Abstract
The armament of neutrophil-mediated host defense against pathogens includes the extrusion of a lattice of DNA and microbicidal enzymes known as Neutrophil Extracellular Traps (NETs). The receptor:ligand interactions and intracellular signaling mechanisms responsible for elaborating NETs were determined for the response to Candida albicans. Since the host response of extravasated neutrophils to mycotic infections within tissues necessitates contact with ECM, this study also identified a novel and significant regulatory role for the ubiquitous matrix component fibronectin (Fn) in NET release. We report that recognition of purified fungal pathogen-associated molecular pattern β-glucan by human neutrophils causes rapid (≤ 30 mins) homotypic aggregation and NET release by a mechanism that requires Fn. Alone, immobilized β-glucan induces reactive oxygen species (ROS) production but not NET release, whereas in the context of Fn, ROS production is suppressed and NETs are extruded. NET release to Fn + β-glucan is robust, accounting for 17.2 ± 3.4% of total DNA in the cell population. Release is dependent on β-glucan recognition by CR3 (CD11b/CD18), but not Dectin-1, or ROS. The process of NET release included filling of intracellular vesicles with nuclear material that was eventually extruded. We identify a role for ERK in homotypic aggregation and NET release. NET formation to C. albicans hyphae was also found to depend on β-glucan recognition by CR3, require Fn and ERK but not ROS, and result in hyphal destruction. We report a new regulatory mechanism of NETosis in which the extracellular matrix is a key component of the rapid anti-fungal response.
INTRODUCTION
Polymorphonuclear leukocytes (PMNs, neutrophils) are innate immune cells responsible for host defense against opportunistic fungal infections such as Candida albicans. Patients who have a reduction in the number of peripheral blood PMNs are at risk for acquiring invasive candidiasis, a nosocomial infection that is associated with 40–80% mortality and causes approximately 10,000 deaths per year in the United States.1–6 Neutrophil antimicrobial defense mechanisms are well-studied and include phagocytosis, production of reactive oxygen species (ROS),7–9 production of anti-microbial peptides,10 degranulation, and extrusion of Neutrophil Extracellular Traps (NETs).11 NET production, or NETosis, is a cell death pathway that involves the destruction of the cytoplasmic membrane by a process that is different from apoptosis or necrosis, releasing NETs from within the cell.11–14 NETs are a combination of DNA fibers and granular enzymes15 such as elastase and myeloperoxidase.5,16,17 These fibers immobilize extracellular organisms, including fungi18, and are able to induce effective death of the microbe due to the high central concentration of anti-microbial molecules.15 In the context of invasive fungal disease, NET release plays an important role in anti-fungal defense.16,18 It has previously been shown that NET extrusion is dependent on the respiratory burst, which has been reported to correlate with time points beyond one hour post PMA-stimulation.11,13,15,17,19 Additionally, rapid extrusion of NETs in response to Staphylococcus aureus has been reported to occur by an oxidant-independent mechanism.20
Pathogenic fungi such as C. albicans undergo a dimorphic switch between cellular and hyphal forms.18 Whereas blastoconidia can be cleared by phagocytosis, hyphal forms are too large to be internalized, obviating this signature mechanism of anti-microbial host defense.16 In this regard, NET release provides the host with an effective, extracellular anti-fungal defense. The key receptor-ligand recognition events required for neutrophilic elaboration of NETs in response to fungal pathogens is not known and is the subject of this study.
β-glucan, a class of long-chain polymers of glucose, is a major structural component of the fungal cell wall that allows neutrophils to recognize fungi without the need for opsonization and, as such, is a pathogen-associated molecular pattern (PAMP).21–24 The β2 integrin complement receptor 3 (CR3, αmβ2, Mac-1, CD11b/CD18) is a receptor for fungal β-glucan recognition by human PMNs.21–27 Leukocyte Adhesion Deficiency patients that lack CR3 and other β2 integrins have recurrent localized and systemic candidiasis.28 Therefore, we hypothesized that CR3 may also play an important role in fungal-stimulated NET release.
Anti-mycotic immune responses occur within infected tissues necessitating contact of extravasated PMNs with matrix components. Prior work from our laboratory identified a regulatory effect of extracellular matrix that modifies the CR3-dependent response to fungal β-glucan.23,29 In this regard, presentation of β-glucan to neutrophils migrating on fibronectin (Fn) induced a conversion from random to directed migration to otherwise sub-optimal concentrations of fMLF or IL-8, extending the range of chemoattractant detection and driving cell navigation to a point source.26,29 In a subsequent report that is of particular relevance to the current study we showed that neutrophils undergo a robust respiratory burst to immobilized, purified fungal β-glucan, which is actively suppressed by extracellular matrix.23 This suppression was hypothesized to limit consequent tissue damage of migrating neutrophils until multifocal contact with hyphae is established. Herein, we tested the hypothesis that ECM plays a regulatory role in fungal NET formation under conditions that we showed previously prevents generation of a respiratory burst. We now demonstrate that CR3 recognition of fungal β-glucan results in homotypic cell aggregation and rapid NET release, but only when β-glucan is presented to PMNs together with matrix. We thereby report a requirement for extracellular matrix, but not ROS, in mediating the rapid NET formation to purified, immobilized β-glucan as well as to the β-glucan expressed in the cell wall of C. albicans hyphae.
MATERIALS AND METHODS
Reagents
Antibodies: anti-phospho-44/42 MAPK (ERK 1/2) (Thr202/Tyr204), anti-total 44/42 MAPK (ERK 1/2) and anti-phospho-tyrosine (p-Tyr-100) (Cell Signaling, Danvers, MA); activating anti-CD11b (VIM12 F(ab’)2) (Caltag Laboratories, Burlingame, CA); blocking anti-CD11b Clone 44 abc, hybridoma from ATCC; blocking antibody to human Dectin-1, GE2 (AbCam, Cambridge, MA). Dulbecco’s PBS (dPBS); Lebovitz’s L15 medium (L-15), RPMI 1640, Sytox Green, CM-H2DCFDA (2’,7’- dichlorodihydrofluorescein diacetate) and IL-8 were from Invitrogen (Carlsbad, CA). PicoGreen dsDNA quantitation reagent was from Molecular Probes (Eugene, OR), TNFα was from R&D Systems (Minneapolis, MN). Medium 199 with Earle’s balanced salt solution, L-glutamine, and 25 mM HEPES was from Lonza (Walkersville, MD). Pharmaceutical grade purified, endotoxin-free, soluble yeast β-glucan (ImPrime PGG™), anti-glucan mAb (clone BFDiv) and whole glucan particles (Wellmune, WGP) isolated from Saccharomyces cerevisiae were provided by Biothera (Eagan, MN). The β-glucan preparation contained <0.02% protein, <0.01% mannan and <1% glucosamine. Human fibronectin (Fn) was from BD Biosciences (Bedford, MA). All other reagents were from Sigma Aldrich unless otherwise noted.
Neutrophil isolation
Blood was obtained from healthy human volunteers with approval of Rhode Island Hospital Institutional Review Board. Blood was collected in EDTA-containing Vacutainer tubes (BD Biosciences, San Jose, CA) and used within 5 min of venipuncture. Histopaque - 1077 was used for initial cell separation followed by sedimentation through 3% dextran (400–500 kDa molecular weight). Contaminating erythrocytes were removed by hypotonic lysis, yielding a >95% pure neutrophil preparation of >90% viability by trypan dye exclusion. Neutrophils were suspended in HBSS (without Ca+2/Mg+2) and placed on ice until use.
Neutrophil adhesion
6-well tissue culture plates (Falcon Labware, Becton Dickinson) were coated overnight with purified, endotoxin-free human Fn at a concentration of 6 µg/ml in TBS (25mM Tris pH 7.2, 150 mM NaCl) pH 9.0 and/or 1 mg/ml of β-glucan and air-dried. Neutrophils were resuspended to a concentration of 3.5×106 cells/ml in L-15 medium supplemented with 2 mg/ml glucose (L-15/2 mg/ml glucose) and 2 mls added to each well. Where indicated, cells were pre-incubated with 20 µg/ml blocking Ab or isotype control; U0126 (50 µM) or PD 98059 (30 µM) (Calbiochem, EMD Chemicals Inc., San Diego, CA); or DPI (6.25 µM) on ice for 20 minutes. Cells were pre-treated on ice with fMLF (10−9 M) for 20 min and/or 1 mM Mn+2 was added to cells immediately before plating.
Microscopy
Images were captured using a Nikon TE-2000U inverted microscope (Nikon, Melville, NY) coupled to an iXonEM + 897E back illuminated EMCCD camera (Andor, Belfast, UK), outfitted with a Bioptechs Inc. (Butler, PA) stage heater and 10×, 20×, and 40× Nikon Plan Apochromat objectives. DIC images were captured using Elements program (Nikon). For fluorescence microscopy, a xenon lamp illuminated cells through a 33 mm ND4 filter and 10×, 20×, and 40× Nikon Plan Apochromat objectives using a Nikon B2-A longpass emission filter set cube.
