Abstract
Context
Previous epidemiological, animal, and human cognitive neuroscience research suggests that maternal smoking during pregnancy causes increased risk of offspring substance use/problems.
Objective
To determine the extent to which the association between SDP and offspring substance use/problems depends on confounded familial background factors by using a quasi-experimental design.
Design
We used two separate samples, from the United States and from Sweden, respectively. The analyses prospectively predicted multiple indices of substance use and problems while controlling for statistical covariates and comparing differentially exposed siblings to minimize confounding.
Setting
Sample 1: Offspring of a representative sample of women in the United States. Sample 2: The total Swedish population born over 13 years.
Patients or Other Participants
Sample 1: Adolescent offspring of the women in the National Longitudinal Survey of Youth 1979 (n=6,094). Sample 2: All offspring born in Sweden from 1983 through 1995 (n=1,187,360).
Main Outcome Measures
Sample 1: Self-reported adolescent alcohol, cigarette, and marijuana use, and early onset (before age 14 years) of each substance. Sample 2: Substance-related convictions and hospitalizations for an alcohol- or drug-related problem.
Results
The same pattern emerged for each index of substance use/problems across the two samples. At the population level maternal smoking during pregnancy predicted every measure of offspring substance use/problems in both samples, ranging from adolescent alcohol use (HRmoderate=1.32, CI=1.22–1.43; HRhigh=1.33, CI=1.17=1.53) to a narcotic convictions (HRmoderate=2.23, CI=2.14–2.31; HRhigh=2.97, CI=2.86–3.09). When comparing differentially exposed siblings to minimize genetic and environmental confounds, however, the association between SDP and each measure of substance use/problems was minimal and not statistically significant.
Conclusions
The association between maternal smoking during pregnancy and offspring substance use/problems was likely due to familial background factors, not a causal influence, because siblings had similar rates of substance use and problems regardless of their specific exposure to smoking during pregnancy.
Maternal smoking during pregnancy (SDP) is associated with pregnancy-related problems and poor offspring functioning across a number of domains,1–3 including increased risk for substance use problems during adolescence and adulthood.4 For instance, maternal SDP predicts greater offspring cigarette, alcohol, marijuana, and other illicit substance use during adolescence and early adulthood.5–9 Offspring exposed to SDP also are more likely to have substance use problems.7, 10–14, 15 Most epidemiological research has found robust statistical associations between SDP and substance-use problems in offspring when controlling for measured covariates,4 which is consistent with a causal inference, although some studies suggest that these associations are accounted for by correlated risks.12–13
Animal studies and human cognitive neuroscience research have documented plausible neural pathways through which exposure to maternal SDP could cause offspring substance use/problems.16–17 First, the hypothalamic-pituitary-adrenal axis, which has been implicated in drug-seeking behavior,18 may be altered prenatally by SDP exposure through nicotine binding to acetylcholine receptors.19 Second, the mesolimbic system, in which drugs exert their reinforcing effects, has been implicated from animals models of prenatal drug exposure.20–21 Third, the orbitofrontal cortex is involved in decision-making and responsiveness to drug-related stimuli,22 and exposure to SDP has been correlated with decreased volume in this structure in adolescent humans.8
Numerous studies, however, indicate that maternal SDP frequently co-occurs with other environmental and genetic risks for offspring substance use and problems.2–3 To provide strong tests of the causal inference research designs that can rule out plausible alternative explanations are necessary.23–25 Recent quasi-experimental studies (i.e. studies using design features to exclude plausible alternative mechanisms) suggest that the association between maternal SDP and characteristics related to substance use and problems, such as child conduct problems,26–28 Attention Deficit Hyperactivity Disorder,29–30 lower intellectual abilities and academic achievement,31–33 and adolescent and young-adult criminality,34–35 are due to confounded background familial factors, not a causal influence of SDP.
Although these studies suggest that the association between maternal SDP and offspring substance use and problems may be confounded, there is considerable disagreement regarding the findings form previous quasi-experimental reasearch. In particular, several researchers have suggested that the limitations of the quasi-experimental studies greatly hinder the generalizability of the results and that the existing quasi-experimental research does not negate, “the numerous studies that have shown long-term adverse effects of prenatal nictonie exposure.”36 Furthermore, to our knowledge, no published quasi-experimental studies have specifically predicted offspring substance use and problems from maternal SDP.
We tested the hypothesis that maternal SDP has a causal effect on numerous indices of subsequent offspring adolescent substance use and problems. To explicitly address concerns about the generalizability of the findings from previous sibling-comparison studies, the current study included prevalent behaviors, such as alcohol, tobacco, and marijuana use during adolescence, and less common indices of substance problems, including early onset of use,37–40 narcotic-related convictions, and hospitalization for substance-related problems. We used two methods to test for confounding factors: we (a) controlled for measured covariates that were correlated with maternal SDP and (b) compared siblings within nuclear families who were differentially exposed to maternal SDP, a design that rules out some confounding factors, such as environmental and genetic factors that make siblings similar.41–42 Data were drawn from two different studies and countries, which provided the opportunity to find converging evidence.
Methods
United States Study
Sample
The National Longitudinal Survey of Youth 1979 (NLSY79) was funded by the Bureau of Labor Statistics to study characteristics of individuals in the U.S. workforce. A nationally representative household sample of 6,111 14–22 year old males and females who were not in the military was selected for the NLSY79, using a complex survey design.43 An additional 3,652 African-American and Hispanic youths was selected for the NLSY79 mother-generation sample to oversample from these ethnicity groups. The response rate for the initial NLSY79 assessment was 90% of the eligible sample. Participants were interviewed annually from 1979 through 1994 and have been interviewed biennially since 1995. Retention rates for the NLSY79 during follow-up assessments were ≥90% during the first 16 waves and >80% in subsequent waves. To date, 4,926 NLSY79 females (1,472 African Americans; 977 Hispanics; 2,477 non-Hispanic Caucasians and other ethnicities) have given birth. The sample included in the analyses comprised 3,168 mothers with one or more offspring at least 14 years old.
