Abstract
Iron(II) bromide catalyzes the transformation of ortho-substituted aryl azides into 2,3-disubstituted indoles through a tandem ethereal C–H bond amination–[1,2]-shift reaction. The preference for the 1,2-shift component of the tandem reaction was established to be Me < 1° < 2° < Ph.
The ability of tandem reactions to rapidly increase the molecular complexity of simple substrates continues to inspire the efforts of synthetic groups to incorporate new reactions into these cascades.1,2 While transition metal-catalyzed C–H bond amination is emerging as a useful synthetic process,3,4 this reaction has never been harnessed to initiate a cascade reaction. Further, the incorporation of migratorial processes into these cascade sequences remains rare despite the potential of these processes to transform simple substrates into complex, functionalized products.5 We have demonstrated that metal nitrenes originating from ortho-alkenyl-substituted aryl azides can engage in cascade reactions where electro-cyclization of the rhodium nitrene triggers a subsequent, selective 1,2-shift.6 Initiating these tandem reactions with a C–H bond amination reaction—ideally using an inexpensive, non-toxic first row transition metal catalyst—would be highly appealing because it would minimize the amount of functionality required in the starting material. Towards this goal, we report our development of an iron(II) bromide-catalyzed ethereal C–H bond amination-1,2-migration tandem reaction that efficiently and selectively transforms ortho-substituted aryl azides into 2,3-disubstituted indoles.
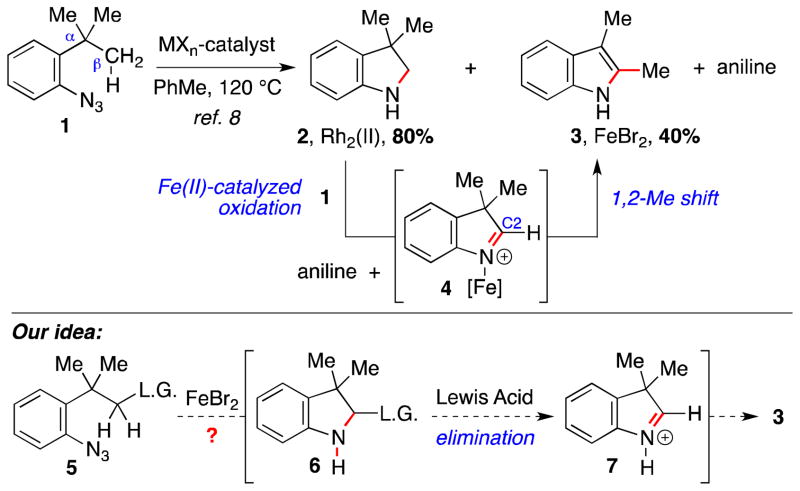
An unexpected observation during our optimization study into the formation of indoline 2 from aryl azide 1 prompted our interest into using an amination reaction to initiate a tandem reaction sequence (Scheme 1). A screen of transition metal complexes identified Rh2(esp)2 to be the most efficient catalyst for the intramolecular C–H bond amination of 1, which provided indoline 2.8 This screen also revealed that FeBr2 decomposed the aryl azide. The expected indoline, however, was not observed. Instead, a mixture of 2,3- dimethylindole 3 and aniline were formed. We attributed the formation of these products to an Fe-mediated oxidation of indoline 2,9 which would produce iminium ion 4 and aniline if the aryl azide was the oxidant.10 A 1,2-methyl shift from 4 would then produce the observed indole. We anticipated that this tandem amination-migration process might be rendered a viable synthetic method if the mechanism for iminium ion formation were changed from an oxidative process (requiring a stoichiometric oxidant, azide) to an elimination step. We envisioned that this modification could be achieved if one of the β-hydrogen atoms in 1 were replaced with a leaving group. Transition metal-catalyzed C–H bond amination of 5 would form indoline 6, which could undergo Lewis acid-catalyzed elimination of the leaving group to form iminium ion 7 and trigger the desired 1,2-migratorial process.11
Scheme 1.
Observation of a Fe(II)-promoted Tandem Reaction.
Our pursuit of triggering a tandem C–H bond amination-elimination- migration sequence started by investigating the reactivity of aryl azides 8 toward transition metal complexes (Table 1). We began by substituting the β-hydrogen atom in 1 with an alkoxy group and examining the reactivity of the resulting azides toward iron(II) bromide.11–13 While the use of an acetate lead only to aniline formation (entry 1), changing R to Et led to nearly complete 2,3-dimethylindole formation (entry 2). The reaction conversion was dependent on both the temperature as well as catalyst loading with severe attenuation of indole formation observed when either was reduced (entries 2 – 4).
Table 1.
Development of Optimal Conditions.

