Abstract
Premalignant oral lesions have a high incidence of recurrence and progression to malignant disease and, although studies have shown the contribution of transforming growth factor β (TGF-β) to cancer progression, none have been conducted with premalignant oral lesion cells to determine the impact of TGF-β in stimulating properties that are characteristic of more invasive cells. The present study focused on TGF-β-modulation of paxillin and the serine/threonine protein phosphatase PP-1, and the impact on cellular motility. These studies show that TGF-β stimulates premalignant lesion cell motility and up regulates expression of paxillin, as well as its co-localization with PP-1, while concurrently diminishing the level of paxillin serine phosphorylation. The TGF-β-mediated up regulation of paxillin and co-localization with actin, as well as the TGF-β-stimulated motility of premalignant lesion cells, were all blocked by inhibiting PP-1, indicating their dependence on PP-1 activity. These studies suggest interplay between TGF-β and PP-1 in promoting a more malignant phenotype in premalignant oral lesion cells.
Keywords: Cytoskeleton, paxillin, phosphatase, PP-1, TGF-β
Prior studies have shown transforming growth factor β (TGF-β) to be a mediator in cancer progression. For example, TGF-β can promote the epithelial to mesenchymal transition, stimulate motility and prevent cell cycle arrest in malignant prostatic epithelial cells and esophageal cells (1, 2). Blocking TGF-β signaling reduces the motility of bladder cancer cells (3). The motility and invasiveness of pancreatic cancer cells is stimulated by TGF-β through its down-regulation of phosphatase and tensin homolog (PTEN) expression (4).
Premalignant oral lesions, the most common being leukoplakias, have a high incidence of recurrence and progression to malignant disease. However, despite studies showing the contribution of TGF-β to cancer progression, studies have not been conducted with premalignant oral lesion cells to determine the impact of TGF-β in stimulating properties that are characteristic of more invasive cells. These properties include an increased capacity to migrate and to invade. Tumor invasion requires dissociation from other cells and degradation of the extracellular matrix (5). Motility is also critical to invasion. Studies with various tumor types have shown that more invasive tumor cells are more highly motile (6-8). Motility involves assembly and dissolution of focal adhesions, where the actin skeleton converges with integrins and an interconnection of proteins such as α-actinin, vinculin and paxillin (9, 10). This increased motility also involves an increase in cytoskeletal polymerization and depolymerization. Alterations to the cytoskeleton or cytoskeletal-associated proteins in turn influence cancer spreading (11, 12).
The integrity of the cytoskeletal architecture and focal adhesions is tightly regulated by phosphorylation reactions. These include the formation of complexes between kinases such as Src, focal adhesion kinase (FAK) and the scaffolding protein paxillin (13-15). Dephosphorylation reactions mediated by protein phosphatases also regulate the cytoskeletal integrity and, in turn, motility. For example, cell spreading and migration involve a complex interaction between the scaffolding protein paxillin and the protein tyrosine phosphatase PTP-PEST (10). The serine/threonine protein phosphatase PP-2A co-localizes with focal adhesion complexes, but a decline in PP-2A causes their destabilization and increased cell motility (13, 16). Less attention has been given to the serine/threonine protein phosphatase PP-1 and its regulation of the cytoskeletal organization and motility of cancer cells. However, studies with endothelial cells have shown that the inter-relationship between paxillin and PP-1 regulates cell motility, with paxillin being a direct target for PP-1-mediate dephosphorylation (17).
The present study aimed to assess the inter-relationship between the cytoskeletal scaffolding protein paxillin and PP-1. Since TGF-β has a prominent role in cancer progression, this study focused on the modulation of paxillin and PP-1 in premalignant oral lesion cells in the context of the impact on cellular motility.