Biennial assessments of the biological children of women in the NLSY79, referred to as the CNLSY study, began in 1986.44 In 1986, 95% of the offspring of NLSY79 mothers were assessed with an average retention rate of approximately 90% in assessments occurring through 2008. In each wave, self-report questionnaires were administered to older children and adolescents based on the age of the offspring. Adolescents and young adults (aged 14–30 years) completed the Young-Adult Computer Assisted Personal Interview on academic, social, and emotional development during adolescence and the transition into adulthood. As of the 2008 wave of assessment, 11,506 offspring have been born to women in the NLSY79. Offspring who were missing maternal identification number (n=11); assessment of maternal SDP (1,244), due mostly to an incorrect skip pattern in the assessment procedure in one wave26; or who were younger than 14 years old (3,347) were sequentially excluded from the dataset, resulting in a sample of 6,094 offspring. The offspring were 22.1 years old on average (SD=4.92) at the latest assessment (2008).
The NLSY79 provides sample weights, indicating the inverse of the probability of each participant being selected into the sample, based on the clustered, unequal selection probability design. We applied these weights, which apply equally to all offspring of a given mother, to all analyses to better approximate population-based estimates. More details about the sample and measurement are available elsewhere.45–46
Measures
Risk Factors
In the first assessment wave of the CNLSY study after the birth of a child mothers reported on their SDP frequency on a four-point ordinal scale: none, <1 pack/day, 1–2 packs/day, >2 packs/day. Less than 1 pack/day was considered moderate use (n=1,387, 20.1%) and the highest two categories were combined to indicate high use, n=520, 7.5%).
Demographic characteristics of the CNLSY sample are presented in Table 1. For each measure, individuals at higher risk or with missing values were compared to individuals with low risk using ordinal or binary scales. Offspring gender, birth order, maternal age childbearing, and maternal alcohol consumption during pregnancy 45 were used as measured offspring-specific covariates.
Table 1.
Demographic Characteristics of Offspring and Families in the United States (NLSY) Dataset.
| Variable | N | Percentage |
|---|---|---|
| Offspring-Specific Covariatesa | ||
| Femalec | 3356 | 48.6 |
| Birth Order | ||
| Onec | 3164 | 45.8 |
| Two | 2221 | 32.2 |
| Three | 1015 | 14.7 |
| Four plus | 504 | 7.3 |
| Maternal Teenage Childbearing | ||
| Noc | 3856 | 55.9 |
| Yes | 2926 | 42.4 |
| Missing | 122 | 1.8 |
| Maternal Alcohol Consumption during Pregnancy (frequency of use) | ||
| Neverc | 4777 | 69.2 |
| Less than once/month | 1015 | 14.7 |
| Once/month | 503 | 7.3 |
| Three-Four days/month | 277 | 4.0 |
| One-Two days/week | 250 | 3.6 |
| Three-Four days/week | 43 | 0.6 |
| Nearly every day or more | 28 | 0.4 |
| Missing | 11 | 0.2 |
| Maternal/Familial Covariatesb | ||
| Mother Adolescent Antisocial Behavior | ||
| Lowc | 752 | 23.7 |
| Medium Low | 714 | 22.5 |
| Medium High | 745 | 23.5 |
| High | 803 | 25.4 |
| Missing | 154 | 4.9 |
| Maternal Intellectual Abilities | ||
| Low | 642 | 20.3 |
| Medium Low | 775 | 24.5 |
| Medium High | 783 | 24.7 |
| Highc | 842 | 26.6 |
| Missing | 126 | 4.0 |
| Maternal Educational Attainment | ||
| 12 years or lessc | 1165 | 36.8 |
| 13 to 15 years | 1000 | 31.6 |
| 16 years or more | 1003 | 31.7 |
| Family Income | ||
| Lowc | 672 | 21.2 |
| Medium Low | 697 | 22.0 |
| Medium High | 754 | 23.1 |
| Highc | 793 | 25.0 |
| Missing | 252 | 7.9 |
| Lifetime History of Binge Drinking | ||
| Noc | 2655 | 83.8 |
| Yes | 513 | 16.2 |
| Lifetime History of Alcohol Abuse/Dependence Problems | ||
| Noc | 2564 | 80.9 |
| Yes | 502 | 15.9 |
| Missing | 102 | 3.2 |
| Maternal Adolescent Substance Use | ||
| Noc | 2448 | 77.3 |
| Yes | 662 | 20.9 |
| Missing | 58 | 1.8 |
| Family Race/Ethnicity | ||
| Caucasianc | 1536 | 48.5 |
| African American | 1005 | 31.7 |
| Hispanic | 627 | 19.8 |
Note.
Based on 6,904 offspring.
Based on 3,168 unique mothers.
Used as the reference group in the analyses.
The study also included measured traits of the mothers and the families. Maternal adolescent antisocial behavior was based on maternal self-reports.47 An index of low maternal intellectual abilities was based on a composite derived from the Armed Services Vocational Aptitude Battery given in 1980. Maternal education was based on highest completed grade. Total family income was based on assessments when the mothers were 30 years old. The mothers also completed a detailed assessment of lifetime history of alcohol problems in 1994. If a woman ever reported binge drinking or any history of alcohol abuse or dependence problems (from a 25 item assessment), we considered them to be at higher risk of alcohol problems. The mothers also reported on their use of any illicit substances during adolescence. The women’s race/ethnicity was divided into three categories: Caucasian, African American, and Hispanic.
Offspring Outcomes
At each childhood assessment wave (ages 10–13), participants were asked, “Have you ever drunk alcohol, other than just a sip or two? (Do not include childhood sips that you might have had from an older person’s drink.)” Participants responding “yes” were asked to report how old they were the first time they had “a glass of beer or wine or a drink of liquor, such as whiskey, gin, scotch, etc.” At each young adult assessment wave (ages 14–30), participants were asked if they ever drank alcohol in the past 12 months, and their age at first alcoholic drink. If participants reported varying ages at first drink across assessment waves, an average reported age at first drink was calculated. Reported age at first drink less than 7 years old was also coded as missing due to low prevalence rates and concerns about reporting accuracy. Because the present analyses focused on adolescent alcohol use, we created an indicator of drinking before the age of 20. For offspring below age 20, time-to-event information was based on their last age of assessment. Due to missing values (n=233, 3%), analyses were run with 6,671 offspring. Kaplan-Meier estimates indicated 87% of offspring used alcohol during adolescence. Because previous studies have shown that early onset of alcohol use (i.e., before the age of 14) was a strong predictor of subsequent alcohol problems,37–38 we identified offspring who reported being less than 14 years old at their first drink (n=1,470, 22%) to index problematic use.
