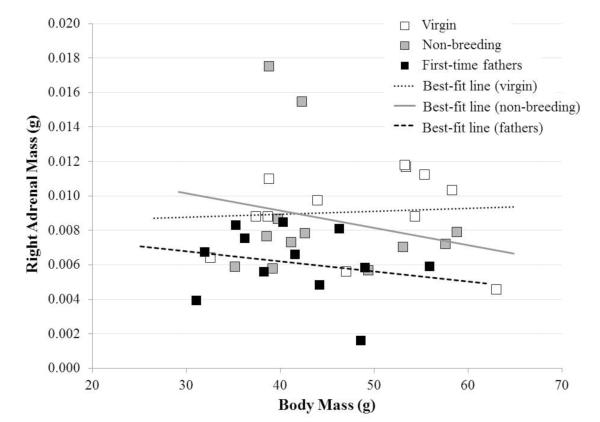
Body mass did not differ among groups on the day prior to dissection (44.76 ± 1.43 g, range 31.10-62.99 g; F2,33=2.28, P=0.119), and this variable was used as a covariate for organ-mass analyses. Adrenal mass data did not pass the Shapiro-Wilk test and were transformed by taking values to the 0.5 power. Initially, ANCOVAs were computed using organ mass, body mass, and the organ mass*body mass interaction. For both organs of interest the interaction term was not significant and was dropped from the model; body mass remained in the model as a covariate, even though it was not a significant term in either instance.
Adrenal mass differed significantly among reproductive conditions (F=4.70, P=0.016; Fig. 4). Pairwise comparisons showed that adrenal mass was lower in fathers than in both virgin (t=2.86, P=0.007) and non-breeding (t=2.50, P=0.024) males but did not differ between the latter two groups (P=0.522; Fisher’s LSD pairwise comparisons). Right testis mass did not differ significantly among the three reproductive conditions in a two-tailed test (F=2.92, P=0.068), but non-breeding males tended to have heavier testes than the other two reproductive conditions (non-breeding males: 0.216 ± 0.016; virgin males: 0.163 ± 0.019; first-time fathers: 0.175 ± 0.013 g).
Figure 4.
Right adrenal mass vs. body mass for adult, male California mice (n=12 per group). Body mass was used as a covariate but was not significant in the analysis model; for this reason the lines graphed above are best-fit lines and not ANCOVA lines. Adrenal glands from new fathers weighed less than did adrenals from non-breeding (P=0.024) and virgin males (P=0.007).
4. Discussion
This experiment addressed several predictions related to the behavior hypothesis of glucocorticoid regulation. We predicted that first-time California mouse fathers would show decreased amplitude of the CORT diurnal rhythm, a blunted CORT increase following exposure to an acute predator-odor stressor, increased sensitivity to glucocorticoid negative feedback, and/or a blunted CORT response to CRH, as compared to virgin males and non-breeding males. In addition, we predicted that first-time fathers would be more resistant to CORT-induced suppression of testosterone levels when compared to virgin or non-breeding males, as testosterone promotes paternal behavior in this species [47,48]. Contrary to our predictions, first-time fathers did not show any differences in baseline, post-stress, DEX-suppressed, or CRH-stimulated CORT concentrations when compared to non-breeding and virgin males; CORT levels of the three groups were statistically indistinguishable at each time point measured. Additionally, circulating levels of testosterone did not differ among groups, but testosterone values were positively correlated with post-CRH CORT values at both 45 and 90 min post-CRH injection. However, since we were not able to measure baseline testosterone concentrations we cannot be certain that virgin males, non-breeding males and first-time fathers had equivalent resting testosterone values. Therefore, despite the lack of difference in testosterone concentrations between the groups at 90 min post-CRH injection, we cannot determine the magnitude of change in the face of acute CORT elevation.
