Abstract
Many studies have tested the consumption of foods and supplements to reduce exercise-induced muscle damage, but fasting itself is also worthy of investigation due to reports of beneficial effects of caloric restriction and/or intermittent fasting on inflammation and oxidative stress. This preliminary investigation compared indicators of exercise-induced muscle damage between upper-body untrained participants (N = 29, 22 yrs old (SD = 3.34), 12 women) who completed 8 hour water-only fasts or ate a controlled diet in the eight hours prior to five consecutive laboratory sessions. All sessions were conducted in the afternoon hours (i.e., post meridiem) and the women completed the first session while in the follicular phase of their menstrual cycles. Measures of muscle pain, resting elbow extension, upper arm girth, isometric strength, myoglobin (Mb), total nitric oxide (NO), interleukin 1beta (IL1b), and tumor necrosis factor alpha (TNFa) were collected before and after eccentric contractions of the non-dominant elbow flexors were completed. The fasting group’s loss of elbow extension was less than the post-prandial group (p < .05, eta2 = .10), but the groups did not change differently across time for any other outcome measures. However, significantly higher NO (p < .05, eta2 = .22) and lower TNFa (p < .001, eta2 = .53) were detected in the fasting group than the post-prandial group regardless of time. These results suggest intermittent fasting does not robustly inhibit the signs and symptoms of exercise-induced muscle damage, but such fasting may generally affect common indirect markers of muscle damage.
Keywords: delayed-onset muscle soreness, stretch injury
Introduction
A myriad of dietary interventions for exercise-induced muscle damage have been investigated. In these studies, participants are often consuming foods or supplements of interest or placebos during a controlled diet. However, the effects of fasting on exercise-induced muscle damage are worthy of investigation.
Increasing evidence supports a strong relationship between energy metabolism and immune function (Matarese and La Cava, 2004). For example, numerous studies have shown that a high-fat meal increases markers of inflammation and/or oxidative stress – even in healthy humans (Derosa et al., 2009; Nappo et al., 2002; Tsai et al., 2004). In contrast, overweight adult asthmatics reduction of caloric input on alternate days over eight weeks was associated with decreased oxidative stress and inflammatory markers (Johnson et al., 2007). Also, in healthy adults, two months of a one-meal per day diet induced peripheral blood mononuclear cells to produce lower levels of cytokines than a three-meal per day diet, but serum levels of inflammatory mediators were not differentially affected (Dixit et al., 2011). The results of these fasting studies are intriguing, but fasting interventions for months require participants with a high level of motivation. Accordingly, short-term fasting interventions would be more useful.
Unfortunately, studies of the effects of short-term fasting on inflammation and/or oxidative stress in humans are uncommon. Two studies of such short-term fasting in preparation for surgical procedures have produced mixed results. In one study, patients who consumed carbohydrate drinks while fasting from solid foods for 6 hours had a smaller decrease in post-cholecystecomy or inguinal herniorraphy C-reactive protein levels than patients who consumed only water for 6 hours (Perrone et al., 2011). In the other study, a 3-day calorie restricted diet followed by a 1-day fast was associated with lower numbers of circulating leukocytes and less tumor necrosis factor alpha (TNFa) production after kidney donation, but higher serum levels of interleukin-8 (van Ginhoven et al., 2011). Thus, studies of both short- and long-term fasting have detected beneficial effects on markers of inflammation and/or oxidative stress.
The objective of this preliminary study was to test the effects of a controlled diet and intermittent fasting on exercise-induced muscle damage. Based on the literature showing beneficial effects of fasting on markers of inflammation and oxidative status, we hypothesized that fasting for eight hours prior would produce lower indicators of muscle damage than eating a controlled diet eight hours prior. Support for or against the hypothesis will clarify the importance of controlling diets to reduce variability in studies of exercise-induced muscle damage, which has been noted by many researchers (Beaton et al., 2002; Chen, 2006; Gulbin and Gaffney, 2002; Hubal et al., 2007).
Methods
Experimental Approach to the Problem
The objective of this preliminary study was to test the effects of fasting and a controlled diet during the eight hours prior to data collection on each day of a 5-day investigation of exercise-induced muscle damage. We focused on the eight hours prior because such a duration is similar to the reviewed pre-surgical studies and an 8-hour fast could be easily administered by skipping breakfast and/or lunch. The typical signs and symptoms of exercise-induced muscle damage were measured along with circulating levels of muscle proteins, inflammation, and oxidative status before and after isokinetic eccentric contractions of the non-dominant elbow flexors were completed.