At each childhood and young adult assessment wave, participants were asked to report whether they had ever smoked a cigarette or used marijuana, respectively. Participants responding “yes” to each item were subsequently asked to report age of first use. Following the same pattern as with the alcohol variables, we created measures of cigarette and marijuana use. Kaplan-Meier estimates indicated 58% tried cigarettes during adolescence (from a sample of 6,620 offspring with valid information), and 23% had early cigarette use (n=1,501).39 Kaplan-Meier estimates indicated that 53% had used marijuana during adolescence (from a sample of 6,708 offspring), and 10% reported an age of first marijuana use less than 14 (n=685).40 The reported ages of onset for each substance were quite reliable (correlations ranged from 0.89 to 0.97 based on multiple reports), and the estimates of drug use in the CNLSY are very consistent with recent epidemiological studies in the United States.48 More details about the assessment are available elsewhere.26
Swedish Study
Sample
The analyses were based on all offspring born in Sweden between 1983 and 1995. All data were obtained by merging information available from seven government-maintained population registers: (1) the Swedish Medical Birth Registry, kept by the National Board of Health and Welfare, included data on more than 99% of pregnancies in Sweden from 1973 onwards;49–50 (2) the Multi-Generation Register, held by Statistics Sweden, contained information about biological and adoptive relationships for individuals living in Sweden since 1933;51 (3) the National Crime Register, held by the National Council for Crime Prevention, included detailed information about all criminal convictions since 1973 for those aged 15 and older;52 (4) the Inpatient Registry (National Board of Health and Welfare), provided data on all hospital admissions for psychiatric disorder in Sweden since 1973;53 (5) the Education Register (Statistics Sweden), contained information on highest level of completed formal education;54 (6) the Cause of Death Register (National Board of Health and Welfare), provided data on principal and contributing causes of death since 1958; and (7) the Migration Register (Statistics Sweden) supplied data on dates for migration into or out of Sweden.
Between 1983 and 1995, data for 1,411,134 children were included in the Medical Birth Registry. Children who had serious malformations at birth (n=57,555), were stillborn either before or during delivery (n=4,939), were from multiple births (n=33,061), died before the age of 15 (n=7,861), or emigrated from the country before the age of 15 (n=49,733) were not eligible for inclusion. Of the remaining 1,256,983 offspring, those who were missing data on SDP (n=77,228), offspring sex (n=36), or the identification number of their mother (n=84) were excluded from the analyses. The resulting sample of 1,187,360 offspring represents 94.5 % of the targeted population. The final sample includes offspring born to 743,673 different mothers; the siblings in our analyses share the same mother. More details about the sample and measurement are available elsewhere.32, 34
Measures
Risk Factors
Maternal SDP was based on self-report of daily tobacco use at the first antenatal visit, which typically occurred during the first trimester, using the following responses: no smoking, 1–9 cigarettes/day (moderate SDP, n=186,342, 15.7%), or 10 or more cigarettes/day (high SDP, n=114,351, 9.6%). Previous studies indicate that the validity of this measure is high.55 SDP was converted into two dummy codes to compare moderate and high levels of SDP to no smoking.
Demographic characteristics of the Sweden sample are presented in Table 2. Sex, birth parity, maternal age at childbearing, and paternal age at childbearing, and maternal cohabitation with the father at childbirth were offspring-specific risk factors included in the analyses. Low maternal and paternal highest level of education (indexed by ≤ 1–2 years of upper secondary education), history of any criminal conviction, and history of ever being hospitalized for a substance-related problem were included as familial risk factors. Dummy codes were created to compare high-risk groups and those with missing values (if present) to the low-risk groups.
Table 2.
Demographic Characteristics of Offspring and Families in the Sweden Dataset.
| Variable | N | % |
|---|---|---|
| Offspring-Specific Covariatesa | ||
| Female | 578721 | 48.7 |
| Birth order | ||
| First born | 485857 | 40.9 |
| Second born | 427318 | 36.0 |
| Third born | 193692 | 16.3 |
| Fourth+ | 80493 | 6.8 |
| Maternal age at childbirth | ||
| Less than 20 years old | 33085 | 2.8 |
| 20 to 25 years old | 267409 | 22.5 |
| 25 to 30 years oldc | 445367 | 37.5 |
| 30 to 35 years old | 302946 | 25.5 |
| 35 years or older | 138553 | 11.7 |
| Paternal age at childbirth | ||
| Less than 20 years old | 7843 | 0.7 |
| 20 to 25 years old | 135851 | 11.4 |
| 25 to 30 years oldc | 375561 | 31.6 |
| 30 to 35 years old | 362932 | 30.6 |
| 35 years or older | 299351 | 25.2 |
| Missing | 5822 | 0.5 |
| Cohabitating at time of childbirth | ||
| Cohabitatingc | 1073686 | 90.4 |
| Not Cohabitating | 59781 | 5.0 |
| Missing | 53893 | 4.5 |
| Maternal Covariatesb | ||
| Low Education | ||
| Less than 1–2 years of upper secondary education | 88034 | 11.8 |
| Greater than 1–2 years of upper secondary educationc | 654398 | 88.0 |
| Missing | 1241 | 0.2 |
| Criminal Conviction | ||
| Any Lifetime Conviction | 94734 | 12.7 |
| No History of Convictionc | 648939 | 87.3 |
| Substance-Related Hospitalization | ||
| Any Lifetime Hospitalization | 16170 | 2.2 |
| No History of Hospitalizationc | 727503 | 97.8 |
| Paternal Covariatesb | ||
| Low Education | ||
| Less than 1–2 years of upper secondary education | 143687 | 19.3 |
| Greater than 1–2 years of upper secondary educationc | 591499 | 79.5 |
| Missing | 8487 | 1.1 |
| Criminal Conviction | ||
| Any Lifetime Conviction | 318624 | 42.8 |
| No History of Convictionc | 420086 | 56.5 |
| Missing | 4963 | 0.7 |
| Substance-Related Hospitalization | ||
| Any Lifetime Hospitalization | 32251 | 4.3 |
| No History of Hospitalizationc | 706459 | 95.0 |
| Missing | 4963 | 0.7 |
Note.