Materials and Methods
Cells and media
A primary epithelial cell line was generated from premalignant oral lesions induced in C57BL/6 mice by 4-nitroquinolone oxide exposure and was used for all studies. Cells were grown in Dulbecco's Modified Eagle's Medium (DMEM) (Invitrogen, Carlsbad, VA, USA) supplemented with 10% heat-inactivated fetal bovine serum (FBS), 100 U/ml penicillin, 100 μg/ml streptomycin, 0.02 M HEPES buffer, 2 mM L glutamine, and 5×105 M 2 mercaptoethanol in a water-jacketed incubator at 37°C in 5% CO2. Cells were passaged at near-confluence. A 0.05% trypsin, 0.53 mM EDTA solution (Invitrogen) was used to detach cells from the culture flasks for regular cell passage.
Treatments
Prior to use, premalignant lesion cells were cultured for 24 hours in reduced-serum DMEM containing 0.5% FBS. The cells were then treated with recombinant human TGF-β1 (R&D Systems, Minneapolis, MN, USA) and/or with 500 nM tautomycetin (Tocris, Ellisville, MO, USA) as a selective PP-1 inhibitor. The DMEM was used as the diluent control for tautomycetin.
Transwell migration assay
Premalignant lesion cells that were incubated in serum-reduced medium for 24 hours were detached with Accutase (Invitrogen) and plated at a density of 5X104 cells into the top compartment of a transwell migration chamber. Both the upper and lower wells contained diluent or TGF-β and/or 500 nM tautomycetin in reduced-serum DMEM. After overnight migration, the premalignant cells were collected from the lower compartment of the chamber and the relative number of cells was determined using CellTiter 96 AQueous Non-Radioactive Cell Proliferation Assay (Promega, Madison, WI, USA). The relative optical density of the colored product correlates to the number of viable cells.
Phosphatase assay
PP-1 activity was measured using the ProFluor serine/threonine phosphatase assay kit (Promega) containing appropriate salts selective for PP-1 activity. Purified PP-1 (Millipore, Millerica, MA, USA) was used for the standard curve. Endothelial cell lysates were generated in RIPA buffer on ice. Lysates were measured for protein content and equal quantities of protein were diluted in the provided dilution buffer containing appropriate salts for PP-1. A selective inhibitor of PP-2A was added to lysates to eliminate non-specific activity. To each sample and standard, a fluorescently labeled substrate was added for 10 minutes. The reaction was stopped using the supplied protease buffer, releasing the fluorescent tag from dephosphorylated substrates. Fluorescence was measured on a SpectraMax M2 microplate reader (Molecular Devices, Sunnyvale, CA, USA). Phosphatase activity was calculated as mU/μl. In this assay 1 U is equivalent to 1 nmol phosphate released per minute.
Immunoblotting
Premalignant lesion cells were rinsed with cold PBS and removed by scraping in the presence of RIPA buffer containing 1× Phos STOP and MiniComplete phosphatase protease inhibitor cocktails (Roche, Basel, Switzerland). Lysates were vortexed and incubated for 10 minutes on ice. Cell debris was pelleted by centrifugation at 14,000 rpm for 30 minutes at 4°C. Protein content of the lysates was measured using a BCA protein assay (Pierce, Rockford, IL, USA) to equalize protein levels. For lysates being used for immunoprecipitation, 250 μg of sample was mixed with 50 μl magnetically labeled Protein G microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany) and 2 μg of mouse anti paxillin antibody (BD Biosciences, San Diego, CA, USA). The mixture was incubated for 30 minutes on ice. The microbeads were placed on a magnetic separation column and flow through fractions were collected. The beads were washed four times with RIPA buffer and twice with 25 mM TRIS buffer, and then eluted for separation by sodium dodecyl sulfate polyacrylamide gel electrophoresis in hot Laemelli sample buffer. Protein bands were resolved on 10% or 7.5% polyacrylamide gel after passing through a 3% stacking gel. Proteins were transferred to nitrocellulose membranes for immunoblotting. Membranes were blocked for 30 minutes at 37°C in 5% BSA in TRIS-buffered saline with 0.5% Tween-20 (TBST). Primary antibodies were added to the blocking solution and incubated overnight at 4° C. Membranes were washed three times for 10 minutes in TBST and then incubated for one hour at room temperature with the appropriate horseradish peroxidase (HRP) tagged secondary antibody dissolved in a 5% solution of BSA in TBST. Antibodies used in these studies were against β actin (1:1,000; Santa Cruz Biotechnology), phosphoserine (1:500, Calbiochem, San Diego, CA, USA), paxillin (1:2,000; BD Biosciences), pan-specific PP-1c (1:200; Santa Cruz Biotechnology, Santa Cruz, CA, USA), HRP-goat anti-mouse IgG (1:1,000; Millipore), and HRP-goat anti mouse IgM (1:1,000; Invitrogen). An enhanced chemiluminescence (ECL) reagent was used to detect antibody tagged proteins using the VersaDoc Imaging System (Bio Rad, Hercules, CA, USA).
