Abstract
Purpose
Ofatumumab (OFA) is an anti-CD20 antibody recently approved for treatment of fludarabine and alemtuzumab refractory chronic lymphocytic lymphomia (CLL); it mediates much stronger complement-dependent cytotoxicity (CDC) than Rituximab (RTX). Human CD59, a key membrane complement regulator that inhibits CDC, is highly expressed in B-cell malignancies and its up-regulation is an important determinant of the sensitivity of B-cell malignancies to RTX treatment. Previously, we have demonstrated that the potent CD59 inhibitor rILYd4 sensitizes RTX-resistant lymphoma cells to RTX-mediated CDC. Here, we further investigated whether rILYd4 can sensitize B-cell malignancies to OFA-mediated CDC and whether either OFA-mediated CDC or rILYd4-enhanced OFA-mediated CDC correlates with CD20 or CD59 expression, known biomarkers involved in RTX activity.
Experimental Design
RTX-resistant cell lines and primary CLL cells were used to investigate the antitumor efficacy of the combination of rILYd4 with OFA or RTX. Propidium iodide staining or Alamar blue assay were employed to evaluate the CDC effect. The levels of CD20 and CD59 on the cell membrane were analyzed by flow cytometry.
Results
rILYd4 enhanced CDC effects mediated by OFA or RTX on RTX-resistant lymphoma cells and primary CLL cells in vitro. The sensitivity to CDC effects mediated by OFA positively correlated with the ratio of CD20/CD59 and negatively correlated with CD59 levels on CLL cells. The degree to which rILYd4 enhanced CDC correlated positively with the CD59 levels on CLL cells.
Conclusions
These data suggest that rILYd4 may enhance the anti-cancer activity of OFA and RTX in B-cell malignancies that have relapsed after prior antibody-based therapies.
Keywords: Ofatumumab, Rituximab, Complement, CD59, Intermedilysin, chronic lymphocytic leukemia
Background
In the past 10 years, Rituximab (RTX), a chimeric anti-CD20 antibody, has led to significant progress in treating B-cell malignancies(1–5). However, RTX efficacy remains variable and often modest when used as a single agent. Half of Non-Hodgkin’s Lymphoma (NHL) patients are unresponsive to RTX(4, 6). Some responsive NHL patients develop resistance to further treatment(4, 7). When used as a single agent, RTX in chronic lymphocytic leukemia (CLL) is less efficacious than in indolent NHL, and in the relapsed setting has little activity(3, 8–14). Although the addition of RTX to regimens such as fludarabine with or without cyclophosphamide has been demonstrated to improve the overall and complete response rates and prolong survival in patients with CLL, the disease remains incurable(5).
Recently, a new, human IgG1 anti-CD20 monoclonal antibody, Ofatumumab (OFA), has been developed. In October, 2009, the U.S. Food and Drug Administration (FDA) granted accelerated approval to OFA for the treatment of patients with CLL refractory to fludarabine and alemtuzumab(15). The most recent clinical trial demonstrated that single-agent OFA was well tolerated with an overall response rate of 11% in heavily pretreated patients with relapsed or progressive NHL, nearly all of whom had received prior RTX therapy(16). The overall response rate with single-agent OFA was 51% in CLL patients refractory to fludarabine and alemtuzumab and 44% in the CLL patients refractory to fludarabine with bulky (>5 cm) lymphadenopathy. Although OFA has a high response rate in the treatment of CLL, the progression free survival remains quite short(17). NHL and CLL patients inevitably relapse and become increasingly refractory to further treatment(2, 16, 17). A current strategy to enhance the efficacy of OFA and RTX in the treatment of B-cell malignancies is to combine with other agents such as chlorambucil, cyclophosphamide, doxorubicin, vincristine, ifosfamide, carboplatin, cisplatin, and others(11).
OFA targets a membrane-proximal epitope encompassing both the large and the small loops of the CD20 molecule. RTX targets a different region involving only the large loop(18, 19). The mechanisms by which OFA kills B-cell cancers are suspected to involve complement-dependent cytotoxicity (CDC), antibody-dependent cellular cytotoxicity (ADCC) and direct cell death(1, 11, 20–22). Although RTX and OFA have comparable binding affinities to CD20, OFA induces much stronger CDC than RTX(18, 19). This may be due to the lower off rate from CD20 of OFA compared to RTX(18, 19). Additionally, OFA induces ADCC and direct cell death at levels comparable to RTX(18, 19). In vitro, OFA requires approximately 10-fold fewer cell surface CD20 molecules than RTX to induce detectable CDC(9, 19, 23, 24). A comparison study in a xenograft mouse tumor model showed that OFA is more effective in controlling lymphoma growth than RTX(25). These results indicate that OFA’s ability to induce CDC may play a critical role in OFA-mediated cancer therapy.
When a therapeutic antibody activates the classical complement pathway, it triggers the formation of complement membrane attack complex (MAC) on cancer cells leading to the killing of cells through CDC(4). CD59, a critical membrane complement regulator, inhibits MAC formation by binding to complement proteins 8 and 9 (C8 and C9). CD59 is universally expressed on normal cells and also expressed on many kinds of cancer cells including NHL and CLL(4). Extensive evidence indicates that CD59 is highly effective at protecting NHL and CLL cells from antibody-mediated CDC(10, 26–38). Up-regulation of CD59 is an important determinant of sensitivity to antibody (RTX) treatment in CLL(10, 26–36, 39). Therefore, we developed a novel, potent, and specific human CD59 inhibitor, a recombinant 114 amino acid peptide consisting of domain 4 of intermedilysin (rILYd4), the cytolytic toxin secreted by Streptococcus intermedius(39–41). rILYd4 sensitized RTX-resistant lymphoma cells and primary CLL cells from 6 patients to RTX treatment through enhanced CDC effect, indicating that rILYd4 may be a therapeutic candidate for the treatment of antibody-resistant NHL(39). Here, using qualitative and quantitative flow cytometry, we further investigated whether rILYd4 sensitizes RTX-resistant cells and primary CLL cells from 26 patients to CDC induced by OFA. We correlated either OFA-mediated CDC or rILYd4-enhanced OFA-mediated CDC with known biomarkers involved in RTX activity, i.e. CD20 and CD59 expression.
Materials and Methods
Additional information is available in Supplementary Materials and Methods.