Participants
Participants (N = 29, 22 yrs old (SD = 3.34), 12 women) provided written informed consent to participate in a six-session protocol that was approved by the University of Missouri’s Health Science Institutional Review Board. Of these participants, 10 (24 yrs old (SD = 1.50), 5 women) were recruited for this preliminary study and assigned to the post-prandial group. The fasting group was composed of 19 participants (21 yrs old (SD = 5.09), 7 women), who had previously completed afternoon sessions after daily 8-hr water only fasts as part of an investigation of sex differences in muscle damage using the same methodology. The restrictions for participation were the following: (a) had not engaged in upper body strength training on a regular basis (i.e., two times per week) for consecutive weeks within the previous six months, (b) were not currently experiencing arm pain, (c) had no history of upper arm injury within the previous six months, and (d) no chronic pain conditions. In addition, participants were screened for potential risk factors to the exercise protocol (e.g., excessive swelling, loss of range or motion, exertional rhabdomyolysis). Furthermore, participants were restricted from the following behaviors: smoking 3 hours prior to a session, caffeine for 3 hours prior to a session, and performing self-care behaviors for musculoskeletal pain (e.g., taking analgesics) throughout the study period. Only one of the 5 women in the fasting group and only one of the 7 women in the post-prandial group were consuming oral contraceptives.
Procedures
A familiarization session was held to determine the participants’ heights and weights and to acclimate the participants to the sensory tests and arm girth and elbow joint angle measures. After the familiarization session, participants visited the laboratory during afternoon hours (i.e., post meridiem) for five consecutive days. The participants in the fasting group consumed only water for eight hours before each of the five sessions. The participants in the post-prandial group consumed only a supplied meal at 4–5 hours before each of the five sessions. The supplied meal consisted of 810–860 kcal, 250–320 fat kcal, 28–35g fat, 10–13g sat fat, 40–45mg cholesterol, 6–7g fiber, 101–104g carbohydrate, and 32–34g protein, which reflects a typical lunch for males between the ages of 20–29 years of age (U.S. Department of Agriculture et al., 2010). Also, the first session was scheduled when women were in the follicular phase of their menstrual cycles (Cole et al., 2009).
At the beginning of the first session, participants were seated and asked to complete questionnaires regarding their adherence to the study restrictions. Next, non-dominant upper arm muscle pain during rest and movement and pressure pain thresholds were collected. Then, after participants had been seated for about 15 min, 10 mL of blood was collected from venous access on the dominant arm. Subsequently, non-dominant upper arm girth and resting elbow extension were measured before participants were positioned in a muscle testing apparatus (Biodex System 3; Biodex Medical Systems, Shirley, NY). While seated in the muscle testing apparatus, a 5-repetition maximal isometric strength test was completed and ratings of perceived exertion and muscle pain during the test were collected.
Following the isometric strength test, an eccentric strength test was completed. Next, the participants performed 3 sets of 12 maximal eccentric contractions to induce temporary muscle damage. All of the eccentric contractions were completed at a velocity of 90°/s through the participants’ active range of motion with a rest period of 60 s in between each set.
During the next hour, participants were instructed to continue their adherence to the pre-session restrictions, but they were allowed to sit quietly outside the laboratory if they desired. After the 1-hr delay, arm muscle pain, pressure pain thresholds, arm girth, resting elbow extension, and 10 mL of blood were collected again in the same manner as before the eccentric exercise. Finally, the session was terminated and participants were reminded of the schedule and restrictions for the subsequent sessions, which included avoiding any self-care behaviors for musculoskeletal pain (e.g., ice or heat application, stretching, massage, etc.).
Participants returned to the laboratory at one, two, three, and four days after the eccentric exercise with all of these follow-up sessions held in the afternoon hours. During the follow-up sessions, participants’ adherence to the study restrictions were checked by questionnaire again and arm muscle pain, pressure pain thresholds, arm girth, resting elbow extension, and 10 mL of blood were collected again.
Measures
Height and weight
The height and weight of participants were measured using an upright platform and balance beam scale (Health O Meter Professional; Sunbeam Products, Inc.; Boca Raton, FL). Based upon the ratio of height and weight, body mass index (BMI; kg/m2) was calculated.
Exercise Behavior
The exercise behavior of participants during a typical week was assessed by the Leisure-Time Exercise Questionnaire (LTEQ)(Godin and Shepard, 1985). The LTEQ is composed of three items that assess the frequency of performing strenuous, moderate, and mild exercise during leisure-time. The correlation between the LTEQ’s total weighted score and maximal oxygen intake was r = .56 (p < .05), which was the highest correlation among 10 other self-report measures of exercise behavior (Jacobs et al., 1993).
Numeric muscle pain ratings
Ratings of non-dominant arm muscle pain intensity were assessed before and after the eccentric exercise with 0–100 numeric scales. More specifically, these ratings were collected while the non-dominant arm was (1) stationary at approximately 90° of elbow flexion, (2) moving through active range of motion without applied load, and (3) completing the isometric strength test. The anchors of the pain intensity rating scale were “no pain” and “most intense pain sensation imaginable.” The anchors of the pain unpleasantness rating scale were “no unpleasantness” and “most unpleasant imaginable.” Numeric pain scales have been found to be reliable and valid (Jensen and Karoly, 2001).