| |||||
|---|---|---|---|---|---|
| entry | catalystb | mol % | R | Temp | yield, %a |
| 1 | FeBr2 | 20 | Ac | 120 | 10c |
| 2 | FeBr2 | 20 | Et | 120 | 75 |
| 3 | FeBr2 | 10 | Et | 120 | 16 |
| 4 | FeBr2 | 20 | Et | 140 | 85 |
| 5 | Rh2(esp)2 | 20 | Et | 120 | 0 |
| 6 | CoTTP | 20 | Et | 120 | 0 |
| 7 | RuCl3•nH2O | 20 | Et | 120 | 0 |
| 8 | CuI | 20 | Et | 120 | 0 |
| 9 | ZnI2 | 20 | Et | 120 | trace |
| 10 | FeCl2 | 20 | Et | 120 | 0 |
| 11 | FeBr3 | 20 | Et | 120 | 20 |
As determined using 1H NMR spectroscopy using CH2Br2 as the internal standard.
No desired product was observed in the absence of a transition metal catalyst; only decomposition of the azide was obtained.
Aniline formed.
Upon completion of our initial optimization studies using iron(II) bromide, other transition metal complexes were examined to determine if they could catalyze this tandem reaction (Table 1). Despite their proven ability to catalyze N-atom transfer reactions from azides, our survey of Rh2(II)-,14 Ir(I),15 Co(I),16 Ru(III)-17 or Cu(I)-complexes18 did not identify any competent catalyst for our process (entries 5 – 8). The reaction also proved sensitive to the Lewis acidity of the iron salt:19 no reaction was observed if the counterion or the oxidation state was changed (entries 9 – 11). From our studies, iron(II) bromide appears unique in its ability to catalyze the C–H bond amination reaction, elimination and the 1,2-methyl shift with the optimal conditions to be 20 mol % catalyst loading in toluene at 140 °C.
Using these optimal conditions, the scope of our iron(II) bromide-catalyzed tandem C–H bond amination-elimination-1,2-methyl migration reaction was investigated (Table 2). We found that the reaction yield was not affected by the electronic nature of the aryl azide with consistent yields of the 2,3-dimethylindole obtained for both electron-releasing as well as electron-withdrawing groups. Despite the established reactivity of olefins with iron nitrenes,9c we found that aryl azide 8e bearing a styryl group was transformed into the indole product, albeit with a diminished yield (entry 5). Our reaction enables the synthesis of 6-substituted indoles (e.g. 10g), which cannot be made regioselectively using the Fischer indole reaction.20 These results indicate that changing the electronic nature of the aryl azide is not detrimental to the outcome of our tandem reaction.
Table 2.
Scope of Fe(II)-Catalyzed Tandem Reaction.

| ||||
|---|---|---|---|---|
| entry | # | R1 | R2 | yield, %a |
| 1 | a | H | H | 85 |
| 2 | b | OMe | H | 70 |
| 3 | c | Me | H | 98 |
| 4 | d | Ph | H | 85 |
| 5 | e | PhCH=CH | H | 50 |
| 6 | f | Br | H | 81 |
| 7 | g | H | Br | 79 |
Isolated after silica gel chromatography.
Next, the effect of changing the identity of the migrating group on the Fe(II)-catalyzed C–H bond amination-1,2-migration reaction was investigated (Table 3). We found that our reaction was not limited to 1,2-methyl shifts, but that ethyl group migrations as well as ring expansions could be triggered (entries 1 – 4). For the latter, the reaction was not constrained by the alleviation of ring strain: the highest reaction yield was obtained from the expansion the cyclohexyl substituted aryl azide 11d in comparison to cyclobutyl- and cyclopentyl substrates (entries 2 – 4).
Table 3.
Effect of Changing the Identity of the Migrating Group on the Tandem Reaction.