Confocal microscopy was performed at the Brown University Leduc Bioimaging Facility. Z-stacks of both DIC and fluorescence images were acquired with a z-step of 0.9 µm, using a Zeiss LSM 710 confocal laser scanning microscope equipped with a 34-channel QUASAR detector running ZEN 2009 software (Carl Zeiss, Jena, Germany). The confocal module was mounted on a Zeiss Axio Observer Z1 inverted microscope outfitted with 37° C heated stage and 20× Plan-Apochromat objective (NA 1.2). Sytox Green was visualized using 488 nm laser excitation at 25% and 500 gain. Fluorescence images were imported into AutoQuant v.9 software (MediaCybernetics, Bethesda, MD) as a z-stack series of images and processed using a blind deconvolution algorithm with 20 iterations. Composite images of DIC and deconvoluted fluorescence sum projections, as well as z-stack video reconstructions, were generated using ImageJ v.1.41o (U.S. National Institutes of Health, Bethesda, MD).
Western Blot Analysis
Adherent neutrophils were scraped and centrifuged at 700 × g for 5 min. Pellets were lysed using RIPA buffer (100 mM Tris-HCl, 150 mM NaCl, 1% deoxycholic acid, 1% Triton X-100, 0.1% SDS with protease and phosphatase inhibitors) and subjected to 10–12% SDS-PAGE gradient gels and transferred to nitrocellulose membranes. Membranes were blocked in TBST (25 mM Tris pH 7.2, 150 mM NaCl, 0.1% Tween) with 3% non-fat dry milk for 1 h at room temperature and incubated overnight at 4° C with anti-pERK 1/2 (Thr202/Tyr204) (1:1,000), anti-total ERK (1:1,000) or anti-actin (1:5,000) Ab in blocking buffer. Thereafter, membranes were washed and incubated with peroxidase-conjugated anti-mouse or anti-rabbit IgG (1:10,000) in blocking buffer. A chemiluminescence kit (Thermo Fisher Scientific, Philadelphia, PA) was used to detect HRP. Relative intensities of immunoreactive bands were quantified by scanning densitometry using NIH ImageJ v.1.41o analysis software.
Visualization of PMN NETs
Neutrophils were adhered as previously described to Fn ± β-glucan coated plates. After aggregation, 150 U/ml of DNaseI (Promega, Madison, WI) was added when indicated. NETs were visualized on adherent PMNs by addition of 5 µM Sytox Green.
C. albicans (SC5314, ATCC) was cultured overnight at 37°C with agitation in yeast extract-peptone-dextrose medium consisting of 1% yeast extract, 2% bactopeptone (both from Difco), and 2% dextrose. Coverslips/culture dishes (MatTek Corporation, Ashland, MA) were coated with 40 µg/ml Fn or poly-L-lysine in TBS pH 9.0 overnight at room temperature. C. albicans was added onto pre-coated coverslips/culture dishes and incubated in Medium 199 supplemented with Earle’s balanced salt solution, L-glutamine, and 25 mM HEPES to induce differentiation into hyphae at 37° C for 4–5 h. Filamentous phenotype was confirmed by light microscopy. Coverslip was placed in a Delta T dish (Bioptechs Inc., Butler, PA) and neutrophils added. When indicated, anti-glucan mAb (BFDiv) was added to hyphae prior to the addition of neutrophils. NETs were visualized by fluorescent microscopy using 5 µM Sytox Green.
Transmission electron microscopy
Samples for TEM were fixed by gently layering 2.5% glutaraldehyde in 0.15 M sodium cacodylate buffer, rinsed with buffer and post fixed with 1% osmium tetroxide. Slides were rinsed, dehydrated and covered with resin and placed over Epox 812 filled slide-duplicating molds (EMS, Hatfield, PA) overnight. Selected areas of interest were mounted on blocks for sectioning. Ultra-thin sections (50–60 nm) were prepared using a Reichert Ultracut S microtome (Leica, Wetzlar, Germany), retrieved onto 300 mesh copper grids, and contrasted with uranyl acetate and lead citrate. Sections were examined using a Morgagni 268 transmission electron microscope (FEI, Hillsboro, OR) and images were collected with an AMT Advantage 542 CCD camera system (Advanced Microscopy Techniques, Danvers, MA).
NET Quantification
Neutrophils were adhered as described above to Fn ± β-glucan coated plates. NETs were quantified after staining extracellular DNA with 2.5 µl PicoGreen stock reagent per ml, incubated for six min and fluorescence was measured using an FL800 Microplate Fluorescence Reader (BIO-TEK Instructions) at 485 nm excitation/ 535 nm emission. Fluorescence measurements were determined from 4 to 6 independent experiments representing at least 3 donors. Blank reagent well readings were subtracted from each experimental well to obtain reported fluorescence and results were confirmed by microscopy. Alternatively, after NET formation on Fn + β-glucan coated plates, NETs were harvested by 30 min digestion at 37°C using 5 Gel Units/ml of micrococcal nuclease (New England Biolabs, Ipswich, MA) and the DNA from the resultant supernatants or the equivalent number of total cells were isolated using a DNeasy Blood and Tissue kit (Qiagen, Hilden, Germany). Isolated NET DNA content and total cellular DNA content from 2–4 replicate wells from 5 independent experiments were quantified by PicoGreen according to manufacturer’s protocols using a Perkin Elmer LS50B Luminescence Spectrometer (Waltham, MA) at 480 nm excitation/ 520 nm emission. The percentage extracellular DNA was determined by dividing the amount of isolated NET DNA by the total DNA.
NET Killing Assay
Neutrophils were adhered as described above to either Fn + β-glucan coated plates or Fn-coated plates which had been lightly seeded with C. albicans that was allowed to differentiate overnight. Where indicated, cells were pretreated with 3% autologus serum or 6.25 µM DPI. After 30 min at 37° C neutrophil-containing wells and control wells containing only Fn + β-glucan or hyphae were washed twice with RPMI 1640. For the hyphal system, 2 ml RPMI 1640 was added and hyphae was allowed to grow overnight at 37°C. For the immobilized system, 2 ml RPMI 1640 containing 70,000 C. albicans blastoconidia and 10 µM cytochalasin D (Sigma) were added to each well representing an MOI of 1:100 and allowed to grow overnight at 37°C. Where indicated, overnight incubations were additionally carried out in the presence of 3% autologus serum, 6.25 µM DPI, 300 U/ mL catalase (Sigma), 300 U/ mL superoxide dismutase (Sigma), 1:1,000 dilution micrococcal nuclease (Sigma), 150 U/ ml DNaseI (Source), or equivalent PMN hypotonic lysate. Cells were scored microscopically for hyphal growth and viability was quantified by an MTT reduction assay.30 Briefly, after overnight growth, wells were washed with indicatorless RPMI 1640, incubated for 5 min at RT with 0.5% sodium deoxycholate (Sigma) to lyse remaining PMNs, and washed with indicatorless RPMI 1640. 1 mL 0.01 g/ mL MTT was added to each well and allowed to incubate at 37°C for 2 hours. Wells were washed twice with indicatorless RPMI 1640, resultant formazan crystals were solubilized in 0.2 mL DMSO for 5 min at 37°C, diluted with 0.4 mL acidified isopropanol, and 4 to 6 100 µL replicates per well were transferred to a 96 well plate and assayed at 540 nm using a Microplate BIO Kinetics Reader (BIO-TEK Instruments), running DeltaSoft3 software. Data was obtained from 4 to 24 independent experiments representing at least 3 donors for each treatment condition.
Oxidative Burst Assay
Respiratory burst activity was determined by measuring the colorimetric change caused by superoxide anion reduction of ferricytochrome C. Cells were plated at a concentration of 3 × 105 cells/well, in replicates of 3–6 wells, at a volume of 100 µl/well onto 96-well tissue culture plates. When indicated, PMNs were assayed in the presence of fMLF, DPI (diphenyl iodonium), and/ or WGPs as an additional stimulant added at time zero of the assay. WGPs were sonicated before use to produce single particle suspensions. Stimulus concentrations of 1.4 × 107 WGPs/well were from published reports and optimized for maximal generation of superoxide through preliminary work. 20 nM PMA was used as a positive control for superoxide production and wells blocked with endotoxin-free BSA and untreated cells served as negative controls. For Ab-blocking experiments, cells were preincubated with 10−9 M fMLF for 10 minutes at room temperature. Cells were then incubated with 20 µg/ml indicated mAb or isotype control for 20 minutes on ice before being plated. Finally, 100 µl/well of 100 µM ferricytochrome C was added to each well and absorbance was measured every 10 minutes for 90 minutes at dual wavelengths of 550 and 630 nm at 37° C using a Microplate BIO Kinetics Reader (BIO-TEK Instruments), running DeltaSoft3 software. Superoxide production was calculated for 60 min with the following formula: (ΔE550–630nm) × 15.166 = nM/well; 15.166 is a predetermined absorbance constant and the final units are nanomoles superoxide anion/3 × 105 cells/h. To account for variability in donor response to WGPs, superoxide production for each donor is expressed as percentage of donor superoxide production to WGPs.