Pressure Pain Threshold
Pressure pain threshold (PPT) was defined as the point at which a pressure stimulus first became painful. The pressure stimulus was applied on the belly of the biceps brachii at 25% of the distance from the cubital fossa to the greater tuberosity of the humerus while the non-dominant arm was stationary at approximately 90° of elbow flexion. Using a hand-held 10 kg dolorimeter with a 1 cm rubber tip (Pain Diagnostics Inc., Great Neck, NY), pressure was increased at a rate of about 1 kg/s until participants first reported feeling pain. The average of two repeated measurements was analyzed.
Upper arm girth
In order to measure swelling of the non-dominant upper arm, girth was measured at 5 cm and 10 cm proximal from the olecranon of the ulna with a Gulick tape measure (Creative Health Products, Inc., Plymouth, MI) while participants stood with their elbow extended and shoulder flexed and abducted to 90°. The measurements from the two sites were averaged.
Resting elbow extension
In order to measure joint angle of the non-dominant arm, participants were instructed to stand and let their arm hang at their sides with their forearm fully supinated. Elbow extension was measured using a standard 12″ goniometer that was positioned with the stationary arm proximal along the humerus, the center of the goniometer on the elbow joint space, and the moving arm distal along the radius.
Elbow flexor strength
The isometric and eccentric strength of the non-dominant elbow flexors were determined using the Biodex System 3 (Biodex Medical Systems, Inc., Shirley, NY). Isometric strength was defined as the maximum peak force achieved during five maximal repetitions at 90°of elbow flexion. Isometric strength is typically interpreted as an indicator of muscle damage (Warren et al., 1999). Eccentric strength was defined as the maximum peak torque achieved during five maximal repetitions at 90°/s through AROM. Eccentric strength was measured to interpret the average peak torque produced during the eccentric exercise for inducing muscle damage.
Ratings of perceived exertion (RPE)
The amount of effort that participants exerted during isometric and eccentric contractions was measured using a 0–10 category ratio scale, which was previously reported to relate to blood and muscle lactates and heart rate (Noble et al., 1983).
Serum measures
The 10mL blood samples were collected from venous access on the dominant arm (i.e., the arm that did not complete the eccentric exercise). The samples were allowed to clot at room temperature for 30 min before centrifugation at 1600 g for 30 min. Then the serum was stored at −80° C until analysis. Myoglobin (Mb), tumor necrosis factor alpha (TNFa), interleukin 1beta (IL1b), and total Nitric Oxide (NO) were measured by enzyme linked immunosorbent assay (ELISA) using commercially available kits (Mb: BioCheck, Inc., Foster City, CA; TNFa and IL1b: QuantiGlo, R&D Systems, Inc., Minneapolis, MN; NO: Assay Designs, Inc., Ann Arbor, MI) according to the manufacturer’s instructions. Samples were analyzed in duplicates. According to the manufacturers, the minimal detection limits of the assays were .005 μg/mL, 0.391 pg/mL, 0.160 pg/mL, and 0.625 μmole/L for Mb, TNFa, IL1b, and NO, respectively. The intra-assay coefficients of variation (CV) were as follows: 7.7%–8.3% for Mb, 4.6%–8.4% for TNFa, 13.3%–14.3% for IL1b, and 3.0%–6.4% for NO.
Statistical Analyses
Group differences in baseline characteristics (age, BMI, and exercise behavior) and average eccentric exercise parameters across sets (percentage of eccentric strength produced, elbow extension, and ratings of perceived exertion) were checked with independent t-tests. Group differences in muscle damage indicators across time were tested by conducting repeated mixed-model analyses of variance (ANOVAs) with the factors of GROUP (fasting and post-prandial) and TIME (pre-eccentrics, 1-hr post-eccentrics, 1 day, 2 days, 3 days, 4 days). The dependent variables were arm muscle pain ratings, PPTs, upper arm girth, elbow resting extension, isometric strength, Mb, TNFa, IL1b, and NO. Significant 2-way interactions were followed up with independent t-tests for GROUP differences at each time point.
All analyses were conducted using SPSS software (SPSS, Inc., Chicago, IL) with Greenhouse-Geisser correction of degrees of freedom to adjust for violations of sphericity. Statistical significance was defined as p < .05 and the meaningfulness of results was determined by calculated effect sizes (i.e., eta squared and Cohen’s d). Eta squared (η 2) values of .01, .06, and .14 are typically interpreted as thresholds for small, medium, and large effect sizes, respectively. Cohen’s d values of .20, .50, and .80 are typically interpreted as thresholds for small, medium, and large effect sizes, respectively (Cohen, 1988).