Although the three reproductive groups did not show differences in circulating hormone levels, differences in adrenal mass were detected: adrenal glands of breeding males weighed significantly less than those of non-breeding and virgin males. Previous studies of other rodents have noted differences in adrenal mass with reproductive condition and season, but results are mixed. Sexually active male mountain voles (Microtus montanus) had significantly smaller adrenal glands than sexually inactive males [56], whereas adrenal mass of male red-backed voles (Clethrionomys rutilus) remained relatively consistent throughout the year [57]. Conversely, adrenal glands of male pine voles (M. pinetorum) were larger during the reproductive period than during the nonbreeding season, and it was hypothesized that this increase was due to increased social stress during the breeding season [58]. The functional significance of changes in adrenal size is not entirely known, but adrenal size has been shown to correlate with circulating levels of GC in some species [for a brief review see 12]. However, despite a difference in adrenal mass, CORT levels did not differ between reproductive conditions in this study. Without histological analysis to determine which portion(s) of the adrenal gland (medulla and/or one or more of the three cortical layers) differs among reproductive groups, few conclusions can be drawn from adrenal-mass results at this time. Future studies could characterize adrenal histology or measure aldosterone and/or DHEA levels, as both of these hormones are produced in the adrenal cortex and have been suggested to change with parental status in males of biparental species [59,60].
The findings from our study of California mice suggest that the behavior hypothesis does not explain HPA function in this biparental rodent. Reproductive status does not appear to modulate circulating CORT concentrations or HPA-axis dynamics in males, and changes in CORT levels do not appear to be necessary for breeding or expression of parental behavior. This conclusion is further supported by a previous experiment showing that injecting new California mouse fathers with a supra-physiological dose of CORT does not reduce paternal care or influence pup survivorship [61]. Additionally, neither morning basal nor post-stressor (predator-urine exposure) CORT levels differed among first-time fathers, vasectomized males housed with a female, and virgin males housed with another male [30]. In the latter study, the fathers’ pups (and pairmate) were present when the fathers were exposed to predator odor. In other mammals, offspring presence either increases [rats; 52] or decreases [sheep; 62] the mother’s HPA response to an acute stressor. In the current study pups were not present during predator-urine stress, but there was no difference in post-urine-exposure CORT levels between virgin males, non-breeding males and first-time fathers. In sum, these previous and current findings suggest that paternal care in California mice is not likely to be mediated by GCs, and that male reproductive status does not alter basal or stress-induced CORT release or HPA dynamics.
Most of the data in support of the behavior hypothesis have come from a variety of bird species analyzed during the breeding season, and some of the most convincing data come from extreme environments, e.g. the Arctic [see 5,9,12]. A review of the mammalian literature, with particular focus on males, suggests that the behavior hypothesis does not seem to be as well supported as in birds. In biparental prairie voles [63], no differences in plasma CORT were found among virgin males, paired males, and fathers, mirroring our findings in California mice. Male striped mice (Rhabdomys pumilio) switch between three different reproductive tactics – philopatric (alloparental), roamers (not paternal), and breeders (paternal) – and longitudinal data show that baseline plasma CORT levels are higher during life history states that involve care of pups, contrary to the behavior hypothesis [64]. In biparental golden lion tamarins (Leontopithecus rosalia), fecal GC levels did not differ across male reproductive conditions or between the mating and infant-care seasons [65]. Similarly, in biparental common marmosets (Callithrix jacchus), plasma cortisol levels did not differ among males that were singly housed, pair-housed with a female, or family-housed with a mate and offspring [66].
These studies are consistent with our findings that reproduction is not associated with decreased CORT levels in California mice. However, a study on human males showed that fathers had lower salivary cortisol levels than did non-fathers [67]. Similarly, parenting-oriented (pairbonded or fathers) men had lower morning and evening salivary cortisol levels than did mating-oriented (non-pairbonded, non-fathers) men [68]. The majority of studies on male mammals have been correlational or have focused on changes in hormone levels either before, during, or immediately following a bout of paternal care. Additional studies are needed in order to provide a more comprehensive data set on the relationship between reproductive status and GC modulation in mammalian fathers.