To measure the generation of reactive oxygen species by PMN against live yeast, PMNs were suspended in HBSS (5 × 106/ml) containing 8 µM CM-H2DCFDA and equilibrated at room temperature in the dark for 30 min. CM-H2DCFDA is a cell permeable indicator for the respiratory burst that becomes fluorescent upon oxidation. Cells were washed once in an excess volume of HBSS without cations and suspended to a concentration of 3.5 × 106/ml in L-15/2 mg/ml glucose. Seven million cells were added to C. albicans hyphae that were differentiated on 6-well tissue culture plates coated with Fn to promote adhesion to the surface and minimize disturbance through multiple assay steps as earlier described and visualized microscopically as explained above.
FACS Analysis
Samples of 1 × 106 isolated PMNs were blocked in ice cold PBS containing 1% normal goat serum and Fc Block (Accurate Chemical Co., Westbury, NY) for 30 min on ice. Cells were stained with 20 µg/ml purified mAb for 1 h on ice in a total volume of 100 µl. Cells were then washed twice and incubated with 30 µg/ml PE-labeled goat F(ab’)2 anti-mouse IgG (Sigma) for 30 min on ice. Cells were washed twice and resuspended in 1% paraformaldehyde in PBS. Analysis was performed on a FACScan (Becton Dickinson) using Becton Dickinson Lysis II software and FlowJo software (Tree Star, Ashland, OR) and gated on neutrophils.
Statistical Analysis
Data was pooled from a minimum of three independent experiments representing at least three different donors, as indicated. ANOVA analysis with Newman-Keuls posthoc analysis or paired-sample Student’s t-test as appropriate were performed using MATLAB (Mathworks, Natick, MA) or Excel (Microsoft, Redmond, WA) running the statistiXL data package (statistiXL, Nedlands, Australia). The null hypothesis was rejected if p < 0.01.
RESULTS
Neutrophil adhesion to the fungal PAMP β-glucan results in rapid homotypic aggregation by a matrix-dependent mechanism
To model immune recognition of β-glucan in fungal hyphae, which are too large for internalization, soluble β-glucan was immobilized onto a tissue culture surface. This reductionist system allowed for the interrogation of neutrophil binding and responsiveness in the absence of phagocytosis. Since neutrophil recognition of fungal hyphae during the response to mycotic tissue necessarily involves contact with extracellular matrix, the cellular effect of binding to β-glucan was studied in the presence and absence of the ubiquitous tissue matrix component Fn. When human neutrophils were primed with 1 nM fMLF in the presence of Mn+2 (0.5–1 mM), rapid homotypic cell aggregation was observed in response to Fn + β-glucan, but not Fn or β-glucan alone (Figure 1A). Neutrophil aggregation was β-glucan specific and required co-recognition of Fn, as primed cells that were adhered to plastic, BSA or fibrinogen ± β-glucan or Fn ± dextran or mannan did not aggregate (data not shown). Time course studies identified a 30 min time point in which 90–95% of the cells exposed to Fn + β-glucan were involved in aggregates (Figure S1, Video 1). Again, it is noteworthy that cell aggregation does not occur to either Fn or β-glucan alone at 30 mins (Figure 1A, Figure S1), which was the time point used for the balance of the studies described. Additionally, we determined that there is a requirement for both fMLF and Mn+2 to promote PMN aggregation responding to Fn + β-glucan (data not shown). Since neutrophils reach a primed state upon diapedesis, fMLF was used as a canonical priming agent, however, rapid aggregate formation on Fn + β-glucan (but not to either ligand alone) was also observed when cells were primed with TNFα (30 ng/ml) or IL-8 (7 ng/ml) (data not shown). Therefore, findings are not a unique consequence of fMLF stimulation, but are a generalized response of the primed neutrophil to β-glucan in the context of Fn. Taken together, this aggregation to fungal β-glucan requires conditions consistent with a tissue-based extravasated neutrophil including extracellular matrix, priming and Mn+2.
Figure 1. Primed neutrophils in the context of Fn + β-glucan, but not Fn or β-glucan alone, form aggregates and Ab inhibition and activation of β2 integrin CR3 modulates cell aggregation.
(A–C) Micrographs show PMNs that were adhered to Fn (6 µg/ml), β-glucan (1 mg/ml) or Fn + β-glucan pre-coated wells. (A) PMNs were pre-treated with 10−9 M fMLF for 20 min on ice and Mn+2 immediately before adhering cells. Cells formed aggregates in the context of Fn + β-glucan, but not Fn or β-glucan alone. Additionally, aggregation required priming with both fMLF and Mn+2 (data not shown). All experiments were incubated for 30 min at 37° C. Data represents at least ten separate experiments done using neutrophils from different individual donors. (B) Aggregation is inhibited with CR3, but not Dectin-1 blocking mAb. PMNs were pre-treated as described in (A) before adhering cells to Fn ± β-glucan coated wells for 30 min at 37° C. When PMNs were pretreated with 20 µg/ml of anti-CR3 blocking mAb (Clone 44abc), cell aggregation was prevented; anti-Dectin blocking mAb (GE2) and IgG1, which was used as an isotype control, have no effect on neutrophil aggregation; and (C) fMLF and Mn+2 primed cells were pre-treated with an anti-CR3 activating mAb (VIM12 F(ab’)2) which mimics β-glucan. Treated cells aggregate on Fn-coated wells and exhibit an exaggerated cell aggregation phenotype on wells coated with Fn + β-glucan. These data represent at least four independent experiments using neutrophils from different individual donors. All images were taken at 20× magnification (Bar=100 µm).
Neutrophil aggregation is dependent on Complement Receptor 3 (CR3)
To determine the neutrophil receptor responsible for β-glucan-induced aggregation, cells were pretreated with a CR3-blocking mAb before exposure to Fn + β-glucan. As shown in Figure 1B cell aggregation was inhibited by a CR3 function-blocking mAb (Clone 44 abc). A Dectin-1 function blocking mAb GE2 had no effect on neutrophil aggregation on Fn + β-glucan when used under conditions where Ab binding was demonstrable by FACS and effected a partial inhibition of the respiratory burst to WGPs (as reported by others31) (Figure 1B; Figure S2).
β-glucan binding to CR3 has been mapped to the lectin-like site of CD11b that is recognized by the anti-CD11b activating mAb, VIM12.32 In the presence of Fn alone, VIM12 activation mimicked β-glucan binding by causing cell aggregation (Figure 1C). Interestingly, in the presence of Fn + β-glucan, VIM12 mAb activation showed a synergistic effect, forming cell aggregates that were highly enlarged relative to either agonist alone (Figure 1C), suggesting that VIM12 is binding to a subset of CR3 integrins that are not fully occupied by β-glucan. These data together support a CR3- dependent mechanism of neutrophil homotypic aggregation in response to Fn + β-glucan.
ERK MAPK is regulated depending on differential ligation of neutrophils
To characterize the intracellular signaling mechanisms by which primed neutrophils formed aggregates in response to differential ligation by Fn vs. Fn + β-glucan, a global tyrosine phosphoproteomic analysis was undertaken (Reichner, unpublished data). Western blotting validated the proteomic finding of elevated ERK phosphorylation in cells exposed to Fn + β-glucan as compared to Fn alone (Figures 2A–2B). To assess a functional role for ERK in neutrophil aggregation, the upstream kinase responsible for ERK phosphorylation, MEK, was inhibited. Neutrophils pretreated with inhibitors of MEK (UO126 or PD 98059) failed to form aggregates (Figure 2C), supporting a role for ERK phosphorylation in the aggregation of primed neutrophils exposed to Fn + β-glucan. Figure S3 demonstrates complete pharmacologic inhibition of pERK under the experimental conditions employed.
Figure 2. Phosphorylated ERK levels are increased in Fn + β-glucan treated PMNs.
(A) PMNs were pre-treated as previously described in the legend for Figure 1A before they were adhered to Fn ± β-glucan pre-coated wells and incubated at 37 °C for 5, 10, 20, and 30 min. Cells were harvested at the appropriate time point, lysed and separated on 10% SDS-PAGE gels and transferred to a nitrocellulose membrane. Membranes were immunoblotted with anti- pERK, anti-total ERK, and anti-actin mAb. White bar indicates noncontiguous samples that were run on the same gel. (B) A representative densitometric analysis was determined by scanning densitometry using Image J analysis software and expressed as phosphorylated ERK over actin. (C) Neutrophils were incubated on ice for 20 min with MEK inhibitors U0126 (50 µM) and PD 98059 (30 µM) and subsequently pre-treated as previously described in the legend for Figure 1A before they were adhered to Fn ± β-glucan pre-coated wells and incubated at 37 °C for 30 min. Inhibition of ERK phosphorylation prevented PMN aggregation when adhered to Fn + β-glucan. Images were taken at 20× magnification (Bar=100 µm). Blots were derived from the same protein samples. These results represent at least four independent experiments using neutrophils from different individual donors.