Results
Baseline and Eccentric Exercise Characteristics
The age, BMI, and exercise behavior of the groups were similar (p = .17, d = .76; p = .87, d = .07; and p = .53, d = .26, respectively). Also, the average percentage of eccentric strength produced and elbow range of motion during the eccentric exercise were comparable for both groups (p = .81, d = .13 and p = .09, d = .57, respectively). However, the average perceived exertion during the eccentric exercise tended to be lower for the fasting group than the post-prandial group (p = .05, d = .75). (See Table 1.)
Table 1.
Means (M) and standard deviations (SD) for the groups’ baseline characteristics.
| Variable | Fasting (n = 10) | Post-prandial (n = 19) | ||
|---|---|---|---|---|
| M | SD | M | SD | |
| Age (years) | 21.47 | 1.50 | 23.90 | 5.09 |
| BMI | 24.79 | 4.15 | 25.52 | 4.10 |
| Leisure-Time Exercise Behavior (total) | 30.53 | 18.17 | 25.40 | 21.52 |
| Average percentage of eccentric strength during eccentric exercise | 70.83 | 8.79 | 71.56 | 6.87 |
| Average range of motion during eccentric exercise (°) | 126.85 | 12.25 | 120.75 | 6.20 |
| Average exertion during eccentric exercise | 9.13 | 1.38 | 10.10 | 1.07 |
Muscle Pain Ratings and PPTs
Arm muscle pain ratings at rest and with movement increased significantly after eccentric exercise (p < .01, η 2 = .19 and p < .01, η 2 = .46, respectively) while PPTs decreased (p < .01, η 2 = .17), which indicated the induction of muscle damage. However, the ratings did not change differently between the groups (i.e., no significant GROUP by TIME interactions). Also, the arm muscle pain ratings during isometric contractions did not change after eccentric exercise. Additionally, when all the measurement time points were collapsed, no group differences were detected (i.e., no significant main effect of GROUP) for any of the pain measures. (See Table 2.)
Table 2.
Means (M) and standard deviations (SD) for the dependent variables within each group at each time point.
| Variable | Session 1 - Pre | Session 1 - Post | Session 2 | Session 3 | Session 4 | Session 5 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | M | SD | M | SD | M | SD | |
| Fasting Group | ||||||||||||
| Pain Intensity | ||||||||||||
| resting (0–100 scale) | 0.00 | 0.00 | 1.05 | 1.84 | 5.78 | 7.43 | 12.11 | 17.03 | 9.58 | 15.64 | 6.26 | 9.44 |
| moving (0–100 scale) | 0.00 | 0.00 | 5.00 | 11.18 | 20.47 | 15.80 | 29.05 | 22.74 | 25.68 | 23.41 | 19.79 | 19.77 |
| isometrics (0–100 scale) | 4.56 | 7.50 | 4.42 | 6.49 | 10.38 | 14.55 | 15.17 | 17.66 | 15.42 | 18.04 | 15.67 | 19.18 |
| PPTs (kg) | 2.25 | 0.63 | 2.32 | 0.68 | 1.98 | 0.81 | 1.95 | 0.79 | 2.09 | 0.74 | 2.29 | 0.86 |
| Upper arm girth (cm) | 28.20 | 2.76 | 28.45 | 2.72 | 28.59 | 2.66 | 28.73 | 2.61 | 28.95 | 2.59 | 29.03 | 2.58 |
| Isometric strength (Nm) | 52.81 | 17.57 | 41.47 | 16.21 | 37.79 | 15.89 | 38.95 | 17.67 | 37.87 | 15.26 | 40.35 | 16.40 |
| Mb (ng/mL) | 19.12 | 6.24 | 25.40 | 9.84 | 56.20 | 91.11 | 113.36 | 338.58 | 179.91 | 282.60 | 162.93 | 247.23 |
| IL1b (pg/mL) | 0.48 | 1.22 | 0.49 | 1.38 | 0.58 | 1.67 | 0.56 | 1.66 | 0.46 | 1.03 | 0.59 | 1.89 |
| Post-prandial Group | ||||||||||||
| Pain Intensity | ||||||||||||
| resting (0–100 scale) | 0.00 | 0.00 | 3.20 | 3.29 | 12.10 | 20.89 | 10.00 | 13.12 | 4.90 | 3.54 | 4.55 | 7.89 |
| moving (0–100 scale) | 0.20 | 0.63 | 8.50 | 7.00 | 25.60 | 22.25 | 27.00 | 15.13 | 22.30 | 14.03 | 14.90 | 11.50 |
| isometrics (0–100 scale) | 13.80 | 13.25 | 11.00 | 13.17 | 15.10 | 12.39 | 15.70 | 9.15 | 16.95 | 13.02 | 11.89 | 9.73 |
| PPTs (kg) | 2.43 | 0.83 | 2.23 | 0.63 | 2.05 | 0.84 | 2.01 | 0.90 | 1.90 | 0.83 | 2.03 | 0.82 |
| Upper arm girth (cm) | 28.00 | 3.90 | 28.29 | 4.01 | 28.59 | 4.10 | 28.78 | 4.21 | 28.84 | 4.26 | 28.63 | 4.26 |
| Isometric strength (Nm) | 53.92 | 26.22 | 43.62 | 20.16 | 42.39 | 17.74 | 40.48 | 16.34 | 40.98 | 16.74 | 43.94 | 18.90 |
| Mb (ng/mL) | 24.58 | 7.14 | 31.96 | 12.89 | 101.40 | 244.29 | 302.82 | 843.32 | 312.56 | 648.05 | 260.39 | 414.57 |
| IL1b (pg/mL) | 0.35 | 0.16 | 0.36 | 0.16 | 0.38 | 0.12 | 0.39 | 0.18 | 0.43 | 0.21 | 0.44 | 0.17 |
Note. Significant (p < .05) changes across time (i.e., main effects of TIME) are noted in bold.