The absence of detectable differences in CORT levels among male reproductive groups in California mice may be related to features of this species’ physiology and/or life history. First, despite the fact that we used a highly controlled experimental design, it is possible that our measure of HPA function was not specific enough. California mice have high levels of circulating GCs as compared to most mammals, and are somewhat resistant to GC negative feedback [51]. Corticosteroid receptors in this species might have low affinity for GCs, as reported in other species with high GC concentrations [prairie voles; 69; New World primates and guinea pigs (Cavia sp.); 70]. Thus, it is possible that corticosteroid receptors, and not plasma levels of CORT, are the main site of modulation of HPA function. Supra-physiological doses of CORT do not disrupt paternal behavior in California mice, and daily peak basal levels of CORT are higher than some post-stress concentrations [51,61], further suggesting that circulating CORT levels may not be the major site of GC-activity regulation. Additionally, corticosteroid-binding globulin (CBG) and/or 11β-hydroxysteroid dehydrogenase, the enzyme that converts CORT to its inactive form, might also play a role in modulating functional levels of CORT [71]. Analysis of receptor number and density, as well as CBG and 11βHSD activity, might provide illuminating results.
A second possible explanation involves the social organization of California mice. In nature, California mice mate for life, breed almost year-round, and are thus almost always in a pair-bonded situation with a single partner [25, 31-33]. Thus, pairing, and not birth of offspring, may trigger modulation of the HPA axis. All mice in our experiment were pair-housed with either a female or another male, but in the wild these animals do not naturally live in male-male pairs, and any dyad of wild mice is likely to be breeding and caring for young. It is possible that using any socially housed animal in the lab mimics the natural living conditions of a reproductive pair, thus making our treatment groups almost indistinguishable. Previous data on California mice lend support to this possibility. CRH and Fos expression in the paraventricular nucleus of the hypothalamus, both under baseline conditions and in response to a predator-odor stressor, did not differ between males that were pair-housed with either another male or a female (with or without pups), but did differ between these groups of pair-housed males and singly housed males [43]. Moreover, socially isolated males had higher baseline CORT levels than males paired with a female [29]. It could be that laboratory breeding has selected for males that form amicable male-male pairs, and that these males respond to being paired with another male in a similar manner as they would if the pairmate were female, at least with respect to HPA function.
Lastly, California mice are naturally found in areas with mild climates – the mountains of central and southern California – and can (and do) breed almost year-round [72,73]. Therefore, it is possible that these mice do not need to fine-tune the HPA response because they rarely experience periods of extreme conditions, at least in comparison to species breeding in very severe (e.g., polar or highly seasonal) climates. Support for this possibility has been found in birds; HPA modulation is more pronounced in species from harsh or extreme habitats as compared to their more temperately located relatives [9,12,74].
Highlights.