Immobilized Fn + β-glucan induces PMN NET formation
Given that human neutrophils release NETs as part of the response to fungal pathogens,16,18 we tested the hypothesis that homotypic cell aggregation to the fungal PAMP β-glucan is caused wholly or in part by NET release following β-glucan recognition. Supporting this hypothesis, when neutrophil aggregates that were formed in response to Fn + β-glucan were subsequently treated with DNaseI, aggregates were significantly disrupted, suggesting extracellular DNA may be entrapping PMNs into aggregates (Figure 3A). A significant increase in extracellular NET extrusion from neutrophils exposed to Fn + β-glucan compared to Fn alone was observed by confocal microcopy using Sytox Green, a molecule that is fluorescent when bound to double stranded DNA (Figure 3B, Video 1).20,33 Z-stack reconstruction of this can be seen in Videos 2–3. NET production in response to Fn ± β-glucan was quantified under our assay conditions using the highly sensitive PicoGreen dsDNA staining revealing a 17 fold increase in extracellular DNA on Fn + β-glucan as compared to Fn alone (Figure 3B bar graph). Using an alternative method to quantitate NET release, extracellular DNA from cells on Fn + β-glucan was harvested by micrococcal nuclease digestion, purified, and stained with PicoGreen (as described in Methods). DNA concentration was determined by fluorometery and the DNA released on Fn + β-glucan was determined to represent 17.2 ± 3.4% (n=5 independent experiments) of the total cellular DNA. Figure 4 shows intracellular NET formation and release from within neutrophils adhered to Fn + β-glucan by transmission electron microscopy (TEM, arrowhead). In concert with other reports, neutrophils showed characteristic rounded and condensed multilobular nuclei, blebbing with extensive dilation between the inner and outer nuclear membranes and budding vesicles, which contains strands of DNA with attached nucleosomes. The nuclei are circular with homogenous condensed chromatin simultaneous with vesicles continuing to bud from the nuclear envelope. The intact vesicles extrude into the extracellular space, rupture, and release chromatin. Along the plasma membrane are dense cytoplasmic granules, which also release their contents in the extracellular space contributing to the granule content of NETs. NETs can be seen in the cytoplasm and extracellularly (Figure 4Ai, increased magnification) as well as in cytoplasmic vesicles20 (Figure 4Aii, increased magnification).
Figure 3. Rapid PMN NET formation of PMNs adhered to immobilized Fn + β-glucan.
PMNs were pre-treated as previously described in the legend for Figure 1A before they were adhered to Fn ± β-glucan pre-coated wells and incubated at 37 °C for 30 min. (A) After aggregates formed, DNaseI was added directly to the wells, which significantly disrupted PMN aggregates. Images were taken at 20× magnification (Bar = 100 µm). (B) Sytox Green staining shows NET formation in the context of Fn + β-glucan, corresponding to PMN aggregates. Aggregates and NET formation are not seen in cells responding to Fn alone. Images were taken at 20× magnification using confocal microscopy as described (see Materials and Methods) (Bar=100 µm). Bar graph shows 17 fold increase in extracellular DNA on Fn + β-glucan as compared to Fn alone as quantified by plate fluorometer under our assay conditions using PicoGreen dsDNA staining. Error bars represent SEM; *p<0.01, paired sample Student’s t test. These results represent six independent experiments using neutrophils from at least three individual donors.
Figure 4. NETs formed by PMNs adhered to immobilized Fn + β-glucan decrease yeast viability.
(A) Neutrophils were prepared as previously described in the legend for Figure 1A, and following glutaraldehyde fixation, samples were processed and examined with transmission electron microscopy. Visualization of changes in the nuclear envelope and NET formation (arrowhead) at a direct magnification of 7,100× (Bar=2 µm). The nuclear envelope undergoes a division of the inner nuclear membrane (INM) and the outer nuclear membrane (ONM). (i) DNA material of NETs shown extracellularly at a direct magnification of 44,000× (Bar=100 nm) and (ii) Enlarged view of cytoplasmic vesicles containing NETs shown at a direct magnification of 36,000× (Bar=500 nm) using a 80 kV voltage. Sections were stained with uranyl acetate and lead citrate (see Materials and Methods). These results represent at least four independent experiments using neutrophils from different individual donors. (B) Yeast viability by reduction of MTT. PMNs were pre-treated as described in the legend Figure 1A before they were adhered to Fn + β-glucan pre-coated wells and incubated at 37 °C for 30 min. Wells were washed and RPMI, C. albicans blastoconidia, and 10 µM cytochalasin were added to wells ± DNaseI and incubated at 37°C overnight. For comparison, blastoconidia were incubated with the hypotonic lysate of an equivalent number of PMNs. Wells were scored microscopically for yeast growth (left) and viability was quantified by MTT reduction (bar graph). * p<0.01 vs. PMN lysate; ** p<0.01 PMN NETs ± DNaseI, ANOVA full factorial, post hoc Newman-Keuls; error bars represent SEM. Results represent 6 to 24 independent experiments from at least three donors. (Bar=100 µm).
Immobilized Fn + β-glucan induced PMN NET formation has fungicidal activity against Candida albicans
The importance of NETosis in killing C. albicans has been shown by others.16,18 We sought out to extrapolate the mechanisms driving this response. We have reported previously that the respiratory burst produced by neutrophils in response to immobilized β-glucan is suppressed to undetectable levels by Fn,23 conditions that were shown herein to be permissive for homotypic cell aggregation and NET formation. We assayed NET killing to confirm that NETs released by PMNs exposed to immobilized Fn + β-glucan and where ROS production is inhibited, the NET composition maintained its expected fungicidal activity. As shown in Figure 4B, there is NET –dependent killing of C. albicans. Yeast grown in the presence of PMN NETs elaborated on Fn + β-glucan resulted in a significant decrease in viability (62.8% ± 11.2%) as measured by MTT reduction when compared to yeast grown in the presence of the hypotonic lysate of an equal number of PMNs. Additionally, when NET integrity is disrupted by the addition of DNaseI there is a significant increase (100.8% ± 13.2%) in yeast viability supporting a NET specific mechanism of killing. In this system, neutrophils were additionally treated with cytochalasin to minimize the phagocytic component of killing.
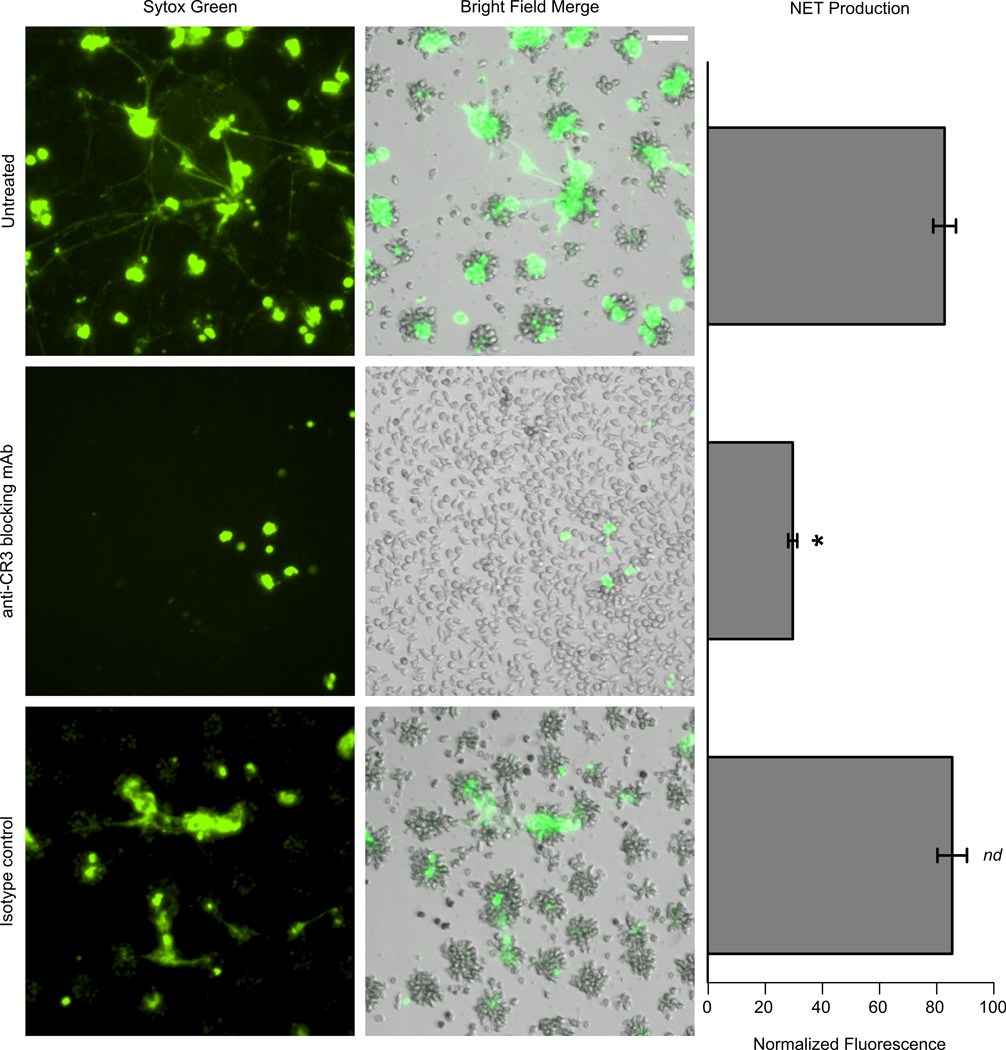
CR3 blockade significantly attenuates neutrophil NET formation to immobilized Fn + β-glucan
As shown in Figure 1B, cell aggregation in response to Fn + β-glucan was abrogated when pretreated with a CR3 blocking mAb. Figure 5 shows that NET formation in response to Fn + β-glucan was also significantly reduced when cells were pretreated with a CR3 blocking mAb but not an isotype control, as measured by fluorescent plate reader using PicoGreen dsDNA stain (Figure 5). Taken together, our data suggests that CR3 mediates aggregation and NET formation to fungal β-glucan presented in the context of Fn.