The only GROUP × TIME interaction was found for resting arm joint angle
Arm Girth, Resting Joint angle, and Isometric Strength
Upper arm girth increased significantly (p < .01, η 2 = .31) while arm extension and isometric strength both decreased significantly across time (p < .01, η 2 = .16 and p < .01, η 2 = .31, respectively), which indicated the induction of muscle damage. However, these measures did not change differently between the groups. (See Table 2.) In contrast, the loss of elbow extension peaked significantly more slowly for the fasting group than the post-prandial group (p < .05, η 2 = .10). (See Figure 1.) When all the measurement time points were collapsed, no group differences were detected for any of these measures. (See Table 2.)
Figure 1.
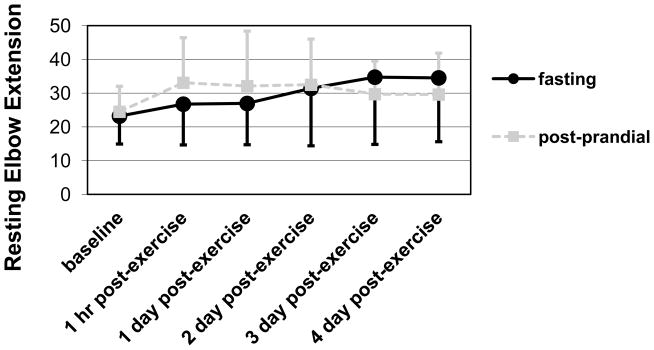
Means and standard deviations for resting joint angle showing how the loss of elbow extension peaked significantly more slowly for the fasting group than the post-prandial group (GROUP by TIME interaction; p < .05, η 2 = .10).
Mb, TNFa, IL1b, and NO
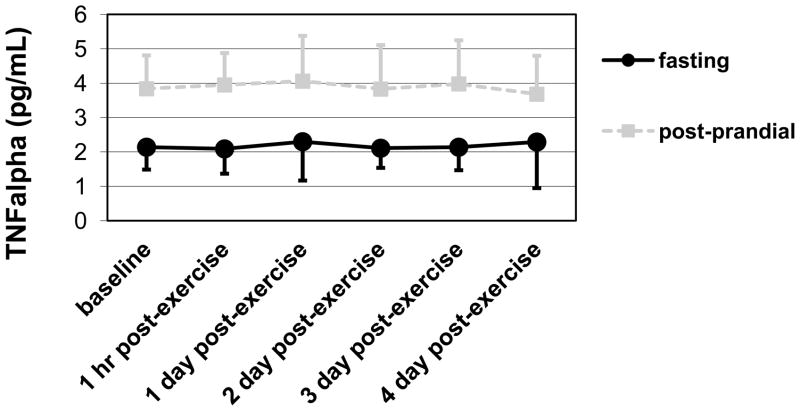
Mb increased significantly across time (p < .05, η 2 = .13), which indicated induction of muscle damage. However, the change was not different between the two groups. No markers of inflammation or oxidative stress increased across time. (See Table 2.) However, the fasting group had higher NO and lower TNFa levels than the post-prandial group regardless of time (p = .01, η 2 = .22 and p < .01, η 2 = .53, respectively). (See Figures 2 and 3.)
Figure 2.