Original and resistant B-cell malignancy cell lines, primary CLL cells and cell culture
The human B-cell lymphoma cell lines ARH-77, RL, Daudi and Raji were purchased from and authenticated by the ATCC (Manassas, VA), and passaged less than 50 times. Original and resistant B-cell malignancy cell lines were cultured in RPMI-1640 medium (Invitrogen, Carlsbad, CA) and RTX-resistant Ramos, Daudi and Raji cell lines were generated with previously published method(33, 39). Those resistant cell lines that survived complement attack induced by RTX at concentrations of 51.2, 20 and 20 μg/ml in the presence of 10% human serum (Valley Biomedical, Winchester, VA) as a source of complement, were named as RamosR51.2, DaudiR20 and RajiR20, respectively.
The CLL patients had been previously enrolled on Dana-Farber Harvard Cancer Center (DF/HCC) protocol 99–224, a tissue banking study that links samples to clinical information. The protocol was approved by the DF/HCC Institutional Review Board and all patients signed written informed consent. The blood from 26 CLL patients (Supplementary Table 1) was then separated by density centrifugation through a Ficoll gradient and peripheral blood mononuclear cells (PBMCs) were frozen for subsequent use. The CLL cells were cultured as described in our previous publication(39).
Flow cytometric analysis of CD20 and CD59 levels
The cells at a density of 5 × 106 cells/ml were harvested and washed twice with Phosphate Buffered Saline (PBS). The cells suspended in 3% bovine serum albumin (BSA)/PBS ware incubated with 1:100 diluted primary mouse mAbs against CD20 or CD59 at room temperature for 30 minutes, washed in 3% BSA/PBS, and then incubated with the secondary Ab (goat anti-mouse IgG conjugated FITC) at room temperature for another 30 minutes. The cells were washed in PBS before analysis. Flow cytometry was performed using a FACScan (Becton Dickinson, FranklincLakes, NJ) and mean fluorescence intensities were converted to molecules of equivalent soluble fluorochrome (MESF) using calibrated beads (Spherotech, Lake Forest, IL). We divided the MESF of CD20 by the MESF of CD59 to calculate the ratio of CD20/CD59.
CDC assays
Cell viability was determined by either PI staining or Alamar blue assay as described(32, 39). Briefly, 105 cells were treated with RTX or OFA with or without rILYd4 in the presence of normal human serum (NHS) as a source of complement for 2 hours at 37 °C. Since ARH and RL were more resistant to RTX-mediated CDC effects(18, 39, 42) than Daudi and Raji(42), 20% or 5% of NHS were used as a source of complement to perform the experiments with ARH-77 and RL cell lines or Daudi and Raji cell lines, respectively. We used 25% final NHS for CLL cells because of the resistance of CLL to RTX. After washing with 1% BSA/PBS, the cells (in 100 μl) were incubated with 10 μl PI (50 μg/ml) at room temperature for 5 min and immediately analyzed on the FACScan. The PI negative population was regarded as live cells. Percentage of cell death was calculated using the following formula: (%) = 100 × [1 − (live cells in treated sample/live cells in untreated control)]. The percentage of rILYd4-enhanced effect (%) on RTX or OFA-mediated CDC was calculated as: (%) = [% of dead cells in RTX or OFA with rILYd4 sample/% of dead cells in RTX or OFA alone sample − 1] × 100%. For the Alamar blue assay, we followed the protocol as we described previously in(39).
Determination of C1q binding, C3b(i) and C9 deposition
After cells were challenged with human complement in the CDC assay, followed by washing with 1% BSA/PBS, they were stained with FITC-1H8 (anti-C3b(i) antibody), polyclonal rabbit anti-human C1q antibody, or polyclonal goat anti-human C9 antibody followed by FITC labeled secondary antibody, respectively. After washing with PBS, the cells were analyzed with flow cytometry and mean fluorescence intensities were converted to molecules of equivalent soluble fluorochrome (MESF) using calibrated beads(9).
Statistical analysis
Linear regression was used to evaluate the correlation between the degree of CDC induction and CD20 or CD59 expression, or the ratio of the two using SPSS 11.5 or GraphPad Prism 4.0 software. The differences in the means of paired samples in the CDC assay on primary CLL cells was evaluated by Wilcoxon’s signed rank test. Most results were expressed as the mean ± SD of data obtained from three to four separate experiments. The statistical significance of the differences between the group means was determined using one-way ANOVA to compare variance. A P value <0.05 was considered significant.
Results
rILYd4 enhances OFA-mediated CDC on B-cell malignancy cell lines and sensitizes the RTX-resistant cell lines to OFA
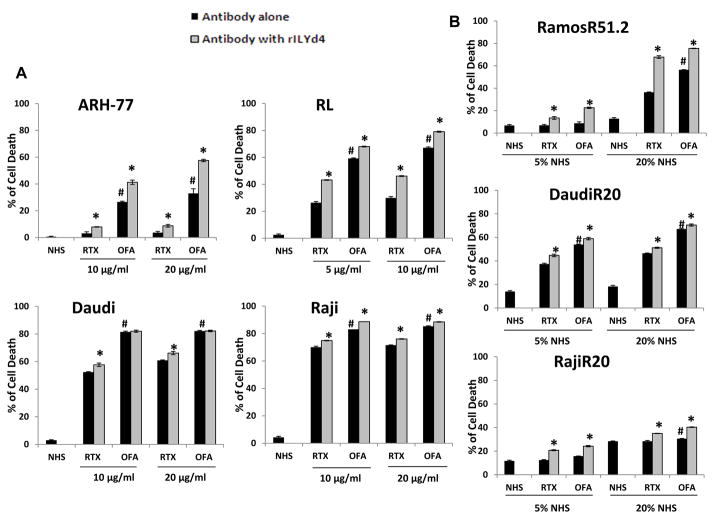
To test whether rILYd4 enhances OFA-mediated CDC, we used ARH-77, RL, Daudi and Raji cell lines, which expressed CD20 and CD59 at different levels (Supplemental fig. 1). We also compared the CDC effect of OFA with that of RTX. We used Alamar blue assay to identify the optimal concentrations of RTX and OFA (20 μg/ml for ARH-77 and 10 μg/ml for RL, Daudi and Raji cells) for each of the cell lines (Supplemental fig. 2) and to determine the appropriate rILYd4 concentration (Supplemental fig. 3). To perform CDC experiments, we selected the final rILYd4 concentration to be 1074 nM, which was the minimal concentration required for mediating maximal cell lysis for all cells tested. Importantly, heat inactivated human serum (IHS) did not mediate any lysis (Supplemental fig. 4), confirming the nature of complement dependent cell death observed in the CDC assay with NHS. In addition, rILYd4 alone did not mediate any lysis (Supplemental fig. 4), further confirming our previous finding that rILYd4 alone has no direct lytic effect on CD59-expressing cells(40). rILY3, a nonfunctional isotype of rILYd4, also did not mediate any more CDC than vehicle buffer (Supplemental fig. 4), confirming the specific effect of rILYd4.