Reproductive condition can alter the stress response and glucocorticoid release
Tested the behavior hypothesis in males - virgins, non-breeding, first-time fathers
Groups did not differ in baseline, post-stress, post-DEX, or post-CRH CORT levels
Adrenal mass was lower in new fathers than in virgin or non-breeding males
The behavior hypothesis (need for parental behavior drives HPA change) - not supported
Acknowledgements
We would like to thank the UCR vivarium staff for their help in animal care. We also thank Vanessa Yang, Julia Cho, Omar Aldaas, Aaron Stamp, Dr. Trynke de Jong, Juan Pablo Perea-Rodriguez, Gavrielle Concepcion, and Dr. Miyetani Chauke for help with various aspects of experimental preparation and data collection, and Brian Gray, Dr. Kris Kaiser, Dr. Zach Hohman, and Dr. Mark Chappell for helpful comments on a previous draft of this manuscript. This work was supported by funds from the University of California, Riverside, and from NIH grant 1R21MH087806.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].McGrady AV. Effects of psychological stress on male reproduction: a review. Arch Androl. 1984;13:1–7. doi: 10.3109/01485018408987495. [DOI] [PubMed] [Google Scholar]
- [2].Moore FL, Miller LJ. Stress-induced inhibition of sexual behavior: Corticosterone inhibits courtship behavior of a male amphibian (Taricha granulosa) Horm Behav. 1984;18:400–410. doi: 10.1016/0018-506x(84)90026-6. [DOI] [PubMed] [Google Scholar]
- [3].Tilbrook AJ, Turner AI, Clarke IJ. Effects of stress on reproduction in non-rodent mammals: the role of glucocorticoids and sex differences. Rev Reprod. 2000;5:105–113. doi: 10.1530/ror.0.0050105. [DOI] [PubMed] [Google Scholar]
- [4].Sapolsky RM. Endocrinology of the Stress-Response. In: Becker JB, Breedlove SM, Crews D, McCarthy MM, editors. Behavioral Endocrinology. Bradford Book: Massachusetts Institute of Technology; Cambridge, Massachusetts: 2002. pp. 409–450. [Google Scholar]
- [5].Wingfield JC, Sapolsky RM. Reproduction and resistance to stress: when and how. J Neuroendocrinol. 2003;15:711–724. doi: 10.1046/j.1365-2826.2003.01033.x. [DOI] [PubMed] [Google Scholar]
- [6].Wilson BS, Wingfield JC. Correlation between females reproductive condition and plasma corticosterone in the lizard Uta stansburiana. Copeia. 1992;3:691–697. [Google Scholar]
- [7].Saltzman W, Schultz-Darken NJ, Scheffler G, Wegner FH, Abbott DH. Social and reproductive influences on plasma cortisol in female marmoset monkeys. Physiol Behav. 1994;56:801–810. doi: 10.1016/0031-9384(94)90246-1. [DOI] [PubMed] [Google Scholar]
- [8].Saltzman W, Schultz-Darken NJ, Wegner FH, Wittwer DJ, Abbott DH. Suppression of cortisol levels in subordinate female marmosets: Reproductive and social contributions. Horm Behav. 1998;33:58–74. doi: 10.1006/hbeh.1998.1436. [DOI] [PubMed] [Google Scholar]
- [9].Wingfield JC, Romero LM. Adrenocortical responses to stress and their modulation in free-living vertebrates. In: McEwen BS, Goodman HM, editors. Handbook of Physiology; Section 7: The Endocrine System. Volume IV: Coping with the Environment: Neural and Endocrine Mechanisms. Oxford Univ. Press; New York: 2001. pp. 211–234. [Google Scholar]
- [10].Creel S. Social dominance and stress hormones. Trends Ecol Evol. 2001;16:491–497. [Google Scholar]
- [11].Creel S. Dominance, aggression, and glucocorticoid levels in social carnivores. J Mammal. 2005;86:255–264. [Google Scholar]
- [12].Romero LM. Seasonal changes in plasma glucocorticoid concentrations in free-living vertebrates. Gen Comp Endocrinol. 2002;128:1–24. doi: 10.1016/s0016-6480(02)00064-3. [DOI] [PubMed] [Google Scholar]