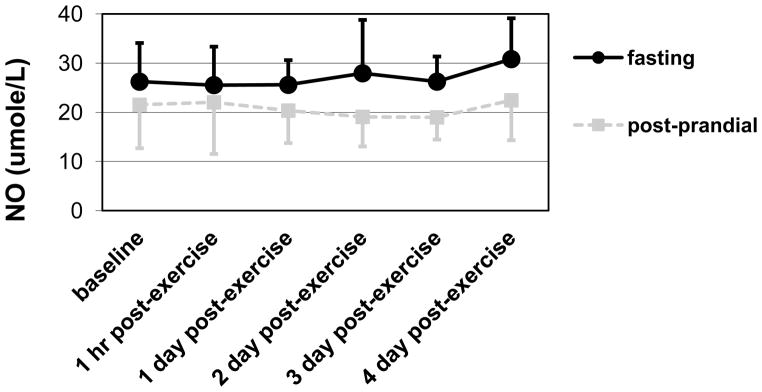
Means and standard deviations for NO showing higher levels in the fasting group than the post-prandial group regardless of time (main effect of GROUP; p = .01, η2 = .22).
Figure 3.
Means and standard deviations for TNFa showing lower levels in the fasting group than the post-prandial group regardless of time (main effect of GROUP; p < .01, η 2 = .53).
Discussion
In this study, we found increased ratings of arm muscle pain, arm girth, and Mb levels and decreased PPTs, resting joint angle, and strength, which supported the successful induction of muscle damage after the eccentric exercise. The absence of significant change in markers of inflammation and oxidative stress after the eccentric exercise was unexpected, but similar findings have been reported by previous studies of eccentric resistance exercise by the elbow flexors (Hirose et al., 2004; Nosaka and Clarkson, 1996). For example, Nosaka and Clarkson detected post-eccentric exercise changes in arm circumference, arm imaging using ultrasonography and magnetic resonance imaging, maximal isometric force, range of motion, muscle soreness, and plasma creatine kinase, aspartate aminotransferase, and lactate dehydrogenase, but no significant changes in plasma levels of interleukin 1 alpha, IL1b, interleukin 2, interleukin 6, TNFa, and C-reactive protein (Nosaka and Clarkson, 1996). The lack of observed change in inflammation and oxidative stress in our study may have just been due to inadequately strenuous eccentric exercise in a small muscle group.
We hypothesized that fasting for eight hours during five consecutive days would protect participants from the sequelae of eccentric exercise. In fact, we found the loss of elbow extension peaked significantly more slowly for the fasting group than the post-prandial group as if fasting had a protective effect. However, the groups did not change differently across time for any other outcome measures. Thus, it appears that fasting did not offer robust protection from exercise-induced muscle damage.
Another important finding was the higher NO and lower TNFa levels in the fasting group than the post-prandial group. These results resemble the effects of short-term fasting on inflammation in surgery patients (Perrone et al., 2011; van Ginhoven et al., in press) and suggest the nutritional intake of the two groups was indeed sufficiently different for lower inflammation and oxidative stress regardless of the eccentric exercise. The fasting may have produced hypometabolism (Bursztein et al., 1980), which increased NO (Aliev et al., 2011), and then NO acted in a pro-apoptotic manner to reduce TNFa (Maskrey et al., 2011). Such results suggest future investigations of these markers in humans should control for participants’ eating behavior during the eight hours prior to measurements. For example, skipping breakfast after an overnight fast might be influential. However, because we focused on changes from exercise-induced muscle damage, no markers were measured before the fasting was initiated so it remains possible the groups were inherently different at baseline for some unknown reason.
Several limitations of this preliminary study’s methodology may have affected the results. Due to our focus on the eight hours proceeding measurements, we did not monitor the total caloric intake and hydration of the participants throughout the study. However, it is noteworthy that the few studies of the effects of dehydration on the sequelae of eccentric exercise have supported that dehydration only has negative effects when the exercise is completed in hot and humid environments (Cleary et al., 2006; Cleary et al., 2005). Also, the meal we supplied to participants was not individualized based on participants’ age, sex, or size. In addition, participants’ typical diet was not assessed to evaluate potential psychological and placebo/nocebo effects of fasting. Plus the 3-hour caffeine and smoking restrictions may need to be extended in future studies, but it is noteworthy that the restrictions were actually for 8 hours in the water-only fasting group due to the fast. Furthermore, our use of previously collected data from fasting participants was not as methodologically sound as random assignment and we checked participants’ adherence to the dietary restrictions by questionnaire instead of measuring blood levels of glucose. Finally, these results are likely to be specific to the eccentric exercise protocol performed and the duration and/or frequency of the daily fasts. Beginning the fasts for several days before the eccentric exercise in a subsequent study might yield different results.
With consideration of these limitations, our preliminary results suggest that intermittent fasting does not inhibit the signs and symptoms of exercise-induced muscle damage. However, eight hour water-only fasts on five consecutive days may generally affect inflammation and oxidative stress. Investigators interested in dietary interventions for the sequelae of muscle damaging exercise should consider pursuing additional studies of fasting based on these findings and the issues raised here. A large volume of research has supported effects of caloric restriction and/or intermittent fasting on inflammation and oxidative stress (Dirks and Leeuwenburgh, 2006; Martin et al., 2006; Mattson and Wan, 2005), but intermittent fasting protocols are probably more tolerable for the general population and such protocols could still enable people to maintain their necessary nutritional intake.