Figure 5. CR3 blockade attenuates neutrophil aggregation and rapid NET formation of PMNs adhered to immobilized Fn + β-glucan.
PMNs were pretreated as previously described in the legend for Figure 1A. PMNs additionally pre-treated with 20 µg/ml of anti-CR3 blocking mAb (Clone 44abc) did not aggregate. Sytox Green staining after the samples were incubated at 37°C for 30 min demonstrated attenuation of NET formation; IgG1, which was used as an isotype control, has no effect on neutrophil aggregation or NET formation (Bar=100 µm). Bar graph shows significant inhibition of NET production on Fn + β-glucan when cells are pretreated with 20 µg/ml of anti-CR3 blocking mAb (Clone 44abc), as quantified by plate fluorometer using PicoGreen dsDNA staining. Error bars represent SEM; *p<0.01 untreated vs. anti-CR3 blocking mAb; nd no significant difference vs. untreated ANOVA full factorial, post-hoc Newman-Kuels. These results represent at least three independent experiments using neutrophils from different individual donors.
Candida albicans in the presence of Fn induces PMN NET formation through recognition of hyphal β-glucan by neutrophil CR3
Prior work in our laboratory showed that when C. albicans infection was established by direct inoculation of rat kidney, a robust neutrophilic response included pronounced cell aggregates surrounding the hyphal filaments which are visually similar to the aggregates we observed on Fn + β-glucan coated surfaces.22 It has also been demonstrated that NETs enhance bacteria trapping and interact with C. albicans in vivo.18,33,34 As mentioned above, the response of neutrophils to Candida-infected tissues includes contact with matrix; therefore, Candida hyphae were grown on Fn to track NET formation to the intact pathogen. Shown in Figure 6 and Video 4, neutrophils aggregate and form NETs rapidly (within 30–50 min) in response to C. albicans hyphae grown on Fn. Additional representative fields of this observation can be seen in Figure S4 and complimentary z-stack images can be viewed in Video 5.
Figure 6. C. albicans induced rapid PMN NETs that are inhibited by anti-CR3 blocking mAb.
C. albicans hyphae were grown on culture dishes coated with 40 µg/ml Fn. Neutrophils were pre-treated as previously described in Figure 1A and blocked with anti-CR3 mAb (Clone 44abc) for 20 min on ice before adding them to the dishes containing yeast hyphae and incubated at 37 °C for 30–50 min. Neutrophils conform to the hyphae and confocal microscopy shows NET formation by Sytox Green staining except when inhibited by anti-CR3 mAb indicating a functional role for CR3. Images were taken at 20× magnification using confocal microscopy as described (see Materials and Methods) (Bar=100 µm). These results represent at least five independent experiments using neutrophils from different individual donors.
The receptor-ligand interactions leading to NET release in response to fungal hyphae have not yet been determined. To assess the role of CR3 recognition of β-glucan in mediating NET formation, neutrophils were pretreated with an anti-CR3 blocking mAb before adding them to C. albicans hyphae. As shown in Figure 6 and Video 6, CR3 receptor blocking significantly reduced the formation of diffuse NETs in response to C. albicans hyphae that was consistent with our finding showing CR3 to be essential for aggregate and NET formation to immobilized Fn + β-glucan (Figures 1B and 5). By observation, blocking CR3 seemingly reduces PMN binding to the hyphae, but does not obliterate binding as observed when β-glucan in the hyphae cell wall is blocked. More interestingly, although there is still cell binding to the hyphae when CR3 is blocked, it’s insufficient to promote NET extrusion. This suggests that there are receptors other than CR3 that mediate cell adhesion to intact hyphal filaments, but do not necessarily lead to NET release.
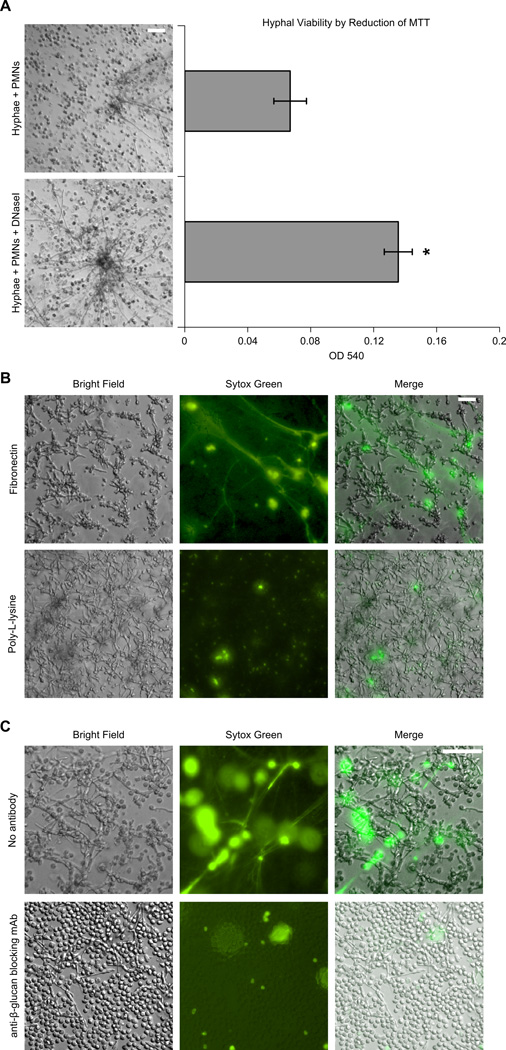
Consistent with yeast viability data obtained using the immobilized system (Figure 4B), PMNs added directly to C. albicans hyphae under NET producing conditions similarly restrict hyphal viability (Figure 7A). Supporting a NET specific mechanism of killing, there is a significant increase in hyphal viability (102.6% ± 17.3% ) when DNaseI is added to disrupt PMNs NETs using the hyphal system. Taken together, this data confirms the fungicidal capacity of NETs elicited under our experimental conditions.
Figure 7. Rapid PMN NET formation in response to C. albicans decreases hyphal viability, is matrix-dependent, and is inhibited by a β-glucan-specific mAb.
(A) Hyphal viability by reduction of MTT. Neutrophils were pre-treated as previously described in Figure 1A and added to lightly seeded C. albicans hyphae grown on Fn coated wells and incubated at 37 °C for 30 min. Wells were washed and RPMI ± DNaseI was added and incubated at 37°C overnight. Wells were scored microscopically for yeast growth (left) and viability was quantified by MTT reduction (bar graph). Error bars represent SEM; * p<0.01 vs. hyphae + PMNs, paired sample Student’s t test. These data represent eight independent experiments with at least three donors. (B–C) C. albicans hyphae were grown on coverslips coated with 40 µg/ml Fn. Neutrophils were pre-treated as previously described in Figure 1A, added to the hyphae and visualized by Sytox Green staining. (B) PMN NET formation is induced when hyphae and neutrophils are adhered to Fn, but not poly-L-lysine. (C) Neutrophil NET formation is prevented when fungal β-glucan is pre-blocked by the β-glucan-specific mAb BFDiv before PMNs were added to hyphae and incubated at 37 °C for 30 min. Samples were stained and visualized at 20× magnification (Bar=100 µm). These data represent four independent experiments using neutrophils from different individual donors.
To further determine the regulatory role of extracellular matrix, C. albicans hyphae were grown on poly-L-lysine as a nonspecific adhesive control and were found not to support rapid NET formation (Figure 7B).