- [13].Wingfield JC. Organization of vertebrate annual cycles: implications for control mechanisms. Phil Trans R Soc B. 2008;363:425–441. doi: 10.1098/rstb.2007.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Moore IT, Jessop TS. Stress, reproduction, and adrenocortical modulation in amphibians and reptiles. Horm Behav. 2003;43:39–47. doi: 10.1016/s0018-506x(02)00038-7. [DOI] [PubMed] [Google Scholar]
- [15].Silverin B. Corticosterone-binding proteins and behavioral effects of high levels of corticosterone during the breeding period in the pied flycatcher. Gen Comp Endocrinol. 1986;64:67–74. doi: 10.1016/0016-6480(86)90029-8. [DOI] [PubMed] [Google Scholar]
- [16].Silverin B. Behavioural and hormonal responses of the pied flycatcher to environmental stressors. Anim Behav. 1998;55:1411–1420. doi: 10.1006/anbe.1997.0717. [DOI] [PubMed] [Google Scholar]
- [17].Saltzman W, Abbott DH. Effects of elevated circulating cortisol concentrations on maternal behavior in common marmoset monkeys. Psychoneuroendocrinology. 2009;34:1222–1234. doi: 10.1016/j.psyneuen.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].O’Connor CM, Gilmour KM, Arlinghaus R, Van Der Kraak G, Cooke SJ. Stress and parental care in a wild teleost fish: Insights from exogenous supraphysiological cortisol implants. Physiol Biochem Zool. 2009;82:709–719. doi: 10.1086/605914. [DOI] [PubMed] [Google Scholar]
- [19].Wasser SK, Barash DP. Reproductive suppression among female mammals: Implications for biomedicine and sexual selection theory. Q Rev Biol. 1983;58:513–538. doi: 10.1086/413545. [DOI] [PubMed] [Google Scholar]
- [20].Lightman S, Windle R, Wood S, Kershaw Y, Shanks N, Ingram C. Peripartum plasticity within the hypothalamo-pituitary-adrenal axis. Prog Brain Res. 2001;133:111–129. doi: 10.1016/s0079-6123(01)33009-1. [DOI] [PubMed] [Google Scholar]
- [21].Numan M, Insel TR. The neurobiology of parental behavior. Springer-Verlag; New York: 2003. [Google Scholar]
- [22].Tu MT, Lupien SJ, Walker CD. Measuring stress responses in postpartum mothers: Perspectives from studies in human and animal populations. Stress. 2005;8:19–34. doi: 10.1080/10253890500103806. [DOI] [PubMed] [Google Scholar]
- [23].Brunton P, Russell J, Douglas A. Adaptive responses of the maternal hypothalamic-pituitary-adrenal axis during pregnancy and lactation. J Neuroendocrinol. 2008;20:764–776. doi: 10.1111/j.1365-2826.2008.01735.x. [DOI] [PubMed] [Google Scholar]
- [24].Kleiman DG, Malcolm J. The evolution of male parental investment in mammals. In: Gubernick DJ, Klopfer PH, editors. Parental care in mammals. Plenum Press; New York: 1981. pp. 347–387. [Google Scholar]
- [25].Gubernick DJ, Teferi T. Adaptive significance of male paternal care in a monogamous mammal. Proc Biol Sci. 2000;267:147–150. doi: 10.1098/rspb.2000.0979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Wright SL, Brown RE. The importance of paternal care on pup survival and pup growth in Peromyscus californicus when required to work for food. Behav Process. 2002;60:41–52. doi: 10.1016/s0376-6357(02)00101-8. [DOI] [PubMed] [Google Scholar]
- [27].Schradin C, Pillay N. The influence of the father on offspring development in the striped mouse. Behav Ecol. 2004;16:450–455. [Google Scholar]
- [28].Bosch OJ, Nair HP, Ahern TH, Neumann ID, Young LJ. The CRF System Mediates Increased Passive Stress-Coping Behavior Following the Loss of a Bonded Partner in a Monogamous Rodent. Neuropsychopharmacology. 2009;34:1406–1415. doi: 10.1038/npp.2008.154. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Glasper ER, DeVries AC. Social structure influences effects of pair-housing on wound healing. Brain Behav Immun. 2005;19:61–68. doi: 10.1016/j.bbi.2004.03.002. [DOI] [PubMed] [Google Scholar]