PI staining, a well established flow cytometry method for quantitative analysis of cell viability(43, 44), was used to assess cell death. Addition of 1074nm rILYd4 to 5% or 20% NHS for Daudi and Raji or ARH-77 and RL respectively led to statistically significantly higher CDC at two different concentrations of RTX or OFA (Fig. 1A). OFA mediated significantly higher CDC effects than RTX in the presence or absence of rILYd4 in all 4 original cell lines, respectively (Fig. 1A). In addition, similar results were observed with 50% NHS, a more relevant physiological condition for ARH-77 (Supplemental fig. 5). Furthermore, the results from PI staining were comparable to those from Alamar blue assay (data not shown).
Figure 1. rILYd4 effect on B-cell malignancy cell lines and RTX-resistant cell lines.
(A) ARH-77, RL, Daudi and Raji were treated with different concentrations of RTX or OFA with 20% NHS (for ARH-77 and RL) or 5% NHS (for Daudi and Raji) in the absence or presence of 1074 nM rILYd4. (B) RamosR51.2, DaudiR20 and RajiR20 were treated with 10 μg/ml RTX or OFA together with 5% or 20% NHS in the absence or presence of 1074nM rILYd4. (A-B) Cells were incubated at 37 °C for 2 hours. Cell viability was assessed by flow cytometry. Result are mean ± SD of three different experiments. *: P < 0.01 v.s. no rILYd4 treatment, #: P<0.01 v.s. RTX.
To further investigate whether rILYd4 also sensitizes RTX-resistant B-cell lymphoma cell lines to OFA-mediated CDC, we used our previously established RTX-resistant cell lines, DaudiR20, RajiR20 and RamosR51.2(39). These resistant cells also express higher levels of CD59 than the original cell lines (Supplemental fig. 6A). The presence of rILYd4 resulted in higher CDC mediated by both OFA and RTX than the absence of rILYd4, with either 5% or 20% NHS (Fig. 1B). OFA also induced higher CDC activity than RTX (Fig. 1B). Further, we also observed comparable results with 50% NHS for RamosR51.2 cells (Supplemental fig. 5). The absence of CDC in any experimental condition with IHS as a source of complement indicates that the CDC effects observed in Fig. 1B were complement-dependent (Supplemental Fig. 6B).
Taken together, these results demonstrate that 1) rILYd4 enhances the CDC effects of OFA; 2) rILYd4 sensitizes RTX-resistant cell lines to both OFA and RTX-mediated CDC; and 3) that OFA has more potent CDC activity than RTX.
rILYd4 sensitizes primary CLL cells to OFA and RTX-mediated CDC ex vivo
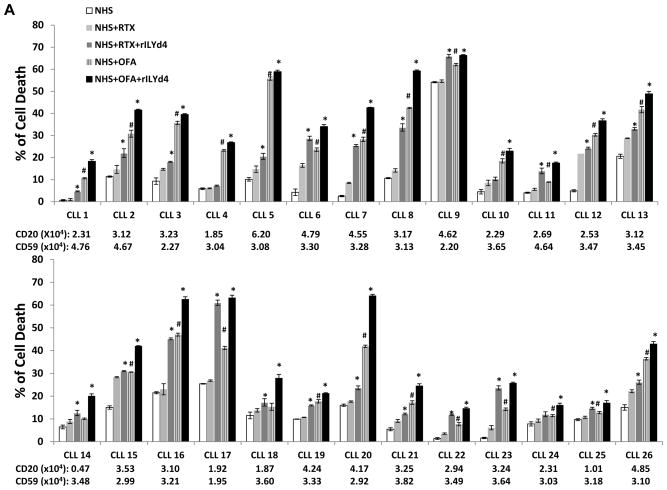
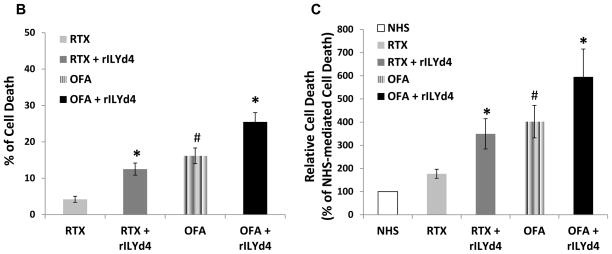
Primary CLL cells are much more resistant to antibody-mediated CDC than NHL cells because of both the lower expression of the target CD20 and higher expression of CD59(3, 8–10). Here, we used primary CLL cells from 26 patients (Supplemental Table 1) to test the effect of rILYd4 on clinical cancer cells. rILYd4 together with OFA or RTX significantly increased the CDC effect on CLL cells from twenty-two out of twenty-six patients compared with OFA or RTX alone (Fig. 2A). The pooled data of the CDC effects obtained from the 26 CLL patients also demonstrated that rILYd4 treatment mediated statistically significant higher OFA or RTX-induced CDC than vehicle treatment (Fig. 2B and 2C). In the presence or absence of rILYd4, 50% NHS, a more relevant physiological condition, achieved stronger CDC effects mediated by RTX or OFA than 25% NHS in four patient CLL samples (Supplemental fig. 7). Of note, the cells from patient CLL9 are very sensitive to NHS alone. Since inactivated HS serum did not lyse the cells, we reason that this phenomenon may result from an increase in complement activation on the patient cells mediated by NHS itself through unknown factors. The levels of CD20 and CD59 on the surface of the CLL cells from each patient were determined by flow cytometric analysis (Fig. 2A). The fact that cell killing was due to CDC was demonstrated in 8 CLLs that showed no cell killing when IHS was used as a source of complement (Supplemental fig. 8). Only 8 samples were tested due to the limiting amount of primary CLL cells. OFA mediated greater CDC than RTX in CLL cells (Fig. 2), consistent with our observations with NHL cell lines. These results indicate that rILYd4 enhances the CDC effect of OFA and RTX on CLL cells and highlights the potential for therapeutic value of rILYd4 in the treatment of CLL.