Highlights.
We tested effects of fasting on exercise-induced muscle damage in humans.
Daily 8-hour fasts were compared to a controlled meal within 4–5 hours of visit.
Indicators of damage were collected before and across five days after exercise.
The fasts did not robustly protect against exercise-induced muscle damage.
However, NO was higher and TNFa was lower in the fasting group across time.
Acknowledgments
Grants: Support was provided by grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (1KO1 AR050146-01A1) and the University of Missouri (Research Council) to Dr. Erin A Dannecker.
The authors would like to thank all the participants who volunteered for this investigation.
Footnotes
Author contributions: The principal investigator was Erin Alice Dannecker, PhD. Ying Liu, MD and R. Scott Rector, PhD were essential to the analysis and interpretation of all blood samples and constructively revising this manuscript. Tom R. Thomas, PhD, Stephen P. Sayers, PhD, Christiian Leeuwenburgh, PhD, and Bimal K. Ray, PhD were involved in the conception and design of the investigation and constructively revising this manuscript.
Conflicts of interest: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Matarese G, La Cava A. The intricate interface between immune system and metabolism. Trends Immunol. 2004;25:193–200. doi: 10.1016/j.it.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Derosa G, Ferrari I, D’Angela A, Salvadeo SA, Fogari E, Gravina A, Mereu R, Palumbo I, Maffioli P, Randazzo S, Cicero AF. Oral fat load effects on inflammation and endothelial stress markers in healthy subjects. Heart Vessels. 2009;24:204–210. doi: 10.1007/s00380-008-1109-y. [DOI] [PubMed] [Google Scholar]
- Nappo F, Esposito K, Cioffi M, Giugliano G, Molinari AM, Paolisso G, Marfella R, Giugliano D. Postprandial endothelial activation in healthy subjects and in type 2 diabetic patients: role of fat and carbohydrate meals. J Am Coll Cardiol. 2002;39:1145–1150. doi: 10.1016/s0735-1097(02)01741-2. [DOI] [PubMed] [Google Scholar]
- Tsai WC, Li YH, Lin CC, Chao TH, Chen JH. Effects of oxidative stress on endothelial function after a high-fat meal. Clin Sci. 2004;106:315–319. doi: 10.1042/CS20030227. [DOI] [PubMed] [Google Scholar]
- Johnson JB, Summer W, Cutler RG, Martin B, Hyun DH, Dixit VD, Pearson M, Nassar M, Telljohann R, Maudsley S, Carlson O, John S, Laub DR, Mattson MP. Alternate day calorie restriction improves clinical findings and reduces markers of oxidative stress and inflammation in overweight adults with moderate asthma. Free Radical Bio Med. 2007;42:665–674. doi: 10.1016/j.freeradbiomed.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixit VD, Yang H, Sayeed KS, Stote KS, Rumpler WV, Baer DJ, Longo DL, Mattson MP, Taub DD. Controlled meal frequency without caloric restriction alters peripheral blood mononuclear cell cytokine production. J Inflamm. 2011;8:6. doi: 10.1186/1476-9255-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrone F, da-Silva-Filho AC, Adôrno IF, Anabuki NT, Leal FS, Colombo T, da Silva BD, Dock-Nascimento DB, Damião A, de Aguilar-Nascimento JE. Effects of preoperative feeding with a whey protein plus carbohydrate drink on the acute phase response and insulin resistance. A randomized trial. Nutr J. 2011;10:66. doi: 10.1186/1475-2891-10-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ginhoven TM, Dik WA, Mitchell JR, Smits-Te Nijenhuis MA, van Holten-Neelen C, Hooijkaas H, Hoeijmakers JH, de Bruin RW, Ijzermans JN. Dietary restriction modifies certain aspects of the postoperative acute phase response. J Surg Res. 2011;171:582–589. doi: 10.1016/j.jss.2010.03.038. [DOI] [PubMed] [Google Scholar]
- Beaton LJ, Tarnopolsky MA, Phillips SM. Variability in estimating eccentric contraction-induced muscle damage and inflammation in humans. Can J Appl Physiol. 2002;27:516–526. doi: 10.1139/h02-028. [DOI] [PubMed] [Google Scholar]
- Chen TC. Variability in muscle damage after eccentric exercise and the repeated bout effect. Res Q Exerc Sport. 2006;77:362–371. doi: 10.1080/02701367.2006.10599370. [DOI] [PubMed] [Google Scholar]
- Gulbin JP, Gaffney PT. Identical twins are discordant for markers of eccentric exercise-induced muscle damage. Int J Sports Med. 2002;23:471–476. doi: 10.1055/s-2002-35076. [DOI] [PubMed] [Google Scholar]