Results
TGF-β treatment up-regulates paxillin expression, phosphorylation and its co-localization with actin in oral premalignant lesion cells
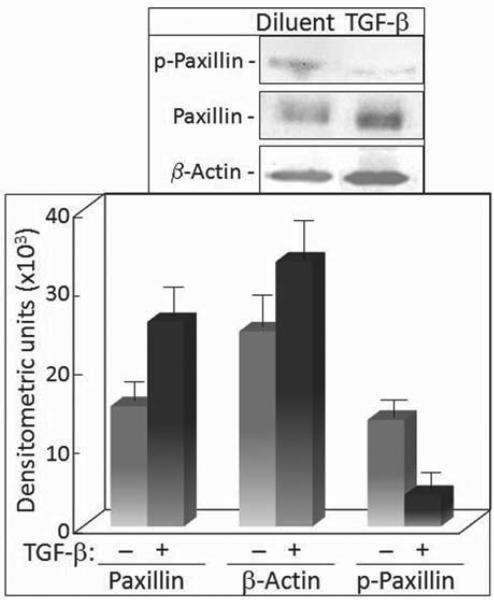
Prior studies showing TGF-β to be a mediator in cancer progression prompted determination of the inter-relationships between TGF-β and key cytoskeletal components, actin and paxillin, which are involved in cellular motility, an important characteristic of malignant cells. This was tested with cells from premalignant oral lesions which have a high frequency of progressing towards malignancy. Premalignant lesion cells were serum deprived and then treated with 5 ng/ml TGF-β, a dose that has previously been shown to be stimulatory of motility of several cell types (1, 17). Paxillin was then immunoprecipitated from cell lysates and analyzed by immunoblotting for the effects of TGF-β on the level of paxillin and co-precipitating actin. Treatment with TGF-β caused a significant increase in levels of paxillin in premalignant lesion cells (Figure 1). Concurrently, there was an increase in levels of actin that co-precipitated with paxillin. While the level of actin that co-precipitated with paxillin increased in TGF-β-treated cells, this increase was not greater than the increase in levels of paxillin. What was unexpected was a prominent decrease in the level of serine phosphorylated paxillin. While paxillin from control premalignant lesion cells had a clearly detectable level of serine phosphorylation, TGF-β treatment caused a prominent loss of phosphorylation.
Figure 1.
Analyses of paxillin immunoprecipitates for levels of paxillin, actin and serine-phosphorylated paxillin in TGF-β-treated cells. Top panel: Representative Western blots following treatment of premalignant oral lesion cells with 5 ng/ml TGF-β for 24 hours. Paxillin immunoprecipitates from these treated cells were separated and blotted with antibodies to paxillin, actin, and phosphoserine. Lower panel: Mean densitometric scans of 3 separate immunoprecipitates and immunoblots from TGF-β-treated premalignant lesion cells. Data shown are means±SD.