Figure 2. rILYd4 enhances OFA or RTX-mediated CDC effects on primary CLL.
(A) The CDC effect on CLL cells of 26 different patients. Primary CLL cells from 26 different patients (CLL-1-CLL-26) were individually subjected to 10μg/ml RTX/OFA-induced CDC with or without 1074nM rILYd4 in the presence of 25% NHS. Results are mean ± SD of three different experiments. Expression levels of CD20 and CD59 on CLL cells were presented by MESF. (B, C) Comparison of the pooled CDC data showing the net increase in cell death (B) or the relative increase in cell death compared to NHS induced cell death, in 26 patients(C). Net increase in cell death equals the cell death treated with antibody or antibody plus rILYd4 subtracting treated by NHS alone individually. The data is presented as mean ± s.e.m from the 26 patients (CLL-1-CLL-26). (A–C) *: P<0.01 v.s. no rLYd4 treatment, #: P<0.01 v.s. RTX.
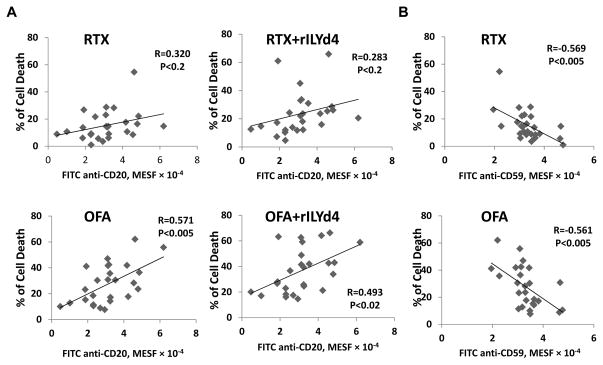
OFA-mediated CDC effects negatively correlate with CD59 levels on the cell surface of B-cell malignancy cell lines
To investigate the underlying mechanism determining the sensitivity to OFA-mediated CDC, we correlated the CDC-induced killing of four original B-cell malignancy cell lines with levels of CD20 or CD59. We used the ratio of CD20/CD59 (divided the MESF of CD20 by the MESF of CD59) to examine if both CD20 and CD59 determine the CDC effect. The CDC effects mediated by either OFA or RTX tended to correlate positively with the level of CD20 among these four cell lines, although these effects did not reach statistical significance (Supplemental fig. 9A). In contrast, CDC effects mediated by OFA correlated negatively with the level of CD59 on the surface of the four B-cell malignancy cell lines (Fig. 3A). Furthermore, CDC effects mediated by antibody positively correlate with the ratio of CD20/CD59, though not reaching statistical significance (Supplemental fig. 9B). Taken together, these results confirm previous findings that CD20 and CD59 are important molecules in determining the sensitivity of lymphoma cells to RTX-mediated CDC.
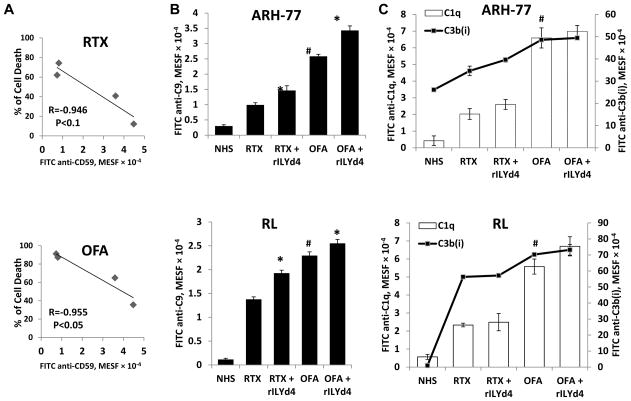
Figure 3. The CDC effect mediated by OFA correlates negatively with CD59 level and rILYd4 enhances C9 deposition on the cell surface of B-cell malignancy cells.
(A) Negative correlation between OFA-mediated CDC and CD59. (B) rILYd4 increases C9 deposition. ARH-77 and RL cells were treated with 10 μg/ml RTX or OFA in the presence of 20% NHS with or without 1074nM rILYd4. (C) C1q binding and C3b(i) deposition were not influenced by rILYd4. ARH-77 and RL cells were treated with 10 μg/ml RTX or OFA in the presence of 20% NHS with or without 1074 nM rILYd4. The levels of C1q binding and C3b(i) and C9 deposition were represented by MESF. (B–C) Result are mean ± SD of three different experiments. *: P<0.01 v.s. no rLYd4 treatment, #: P<0.01 v.s. RTX.
To define the underlying mechanism by which rILYd4 enhances CDC, we quantified deposition of C9 and C3b(i) and C1q binding on the antibody-treated lymphoma cells (ARH-77 and RL) in the presence or absence of rILYd4. We documented that rILYd4 treatment mediated significantly higher levels of C9 deposition but not C1q binding or C3b(i) deposition on the cells than antibody alone (Fig. 3B and Fig. 3C). These results further document that rILYd4 specifically increases MAC formation through inhibiting CD59 and does not influence the level of C1q binding or C3b(i) deposition induced by RTX or OFA. We also found that OFA mediated more C1q binding and more C3b(i) deposition than RTX on the lymphoma cells (Fig. 3C), a result consistent with findings previously reported by Pawluczkowycz et al (9).