Based on 1,187,360 offspring.
Based on 743,673 unique mothers and traits of fathers of the first child of those women.
Used as the reference group in the analyses.
Offspring Outcomes
Two types of offspring substance-related criminality were modeled in the current study: (1) crimes committed by driving a motor vehicle under the influence of alcohol or other substance (Driving Under the Influence), as defined by the Swedish Penal Code, and (2) narcotic drug offenses as defined by the Narcotic Drugs Criminal Act, which includes possession for personal use, supply, and manufacturing. The time-to-event for these outcomes is based on the date of the first criminal act leading to a criminal conviction. In the current sample, 11,231 of the offspring had a driving-related criminal conviction and 16,790 had a drug-related conviction. Kaplan-Meier estimates indicated that by the age of 25 years, 2% of the sample had at least one driving-related conviction and 3% had at least one narcotic drug offense.
Hospitalization for alcohol and drug problems was also employed to indicate substance-related problems. All substance-related hospitalizations in the current study were derived from inpatient records. We restricted our focus to events where the primary diagnosis involved psychoactive substance use as defined by ICD8-10 diagnostic codes. The three categories of offspring substance-related outcomes modeled were (1) any primary or secondary diagnosis of alcohol-related hospitalization or any other, non-nicotine, substance misuse-related hospitalization; (2) only primary alcohol-related diagnosis, (ICD-8/ICD-9: 303; ICD-9: 305A; ICD-10: F10), (3) only primary drug-related diagnosis (excluding nicotine dependence; ICD-8/ICD-9: 304; ICD-9: 305X; ICD-10: F11–F16, F18–F19). Estimated time-to-event and indicator were set to missing if the hospitalization and diagnosis occurred before the age of 12 years old. In the current sample there were 22,092 hospitalizations for either alcohol or drug problems (where the ICD codes could be either the primary or a secondary diagnosis), 14,850 hospitalizations when alcohol was the primary diagnosis, and 5,560 hospitalizations when drugs were the primary diagnosis. Kaplan-Meier estimates indicated 3% had any substance-related hospitalization (primary or secondary), 2% had an alcohol-related (primary) hospitalization, and 1% had a drug-related (primary) hospitalization by the age of 25 years.
Convictions for driving under the influence (OR=9.95, CI=9.44–10.49) and narcotic drug offenses (OR=19.81, CI=19.07–20.58) were highly associated with hospitalization for either an alcohol or drug problem.
Statistical Analyses
Three models were fit to each measure of substance use/problems in the NLSY and Sweden samples, respectively. We used two analytical models. First, we used Cox proportional hazards survival analysis models for all outcomes in which the outcome was right-censored (i.e., not all of the offspring had lived through the risk period). For example, some of the offspring in the CNLSY study had not reached the age of 20 years old. Second, logistic regression models were used when the outcome was a dichotomous measure. All analyses used robust standard errors to account for the nested nature of the data (i.e., cousins and siblings were nested within extended families).
For each outcome, Model 1 (of 3) regressed substance use/problems on maternal SDP, offspring gender, and offspring birth order. This model provided an estimate of the increased risk associated with moderate and high SDP (compared to no SDP) in the entire population. Model 2 included all of the offspring-specific and familial covariates in the model. This model provided the independent association between moderate and high SDP and offspring substance use/problems while statistically controlling for the measured covariates. Model 3, in contrast, introduced a fixed intercept term at the mother-level, thereby estimating the increased risk associated with moderate and his SDP while comparing differentially exposed siblings. These models effectively control for all factors that are shared by siblings, whether those factors are observed or not,56 automatically accounting for all genetic and environmental factors that make siblings similar.41–42 The CNLSY included 1,364 differentially exposed offspring (those experiencing more or less SDP than their siblings) among 462 women. The Sweden study had 141,408 differentially exposed siblings from 60,056 women.
We also conducted a number of sensitivity analyses to examine if the use of the sibling-comparison design with the two datasets resulted in findings consistent with previous quasi-experimental analyses that found an independent association between maternal SDP and offspring low birth weight (below 2,500 grams).1, 3, 26, 28, 57 We fit the three models predicting low birth weight in the United States (13% low birth weight, n=1,318 in the entire sample of 10,251 offspring born to women in the NLSY79 sample) and the Sweden sample (3% low birth weight, n=39,827 in the entire sample of 1,187,360 offspring) while controlling for gestational age.
The Institutional Review Board at Indiana University approved both studies.
Results
United States Study
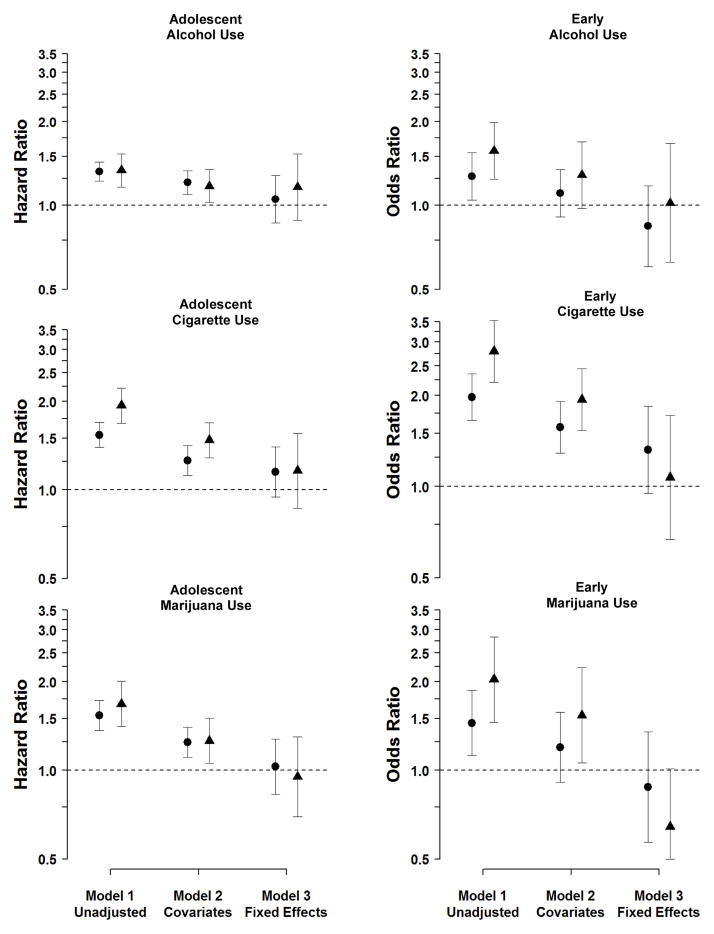
Results of the three analytical models regarding effects of SDP each outcome measure of substance use/problems are presented in Table 3. The corresponding Hazard Ratios (HRs) and Odds Ratios (ORs) (with 95% Confidence Intervals (CIs) for the United States study are shown in Figure 1.