- Hubal MJ, Rubinstein SR, Clarkson PM. Mechanisms of variability in strength loss after muscle-lengthening actions. Med Sci Sport Exer. 2007;39:461–468. doi: 10.1249/01.mss.0000247007.19127.da. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Agriculture, Agricultural Research Service, Beltsville Human Nutrition Research Center, Food Surveys Research Group, U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, & National Center for Health Statistics. What We Eat in America, NHANES 2007–2008 Data. Food Surveys Products and Services; Hyattsville, MD: 2010. [Google Scholar]
- Cole LA, Ladner DG, Byrn FW. The normal variabilities of the menstrual cycle. Fertil Steril. 2009;91:522–527. doi: 10.1016/j.fertnstert.2007.11.073. [DOI] [PubMed] [Google Scholar]
- Godin G, Shepard RJ. A simple method to assess exercise behavior in the community. Can J Appl Sport Sci. 1985;10:141–146. [PubMed] [Google Scholar]
- Jacobs D, Ainsworth B, Hartman T, Leon A. A simultaneous evaluation of 10 commonly used physical activity questionnaires. Med Sci Sport Exer. 1993;25:81–91. doi: 10.1249/00005768-199301000-00012. [DOI] [PubMed] [Google Scholar]
- Jensen MP, Karoly P. Self-report scales and procedures for assessing pain in adults. In: Turk DC, Melzack R, editors. Handbook of Pain Assessment. Guilford Press; New York: 2001. pp. 135–151. [Google Scholar]
- Warren GL, Lowe DA, Armstrong RB. Measurement tools used in the study of eccentric contraction-induced injury. Sports Med. 1999;27:43–59. doi: 10.2165/00007256-199927010-00004. [DOI] [PubMed] [Google Scholar]
- Noble BJ, Borg GA, Jacobs I, Ceci R, Kaiser P. A category ratio perceived exertion scale: Relationship to blood and muscle lactates and heart rate. Med Sci Sport Exer. 1983;15:523–528. [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. Lawrence Erlbaum Associates; Hillsdale, NJ: 1988. [Google Scholar]
- Hirose L, Nosaka K, Newton M, Laveder A, Kano M, Peake J, Suzuki K. Changes in inflammatory mediators following eccentric exercise of the elbow flexors. Exerc Immunol Rev. 2004;10:75–90. [PubMed] [Google Scholar]
- Nosaka K, Clarkson PM. Changes in indicators of inflammation after eccentric exercise of the elbow flexors. Med Sci Sport Exer. 1996;28:953–961. doi: 10.1097/00005768-199608000-00003. [DOI] [PubMed] [Google Scholar]
- Bursztein S, Glaser P, Trichet B, Taitelman U, Nedey R. Utilization of protein, carbohydrate, and fat in fasting and postabsorptive subjects. Am J Clin Nutr. 1980;33:998–1001. doi: 10.1093/ajcn/33.5.998. [DOI] [PubMed] [Google Scholar]
- Aliev G, Li Y, Palacios HH, Obrenovich ME. Oxidative stress induced mitochondrial DNA deletion as a hallmark for the drug development in the context of the cerebrovascular diseases. Recent Pat Cardiovasc Drug Discov. 2011;6:222–241. doi: 10.2174/157489011797376942. [DOI] [PubMed] [Google Scholar]
- Maskrey BH, Megson IL, Whitfield PD, Rossi AG. Mechanisms of resolution of inflammation: a focus on cardiovascular disease. Arterioscler Thromb Vasc Biol. 2011;31:1000–1006. doi: 10.1161/ATVBAHA.110.213850. [DOI] [PubMed] [Google Scholar]
- Cleary MA, Sitler MR, Kendrick ZV. Dehydration and symptoms of delayed-onset muscle soreness in normothermic men. J Athl Training. 2006;41:36–45. [PMC free article] [PubMed] [Google Scholar]
- Cleary MA, Sweeney LA, Kendrick ZV, Sitler MR. Dehydration and symptoms of delayed-onset muscle soreness in hyperthermic males. J Athl Training. 2005;40:288–297. [PMC free article] [PubMed] [Google Scholar]
- Dirks AJ, Leeuwenburgh C. Tumor necrosis factor alpha signaling in skeletal muscle: effects of age and caloric restriction. J Nutr Biochem. 2006;17:501–508. doi: 10.1016/j.jnutbio.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Martin B, Mattson MP, Maudsley S. Caloric restriction and intermittent fasting: two potential diets for successful brain aging. Ageing Res Rev. 2006;5:332–353. doi: 10.1016/j.arr.2006.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Wan R. Beneficial effects of intermittent fasting and caloric restriction on the cardiovascular systems. J Nutr Biochem. 2005;16:129–137. doi: 10.1016/j.jnutbio.2004.12.007. [DOI] [PubMed] [Google Scholar]