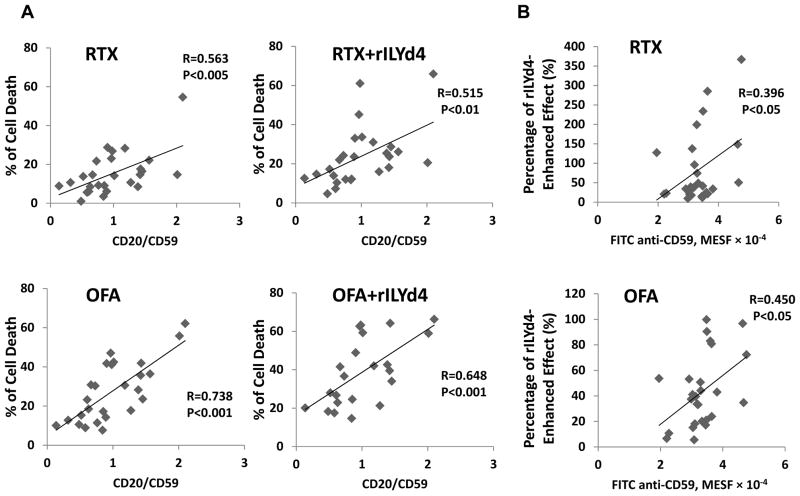
OFA-mediated CDC correlates negatively with CD59 levels and positively with the ratio of CD20/CD59 on the surface of CLL cells
Previous studies by others indicate that RTX-mediated CDC correlates positively with the level of CD20 and negatively with the level of CD59 on the surface of CLL cells(32, 43). OFA mediates stronger CDC effects on CLL than RTX, dependent on the level of CD20 but not complement regulators(45). We document here that the CDC effect induced by OFA correlates positively with the level of CD20 on the surface of CLL cells (Fig. 4A). When combined with rILYd4, the extent of cell death induced by OFA also correlated positively with the level of CD20 (Fig. 4A). RTX-mediated CDC effects with or without rILYd4 tended to correlate with the CD20 level but did not reach statistical significance (Fig. 4A). The sensitivity of CLL to OFA or RTX-mediated CDC correlated negatively with the level of CD59 on the CLL cells (Fig. 4B). Moreover, OFA or RTX-mediated CDC on CLL cells with or without rILYd4 correlated positively with the ratio of CD20/CD59 (Fig. 5A). These correlations were more significant in general in OFA than RTX groups (Fig. 5A). These results indicate that the levels of CD20 and CD59 on the target cell surface are associated with CDC induced by OFA or RTX, and with rILYd4-enhanced CDC induced by OFA or RTX.
Figure 4. Correlations between the level of CD20 or CD59 on the surface of CLL cells and the CDC effect mediated by anti-CD20 antibodies with or without rILYd4.
(A) The extent of cell death induced by OFA correlated significantly with the MESF of CD20 on the CLL cell surface. When combined with rILYd4, the extent of cell death induced by OFA positively correlated with the level of CD20. (B) Sensitivity of CLL cells to CDC induced by OFA or RTX treatment correlated negatively with the MESF of CD59 on CLL cells.
Figure 5. Correlation of OFA-mediated CDC with the ratio of CD20/CD59, and correlation of the percentage of rILYd4-enhanced effect with CD59 level, on the surface of CLL cells.
(A) The sensitivity of CLL cells to RTX or OFA mediated CDC with or without rILYd4 correlates significantly with the ratio of CD20/CD59. (B) The percentage of rILYd4-enhanced effect correlates positively with the level of CD59 on the surface of CLL cells.
We further analyzed the correlation between CD59 levels and the percentage of rILYd4-enhanced effect for either therapeutic antibody on CLL cells. We demonstrated that the percentage of rILYd4-enhanced effect positively correlates with CD59 levels on the CLL cell surface (Fig. 5B). This result directly indicates that rILYd4 enhanced the CDC effects mediated by OFA or RTX through the inhibition of CD59 activity in these primary CLL cells.
Discussion
We have previously reported that rILYd4 enhances RTX-mediated CDC against Ramos cells and primary CLL cells and sensitizes RTX-resistant Ramos cells to RTX treatment in vitro and in vivo(39). These findings are consistent with the results demonstrated here using two other RTX-resistant cell lines (Raji and Daudi) and primary CLL cells. We also demonstrate that the CDC effect mediated by RTX with or without rILYd4 correlated positively with the CD20 level on the surface of the cells and negatively with the CD59 level on the surface of the cells. These results are comparable to the findings previously reported by Golay et al(32, 39, 43).
It has been extensively demonstrated that OFA-mediated CDC effects on both lymphoma cells and primary CLL cells are largely dependent on the level of CD20 on the target cell surface(1, 5, 16, 20). Although CD59 has been widely recognized to reduce the sensitivity of B-cell malignancies to RTX, whether it has a similar role in OFA treatment has not been extensively studied. An ASH meeting abstract reported that OFA-mediated CDC effects on lymphoma cell lines and primary cells derived from diffuse large B-cell lymphoma patients correlated negatively with CD59, but not with CD46 or CD55(45). Here, we demonstrated that 1) CDC mediated by OFA with or without rILYd4 on the CLL cells positively correlated with CD20 levels and CD20/CD59 ratio; and 2) OFA-mediated CDC effect on the original lymphoma cell lines correlated positively and negatively with CD20 and CD59 levels, respectively. These results together with those obtained using RTX with or without rILYd4 shed light on the importance of the inhibition of CD59 for the treatment of B-cell malignancies with RTX or OFA. Furthermore, we also document that rILYd4 is able to enhance OFA-mediated CDC. This result highlights the potential therapeutic uses of rILYd4 or rILYd4 derivatives in the treatment of B-cell malignancies, providing justification for further development and evaluation.
It is notable that the rILYd4-enhanced CDC effect on Daudi and Raji is much less than that on ARH-77 and RL lymphoma cell lines as well as CLL primary cells (Fig. 1A). This is attributable to a much lower level of CD59 on Daudi and Raji cell lines as compared to that on ARH-77 and RL lymphoma cell lines, and CLL cells. This explanation is further supported by the fact that the percentage of rILYd4-enhanced effect on CLL cells positively correlates with the level of CD59 on the CLL cells (Figure. 5B). Further, three RTX-resistant cell lines show variable sensitivity to rILYd4 treatment although they express a high level of CD59 on their surfaces. This observation indicates that in addition to CD59 and CD20, other resistance mechanisms may also contribute to the development of resistance to CDC effect; these alternative mechanisms warrant further investigation.
It is widely accepted that OFA has much more potent CDC than RTX(18, 19). Consistently, we found that OFA mediates much stronger CDC effects than RTX in all cells. The more potent CDC effect mediated by OFA compared to RTX is attributed to increased C1q binding and C3b(i) deposition on the target cells triggered. Previously, Beum et al and Pawluczkowycz et al demonstrated that OFA triggers more C1q binding and more C3b(i) deposition on these Daudi and Raji cells, leading to more MAC attack(9, 23). These results were further confirmed by us with two other lymphoma cell lines expressing high levels of CD59. The application of rILYd4 to OFA or RTX-mediated CDC specifically increases C9 deposition but not C1q binding and C3b(i) deposition on the targeted cells. This result directly highlights the specificity of rILYd4 activity, the inhibition of anti-MAC activity of CD59.