To test the hypothesis that β-glucan within fungal filaments is mediating NET formation, C. albicans hyphae were incubated with β-glucan-specific mAb (BFDiv) to block exposed β-glucan. Shown in Figure 7C, NET formation is abrogated with BFDiv treatment indicating that β-glucan is mediating the PMN NET response to fungal hyphae.
Cell aggregation and NET formation to immobilized Fn + β-glucan and C. albicans are independent of the respiratory burst
A number of initial reports posited that NET formation is functionally coupled to the release of ROS. 11,13,15,17,19 Prior work from our laboratory showed that neutrophils exposed to immobilized β-glucan alone induced a dose-dependent respiratory burst22 whereas our current study demonstrated that β-glucan alone is insufficient for aggregation and hence NET formation (Figure S1). We also reported that addition of Fn to immobilized β-glucan completely suppressed respiratory burst23 under conditions which we now show results in rapid and robust aggregation and NET formation, suggesting that the response is dependent upon Fn, but independent of ROS. This independence of ROS is in agreement with the findings of Pilsczek et al.20 Moreover, in experiments to exclude any possible vestigial respiratory burst activity, we found that addition of the NADPH oxidase inhibitor, DPI (diphenyl iodonium), did not impede aggregation and NET formation on immobilized Fn + β-glucan (Figure 8A–8B). The complete inhibition of the respiratory burst at the concentrations of DPI used here was confirmed both by cytochrome C colorimetric assay as shown in Figure 8C and by using the fluorescent oxidative stress indicator, CM-H2DCFDA (data not shown). Consistent with observations on immobilized Fn + β-glucan, NET formation in response to fungal hyphae was also found to be independent of the PMN respiratory burst and unperturbed by use of DPI (Figure 9A). Treatment with DPI, superoxide dismutase, or catalase did not prevent PMN killing by NET formation. When 6.25µM DPI, 300U/ mL superoxide dismutase, or 300U/ mL catalase were added to the yeast viability assay described in Figure 4B there was no significant change in percent viability after NET exposure as measured by reduction of MTT relative to treatment controls (data not shown; see Materials and Methods).
Figure 8. Neutrophil aggregation and rapid PMN NET formation in the context of immobilized Fn + β-glucan are independent of the PMN respiratory burst.
(A–B) PMNs and yeast hyphae were prepared as previously described in Figure 6. Cells were pretreated with DPI (6.25 µM) on ice for 20 min before they were adhered to Fn ± β-glucan pre-coated wells and incubated at 37 °C for 30–50 min. (A) Inhibition of the respiratory burst with DPI does not prevent PMN aggregation. Images were taken at 10× magnification (Bar=100µM). (B) Inhibition of the respiratory burst with DPI does not attenuate NET formation in the context of Fn + β-glucan. Sytox Green was added to the sample after aggregate formation to assess NET formation. Images were taken at 20× magnification (Bar=100 µm). (C) DPI inhibits the PMN respiratory burst to PMA. Error bars represent SD. These results represent at least four independent experiments using neutrophils from different individual donors.
Figure 9. Rapid PMN NET formation in the context of C. albicans is independent of the PMN respiratory burst.
(A–C) Neutrophils and yeast hyphae were prepared as previously described in Figure 6. (A) Cells were pretreated with DPI (6.25 µM) on ice for 20 min before adding them to coverslips containing yeast hyphae and incubated at 37 °C for 30–50 min. Inhibition of the respiratory burst with DPI does not attenuate NET formation in the context of C. albicans hyphae. NET formation was visualized at 10× magnification (Bar=100 µm). (B) Neutrophils were incubated the MEK inhibitor U0126 for 20 min on ice before adding them to hyphae to prevent ERK phosphorylation. This treatment resulted in a significant decrease in NET formation in response to C. albicans hyphae. Sytox Green was added after the samples were incubated at 37°C for 30–50 min. NET formation was visualized at 20× magnification (Bar=100 µm). (C) PMNs were loaded with the respiratory burst indicator CM-H2DCFDA, treated with U0126, and then added to hyphae. Inhibition of ERK phosphorylation with U0126 does not inhibit the respiratory burst (top) but it does inhibit the formation of NETs (bottom) as visualized by Sytox Green staining. NET formation was visualized at 10× magnification (Bar=100 µm). Data represent four independent experiments with neutrophils from different individual donors.
With respect to the neutrophilic response to intact C. albicans hyphae, inhibition of ERK phosphorylation significantly reduced NET formation (Figure 9B and 9C, lower panels) without affecting the ability of neutrophils to undergo the respiratory burst (Figure 9C, upper panel).
Taken together, these data show that neutrophil rapid NET formation in response to fungal hyphae is mediated by CR3 recognition of fungal β-glucan and co-recognition of Fn. NET formation facilitates neutrophil aggregation, is independent of the PMN respiratory burst, and is modulated in part through ERK phosphorylation.
DISCUSSION
Neutrophils play a central role in host defense against fungal pathogens and, indeed, neutropenia or deficient cell function is a predisposing factor for mycotic infection.1 Unlike phagocytic clearance of unicellular microbes, neutrophils are challenged with the physical reality that fungal hyphae are too large to ingest and must be combated by alternative mechanisms.16 β-glucans are the major structural component of the fungal cell wall and are important PAMPs for innate immune sensing of fungi.21–24 Immobilization of purified β-glucan onto tissue culture surfaces enables mechanistic studies of β-glucan recognition by human neutrophils to be conducted in the absence of phagocytosis, thereby modeling the response to hyphae. Here we report that neutrophils undergo rapid (≤ 30 min) homotypic aggregation and extrusion of NETs in response to β-glucan when immobilized together with the ECM protein Fn, but not to either ligand alone (Figures 1A, 3B; Figures S1; Videos 1–3). The physiological relevance of the regulated response to β-glucan by Fn is considerable since recognition of fungi by extravasated neutrophils occurs within afflicted tissues and necessitates matrix contact.23 Moreover, the rapid release of NETs requires neutrophil priming, again, consistent with the state of the extravasated cell. Since the response to fungi as shown here depends on CR3, the requirement for priming may also allow the translocation of the large intracellular store of this receptor to the cell surface to heighten the response. In a similar vein, the effect of Mn+2 is consistent with an integrin-mediated response as it maintains CR3 in the activated state. Moreover, neither immobilized Fn or Mn+2 is found in the circulation but is demonstrable in damaged or infected tissues and neither one alone effects the cellular response to fungal β-glucan. Together, the combinatorial requirements of matrix, cell priming and CR3 activation for a rapid NET response to fungal components is thoroughly consistent with a host defense mechanism selective for a host response within infected tissues and not within in the peripheral bloodstream.
Work shown here, and reported previously, identifies consistent regulation of neutrophil host defense functions when β-glucan is presented in the context of ECM as compared to cells exposed to either ligand alone. We showed cells migrating on β-glucan together with Fn resulted in a conversion from random to directed motility29 and a suppressed respiratory burst as compared to β-glucan alone.23 We now show promotion of homotypic aggregation and NET formation to ligand combinations suggesting a broad regulatory role for extracellular matrix in mediating host response to a fungal PAMP. Finding that NET formation in response to C. albicans hyphae or to immobilized β-glucan did not occur with poly-L-lysine in place of Fn (Figure 7B), suggests adhesion without matrix activation is an insufficient signal for rapid NET release to intact hyphae. The need for the ECM protein Fn in PMN aggregation and rapid NET formation is an unprecedented finding that renders insight into understanding this host defense mechanism against problematic fungal infections within tissues. It remains to be determined whether matrix proves to be a generalized regulator of the rapid NET response to other microbes.
Urban et al.16 found NETs released from PMA-activated neutrophils capture and kill C. albicans in the absence of exogenous ECM at time points beyond one hour. While their experiments did not include the addition of purified Fn, serum was present so a role for Fn in temporal regulation of NET formation to PMA cannot be dismissed. Moreover, they used a nonphysiological stimulant as opposed to C. albicans used in our current study. Because of this data and the preponderance of literature already showing killing, our contribution is to understand the mechanisms mediating this response. We have shown that NETs formed in response to immobilized Fn + β-glucan, as well as in response to intact C. albicans, have fungicidal activity (Figure 4B and 7A). The role of the respiratory burst is non-essential as inhibition with DPI, SOD, and catalase has no effect on the ability of PMNs to kill C. albicans hyphae (data not shown). In addition, there does not appear to be an essential role for complement, as NET production and hyphal killing experiements in the presence autologus serum showed robust NET production and percentages of hyphal viability that did not statistically differ from those in the absence of serum (data not shown). Future studies will recapitulate these data to corroborate the findings we have reported herein and to further understand the intricacies of NET formation and killing.