TGF-β treatment up-regulates co-localization of PP-1 with paxillin in oral premalignant lesion cells
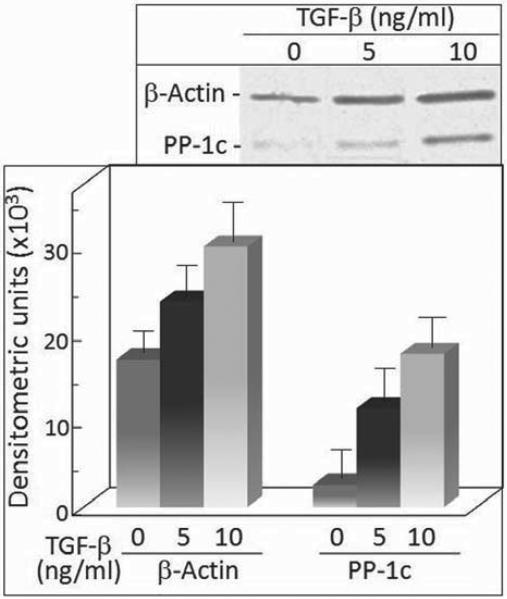
Paxillin has previously been shown to be a target of the serine/threonine phosphatase PP-1 (17). Thus, the TGF-β-induced decrease in the level of serine phosphorylated paxillin described above prompted assessment of whether PP-1 co-localizes with paxillin and the effect of TGF-β on that co-localization. Co-localization of PP-1 with paxillin was detected when probing for the catalytic PP-1 subunit in paxillin immunoprecipitates. As shown in Figure 1, treatment of premalignant lesion cells with TGF-β resulted in an increase in the level of actin that co precipitated with paxillin (Figure 2). Treatment with 10 ng/ml TGF-β further increased the level of actin that co-precipitated with paxillin over the level seen for cells treated with 5 ng/ml TGF-β. Of interest is that PP-1 was shown to co-localize with paxillin and that treatment with TGF-β resulted in a dose-dependent increase in the level of the PP-1 catalytic subunit that co-precipitated with paxillin.
Figure 2.
Analyses of paxillin immunoprecipitates for levels of paxillin and co-precipitated PP-1 in TGF-β-treated cells. Top panel: Representative Western blots following treatment of premalignant oral lesion cells with TGF-β for 24 hours. Paxillin immunoprecipitates from these treated cells were separated and blotted with anti-paxillin and antibody to the catalytic subunit of PP-1. Lower panel: Mean densitometric scans of 3 separate immunoprecipitates and immunoblots from TGF-β-treated premalignant lesion cells. Data shown are means±SD.
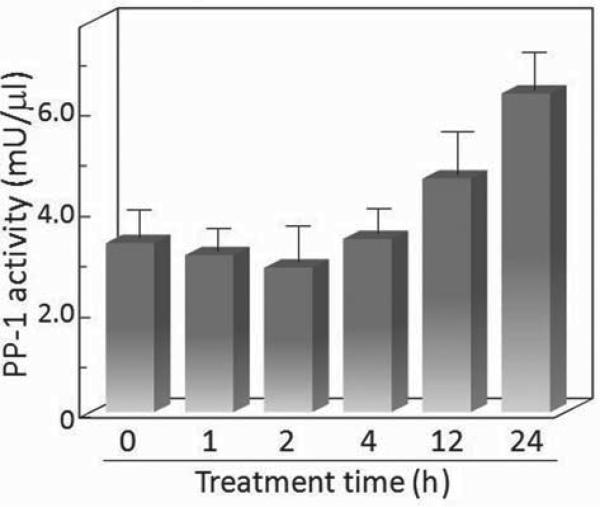
While it would be ideal to determine in a functional manner whether the level of PP-1 enzyme activity that is associated with paxillin increases in accordance with the increase in co-precipitated PP-1, this is not technically feasible. Instead, the overall level of PP-1 enzyme activity in control and TGF-β-treated cells was measured after different durations of exposure to TGF-β (Figure 3). While there was not a significant initial impact of TGF-β treatment on PP-1 activity, there was an increase at 12 hours after initiating treatment. The PP-1 enzyme activity became further elevated after 24 hours of treatment. This was consistent with the increase in protein levels of the PP-1 catalytic subunit associated with paxillin after 24 hours of TGF-β treatment.
Figure 3.
TGF-β increases PP-1 enzyme activity in premalignant oral lesion cells. Premalignant lesion cells were treated with 5 ng/ml TGF-β and, after various time increments, lysed and used to measure PP-1 enzyme activity. Data shown are means of triplicate assays + SEM.