The clinical response to RTX-containing chemotherapy varies between patients. Previously, using a living cell-imaging technique to evaluate the CDC activity of RTX(46), Mishima et al documented that CDC susceptibility of lymphoma cells freshly obtained from patients was strongly associated with response to RTX-containing chemotherapy(46). Here, we also observed variation in response to the CDC effects mediated by OFA or RTX with or without rILYd4. For example, the rILYd4 enhancement effect mediated by both antibodies on some CLL cells such as CLL2, 6, 7, 8, 16, or 17 in Fig. 2A was significantly higher than that of other CLL cells such as CLL4 or 10. rILYd4 was able to enhance the effects of RTX in some of the CLL cells (CLL6, 16, or 17) to the level close to or even higher than that mediated by OFA alone. These observations suggest that different combinatorial strategies may be needed for different patients based on their responses to antibody-mediated CDC and their levels of CD20 and CD59. However, the therapeutic implications of these different responses among CLL patients remain to be determined in the future.
In summary, these results reported here indicate that rILYd4 may be able to function as an adjuvant for therapeutic antibodies in the treatment of cancer, including in particular OFA and RTX in the treatment of B-cell malignancies. However, it is important to note that the impact of rILYd4 on OFA therapy reported here is modest. Therefore, whether rILYd4 would be able to overcome resistance to OFA-based cancer therapy in patients will require further investigation. Clinical testing of rILYd4 will depend on further preclinical development that reduces its immunogenicity and improve its half life.
Supplementary Material
Translational relevance.
The efficacy of Rituximab (RTX) and Ofatumumab (OFA) in cancer therapy depends in part on the induction of complement-dependent cytotoxicity (CDC). Human CD59 is a key complement regulator that inhibits induction of CDC. CD59 is highly expressed in B-cell malignancies such as non-Hodgkin’s lymphoma (NHL) and chronic lymphocytic leukemia (CLL) and up-regulation of CD59 expression is an important determinant of the sensitivity of those cancer cells to RTX treatment. Here, we demonstrate that rILYd4, a potent and non toxic human CD59 inhibitor, enhances CDC effects mediated by OFA or RTX on RTX-resistant B-cell malignancy cell lines and primary CLL cells. The sensitivity to CDC effects mediated by OFA or RTX with or without rILYd4 negatively correlated with the level of CD59. These data suggest that rILYd4 may be a new approach to enhance the anti-cancer activity of OFA and RTX in B-cell malignancies that have relapsed after prior antibody-based therapies.
Acknowledgments
We thank Dr. Ronald P. Taylor for kindly providing C3b/iC3b/C3dg monoclonal antibodies, FITC-1H8, and GSK/Genmab for kindly providing Ofatumumab.
Grant support
NIHRO1AI061174 (XB.Q) and NIHR21CA141324 (XB.Q), Harvard Technology Development Accelerator Fund (XB.Q), and China Scholarship Council (2009628090) (X.G.). Dr Jennifer Brown is supported by NIHK23CA115682, as well as by an ASH Scholar Award and the Leukemia and Lymphoma Society Scholar in Clinical Research Award.
Footnotes
Disclosure of Potential Conflicts of Interest
JRB receives research funding from GSK.
References
- 1.Castillo J, Milani C, Mendez-Allwood D. Ofatumumab, a second-generation anti-CD20 monoclonal antibody, for the treatment of lymphoproliferative and autoimmune disorders. Expert Opin Investig Drugs. 2009;18:491–500. doi: 10.1517/13543780902832679. [DOI] [PubMed] [Google Scholar]
- 2.Maddocks KJ, Lin TS. Update in the management of chronic lymphocytic leukemia. J Hematol Oncol. 2009;2:29. doi: 10.1186/1756-8722-2-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glennie MJ, French RR, Cragg MS, Taylor RP. Mechanisms of killing by anti-CD20 monoclonal antibodies. Mol Immunol. 2007;44:3823–37. doi: 10.1016/j.molimm.2007.06.151. [DOI] [PubMed] [Google Scholar]
- 4.Zhou X, Hu W, Qin X. The role of complement in the mechanism of action of rituximab for B-cell lymphoma: implications for therapy. Oncologist. 2008;13:954–66. doi: 10.1634/theoncologist.2008-0089. [DOI] [PubMed] [Google Scholar]
- 5.Cheson BD. Monoclonal antibody therapy of chronic lymphocytic leukaemia. Best Pract Res Clin Haematol. 2010;23:133–43. doi: 10.1016/j.beha.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 6.Fishelson Z, Donin N, Zell S, Schultz S, Kirschfink M. Obstacles to cancer immunotherapy: expression of membrane complement regulatory proteins (mCRPs) in tumors. Mol Immunol. 2003;40:109–23. doi: 10.1016/s0161-5890(03)00112-3. [DOI] [PubMed] [Google Scholar]
- 7.Bonavida B. Rituximab-induced inhibition of antiapoptotic cell survival pathways: implications in chemo/immunoresistance, rituximab unresponsiveness, prognostic and novel therapeutic interventions. Oncogene. 2007;26:3629–36. doi: 10.1038/sj.onc.1210365. [DOI] [PubMed] [Google Scholar]
- 8.Kennedy AD, Beum PV, Solga MD, DiLillo DJ, Lindorfer MA, Hess CE, et al. Rituximab infusion promotes rapid complement depletion and acute CD20 loss in chronic lymphocytic leukemia. J Immunol. 2004;172:3280–8. doi: 10.4049/jimmunol.172.5.3280. [DOI] [PubMed] [Google Scholar]