Table 3.
Parameter Estimates and Standard Errors Quantifying the Effects of Maternal Smoking During Pregnancy on Offspring Substance Use/Problems in the United States and Sweden Samples.
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| b | SE | b | SE | b | SE | |
| United States Study | ||||||
| Adolescent Alcohol Usea | ||||||
| Moderate SDP | 0.28* | 0.04 | 0.19* | 0.05 | 0.05 | 0.10 |
| High SDP | 0.29* | 0.07 | 0.16* | 0.07 | 0.15 | 0.14 |
| Early Alcohol Useb | ||||||
| Moderate SDP | 0.24* | 0.10 | 0.10 | 0.10 | −0.17 | 0.17 |
| High SDP | 0.45* | 0.12 | 0.27* | 0.13 | 0.02 | 0.25 |
| Adolescent Cigarette Usea | ||||||
| Moderate SDP | 0.43* | 0.05 | 0.23* | 0.06 | 0.14 | 0.10 |
| High SDP | 0.66* | 0.07 | 0.39* | 0.07 | 0.15 | 0.15 |
| Early Cigarette Useb | ||||||
| Moderate SDP | 0.68* | 0.09 | 0.45* | 0.10 | 0.28 | 0.17 |
| High SDP | 1.03* | 0.12 | 0.66* | 0.12 | 0.07 | 0.24 |
| Adolescent Marijuana Usea | ||||||
| Moderate SDP | 0.43* | 0.06 | 0.23* | 0.06 | 0.03 | 0.11 |
| High SDP | 0.52* | 0.09 | 0.24* | 0.09 | −0.05 | 0.16 |
| Early Marijuana Useb | ||||||
| Moderate SDP | 0.37* | 0.13 | 0.18 | 0.14 | −0.13 | 0.22 |
| High SDP | 0.71* | 0.17 | 0.43* | 0.18 | −0.44 | 0.33 |
| Sweden Study | ||||||
| Substance-Related Driving Convictiona | ||||||
| Moderate SDP | 0.73* | 0.02 | 0.38* | 0.02 | −0.03 | 0.10 |
| High SDP | 0.98* | 0.02 | 0.50* | 0.03 | 0.11 | 0.12 |
| Narcotic Convictiona | ||||||
| Moderate SDP | 0.80* | 0.02 | 0.43* | 0.02 | −0.05 | 0.08 |
| High SDP | 1.09* | 0.02 | 0.55* | 0.02 | 0.01 | 0.09 |
| Substance-Related Hospitalization (Primary or Secondary Diagnosis)a | ||||||
| Moderate SDP | 0.60* | 0.02 | 0.35* | 0.02 | 0.06 | 0.06 |
| High SDP | 0.82* | 0.02 | 0.43* | 0.02 | 0.02 | 0.07 |
| Alcohol-Related Hospitalization (Primary Diagnosis)a | ||||||
| Moderate SDP | 0.54* | 0.02 | 0.32* | 0.02 | 0.12 | 0.07 |
| High SDP | 0.73* | 0.02 | 0.40* | 0.03 | 0.04 | 0.08 |
| Drug-Related Hospitalization (Primary Diagnosis)a | ||||||
| Moderate SDP | 0.82* | 0.03 | 0.42* | 0.04 | 0.02 | 0.12 |
| High SDP | 1.15* | 0.03 | 0.56* | 0.04 | 0.08 | 0.14 |
Note. Parameter estimates are log odds ratios or log hazard ratios.
p<.05.
Based on Cox proportional hazard survival models.
Figure 1.
Hazard and Odds Ratio estimates for the Association between Maternal Smoking during Pregnancy and Offspring Substance Use/Problems in the United States Sample
Note. Point estimates for moderate (versus no) smoking are presented as circles and point estimates for high (versus no) smoking are in triangles. Model 1 presents the associations in the entire sample. Model 2 presents the associations in the sample controlling for statistical covariates. Model 3 presents the estimated associations fitting fixed-effects models at the mother level, which compared differentially exposed siblings. The estimated Hazard Ratios for Teenage Alcohol Use, Cigarette Use, and Marijuana Use were based on Cox proportional hazard survival models, which used a sandwich estimator to account for familial clustering. The estimated Odds Ratios for Early Alcohol Use, Early Cigarette Use, and Early Marijuana Use were based on logistic regression models, which used a sandwich estimator to account for familial clustering.
In Model 1 both moderate (HR=1.32) and high (HR=1.33) maternal SDP was associated with approximately a 30% increased odds of drinking during adolescence. In Model 2, when measured covariates were included in the model, the estimates were somewhat attenuated, but moderate (HR=1.20) and high (HR=1.17) maternal SDP were still associated with approximately 20% increased risk. In Model 3, in which differentially exposed siblings were compared, however, moderate (HR=1.05) and high (HR=1.16) SDP did not predict adolescent alcohol use, as the magnitude of the associations were greatly attenuated compared to the unadjusted associations and were not statistically significant. Similarly, for early offspring alcohol use, maternal SDP (ORmoderate=1.27, ORhigh=1.57) was a moderate predictor in Model 1. The inclusion of measured covariates in Model 2 slightly reduced the magnitude of the associations (ORmoderate=1.10, ORhigh=1.30), but maternal SDP was not associated with early alcohol use in the fixed-effects model (Model 3, ORmoderate=0.84, ORhigh=1.02).