PP-1 inhibition blocks TGF-β-mediated up-regulation of paxillin expression and reduces co-localization of paxillin with actin
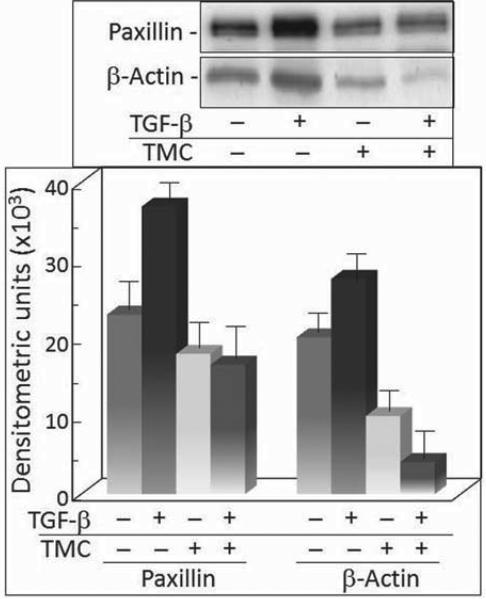
The above studies showing an up-regulation of paxillin and the level of co-precipitated actin and PP-1 following treatment of premalignant oral lesion cells with TGF-β stimulated the assessment of whether the up-regulation of paxillin and the associated actin level was dependent on PP-1 activity. This was determined by treating serum-deprived premalignant lesion cells with TGF-β in the presence or absence of the PP-1 inhibitor tautomycetin. As seen in Figures 1 and 2, TGF-β treatment increased expression of paxillin and the level of actin that co-precipitated with paxillin (Figure 4). Treatment with tautomycetin alone did not affect the protein level of paxillin, but it prevented the TGF-β stimulation of paxillin that was seen in premalignant lesion cells treated with diluent instead of tautomycetin. While actin co-precipitated with paxillin in control premalignant lesion cells, the level of actin that co-precipitated with paxillin in cells treated with tautomycetin was significantly reduced, suggesting a dependence on PP-1 for the actin and paxillin co-localization. Furthermore, treatment of premalignant lesion cells with tautomycetin blocked the TGF-β-stimulated increase in actin co-precipitation with paxillin seen in cells treated with diluent instead of tautomycetin.
Figure 4.
TGF-β-mediated increases in paxillin protein levels and co precipitation of actin are dependent on PP-1. Top panel: Representative Western blots following treatment of premalignant oral lesion cells with 5 ng/ml TGF-β and/or 500 nM tautomycetin (TMC). Paxillin immunoprecipitates were blotted with anti-paxillin and anti-actin antibodies. Bottom panel: Mean densitometric scans of 3 separate immunoprecipitates and immunoblots from TGF-β-treated premalignant lesion cells. Data shown are means±SD.
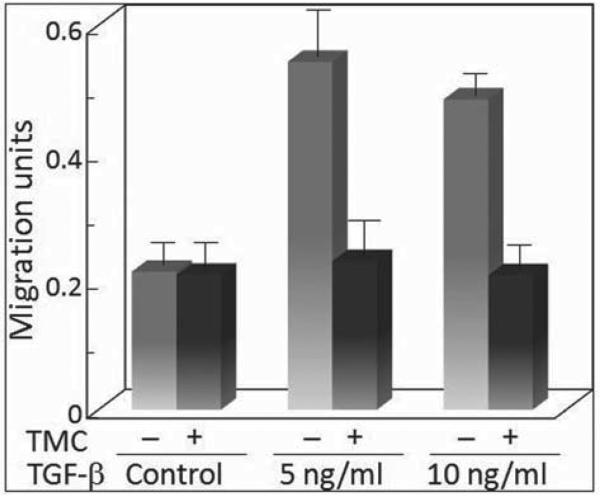
TGF-β stimulation of premalignant lesion cell migration and its dependence on PP-1
Since exposure to TGF-β results in a modulation of co-localization of actin with the cytoskeletal scaffolding protein paxillin in a PP-1-dependent manner, studies assessed the interplay between TGF-β and PP-1 in regulating the motility of premalignant lesion cells. The presence of TGF-β stimulated the motility of premalignant lesion cells (Figure 5). This was seen for both 5 and 10 ng/ml TGF-β, although the level of stimulated motility was not further increased by increasing the TGF-β level from 5 to 10 ng/ml. Inhibition of PP-1 activity by treatment with tautomycetin had no effect on the basal level of premalignant lesion cell motility. However, the motility that was stimulated by either 5 or 10 ng/ml TGF-β was completely blocked by inhibition of PP-1. These results are consistent with the Western blot analyses described above showing that the modulation of actin co-localization of actin with paxillin was dependent on PP-1.