- [30].Chauke M, Malisch JL, Robinson C, de Jong TR, Saltzman W. Effects of reproductive status on behavioral and endocrine responses to acute stress in a biparental rodent, the California mouse (Peromyscus californicus) Horm Behav. 2011;60:128–138. doi: 10.1016/j.yhbeh.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ribble DO, Salvioni M. Social-organization and nest co-occupancy in Peromyscus californicus, a monogamous rodent. Behav Ecol Sociobiol. 1990;26:9–15. [Google Scholar]
- [32].Ribble DO. The monogamous mating system of Peromyscus californicus as revealed by DNA fingerprinting. Behav Ecol Sociobiol. 1991;29:161–166. [Google Scholar]
- [33].Ribble DO. Lifetime reproductive success and its correlates in the monogamous rodent, Peromyscus californicus. J Anim Ecol. 1992;61:457–468. [Google Scholar]
- [34].Gubernick DJ, Alberts JR. The biparental care system of the California mouse, Peromyscus californicus. J Comp Psychol. 1987;101:169–177. [PubMed] [Google Scholar]
- [35].Gubernick DJ, Wright SL, Brown RE. The significance of fathers presence for offspring survival in the monogamous California mouse, Peromyscus californicus. Anim Behav. 1993;46:539–546. [Google Scholar]
- [36].Cantoni D, Brown RE. Male influence on interbirth interval in the monogamous California mouse when required to forage for food. Ann N Y Acad Sci. 1997;807:486–489. doi: 10.1111/j.1749-6632.1997.tb51946.x. 1997. [DOI] [PubMed] [Google Scholar]
- [37].Cantoni D, Brown RE. Paternal investment and reproductive success in the California Mouse, Peromyscus californicus. Anim Behav. 1997;54:377–386. doi: 10.1006/anbe.1996.0583. [DOI] [PubMed] [Google Scholar]
- [38].Gubernick DJ, Schneider KA, Jeannotte LA. Individual differences in the mechanisms underlying the onset and maintenance of paternal behavior and the inhibition of infanticide in the monogamous biparental California mouse, Peromyscus californicus. Behav Ecol Sociobiol. 1994;34:225–231. [Google Scholar]
- [39].Trainor BC, Bird IM, Alday NA, Schlinger BA, Marler CA. Variation in aromatase activity in the medial preoptic area and plasma progesterone is associated with the onset of paternal behavior. Repro Neuroendocrinol. 2003;78:36–44. doi: 10.1159/000071704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].de Jong TR, Chauke M, Harris BN, Saltzman W. From here to paternity: neural correlates of the onset of paternal behavior in California mice (Peromyscus californicus) Horm Behav. 2009;56:220–231. doi: 10.1016/j.yhbeh.2009.05.001. [DOI] [PubMed] [Google Scholar]
- [41].de Jong TR, Measor KR, Chauke M, Harris BN, Saltzman W. Brief pup exposure induces Fos expression in the lateral habenula and serotonergic caudal dorsal raphe nucleus of paternally experienced male California mice (Peromyscus californicus) Neuroscience. 2010;169:1094–1104. doi: 10.1016/j.neuroscience.2010.06.012. [DOI] [PubMed] [Google Scholar]
- [42].de Jong TR, Korosi A, Harris BN, Perea-Rodriguez JP, Saltzman W. Individual variation in paternal responses of virgin California mice (Peromyscus californicus): behavioral and physiological correlates. Physiol Biochem Zool. doi: 10.1086/665831. In press. [DOI] [PubMed] [Google Scholar]
- [43].Chauke M, de Jong TR, Garland T, Jr., Saltzman W. Paternal responsiveness is associated with, but not mediated by reduced neophobia in male California mice (Peromyscus californicus) Physiol Behav. 2012;107:65–75. doi: 10.1016/j.physbeh.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Gubernick DJ, Nelson RJ. Prolactin and paternal behavior in the biparental California mouse, Peromyscus californicus. Horm Behav. 1989;23:203–210. doi: 10.1016/0018-506x(89)90061-5. [DOI] [PubMed] [Google Scholar]