| ||||
|---|---|---|---|---|
| entry | # | aryl azide | indole | yield, %a |
| 1 | a |

|

|
83 |
| 2 | b |

|

|
65b |
| 3 | c |

|

|
69 |
| 4 | d |

|

|
78 |
| 5 | e |

|

|
95 |
| 6 | f |

|

|
58 |
| 7 | g |

|

|
dec |
| 8 | h |

|

|
83 |
| 9 | i |

|

|
60b |
| 10 | j |

|

|
50 |
| 11 | k |

|

|
42c |
Isolated after silica gel chromatography.
Aniline obtained as a by-product.
As determined using 1H NMR spectroscopy using CH2Br2 as the internal standard.
Our next objective was to determine if any selectivity could be observed during the migration component of the tandem reaction. We began this investigation by examining aryl azides that contained both methyl- and aryl groups (Table 3, entries 5 – 7). To our delight, we found that submission of these substrates to reaction conditions resulted in exclusive aryl group migration to afford 2-aryl-3-methylindoles, presumably via a phenonium ion. This reaction, however, was dependent on the electronic nature of the aryl group with only decomposition observed for azide 11g bearing an electron deficient arene. With successful differentiation between sp2- and sp3 carbons, we were curious if our reaction could distinguish between different sp3-substituted migrating carbons (entries 8 and 9). To examine this, we submitted aryl azide 11h to reaction conditions. To our surprise, we found that only ethyl group migration occurred to provide 2-ethyl-3-methylindole as the solitary product. To determine if this high selectivity was general, azide 11i bearing both an isopropyl- and ethyl group was submitted to reaction conditions to afford 2-isopropyl-3-ethylindole as the only product (entry 9). Finally, to test for alkyl- or aryl group migration in the presence of an α-hydrogen azides 11j and 11k were examined (entries 10 and 11). While diminished yields were obtained, only 3-substituted indoles 11j and 11k were observed from these azides revealing that these groups do not migrate when a hydrogen is present.21 From these results, a preliminary migratorial aptitude scale of our reaction can be established to be: Me < 1° < 2° < Ph.
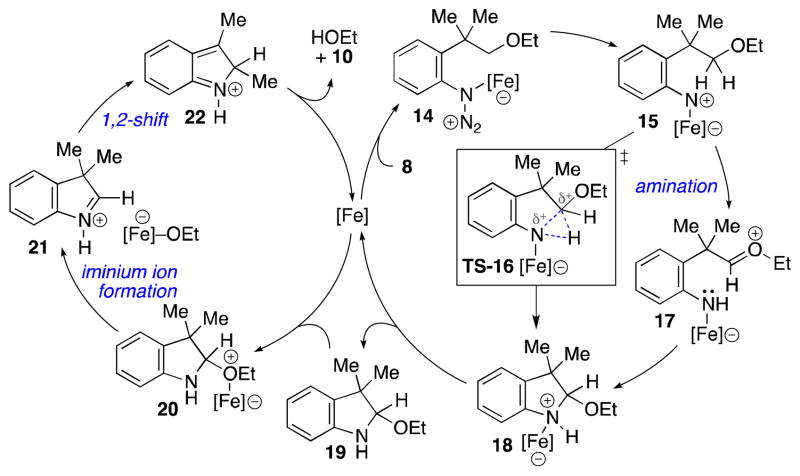
While a number of mechanisms can explain our transformation,21 we have interpreted our results to indicate that iron(II) bromide functions as both an N-atom transfer catalyst as well as a Lewis acid (Scheme 2). Coordination of the iron catalyst to the aryl azide (to form 14)23 triggers the extrusion of N2 to form iron nitrene 15.24 While the ethereal C–H bond amination reaction could be concerted (via TS-16),11a a stepwise process is also possible: hydride transfer from 15 forms oxocarbenium ion 17 that is attacked by the proximal amine to form indoline 18.25 Coordination of the Lewis acidic iron salt to the ethyl ether promotes the generation of iminium ion 21, which triggers the 1,2-shift.26 Subsequent deprotonation of 22 by iron ethoxide completes the catalytic cycle.
Scheme 2.
Possible Mechanisms for Fe-Catalyzed Tandem Reaction.
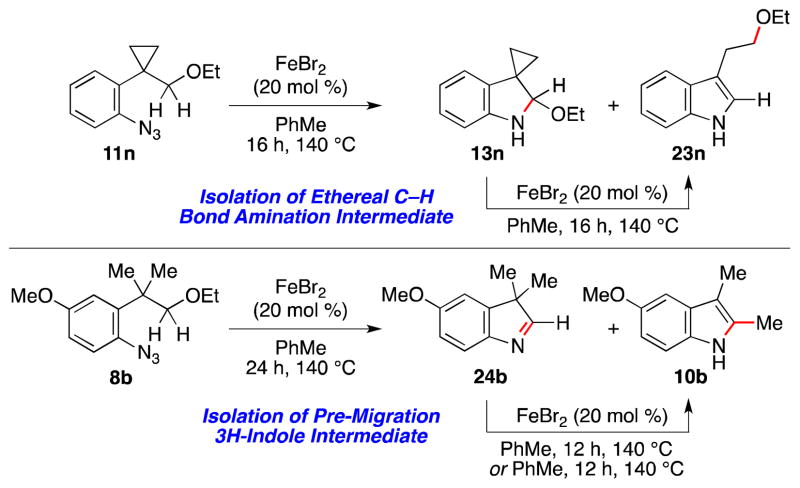