The same pattern of results emerged when predicting offspring cigarette use and early cigarette use. Finally, the results for models predicting adolescent marijuana use were parallel to those for alcohol and cigarette use--maternal SDP was strongly associated with offspring substance use/problems in Model 1, the association was attenuated in Model 2, but maternal SDP did not predict early the substance use/problems measures in Model 3.
Sweden Study
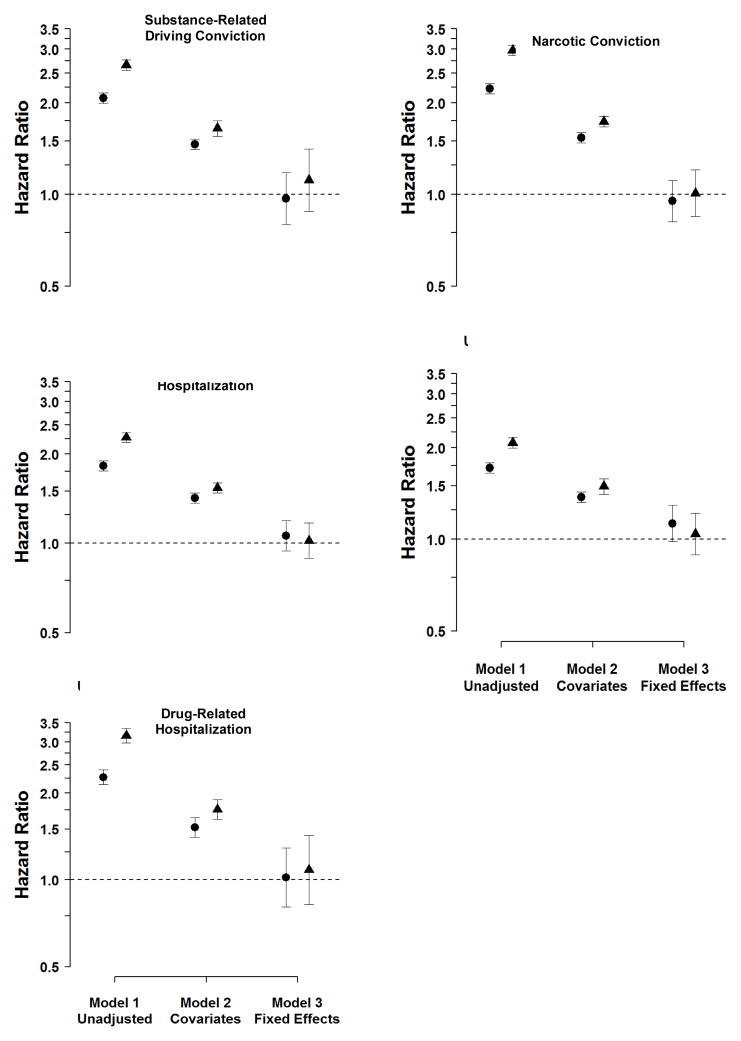
The corresponding Hazard and Odds Ratios (with 95% CIs) for the Sweden study are shown in Figure 2. Maternal SDP was strongly associated with substance-related driving convictions in Sweden (Model 1; HRmoderate=2.07, HRhigh=2.66). In Model 2 (which included statistical covariates), maternal SDP remained a robust predictor (HRmoderate=1.46, HRhigh=1.65), although the magnitude of the association was reduced. In Model 3 (the fixed-effects model), in contrast, the associations were completely attenuated (HRmoderate=0.97, HRhigh=1.12) and not statistically significant. The same pattern occurred when predicting a narcotics-related conviction.
Figure 2.
Hazard Ratios for the Association between Maternal Smoking during Pregnancy and Offspring Substance Use/Problems in the Sweden Sample
Note. Point estimates for moderate (versus no) smoking are presented as circles and point estimates for high (versus no) smoking are in triangles. Model presents the associations in the entire sample. Model 2 presents the associations in the sample controlling for statistical covariates. Model 3 presents the estimated associations fitting fixed-effects models at the mother level, which compared differentially exposed siblings. All estimated Hazard Ratios are based on Cox proportional hazard survival models, which used a sandwich estimator to account for familial clustering.
The same pattern of associations was evident also for all the substance-related hospitalizations, which included all primary and secondary diagnoses of alcohol or drug problems (excluding nicotine dependence), only primary alcohol problems, and only primary drug problems (Table 3, Figure 2).
Sensitivity Analyses
To examine the statistical power and other limitations (e.g., measurement bias in SDP) of the sibling comparison approach (Model 3), we applied the three analytical models described above to the prediction of low birth weight in both samples. In the United States sample, moderate (OR=1.22, 95% CI=1.01–1.47) and high SDP (OR=1.42, 95% CI=1.09–1.84) predicted low birth weight in Model 1. In Model 2, SDP still predicted low birth weight (ORmoderate= 1.20, 95% CI=1.00–1.44; ORhigh=, 1.52, 95% CI=1.17–1.97). Finally, in the sibling comparisons (Model 3), both odds ratios indicated higher risk of low birth weight, although the confidence intervals for moderate SDP included 1.00 (ORmoderate=1.19, 95% CI=0.75–1.91; ORhigh=1.86, 95% CI=1.01–3.41). In the Sweden sample, in model 1 moderate (ORmoderate=1.71, 95% CI=1.65–1.76) and high SDP (ORhigh=2.09, 95% CI=2.02–2.17) predicted low birth weight. The associations were persistent in Model 2 (ORmoderate=1.67, 95% CI=1.62–1.73; ORhigh=1.99, 95% CI=1.91–2.07). In Model 3, SDP still predicted low birth weight (ORmoderate=1.46, 95% CI=1.26–1.69; ORhigh=1.47, 95% CI=1.24–1.74).