- [45].Gubernick DJ, Winslow JT, Jensen P, Jeanotte L, Bowen J. Oxytocin changes in males over the reproductive cycle in the monogamous, biparental California mouse, Peromyscus californicus. Horm Behav. 1995;29:59–73. doi: 10.1006/hbeh.1995.1005. [DOI] [PubMed] [Google Scholar]
- [46].Lee AW, Brown RE. Comparison of medial preoptic, amygdala, and nucleus accumbens lesions on parental behavior in California mice (Peromyscus californicus) Physiol Behav. 2007;92:617–628. doi: 10.1016/j.physbeh.2007.05.008. [DOI] [PubMed] [Google Scholar]
- [47].Trainor BC, Marler CA. Testosterone, paternal behavior, and aggression in the monogamous California mouse (Peromyscus californicus) Horm Behav. 2001;40:32–42. doi: 10.1006/hbeh.2001.1652. [DOI] [PubMed] [Google Scholar]
- [48].Trainor BC, Marler CA. Testosterone promotes paternal behaviour in a monogamous mammal via conversion to oestrogen. Proc Biol Sci. 2002;269:823–829. doi: 10.1098/rspb.2001.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Waite E, Kershaw Y, Spiga F, Lightman SL. A glucocorticoid sensitive biphasic rhythm of testosterone secretion. J Neuroendocrinol. 2009;21:737–741. doi: 10.1111/j.1365-2826.2009.01900.x. [DOI] [PubMed] [Google Scholar]
- [50].Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Meth. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- [51].Harris BN, Saltzman W, de Jong TR, Milnes MR. Hypothalamic-pituitary-adrenal (HPA) axis function in the California mouse (Peromyscus californicus): Changes in baseline activity, reactivity, and fecal excretion of glucocorticoids across the diurnal cycle. Gen Comp Endocrinol. doi: 10.1016/j.ygcen.2012.08.026. In Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Deschamps S, Woodside B, Walker CD. Pups’ presence eliminates the stress hyporesponsiveness of early lactating females to a psychological stress representing a threat to the pups. J Neuroendocrinol. 2003;15:486–497. doi: 10.1046/j.1365-2826.2003.01022.x. [DOI] [PubMed] [Google Scholar]
- [53].Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- [54].Tomkins JL, Simmons LW. Measureing relative investment: a case study of testes investment in species with alternative male reproductive tactics. Anim Behav. 2002;63:1009–1016. [Google Scholar]
- [55].Oyegbile TO, Marler CA. Winning fights elevates testosterone levels in California mice and enhances future ability to win fights. Horm Behav. 2005;48:259–267. doi: 10.1016/j.yhbeh.2005.04.007. [DOI] [PubMed] [Google Scholar]
- [56].McKeever S. Effects of reproductive activity on the weight of adrenal glands in Microtus montanus. Anat Rec. 1959;135:1–5. [Google Scholar]
- [57].Sealander JA. Reproductive status and adrenal size in the northern red-backed vole in relation to season. Int J Biometero. 1967;11:213–220. doi: 10.1007/BF01426850. [DOI] [PubMed] [Google Scholar]
- [58].Valentine GL, Kirkpatrick RL. Seasonal changes in reproductive and related organs in the pine vole, Microtus pinetorum in Southwestern Virginia. J Mammal. 1970;51:553–560. [PubMed] [Google Scholar]
- [59].Schradin C, Wynne-Edwards KE, Timonin ME. Paternal care in rodents: Weakening support of hormonal regulation of the transition to behavioral fatherhood in rodent animal models of biparental care. Horm & Behav. 52:114–121. doi: 10.1016/j.yhbeh.2007.03.018. Comments to. 2007. [DOI] [PubMed] [Google Scholar]; Horm Behav. 2007;52:557–559. doi: 10.1016/j.yhbeh.2007.08.008. [DOI] [PubMed] [Google Scholar]