In the course of our optimization studies, we isolated two potential heterocyclic intermediates, whose reactivity towards the reaction conditions support our proposed mechanism (Scheme 3). When cyclopropyl-substituted aryl azide 11n was exposed to iron(II) bromide, a mixture of indoline 13n and indole 23n was isolated. Isolation of indoline 13n provides support that C–N bond formation occurs through an ethereal C–H bond amination reaction. The lack of fragmentation of the cyclopropane suggests that this amination reaction does not proceed through an H-atom abstraction-radical recombination reaction.22e,22h,27 Resubmission of 13n to reaction conditions produced indole 23n; in the absence of iron(II) bromide no reaction was observed. The reactivity of methoxy-substituted 8b was also consistent with our mechanistic hypothesis. The isolation of 3H-indole 24b indicates that the 1,2-methyl shift occurs after elimination of the ethoxide group. In contrast to 13n, thermolysis of 24b forms the 2,3-dimethylindole product in the absence of the Lewis acid. Together these results suggest that iron(II) bromide is required for both C–H bond amination as well as iminium ion formation, but not for 1,2- alkyl migration.
Scheme 3.
Isolation of Reactive Intermediates.
To probe the mechanism of the 1,2-shift reaction, a double crossover experiment was performed (eq 1). Exposure of a 1:1 mixture of 8a and 11a to reaction conditions resulted in the formation of only two indoles. The lack of crossover products suggests that the 1,2-shift component of our tandem reaction either is a concerted process; or if stepwise, the shift occurs faster than diffusion of the migrating group.
 |
(1) |
In conclusion, we have discovered that iron(II) bromide promotes tandem C–H bond amination-1,2 migration reactions of ortho-substituted aryl azides to enable the formation of 2,3-disubstituted indoles. The 1,2-shift component of our tandem reaction is remarkably selective, and our results enable prediction of the migration aptitude to be Me < 1° < 2° < Ph. Our future studies are aimed at achieving a better understanding of the mechanism of our tandem reaction as well as further exploring iron-catalyzed C–H bond amination reactions.
Supplementary Material
Acknowledgments
Funding Sources
National Institutes of Health NIGMS (R01GM084945) and the University of Illinois at Chicago
We are grateful to the National Institutes of Health NIGMS (R01GM084945) and the University of Illinois at Chicago for their generous financial support. We thank Mr. Furong Sun (UIUC) for high resolution mass spectrometry data.
Footnotes
Author Contributions
The manuscript was written through contributions of all authors.
Supporting Information. Experimental procedures, spectroscopic and analytical data for the products (PDF) are available free of charge via the Internet at http://pubs.acs.org.
References