The NET response to β-glucan and Fn was found to depend on the integrin CR3 (CD11b/CD18) as antibody blockade of the integrin prevented aggregation and NET release (Figure 1B, 5 and 6). Using our reductionist system, we demonstrate that recognition of β-glucan by CR3, and not Dectin-1 (Figure 1B and 1C), in the context of Fn is necessary and sufficient for homotypic aggregation and hence NET production. This is consistent with the finding that purified recombinant Dectin-1 only recognizes β-glucan-containing or unicellular yeast particles and yeast but not hyphae35. Furthermore, blockade of β-glucan within C. albicans hyphae in the presence of Fn substantially reduced the NET response to the intact pathogen demonstrating that β-glucan is a key PAMP in the NET response (Figure 7B). Blockade of neutrophil CR3 prevented NET formation to both immobilized Fn + β-glucan and fungal hyphae (Figure 5 and 6, respectively). This is in support of van Bruggen et al.28 who showed that CR3, not Dectin-1, is the primary receptor on human neutrophils for the phagocytosis and response to β-glucan-containing particles. Therefore, our current study identified both the fungal ligand as well as the neutrophil counter-receptor that leads to NETosis.
Intracellular mechanisms leading to NET formation are not well understood. Neutrophil NET formation has been shown to occur after several hours of PMA treatment by a ROS-dependent mechanism11,15,17,19 even though PMA stimulates the respiratory burst instantaneously.36 An alternate rapid (5–60 min) pathway of NET formation to Staphylococcus aureus that is ROS-independent has also been described20 and resembles the kinetics of NET release we report here. We also find rapid, ROS-independent neutrophil aggregation and NET formation when cells adhere to immobilized Fn + β-glucan in ≤ 30 min and in ≤ 60 min in response to Fn + hyphae. Furthermore, we observe similar changes in the morphology of NET-producing neutrophils as described in that report 20 such as blebbing with extensive dilation between the inner and outer nuclear membranes containing strands of DNA with attached nucleosomes (Figures 4A).
In a previous report that is of particular relevance to the current study we showed that neutrophils undergo a robust respiratory burst to immobilized, purified fungal β-glucan, which is actively suppressed by extracellular matrix. This suppression was hypothesized to limit consequent tissue damage of migrating neutrophils until multifocal contact with hyphae is established.23 Herein, we tested the hypothesis that ECM plays a regulatory role in fungal NET formation under conditions that we showed previously prevents generation of a respiratory burst. Although contrary to other reports,11,13,15,17,19 we provide several lines of evidence for oxidant-independent NET formation. First, we have reported previously that the respiratory burst produced by neutrophils in response to immobilized β-glucan is suppressed to undetectable levels by Fn,23 conditions that were shown herein to be permissive for homotypic cell aggregation and NET formation. The use of DPI to block any vestigial ROS did not affect neutrophil aggregation and NET formation (Figure 8). Since DPI did not limit cell migration, aggregation or NET release, its use with neutrophils to assess the role of ROS in NET production was not pharmacologically contraindicated. Taken together with the biological inhibition of ROS by Fn + β-glucan our conclusions are not solely reliant on chemical inhibition of NADPH oxidase. Secondly, NETs were formed by neutrophils responding to C. albicans hyphae in the presence of DPI under conditions where production of ROS were inhibited (Figure 9A). Thirdly, ERK-inhibited cells responding to C. albicans hyphae were competent for respiratory burst, but NET formation was attenuated (Figure 9C). Interestingly, this data is in support of Dikshit et al. who demonstrate inhibition of ERK phosphorylation does not significantly reduce PMA-induced ROS production, but prevents the release of NETs19. Fourth, cells plated on immobilized β-glucan alone undergo the respiratory burst, but not clustering and hence do not release NETs. These lines of evidence show that the oxidative burst and rapid NET formation are uncoupled with respect to the response to fungal β-glucan. These data support the independence of ROS in mediating the fungicidal activity of NETs as previously discussed. We cannot rule out that at time points beyond one hour, NET formation may include oxidant-dependent mechanisms. It is noteworthy that a recent report shows that the requirement for oxidant production for NET formation is not absolute and depends on the stimulus. Keeping this in mind when contrasting our work to that of Dikshit et al.19 who attest a ROS dependent activation of ERK and MAPK in NET formation, time and stimulus are crucial differences, which might explain the apparent discrepancy in our reported results. Ligand:receptor complexes may ultimately determine the intracellular signaling that leads to NET release either in the presence or absence of ROS.
To begin to understand intracellular signaling mechanisms affected by cells responding to β-glucan supplemented with Fn, as compared to Fn alone, we undertook a quantitative global tyrosine phosphorylation approach (Reichner, unpublished data). Tyrosine kinases, particularly of the Src and Syk families, are activated upon ligation of β1 and β2 integrins and transduce signals that have functional effects on leukocytes38–41 hence phosphorylation of tyrosine is germane to our studies. From our analysis, we characterized phosphorylated ERK (Y204) as a potential regulator of PMN homotypic aggregates and NET formation. To begin to dissect the intracellular signaling mechanisms affected by cells responding to β-glucan supplemented with Fn, as compared to Fn alone, we took advantage of pharmacological inhibitors. For this study and the phosphoproteomic analysis, a 30 min time point was chosen since at this time we observed maximal cellular response to β-glucan supplemented Fn compared to Fn alone. This is considered an early step to mapping the signaling profile of human neutrophils upon binding to a PAMP, characterizing a single time point that will ultimately render an extensive pathway analysis.
In conclusion, we show promotion of homotypic aggregation and NET formation to ligand combinations suggesting a regulatory role for CR3 in mediating the host response to a fungal PAMP. In this work, we have found that this phenomenon depends on ERK MAPK, but is independent of the respiratory burst allowing NET production in the absence of ROS, which in turn may minimize collateral tissue damage. We have successfully shown a correlation between PMN defense mechanisms within a reductionist model and responses to a more physiologically relevant stimulus, C. albicans hyphae. Further work will recapitulate an in vivo representation, mitigating how diverse mechanisms converge, optimizing the host defense against pathological fungal infections.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Dr. Nicole Morin, Dr. Meredith Crane, Annalisa Wilde and Maggie Chung for their technical assistance, Carol Ayala (Core Research Laboratories at Rhode Island Hospital) for TEM, Brown University Leduc Bioimaging Facility, and Drs. Jorge Albina and Crane for critically reading the manuscript.
Footnotes
This work was supported by the NIH grant GM066194 (JSR); A.S. Byrd is supported by UNCF/Merck Graduate Science Research Dissertation Fellowship.
DISCLOSURES
Conflict-of-interest disclosure: The authors claim no competing financial interests.