Comments
We found converging evidence across samples and measures. Consistent with previous research, offspring exposed to maternal SDP were more likely to use and abuse substances during adolescence and young adulthood. The association between maternal SDP and offspring substance use was generally robust to the use of measured statistical covariates, also consistent with most previous research.4 However, siblings within the same nuclear family who were differentially exposed to maternal SDP did not differ in their risk of substance use or abuse. As such, these results strongly suggest that familial confounds account for the increased risk of substance use/abuse among offspring exposed to maternal SDP. It is not possible to accept the null hypothesis regarding SDP, of course, but the present results are not consistent with a causal effect of SDP on offspring substance-use outcomes.
These conclusions are strengthened by the internal and external validity of the current findings.23 First, the study used a powerful design, the comparison of differentially exposed siblings,41–42 to control for unmeasured confounds that could account for the association between maternal SDP and offspring substance use/problems rather than solely relying on the use of measured covariates. Second, the results are based on multiple datasets, including a study of offspring of a representative sample of women in the United States and a population-based study of all offspring born in Sweden over 13 years. These results, therefore, neither depend on using one particular sampling strategy nor apply only to a sample with restricted demographic, racial, or ethnic characteristics. Third, the study addressed concerns regarding the generalizability of previous quasi-experimental research on maternal SDP36 by using multiple indices of substance use and problems. The current manuscript used measures of substance use that (a) were quite common, such as adolescent alcohol, cigarette, and marijuana use; (b) indicated risk for substance-related problems (i.e. early onset of use37–40), and (c) documented serious substance problems problems, including substance-related convictions and hospitalizations. Different methods (e.g., self ratings, register of criminal convictions, and clinical diagnoses) were also used to assess substance use/problems across the two samples. It is quite remarkable, in fact, that the same pattern of results was found for each and every measure across the two samples.
The current manuscript also has a number of limitations that should be considered. Although we were able to replicate the same findings across measures and sample, the same outcomes were not measured in both samples. For instance, we do not have information on adolescent substance use or age of onset in the Swedish sample. The CNLSY study includes measures of alcohol and marijuana impairment for individuals meeting strict gateway criteria. However, ut the prevalence of each was too small to predict, and the assessments did not include usable information about age of onset of such problems. Further, the current analyses of CNLSY sample also were based on a subset of the offspring, as not all offspring had reached adolescence. The current study used sample weights to address concerns about the generalizability of the results from the subset, and prevalence rates from this study are comparable to recent epidemiological studies in the United States.48 There are also a number of assumptions and limitations in the sibling-comparison design.41–42 The statistical power to detect small effects is limited in the sibling-comparison designs, as with all fixed effects models,56 because the estimates rely on the subset of women who varied their smoking across pregnancies. As a result, there are relatively large confidence intervals around the estimates from the sibling-comparison models in the United States sample, although the large Swedish sample allowed for more precise estimates. Family-based quasi-experimental studies also are sensitive to problems with poor measurement reliability in the predictor variable,58 but previous research has shown that self-reported maternal SDP is reliable59 and valid (e.g., compared to serum cotinine levels).60 Research in Sweden61 and the United States62 also suggest that the validity of self-reported SDP has not changed over time. Nevertheless, the study suggests that limitations of the current studies (e.g., the limitations of self-reported SDP) and the assumptions in the sibling-comparison design may not lead to overly conservative estimates of maternal SDP because we replicated the well-established finding of a robust, independent association between maternal SDP and offspring low birth weight1, 3, 26, 28, 57 in both samples.
Future studies also should explore the timing of maternal SDP across pregnancies35 and test the generalizability of findings from women who vary their smoking over time.34 Additional research must also seek to specify the exact familial confounds that increase risk for offspring substance use/problems because sibling comparison studies cannot identify those factors by themselves.41–42 Additional quasi-experimental designs, such as the comparison of full and half siblings,32 children of siblings and twins,26, 32, 57, 63 adopted individuals,64 and offspring conceived through fertility treatments28 are necessary to answer such questions because the confounding factors make siblings within a nuclear family similar. The current studies had limited measures of confounding variables; for example, the CNLSY study did not include paternal characteristics and neither study included measures of postnatal smoking exposure.65 Future studies will need to include extensive measures of family functioning.
The present findings are consistent with a growing body of research on maternal SDP using quasi-experimental designs,3 which strongly suggests that familial background factors are responsible for increased risk of childhood child conduct problems,26–28 Attention Deficit Hyperactivity Disorder,29–30 lower intellectual abilities and academic achievement,31–33 suicidal behavior,66 and adolescent and young adult criminality.34–35 Certainly, the conclusions concerning substance use/problems drawn from the current manuscript will need to be replicated in other studies, particularly other studies that both (a) include precise measures of SDP, covarying environmental risks, and substance use/problems and (b) use design features to rule out plausible alternative processes.23
The recent quasi-experimental studies as a whole, nonetheless, have serious implications for several research areas and intervention efforts.3, 67 First, research needs to focus on the translation of findings regarding SDP from animal studies to human studies and vice versa, as is true in all areas of neuropsychiatric research.68–69 Differences in pregnancies across species, such as factors influencing timing of gestation,70 may limit the generalizability of animal studies of SDP. Second, researchers studying moderating factors, such as gene-environment interactions, must also be aware that maternal SDP may not be a causal environmental risk factor for offspring behavioral and substance use problems, which is necessary for correctly interpreting such studies.71 Third, the results of our analyses predicting low birth weight and other quasi-experimental research on pregnancy and infancy-related problems1, 72 indicate that prevention and intervention efforts should continue to focus on reducing maternal SDP. The present results for offspring substance use/problems, in concert with other quasi-experimental studies,3 however, suggest that solely reducing maternal SDP may not ameliorate offspring cognitive, social, behavioral, or drug problems. Rather, wrap-around services73 that address multiple familial risks that are associated with maternal SDP are necessary.
Acknowledgments
The design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript was supported by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (HD061817 and HD061384), the Swedish Research Council (Medicine), and the Swedish Prison and Probation Services.
Footnotes
There were no financial or other conflicts of interest for any of the authors. The lead author takes responsibility for the integrity of the data and the accuracy of the data analysis. Preliminary results were presented at the June 2011 Behavior Genetics Association Conference in Newport, RI.