- 1.For recent, leading reviews, see: Wasilke JC, Obrey SJ, Baker RT, Bazan GC. Chem Rev. 2005;105:1001. doi: 10.1021/cr020018n.Nicolaou KC, Edmonds DJ, Bulger PG. Angew Chem, Int Ed. 2006;45:7134. doi: 10.1002/anie.200601872.Padwa A. J Org Chem. 2009;74:6421. doi: 10.1021/jo901300x.Grant TN, Rieder CJ, West FG. Chem Commun. 2009:5676. doi: 10.1039/b908515g.Touré BB, Hall DG. Chem Rev. 2009;109:4439. doi: 10.1021/cr800296p.Lu LQ, Chen JR, Xiao WJ. Acc Chem Res. 2012;45:1278. doi: 10.1021/ar200338s.
- 2.For recent reports, cf. Green JC, Pettus TRR. J Am Chem Soc. 2010;133:1603. doi: 10.1021/ja109925g.Zou YQ, Lu LQ, Fu L, Chang NJ, Rong J, Chen JR, Xiao WJ. Angew Chem, Int Ed. 2011;50:7171. doi: 10.1002/anie.201102306.Tietze LF, Hungerland T, Düfert A, Objartel I, Stalke D. Chem Eur J. 2012;18:3286. doi: 10.1002/chem.201103209.Okazaki E, Okamoto R, Shibata Y, Noguchi K, Tanaka K. Angew Chem, Int Ed. 2012;51:6722. doi: 10.1002/anie.201202125.Jansone-Popova S, May JA. J Am Chem Soc. 2012;134:17877. doi: 10.1021/ja308305z.
- 3.For recent reviews of C–H bond amination, see: Davies HML, Manning JR. Nature. 2008;451:417. doi: 10.1038/nature06485.Collet F, Dodd RH, Dauban P. Chem Commun. 2009:5061. doi: 10.1039/b905820f.Driver TG. Org Biomol Chem. 2010;8:3831. doi: 10.1039/c005219c.Du Bois J. Org Process Res Dev. 2011;15:758. doi: 10.1021/op200046v.Davies HML, Du Bois J, Yu JQ. Chem Soc Rev. 2011;40:1855. doi: 10.1039/c1cs90010b.Roizen JL, Harvey ME, Du Bois J. Acc Chem Res. 2012;45:911. doi: 10.1021/ar200318q.
- 4.For leading reports of N-atom transfer, see: Espino CG, Du Bois J. Angew Chem, Int Ed. 2001;40:598. doi: 10.1002/1521-3773(20010202)40:3<598::AID-ANIE598>3.0.CO;2-9.Espino CG, Fiori KW, Kim M, Du Bois J. J Am Chem Soc. 2004;126:15378. doi: 10.1021/ja0446294.Lebel H, Huard K, Lectard S. J Am Chem Soc. 2005;127:14198. doi: 10.1021/ja0552850.Fraunhoffer KJ, White MC. J Am Chem Soc. 2007;129:7274. doi: 10.1021/ja071905g.Liang C, Collet F, Robert-Peillard F, Müller P, Dodd RH, Dauban P. J Am Chem Soc. 2008;130:343. doi: 10.1021/ja076519d.Reed SA, White MC. J Am Chem Soc. 2008;130:3316. doi: 10.1021/ja710206u.Milczek E, Boudet N, Blakey S. Angew Chem, Int Ed. 2008;47:6825. doi: 10.1002/anie.200801445.Nguyen AI, Zarkesh RA, Lacy DC, Thorson MK, Heyduk AF. Chem Sci. 2011;2:166.
- 5.(a) Tsang WCP, Zheng N, Buchwald SL. J Am Chem Soc. 2005;127:14560. doi: 10.1021/ja055353i. [DOI] [PubMed] [Google Scholar]; (b) Thu HY, Yu WY, Che CM. J Am Chem Soc. 2006;128:9048. doi: 10.1021/ja062856v. [DOI] [PubMed] [Google Scholar]; (c) Mei TS, Wang X, Yu JQ. J Am Chem Soc. 2009;131:10806. doi: 10.1021/ja904709b. [DOI] [PubMed] [Google Scholar]; (d) Tan Y, Hartwig JF. J Am Chem Soc. 2010;132:3676. doi: 10.1021/ja100676r. [DOI] [PubMed] [Google Scholar]; (e) Kim JY, Park SH, Ryu J, Cho SH, Kim SH, Chang S. J Am Chem Soc. 2012;134:9110. doi: 10.1021/ja303527m. [DOI] [PubMed] [Google Scholar]
- 6.For leading reports of N-atom transfer to trigger a tandem reaction, see: Bach T, Körber C. J Org Chem. 2000;65:2358. doi: 10.1021/jo991569p.Thornton AR, Blakey SB. J Am Chem Soc. 2008;130:5020. doi: 10.1021/ja7111788.Thornton AR, Martin VI, Blakey SB. J Am Chem Soc. 2009;131:2434. doi: 10.1021/ja809078d.