REFERENCES
- 1.Aratani Y, Koyama H, Nyui S, Suzuki K, Kura F, Maeda N. Severe impairment in early host defense against Candida albicans in mice deficient in myeloperoxidase. Infect Immun. 1999;67:1828–1836. doi: 10.1128/iai.67.4.1828-1836.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bassetti M, Righi E, Costa A, Fasce R, Molinari MP, Rosso R, Pallavicini FB, Viscoli C. Epidemiological trends in nosocomial candidemia in intensive care. BMC Infect Dis. 2006;6:21–26. doi: 10.1186/1471-2334-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng MF, Yang YL, Yao TJ, Lin CY, Liu JS, Tang RB, Yu KW, Fan YH, Hsieh KS, Ho M, Lo HJ. Risk factors for fatal candidemia caused by Candida albicans and non-albicans candida species. BMC Infect Dis. 2005;5:22–26. doi: 10.1186/1471-2334-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fraser VJ, Jones M, Dunkel J, Storfer S, Medoff G, Dunagan WC. Candidemia in a tertiary care hospital: Epidemiology, risk factors, and predictors of mortality. Clin Infect Dis. 1992;15:414–421. doi: 10.1093/clind/15.3.414. [DOI] [PubMed] [Google Scholar]
- 5.Metzler KD, Fuchs TA, Nauseef WM, Reumaux D, Roesler J, Schulze I, Wahn V, Papayannopoulos V, Zychlinsky A. Myeloperoxidase is required for neutrophil extracellular trap formation: Implications for innate immunity. Blood. 2011;117:953–959. doi: 10.1182/blood-2010-06-290171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aratani Y, Kura F, Watanabe H, Akagawa H, Takano Y, Suzuki K, Dinauer MC, Maeda N, Koyama H. Relative contributions of myeloperoxidase and NADPH-oxidase to the early host defense against pulmonary infections with Candida albicans and Aspergillus fumigatus. Med Mycol. 2002;40:557–563. doi: 10.1080/mmy.40.6.557.563. [DOI] [PubMed] [Google Scholar]
- 7.Chen Y, Junger WG. Measurement of oxidative burst in neutrophils. Methods Mol Biol. 2012;844:115–124. doi: 10.1007/978-1-61779-527-5_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nathan C. Neutrophils and immunity: Challenges and opportunities. Nat Rev Immunol. 2006;6:173–182. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- 9.Nathan C, Srimal S, Farber C, Sanchez E, Kabbash L, Asch A, Gailit J, Wright SD. Cytokine-induced respiratory burst of human neutrophils: Dependence on extracellular matrix proteins and CD11/CD18 integrins. J Cell Biol. 1989;109:1341–1349. doi: 10.1083/jcb.109.3.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lehrer RI, Lu W. Alpha-defensins in human innate immunity. Immunol Rev. 2012;245:84–112. doi: 10.1111/j.1600-065X.2011.01082.x. [DOI] [PubMed] [Google Scholar]
- 11.Brinkmann V, Zychlinsky A. Beneficial suicide: Why neutrophils die to make NETs. Nat Rev Microbiol. 2007;5:577–582. doi: 10.1038/nrmicro1710. [DOI] [PubMed] [Google Scholar]
- 12.Amulic B, Hayes G. Neutrophil extracellular traps. Curr Biol. 2011;21:R297–R298. doi: 10.1016/j.cub.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 13.Lee WL, Grinstein S. Immunology. The tangled webs that neutrophils weave. Science. 2004;303:1477–1478. doi: 10.1126/science.1095484. [DOI] [PubMed] [Google Scholar]
- 14.Phillipson M, Kubes P. The neutrophil in vascular inflammation. Nat Med. 2011;17:1381–1390. doi: 10.1038/nm.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, Weinrauch Y, Brinkmann V, Zychlinsky A. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol. 2007;176:231–241. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Urban CF, Reichard U, Brinkmann V, Zychlinsky A. Neutrophil extracellular traps capture and kill Candida albicans yeast and hyphal forms. Cell Microbiol. 2006;8:668–676. doi: 10.1111/j.1462-5822.2005.00659.x. [DOI] [PubMed] [Google Scholar]
- 17.Papayannopoulos V, Metzler KD, Hakkim A, Zychlinsky A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J Cell Biol. 2010;191:677–691. doi: 10.1083/jcb.201006052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Urban CF, Ermert D, Schmid M, Abu-Abed U, Goosmann C, Nacken W, Brinkmann V, Jungblut PR, Zychlinsky A. Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Pathog. 2009;5:e1000639. doi: 10.1371/journal.ppat.1000639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keshari RS, Verma A, Barthwal MK, Dikshit M. Reactive oxygen species induced activation of ERK and p38 MAPK mediates PMA-induced NETs release from human neutrophils. J Cell Biochem. 2013;114:532–540. doi: 10.1002/jcb.24391. [DOI] [PubMed] [Google Scholar]
- 20.Pilsczek FH, Salina D, Poon KK, Fahey C, Yipp BG, Sibley CD, Robbins SM, Green FH, Surette MG, Sugai M, Bowden MG, Hussain M, Zhang K, Kubes P. A novel mechanism of rapid nuclear neutrophil extracellular trap formation in response to Staphylococcus aureus. J Immunol. 2010;185:7413–7425. doi: 10.4049/jimmunol.1000675. [DOI] [PubMed] [Google Scholar]
- 21.Xiang D, Sharma VR, Freter CE, Yan J. Anti-tumor monoclonal antibodies in conjunction with beta-glucans: a novel anti-cancer immunotherapy. Curr Med Chem. 2012;19:4298–4305. doi: 10.2174/092986712802884303. [DOI] [PubMed] [Google Scholar]
- 22.Lavigne LM, Albina JE, Reichner JS. Beta-glucan is a fungal determinant for adhesion-dependent human neutrophil functions. J Immunol. 2006;177:8667–8675. doi: 10.4049/jimmunol.177.12.8667. [DOI] [PubMed] [Google Scholar]
- 23.Lavigne LM, O'Brien XM, Kim M, Janowski JW, Albina JE, Reichner JS. Integrin engagement mediates the human polymorphonuclear leukocyte response to a fungal pathogen-associated molecular pattern. J Immunol. 2007;178:7276–7282. doi: 10.4049/jimmunol.178.11.7276. [DOI] [PubMed] [Google Scholar]
- 24.Goodridge HS, Wolf AJ, Underhill DM. Beta-glucan recognition by the innate immune system. Immunol Rev. 2009;230:38–50. doi: 10.1111/j.1600-065X.2009.00793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O'Brien XM, Heflin KE, Lavigne LM, Yu K, Kim M, Salomon AR, Reichner JR. Lectin site ligation of CR3 induces conformational changes and signaling. J Biol Chem. 2012;287:3337–3348. doi: 10.1074/jbc.M111.298307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsikitis VL, Albina JE, Reichner JS. Beta-glucan affects leukocyte navigation in a complex chemotactic gradient. Surgery. 2004;136:384–389. doi: 10.1016/j.surg.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 27.Leal SM, Jr, Vareechon C, Cowden S, Cobb BA, Latge JP, Momany M, Pearlman E. Fungal antioxidant pathways promote survival against neutrophils during infection. J Clin Invest. 2012;122:2482–2498. doi: 10.1172/JCI63239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Bruggen R, Drewniak A, Jansen M, van Houdt M, Roos D, Chapel H, Verhoeven AJ, Kuijpers TW. Complement receptor 3, not dectin-1, is the major receptor on human neutrophils for beta-glucan-bearing particles. Mol Immunol. 2009;47:575–581. doi: 10.1016/j.molimm.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 29.Harler MB, Wakshull E, Filardo EJ, Albina JE, Reichner JS. Promotion of neutrophil chemotaxis through differential regulation of beta 1 and beta 2 integrins. J Immunol. 1999;162:6792–6799. [PubMed] [Google Scholar]
- 30.Levitz SM, Diamond RD. A rapid colorimetric assay of fungal viability with the tetrazolium salt MTT. J Infect Dis. 1985;152:938–945. doi: 10.1093/infdis/152.5.938. [DOI] [PubMed] [Google Scholar]
- 31.Kennedy AD, Willment JA, Dorward DW, Williams DL, Brown GD, DeLeo FR. Dectin-1 promotes fungicidal activity in human neutrophils. Eur. J. Immunol. 2007;37:467–478. doi: 10.1002/eji.200636653. [DOI] [PubMed] [Google Scholar]
- 32.Whitlock BB, Gardai S, Fadok V, Bratton D, Henson PM. Differential roles for alpha(M)beta(2) integrin clustering or activation in the control of apoptosis via regulation of akt and ERK survival mechanisms. J Cell Biol. 2000;151:1305–1320. doi: 10.1083/jcb.151.6.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clark SR, Ma AC, Tavener SA, McDonald B, Goodarzi Z, Kelly MM, Patel KD, Chakrabarti S, McAvoy E, Sinclair GD, Keys EM, Allen-Vercoe E, Devinney R, Doig CJ, Green FH, Kubes P. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med. 2007;13:463–469. doi: 10.1038/nm1565. [DOI] [PubMed] [Google Scholar]
- 34.Ma AC, Kubes P. Platelets, neutrophils, and neutrophil extracellular traps (NETs) in sepsis. J Thromb Haemost. 2008;6:415–420. doi: 10.1111/j.1538-7836.2007.02865.x. [DOI] [PubMed] [Google Scholar]
- 35.Ganter BN, Simmons RM, Underhill DM. Dectin-1 mediates macrophage recognition of Candida albicans yeast but not filaments. EMBO J. 2005;24:1277–1286. doi: 10.1038/sj.emboj.7600594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Steinberg BE, Grinstein S. Unconventional roles of the NADPH oxidase: Signaling, ion homeostasis, and cell death. Sci STKE. 2007;2007:pe11. doi: 10.1126/stke.3792007pe11. [DOI] [PubMed] [Google Scholar]
- 37.Parker H, Dragunow M, Hampton MB, Kettle AJ, Winterbourn CC. Requirements for NADPH oxidase and myeloperoxidase in neutrophil extracellular trap formation differ depending on the stimulus. J Leukoc Biol. 2012;92:841–849. doi: 10.1189/jlb.1211601. [DOI] [PubMed] [Google Scholar]
- 38.Mocsai A, Zhou M, Meng F, Tybulewicz VL, Lowell CA. Syk is required for integrin signaling in neutrophils. Immunity. 2002;16:547–558. doi: 10.1016/s1074-7613(02)00303-5. [DOI] [PubMed] [Google Scholar]
- 39.Miranti CK, Brugge JS. Sensing the environment: A historical perspective on integrin signal transduction. Nat Cell Biol. 2002;4:E83–E90. doi: 10.1038/ncb0402-e83. [DOI] [PubMed] [Google Scholar]
- 40.Berton G, Lowell CA. Integrin signalling in neutrophils and macrophages. Cell Signal. 1999;11:621–635. doi: 10.1016/s0898-6568(99)00003-0. [DOI] [PubMed] [Google Scholar]
- 41.Gakidis MA, Cullere X, Olson T, Wilsbacher JL, Zhang B, Moores SL, Ley K, Swat W, Mayadas T, Brugge JS. Vav GEFs are required for beta2 integrin-dependent functions of neutrophils. J Cell Biol. 2004;166:273–282. doi: 10.1083/jcb.200404166. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.