Figure 5.
TGF-β–mediated premalignant lesion cell migration is PP-1 dependent. The migration of premalignant lesion cells from the upper compartment to the lower compartment of a transwell migration chamber was assessed in the presence of various doses of TGF-β with or without 500 nM tautomycetin (TMC) to inhibit PP-1 activity. Migration is expressed as densitometric units that are proportional to cell numbers. Error bars represent SEM for triplicate measurements in 3 separate experiments.
Discussion
Since TGF-β has a prominent role in cancer progression, with increased motility being among the hallmarks of more malignant cells, the present study focused on the modulation of motility and the focal adhesion scaffolding protein, paxillin, by TGF-β in premalignant lesion cells. These studies showed that TGF-β stimulates motility of premalignant lesion cells and up regulates paxillin expression, as well as its co-localization with actin and the serine/threonine protein phosphatase, PP-1. In fact, the overall cellular PP-1 activity increased following TGF-β treatment. Consistent with the up-regulation of PP-1 expression and its co-localization with paxillin was a TGF-β-induced reduction in the level of serine phosphorylation of paxillin. Paxillin phosphorylation occurs on tyrosine, serine and threonine residues. Tyrosine phosphorylation has been the primary focus of research as it appears to be most important in the coordination of paxillin with FAK and other kinase and second messenger proteins, with functional consequences on cellular migration (10). The function of serine and threonine phosphorylation has been shown to carry an equally important task of regulating cellular motility and adhesiveness (18, 19). Thus, the balance in the level of paxillin phosphorylation regulates focal adhesions to anchor cells and focal adhesion turnover for cell migration.
Since paxillin has previously been shown to be a target for dephosphorylation by PP-1 and since PP-1 co-localization with paxillin was increased upon TGF-β treatment, studies determined whether the TGF-β-mediated up-regulation of paxillin and increased motility were dependent on PP-1 activity. Studies to block PP-1 activity with tautomycetin showed that the TGF-β-stimulated up-regulation of paxillin levels and its co-localization with actin was dependent on PP-1 activity.
Consistent with this dependence on PP-1 for TGF-β modulation of the co-localization of paxillin with actin was the dependence on PP-1 for the TGF-β stimulation of motility of premalignant lesion cells. Cell motility is a very specific coordination of a multitude of signaling events. The inhibition of PP-1 activity by tautomycetin may dampen the activity needed to form new adhesion sites at the leading edge to allow stimulated migration. Studies to assess this possibility have not yet been conducted.
While the present studies showed that the TGF-β-mediated up-regulation of paxillin and its co-localization with actin and PP-1 are dependent on PP-1 activity, this does not exclude the involvement of other phosphatases in the regulation of cytoskeletal organization and motility of premalignant lesion cells. This includes the serine/threonine phosphatase PP-2A which has been shown to restrict cellular motility, including that of malignant cells and endothelial cells (20-22). Whether or not PP-2A, in addition to PP-1, also regulates TGF-β modulation of cytoskeletal organization and motility in premalignant lesion cells was not addressed in this study. However, this possibility cannot be excluded since in a prior study, we showed that treatment of endothelial cells with TGF-β together with prostaglandin E2 diminished PP-2A activity and stimulated cellular motility (22).