- 9.Pawluczkowycz AW, Beurskens FJ, Beum PV, Lindorfer MA, van de Winkel JG, Parren PW, et al. Binding of submaximal C1q promotes complement-dependent cytotoxicity (CDC) of B cells opsonized with anti-CD20 mAbs ofatumumab (OFA) or rituximab (RTX): considerably higher levels of CDC are induced by OFA than by RTX. J Immunol. 2009;183:749–58. doi: 10.4049/jimmunol.0900632. [DOI] [PubMed] [Google Scholar]
- 10.Golay J, Zaffaroni L, Vaccari T, Lazzari M, Borleri GM, Bernasconi S, et al. Biologic response of B lymphoma cells to anti-CD20 monoclonal antibody rituximab in vitro: CD55 and CD59 regulate complement-mediated cell lysis. Blood. 2000;95:3900–8. [PubMed] [Google Scholar]
- 11.Cheson BD. Ofatumumab, a novel anti-CD20 monoclonal antibody for the treatment of B-cell malignancies. J Clin Oncol. 2010;28:3525–30. doi: 10.1200/JCO.2010.27.9836. [DOI] [PubMed] [Google Scholar]
- 12.Jaglowski SM, Byrd JC. Rituximab in chronic lymphocytic leukemia. Semin Hematol. 2010;47:156–69. doi: 10.1053/j.seminhematol.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 13.Huhn D, von SC, Wilhelm M, Ho AD, Hallek M, Kuse R, et al. Rituximab therapy of patients with B-cell chronic lymphocytic leukemia. Blood. 2001;98:1326–31. doi: 10.1182/blood.v98.5.1326. [DOI] [PubMed] [Google Scholar]
- 14.Klepfish A, Gilles L, Ioannis K, Rachmilewitz EA, Schattner A. Enhancing the action of rituximab in chronic lymphocytic leukemia by adding fresh frozen plasma: complement/rituximab interactions & clinical results in refractory CLL. Ann NY Acad Sci. 2009;1173:865–73. doi: 10.1111/j.1749-6632.2009.04803.x. [DOI] [PubMed] [Google Scholar]
- 15.Lemery SJ, Zhang J, Rothmann MD, Yang J, Earp J, Zhao H, et al. U.S. Food and Drug Administration approval: ofatumumab for the treatment of patients with chronic lymphocytic leukemia refractory to fludarabine and alemtuzumab. Clin Cancer Res. 2010;16:4331–8. doi: 10.1158/1078-0432.CCR-10-0570. [DOI] [PubMed] [Google Scholar]
- 16.Coiffier B, Bosly A, Wu KL, Verhoef G, Koen VE. Ofatumumab Monotherapy for Treatment of Patients with Relapsed/Progressive Diffuse Large B-Cell Lymphoma: Results From a Multicenter Phase II Study. 52nd ASH Annual Meeting and Exposition; 2010. [Google Scholar]
- 17.Wierda WG, Kipps TJ, Mayer J, Robak T. Final Analysis From the International Trial of Single-Agent Ofatumumab In Patients with Fludarabine-Refractory Chronic Lymphocytic Leukemia. 52nd ASH Annual Meeting and Exposition; 2010; 2010. [Google Scholar]
- 18.Teeling JL, French RR, Cragg MS, van den BJ, Pluyter M, Huang H, et al. Characterization of new human CD20 monoclonal antibodies with potent cytolytic activity against non-Hodgkin lymphomas. Blood. 2004;104:1793–800. doi: 10.1182/blood-2004-01-0039. [DOI] [PubMed] [Google Scholar]
- 19.Teeling JL, Mackus WJ, Wiegman LJ, van den Brakel JH, Beers SA, French RR, et al. The biological activity of human CD20 monoclonal antibodies is linked to unique epitopes on CD20. J Immunol. 2006;177:362–71. doi: 10.4049/jimmunol.177.1.362. [DOI] [PubMed] [Google Scholar]
- 20.Robak T. Ofatumumab, a human monoclonal antibody for lymphoid malignancies and autoimmune disorders. Curr Opin Mol Ther. 2008;10:294–309. [PubMed] [Google Scholar]
- 21.Beurskens FJ, Ruuls SR, Engelberts PJ, Vink T, Mackus WJ, van de Winkel JG, et al. Complement activation impacts B-cell depletion by both type I and type II CD20 monoclonal antibodies. Blood. 2008;112:4354–5. doi: 10.1182/blood-2008-07-171082. author reply 5–6. [DOI] [PubMed] [Google Scholar]
- 22.de Haij S, Jansen JH, Boross P, Beurskens FJ, Bakema JE, Bos DL, et al. In vivo cytotoxicity of type I CD20 antibodies critically depends on Fc receptor ITAM signaling. Cancer research. 2010;70:3209–17. doi: 10.1158/0008-5472.CAN-09-4109. [DOI] [PubMed] [Google Scholar]
- 23.Beum PV, Lindorfer MA, Beurskens F, Stukenberg PT, Lokhorst HM, Pawluczkowycz AW, et al. Complement activation on B lymphocytes opsonized with rituximab or ofatumumab produces substantial changes in membrane structure preceding cell lysis. J Immunol. 2008;181:822–32. doi: 10.4049/jimmunol.181.1.822. [DOI] [PubMed] [Google Scholar]
- 24.Li B, Shi S, Qian W, Zhao L, Zhang D, Hou S, et al. Development of novel tetravalent anti-CD20 antibodies with potent antitumor activity. Cancer Res. 2008;68:2400–8. doi: 10.1158/0008-5472.CAN-07-6663. [DOI] [PubMed] [Google Scholar]
- 25.Barth M, Hernandez-Ilizaliturri FJ, Mavis C, Tsai P-C, Gibbs J, Deeb G. Ofatumumab, a Fully Human Monoclonal Antibody Targeting CD20, Demonstrates Activity Against and Potentiates the Anti-Tumor Activity of Chemotherapy Agents In Rituximab-Sensitive Cell Lines (RSCL), Rituximab-Resistant Cell Lines (RRCL), Lymphoma Xenografts, and Primary Tumor Cells Derived From Patients with B-Cell Non-Hodgkin Lymphoma (NHL). 52nd ASH Annual Meeting and Exposition; 2010; 2010. [Google Scholar]