- 7.(a) Sun K, Liu S, Bec PM, Driver TG. Angew Chem, Int Ed. 2011;50:1702. doi: 10.1002/anie.201006917. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Stokes BJ, Liu S, Driver TG. J Am Chem Soc. 2011;133:4702. doi: 10.1021/ja111060q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nguyen Q, Sun K, Driver TG. J Am Chem Soc. 2012;134:7262. doi: 10.1021/ja301519q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.For the use of Fe-catalysts to oxidize C–H bonds, see: Srivastava RS, Khan MA, Nicholas KM. J Am Chem Soc. 1996;118:3311.Chen MS, White MC. Science. 2007;318:783. doi: 10.1126/science.1148597.King ER, Hennessy ET, Betley TA. J Am Chem Soc. 2011;133:4917. doi: 10.1021/ja110066j.Qin C, Zhou W, Chen F, Ou Y, Jiao N. Angew Chem, Int Ed. 2011;50:12595. doi: 10.1002/anie.201106112.Paradine SM, White MC. J Am Chem Soc. 2012;134:2036. doi: 10.1021/ja211600g.
- 10.cf. Cenini S, Gallo E, Penoni A, Ragaini F, Tollari S. Chem Commun. 2000:2265.Ragaini F, Penoni A, Gallo E, Tollari S, Gotti CL, Lapadula M, Mangioni E, Cenini S. Chem—Eur J. 2003;9:249. doi: 10.1002/chem.200390018.
- 11.For reports of ethereal C–H bond amination, see: Fiori KW, Fleming JJ, Du Bois J. Angew Chem, Int Ed. 2004;43:4349. doi: 10.1002/anie.200460791.Huard K, Lebel H. Chem–Eur J. 2008;14:6222. doi: 10.1002/chem.200702027.
- 12.For leading reports of Fe-catalyzed N-atom transfer from azides, see: Bach T, Körber C. Tetrahedron Lett. 1998;39:5015.Bach T, Schlummer B, Harms K. Chem Commun. 2000:287.Shen M, Driver TG. Org Lett. 2008;10:3367. doi: 10.1021/ol801227f.Stokes BJ, Vogel CV, Urnezis LK, Pan M, Driver TG. Org Lett. 2010;12:2884. doi: 10.1021/ol101040p.(from azirines) Jana S, Clements MD, Sharp BK, Zheng N. Org Lett. 2010;12:3736. doi: 10.1021/ol101130e.
- 13.For 1,2-alkyl shifts triggered by iminium ion formation, see: Caballero E, Avendaño C, Menéndez JC. J Org Chem. 2003;68:6944. doi: 10.1021/jo034703l.López-Alvarado P, Caballero E, Avendaño C, Menéndez JC. Org Lett. 2006;8:4303. doi: 10.1021/ol061631m.
- 14.cf. Stokes BJ, Dong H, Leslie BE, Pumphrey AL, Driver TG. J Am Chem Soc. 2007;129:7500. doi: 10.1021/ja072219k.Shen M, Leslie BE, Driver TG. Angew Chem, Int Ed. 2008;47:5056. doi: 10.1002/anie.200800689.
- 15.Sun K, Sachwani R, Richert KJ, Driver TG. Org Lett. 2009;11:3598. doi: 10.1021/ol901317j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.(a) Lu H, Subbarayan V, Tao J, Zhang XP. Organometallics. 2009;29:389. [Google Scholar]; (b) Lu H, Tao J, Jones JE, Wojtas L, Zhang XP. Org Lett. 2010;12:1248. doi: 10.1021/ol100110z. [DOI] [PubMed] [Google Scholar]
- 17.(a) Milczek E, Boudet N, Blakey S. Angew Chem, Int Ed. 2008;47:6825. doi: 10.1002/anie.200801445. [DOI] [PubMed] [Google Scholar]; (b) Shou WG, Li J, Guo T, Lin Z, Jia G. Organometallics. 2009;28:6847. [Google Scholar]; (c) Dong H, Latka RT, Driver TG. Org Lett. 2011;13:2726. doi: 10.1021/ol2008268. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Harvey ME, Musaev DG, Du Bois J. J Am Chem Soc. 2011;133:17207. doi: 10.1021/ja203576p. [DOI] [PubMed] [Google Scholar]