While it was beyond the scope of this study to define the mechanisms by which TGF-β modulated PP-1 levels, it was clearly demonstrated that PP-1 is an intermediate regulator in the modulation of paxillin and its association with the actin cytoskeletal network. It also demonstrated PP-1 as an intermediate in the TGF-β-mediated regulation of premalignant lesion cell motility. Since increased motility is a hallmark of more malignant cells, these studies suggest PP-1 as an intermediate contributor to the role of TGF-β in cancer progression.
Acknowledgements
This study is part of the doctoral dissertation of Jarrett Walsh. This study was supported by the Clinical Science and Biomedical Laboratory Research and Development Services of the Department of Veterans Affairs and by grants R01 DE018168 and R01CA128837 from the National Institutes of Health to MRIY.
Footnotes
This article is freely accessible online.
References
- 1.Rees JR, Onwuegbusi BA, Save VE, Alderson D, Fitzgerald RC. In vivo and in vitro evidence for transforming growth factor-β1-mediated epithelial to mesenchymal transition in esophageal adenocarcinoma. Cancer Res. 2006;66:9583–9590. doi: 10.1158/0008-5472.CAN-06-1842. [DOI] [PubMed] [Google Scholar]
- 2.Ao M, Williams K, Bhowmick NA, Hayward SW. Transforming growth factor-β promotes invasion in tumorigenic but not in nontumorigenic human prostatic epithelial cells. Cancer Res. 2006;66:8007–8016. doi: 10.1158/0008-5472.CAN-05-4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Y, Yang K, Mao Q, Zheng X, Kong D, Xie L. Inhibition of TGF-β receptor I by siRNA suppresses the motility and invasiveness of T24 bladder cancer cells via modulation of integrins and matrix metalloproteinase. Int Urol Nephrol. 2010;42:315–323. doi: 10.1007/s11255-009-9620-3. [DOI] [PubMed] [Google Scholar]
- 4.Chow JYC, Dong H, Quach KT, Van Nguyen PN, Chen K, Carethers JM. TGF-β mediates PTEN suppression and cell motility through calcium-dependent PKC-α activation in pancreatic cancer cells. Am J Physiol Gastrointest Liver Physiol. 2008;294:G899–G905. doi: 10.1152/ajpgi.00411.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim ES, Kim JS, Kim SG, Hwang S, Lee CH, Moon A. Sphingosine 1-phosphate regulates matrix metalloproteinase-9 expression and breast cell invasion through S1P3-Gαq coupling. J Cell Sci. 2011;124:2220–2230. doi: 10.1242/jcs.076794. [DOI] [PubMed] [Google Scholar]
- 6.Young MRI, Liu SW, Meisinger J. Differences in association of the serine/threonine protein phosphatase PP-2A with microtubules of metastatic and nonmetastatic tumor cells. Clin Exp Metastasis. 2001;18:407–413. doi: 10.1023/a:1010934106651. [DOI] [PubMed] [Google Scholar]
- 7.Schonrath K, Pan W, Klein-Szanto AJ, Braunewell KH. Involvement of VILIP-1 (visinin-like protein) and opposite roles of cyclic AMP and GMP signaling in in vitro cell migration of murine skin squamous cell carcinoma. Mol Carcinog. 2011;50:319–333. doi: 10.1002/mc.20707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crosswell HE, Dasgupta A, Alvarado CS, Watt T, Christensen JG, De P, Durden DL, Findley HW. PHA665752, a small-molecule inhibitor of c-Met, inhibits hepatocyte growth factor-stimulated migration and proliferation of c-Met-positive neuroblastoma cells. BMC Cancer. 2009;9:411. doi: 10.1186/1471-2407-9-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laukaitis CM, Webb DJ, Donais K, Horwitz AF. Differential dynamics of α5 integrin, paxillin, and α-actinin during formation and disassembly of adhesions in migrating cells. J Cell Biol. 2001;153:1427–1440. doi: 10.1083/jcb.153.7.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jamieson JS, Tumbarello DA, Halle M, Brown MC, Tremblay ML, Turner CE. Paxillin is essential for PTP-PEST-dependent regulation of cell spreading and motility: a role for paxillin kinase linker. J Cell Sci. 2005;118:5835–5847. doi: 10.1242/jcs.02693. [DOI] [PubMed] [Google Scholar]