- 26.Juhl H, Melmig F, Baltzer K, Kalthoff H, Hennebruns D, Kremer B. Frequent expression of complement resistance factors CD46, CD55, and CD59 on gastrointestinal cancer cells limits the therapeutic potential of monoclonal antibody 17-1A. J Surg Oncol. 1997;64:222–30. doi: 10.1002/(sici)1096-9098(199703)64:3<222::aid-jso9>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 27.Bjorge L, Hakulinen J, Wahlstrom T, Matre R, Meri S. Complement-regulatory proteins in ovarian malignancies. Int J Cancer. 1997;70:14–25. doi: 10.1002/(sici)1097-0215(19970106)70:1<14::aid-ijc3>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 28.Jarvis GA, Li J, Hakulinen J, Brady KA, Nordling S, Dahiya R, et al. Expression and function of the complement membrane attack complex inhibitor protectin (CD59) in human prostate cancer. Int J Cancer. 1997;71:1049–55. doi: 10.1002/(sici)1097-0215(19970611)71:6<1049::aid-ijc22>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 29.Coral S, Fonsatti E, Sigalotti L, De Nardo C, Visintin A, Nardi G, et al. Overexpression of protectin (CD59) down-modulates the susceptibility of human melanoma cells to homologous complement. J Cell Physiol. 2000;185:317–23. doi: 10.1002/1097-4652(200012)185:3<317::AID-JCP1>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 30.Fonsatti E, Altomonte M, Coral S, De Nardo C, Lamaj E, Sigalotti L, et al. Emerging role of protectin (CD59) in humoral immunotherapy of solid malignancies. Clin Ter. 2000;151:187–93. [PubMed] [Google Scholar]
- 31.Harjunpaa A, Junnikkala S, Meri S. Rituximab (anti-CD20) therapy of B-cell lymphomas: direct complement killing is superior to cellular effector mechanisms. Scand J Immunol. 2000;51:634–41. doi: 10.1046/j.1365-3083.2000.00745.x. [DOI] [PubMed] [Google Scholar]
- 32.Golay J, Lazzari M, Facchinetti V, Bernasconi S, Borleri G, Barbui T, et al. CD20 levels determine the in vitro susceptibility to rituximab and complement of B-cell chronic lymphocytic leukemia: further regulation by CD55 and CD59. Blood. 2001;98:3383–9. doi: 10.1182/blood.v98.12.3383. [DOI] [PubMed] [Google Scholar]
- 33.Takei K, Yamazaki T, Sawada U, Ishizuka H, Aizawa S. Analysis of changes in CD20, CD55, and CD59 expression on established rituximab-resistant B-lymphoma cell lines. Leuk Res. 2006;30:625–31. doi: 10.1016/j.leukres.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 34.Bjorge L, Stoiber H, Dierich MP, Meri S. Minimal residual disease in ovarian cancer as a target for complement-mediated mAb immunotherapy. Scand J Immunol. 2006;63:355–64. doi: 10.1111/j.1365-3083.2006.01751.x. [DOI] [PubMed] [Google Scholar]
- 35.Ziller F, Macor P, Bulla R, Sblattero D, Marzari R, Tedesco F. Controlling complement resistance in cancer by using human monoclonal antibodies that neutralize complement-regulatory proteins CD55 and CD59. Eur J Immunol. 2005;35:2175–83. doi: 10.1002/eji.200425920. [DOI] [PubMed] [Google Scholar]
- 36.Macor P, EP, Zorzet S, Tripodo C, Marzari R, Amadori A, FT Neutralizing human antibodies against CD55 and CD59 targeted to lymphoma cells in vivo potentiate the therapeutic effect of Rituximab. Mol Immunol. 2007;44:212. [Google Scholar]
- 37.Dalle S, Dupire S, Brunet-Manquat S, Reslan L, Plesa A, Dumontet C. In vivo model of follicular lymphoma resistant to rituximab. Clin Cancer Res. 2009;15:851–7. doi: 10.1158/1078-0432.CCR-08-1685. [DOI] [PubMed] [Google Scholar]
- 38.Wu G, Hu W, Shahsafaei A, Song W, Dobarro M, Sukhova GK, et al. Complement regulator CD59 protects against atherosclerosis by restricting the formation of complement membrane attack complex. Circ Res. 2009;104:550–8. doi: 10.1161/CIRCRESAHA.108.191361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu W, Ge X, You T, Xu T, Zhang J, Wu G, et al. Human CD59 inhibitor sensitizes rituximab-resistant lymphoma cells to complement-mediated cytolysis. Cancer Res. 2011;71:2298–307. doi: 10.1158/0008-5472.CAN-10-3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hu W, Yu Q, Hu N, Byrd D, Amet T, Shikuma C, et al. A high-affinity inhibitor of human CD59 enhances complement-mediated virolysis of HIV-1: implications for treatment of HIV-1/AIDS. J Immunol. 2010;184:359–68. doi: 10.4049/jimmunol.0902278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.You T, Hu W, Ge X, Shen J, Qin X. Application of a novel inhibitor of human CD59 for the enhancement of complement-dependent cytolysis on cancer cells. Cell Mol Immunol. 2011;8:157–63. doi: 10.1038/cmi.2010.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kennedy AD, Solga MD, Schuman TA, Chi AW, Lindorfer MA, Sutherland WM, et al. An anti-C3b(i) mAb enhances complement activation, C3b(i) deposition, and killing of CD20+ cells by rituximab. Blood. 2003;101:1071–9. doi: 10.1182/blood-2002-03-0876. [DOI] [PubMed] [Google Scholar]
- 43.Manches O, Lui G, Chaperot L, Gressin R, Molens JP, Jacob MC, et al. In vitro mechanisms of action of rituximab on primary non-Hodgkin lymphomas. Blood. 2003;101:949–54. doi: 10.1182/blood-2002-02-0469. [DOI] [PubMed] [Google Scholar]
- 44.Peng W, Zhang X, Mohamed N, Inghirami G, Takeshita K, Pecora A, et al. A DeImmunized chimeric anti-C3b/iC3b monoclonal antibody enhances rituximab-mediated killing in NHL and CLL cells via complement activation. Cancer Immunol Immunother. 2005;54:1172–9. doi: 10.1007/s00262-005-0686-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cilessen S, Mackus WJM, CastricumKcm, et al. Chemotherapy-refractory difuse large B-cell lymphomas (DLBCL) are effectively killed by ofatumumab-induced complement-mediated cytotoxicity. Blood. 2007:110. [Google Scholar]
- 46.Mishima Y, Sugimura N, Matsumoto-Mishima Y, Terui Y, Takeuchi K, Asai S, et al. An imaging-based rapid evaluation method for complement-dependent cytotoxicity discriminated clinical response to rituximab-containing chemotherapy. Clin Cancer Res. 2009;15:3624–32. doi: 10.1158/1078-0432.CCR-08-1536. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.