- 18.cf. Chiba S, Zhang L, Ang GY, Hui BWQ. Org Lett. 2010;12:2052. doi: 10.1021/ol100522z.Chiba S, Zhang L, Lee JY. J Am Chem Soc. 2010;132:7266. doi: 10.1021/ja1027327.Gephart RT, III, Huang DL, Aguila MJB, Schmidt G, Shahu A, Warren TH. Angew Chem, Int Ed. 2012;51:6488. doi: 10.1002/anie.201201921.
- 19.This contrasts with our earlier studies (cf. ref 11d) where FeBr3 showed a similar activity as FeBr2.
- 20.For a discussion on the limits of regioselectivity in the Fischer indole reaction, see: Phillips RR. Org React. 1959;10:1143.Robinson B. Chem Rev. 1969;69:227. doi: 10.1021/cr60262a003. and references therein.
- 21.While hydride migration could account for the formation of indole 13j and 13k, these indoles could be formed from an alternative mechanism involving deprotonation of the α-hydrogen from the iminium ion intermediate. We thank the reviewer for pointing out this possibility.
- 22.For leading mechanistic studies on the mechanism of aliphatic C–H bond amination, see: Ragaini F, Penoni A, Gallo E, Tollari S, Gotti CL, Lapadula M, Mangioni E, Cenini S. Chem—Eur J. 2003;9:249. doi: 10.1002/chem.200390018.Fiori KW, Du Bois J. J Am Chem Soc. 2007;129:562. doi: 10.1021/ja0650450.Zalatan DN, Du Bois J. J Am Chem Soc. 2009;131:7558. doi: 10.1021/ja902893u.Cowley RE, Eckert NA, Vaddadi S, Figg TM, Cundari TR, Holland PL. J Am Chem Soc. 2011;133:9796. doi: 10.1021/ja2005303.Lyaskovskyy V, Suarez AIO, Lu H, Jiang H, Zhang XP, de Bruin B. J Am Chem Soc. 2011;133:12264. doi: 10.1021/ja204800a.Kornecki KP, Berry JF. Chem Eur J. 2011;17:5827. doi: 10.1002/chem.201100708.Musaev DG, Blakey SB. Organometallics. 2012;31:4950.Wiese S, McAfee JL, Pahls DR, McMullin CL, Cundari TR, Warren TH. J Am Chem Soc. 2012;134:10114. doi: 10.1021/ja302149k.
- 23.For leading crystal structures of metal azide complexes, see: Fickes MG, Davis WM, Cummins CC. J Am Chem Soc. 1995;117:6384.Waterman R, Hillhouse GL. J Am Chem Soc. 2008;130:12628. doi: 10.1021/ja805530z.
- 24.For a computational studies on the mechanism of copper nitrenoid formation from azides, see: Badiei YM, Dinescu A, Dai X, Palomino RM, Heinemann FW, Cundari TR, Warren TH. Angew Chem, Int Ed. 2008;47:9961. doi: 10.1002/anie.200804304.
- 25.For reports of related C–H bond functionalization through hydride transfer reactions followed by ring closure, see: McQuaid KM, Sames D. J Am Chem Soc. 2009;131:402. doi: 10.1021/ja806068h.Murarka S, Deb I, Zhang C, Seidel D. J Am Chem Soc. 2009;131:13226. doi: 10.1021/ja905213f.
- 26.For the use of Fe-salts to trigger iminium ion formation, see: Vukovic J, Goodbody AE, Kutney JP, Misawa M. Tetrahedron. 1988;44:325.Ponzo VL, Kaufman TS. Synlett. 1995;1995:1149.Ishikawa H, Colby DA, Seto S, Va P, Tam A, Kakei H, Rayl TJ, Hwang I, Boger DL. J Am Chem Soc. 2009;131:4904. doi: 10.1021/ja809842b.Komeyama K, Yamada T, Igawa R, Takaki K. Chem Commun. 2012;48:6372. doi: 10.1039/c2cc32715e.
- 27.Alternatively, radical recombination to form indoline 13n could occur faster than cyclopropyl carbinyl fragmentation.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.