- 11.Zhu H, Zhao J, Zhu B, Collazo J, Gal J, Shi P, Liu L, Strom AL, Lu X, McCann RO, Toborek M, Kyprianou N. EMMPRIN regulates cytoskeleton reorganization and cell adhesion in prostate cancer. Prostate. 2011 doi: 10.1002/pros.21408. in press (published online May 11, 2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abiatari I, Esposito I, Oliveira TD, Felix K, Xin H, Penzel R, Giese T, Friess H, Kleeff J. Moesin-dependent cytoskeleton remodelling is associated with an anaplastic phenotype of pancreatic cancer. J Cell Mol Med. 2010;14:1166–1179. doi: 10.1111/j.1582-4934.2009.00772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jackson JL, Young MR. Protein phosphatase-2A modulates the serine and tyrosine phosphorylation of paxillin in Lewis lung carcinoma tumor variants. Clin Exp Metastasis. 2002;19:409–415. doi: 10.1023/a:1016385027013. [DOI] [PubMed] [Google Scholar]
- 14.Leve F, de Souza W, Morgado-Diaz JA. A cross-link between protein kinase A and rho-family GTPases signaling mediates cell-cell adhesion and actin cytoskeleton organization in epithelial cancer cells. J Pharmacol Exp Ther. 2008;327:777–788. doi: 10.1124/jpet.108.140798. [DOI] [PubMed] [Google Scholar]
- 15.Franzen CA, Amargo E, Todorovic V, Desai BV, Huda S, Mirzoeva S, Chiu K, Grzybowski BA, Chew TL, Green KJ, Pelling JC. The chemopreventive bioflavonoid apigenin inhibits prostate cancer cell motility through the focal adhesion kinase/Src signaling mechanism. Cancer Prev Res. 2009;2:830–841. doi: 10.1158/1940-6207.CAPR-09-0066. [DOI] [PubMed] [Google Scholar]
- 16.Young MRI, Kolesiak K, Meisinger J. Protein phosphatase-2A regulates endothelial cell motility and both the phosphorylation as well as stability of focal adhesion complexes. Int J Cancer. 2002;100:276–282. doi: 10.1002/ijc.10491. [DOI] [PubMed] [Google Scholar]
- 17.Walsh JE, Young MR. Interrelationship between protein phosphatase 1 and TGF-β in regulating motility and cytoskeletal architecture of endothelial cells. Anticancer Res. 2010;30:4861–4866. [PMC free article] [PubMed] [Google Scholar]
- 18.Romashko AA, Young MRI. Protein phosphatase-2A maintains focal adhesion complexes in keratinocytes and the loss of this regulation in squamous cell carcinomas. Clin Exp Metastasis. 2004;21:371–379. doi: 10.1023/b:clin.0000046178.08043.f8. [DOI] [PubMed] [Google Scholar]
- 19.Young MR, Liu SW, Meisinger J. Protein phosphatase-2A restricts migration of Lewis lung carcinoma cells by modulating the phosphorylation of focal adhesion proteins. Int J Cancer. 2003;103:38–44. doi: 10.1002/ijc.10772. [DOI] [PubMed] [Google Scholar]
- 20.Sontag JM, Sontag E. Regulation of cell adhesion by PP2A and SV40 small tumor antigen: an important link to cell transformation. Cell Mol Life Sci. 2006;63:2979–2991. doi: 10.1007/s00018-006-6300-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu L, Deng X. Suppression of cancer cell migration and invasion by protein phosphatase 2A through dephosphorylation of μ- and m-calpains. J Biol Chem. 2006;281:35567–35575. doi: 10.1074/jbc.M607702200. [DOI] [PubMed] [Google Scholar]
- 22.Young MR. Tumor-derived prostaglandin E2 and transforming growth factor-β stimulate endothelial cell motility through inhibition of protein phosphatase-2A and involvement of PTEN and phosphatidylinositide 3-kinase. Angiogenesis. 2004;7:123–131. doi: 10.1007/s10456-004-1027-2. [DOI] [PubMed] [Google Scholar]