- [60].Bardi M, Franssen CL, Hampton JE, Shea EA, Fanean AP, Lambert KG. Paternal experience and stress responses in California mice (Peromyscus californicus) Comp Med. 2011;61:20–30. [PMC free article] [PubMed] [Google Scholar]
- [61].Harris BN, Perea-Rodriguez JP, Saltzman W. Acute effects of corticosterone injection on paternal behavior in California mouse (Peromyscus californicus) fathers. Horm Behav. 2011;60:666–675. doi: 10.1016/j.yhbeh.2011.09.001. [DOI] [PubMed] [Google Scholar]
- [62].Tilbrook AJ, Turner AI, Ibbott MD, Clarke IJ. Activation of the hypothalamao-pituitary-adrenal axis by isolation and restraint stress during lactation in ewes: effect of the presence of the lamb and suckling. Endocrinol. 2006;147:3501–3509. doi: 10.1210/en.2005-1632. [DOI] [PubMed] [Google Scholar]
- [63].Campbell JC, Laugero KD, van Westerhuyzen JA, Hostetler CM, Cohen JD, Bales KL. Costs of pair-bonding and paternal care in male prairie voles (Microtus ochrogaster) Physiol Behav. 2009;98:367–373. doi: 10.1016/j.physbeh.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Schradin C, Yuen C. Hormone levels of male African striped mice change as they switch between alternative reproductive tactics. Horm Behav. 2011;60:676–680. doi: 10.1016/j.yhbeh.2011.09.002. [DOI] [PubMed] [Google Scholar]
- [65].Bales KL, French JA, McWilliams J, Lake RA, Dietz JM. Effects of social status, age, and season on androgen and cortisol levels in wild male golden lion tamarins (Leontopithecus rosalia) Horm Behav. 2006;49:88–95. doi: 10.1016/j.yhbeh.2005.05.006. [DOI] [PubMed] [Google Scholar]
- [66].Ziegler TE, Schultz-Darken NJ, Scott JJ, Snowdon CT, Ferris CF. Neuroendocrine response to female ovulatory odors depends upon social condition in male common marmosets, Callithrix jacchus. Horm Behav. 2005;47:56–64. doi: 10.1016/j.yhbeh.2004.08.009. [DOI] [PubMed] [Google Scholar]
- [67].Berg SJ, Wynne-Edwards KE. Changes in testosterone, cortisol, and estradiol levels in men becoming fathers. Mayo Clin Proc. 2001;76:582–592. doi: 10.4065/76.6.582. [DOI] [PubMed] [Google Scholar]
- [68].Gettler LT, McDade TW, Kuzawa CW. Cortisol and testosterone in Filipino young adult men: evidence for co-regulation of both hormones by fatherhood and relationship status. Am J Hum Biol. 2011;23:609–620. doi: 10.1002/ajhb.21187. [DOI] [PubMed] [Google Scholar]
- [69].Taymans SE, DeVries AC, DeVries MB, Nelson RJ, Friedman TC, Castro M, Detera-Wadleigh S, Carter CS, Chrousos GP. The hypothalamic-pituitary-adrenal axis of Prairie Voles (Microtus ochrogaster): Evidence for target tissue glucocorticoid resistance. Gen Comp Endocrinol. 1997;106:48–61. doi: 10.1006/gcen.1996.6849. [DOI] [PubMed] [Google Scholar]
- [70].Fuller PJ, Smith BJ, Rogerson FM. Cortisol resistance in the New World revisited. TRENDS Endocrinol Metabo. 2004;15:296–299. doi: 10.1016/j.tem.2004.07.001. [DOI] [PubMed] [Google Scholar]
- [71].Michael AE, Thurston LM, Rae MT. Glucocorticoid metabolism and reproduction: a tale of two enzymes. Reproduction. 2003;126:425–441. doi: 10.1530/rep.0.1260425. [DOI] [PubMed] [Google Scholar]
- [72].Merritt JF. Peromyscus californicus. Mammalian Species. 1978;85:1–6. [Google Scholar]
- [73].Gubernick DJ. Reproduction in the California mouse, Peromyscus californicus. J. Mammal. 1988;69:857–860. [Google Scholar]
- [74].Wingfield JC. Modulation of the adrenocortical response to stress in birds. In: Davey KG, Peter RE, Tobe SS, editors. Perspectives in comparative endocrinology. National Research Council of Canada; Ottawa: 1994. pp. 520–528. [Google Scholar]