References
- 1.Cnattingius S. The epidemiology of smoking during pregnancy: Smoking prevalence, maternal characteristics, and pregnancy outcomes. Nicotine and Tobacco Research. 2004;6:S125–S140. doi: 10.1080/14622200410001669187. [DOI] [PubMed] [Google Scholar]
- 2.Huizink AC, Mulder EJH. Maternal smoking, drinking or cannabis use during pregnancy and neurobehavioral and cognitive functioning in human offspring. Neuroscience and Biobehavioral Reviews. 2006;30:24–41. doi: 10.1016/j.neubiorev.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 3.Knopik VS. Maternal smoking during pregnancy and child outcomes: Real or spurious effect? Developmental Neuropsychology. 2009;34:1–36. doi: 10.1080/87565640802564366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glantz MY, Campbell Champers J. Prenatal drug exposure effects on subsequent vulnerability to drug abuse. Development and Psychopathology. 2006;18:893–922. doi: 10.1017/s0954579406060445. [DOI] [PubMed] [Google Scholar]
- 5.Porath AJ, Fried PA. Effects of prenatal cigarette and marijuana exposure on drug use among offspring. Neurotoxicology and Teratology. 2005;27:267–277. doi: 10.1016/j.ntt.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 6.Agrawal A, Scherrer JF, Grant JD, Sartor CE, Pergadia ML, Duncan AE, Madden PA, Haber JR, Jacob T, Bucholz KK, Xian H. The effects of maternal smoking during pregnancy on offspring outcomes. Preventive medicine. 2010;50(1–2):13–18. doi: 10.1016/j.ypmed.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lieb R, Schreier A, Pfister H, Wittchen HU. Maternal smoking and smoking in adolescents: A prospective community study of adolescents and their mothers. European Addiction Research. 2003;9(3):120–130. doi: 10.1159/000070980. [DOI] [PubMed] [Google Scholar]
- 8.Lotfipour S, Ferguson E, Leonard G, Perron M, Pike B, Richer L, Seguin JR, Toro R, Veillette S, Pausova Z, Paus T. Orbitofrontal cortex and drug use during adolescence: Role of prenatal exposure to maternal smoking and BDNF genotype. Archives of General Psychiatry. 2009;66(11):1244–1252. doi: 10.1001/archgenpsychiatry.2009.124. [DOI] [PubMed] [Google Scholar]
- 9.Noland JS, Singer LT, Short EJ, Minnes S, Ardendt RE, Kirchner HL, Bearer C. Prenatal drug exposure and selective attention in preschoolers. Neurotoxicology & Teratology. 2005;27(3):429–438. doi: 10.1016/j.ntt.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 10.O’Callaghan FV, Al Mamun A, O’Callaghan M, Alati R, Majman JM, Williams GM, Bor W. Maternal smoking during pregnancy predicts nicotine disorder (dependence or withdrawal) in young adults – a birth cohort study. Australian and New Zealand Journal of Public Health. 2009;33(4):371–377. doi: 10.1111/j.1753-6405.2009.00410.x. [DOI] [PubMed] [Google Scholar]
- 11.Ekblad M, Gissler M, Lehtonen L, Korkeila J. Prenatal smoking exposure and the risk of psychiatric morbidity into young adulthood. Archives of General Psychiatry. 2010;67(8):841–849. doi: 10.1001/archgenpsychiatry.2010.92. [DOI] [PubMed] [Google Scholar]
- 12.Fergusson DM, Woodward LJ, Horwood LJ. Maternal smoking during pregnancy and psychiatric adjustment in late adolescence. Archives of General Psychiatry. 1998;55(8):721–727. doi: 10.1001/archpsyc.55.8.721. [DOI] [PubMed] [Google Scholar]
- 13.Nomura Y, Gilman SE, Buka SL. Maternal smoking during pregnancy and risk of alcohol use disorders among adult offspring. Journal of Studies on Alcohol and Drugs. 2011;72(2):199–209. doi: 10.15288/jsad.2011.72.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weissman MM, Warner V, Wickramaratne PJ, Kandel DB. Maternal smoking during pregnancy and psychopathology in offspring followed to adulthood. Journal of the American Academy of Child & Adolescent Psychiatry. 1999;38(7):892–899. doi: 10.1097/00004583-199907000-00020. [DOI] [PubMed] [Google Scholar]
- 15.Brennan PA, Grekin ER, Mortensen EL, Mednick SA. Relationship of maternal smoking during pregnancy with criminal arrest and hospitalization for substance abuse in male and female adult offspring. American Journal of Psychiatry. 2002;159(1):48–54. doi: 10.1176/appi.ajp.159.1.48. [DOI] [PubMed] [Google Scholar]
- 16.Ernst M. Behavioral and neural consequences of prental exposure to nicotine. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40:630–642. doi: 10.1097/00004583-200106000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Shea AK, Steiner M. Cigarette smoking during pregnancy. Nicotine and Tobacco Research. 2008;10:267–278. doi: 10.1080/14622200701825908. [DOI] [PubMed] [Google Scholar]
- 18.Koob GF. Stress, corticotropin releasing ractor, and drug addiction. Annals of the New York Academy of Sciences. 1999;897(1):27–45. doi: 10.1111/j.1749-6632.1999.tb07876.x. [DOI] [PubMed] [Google Scholar]
- 19.Huizink AC, Mulder EJH. Maternal smoking, drinking or cannabis use during pregnancy and neurobehavioral and cognitive functioning in human offspring. Neuroscience and Biobehavioral Reviews. 2006;30 (1):24–41. doi: 10.1016/j.neubiorev.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 20.Ferriero DM, Dempsey DA. Impact of addictive and harmful substances on fetal brain development. Current opinion in neurology. 1999;12(2):161–166. doi: 10.1097/00019052-199904000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Glantz MD, Chambers JC. Prenatal drug exposure effects on subsequent vulnerability to drug abuse. Development and Psychopathology. 2006;18(3):893–922. doi: 10.1017/s0954579406060445. [DOI] [PubMed] [Google Scholar]
- 22.Toro R, Leonard G, Lerner JV, Lerner RM, Perron M, Pike GB, Richer L, Veilette S, Pausova Z, Paus T. Prenatal exposure to maternal cigarette smoking and the adolescent cerebral cortex. Neuropsychopharmacology. 2008;33(5):1019–1027. doi: 10.1038/sj.npp.1301484. [DOI] [PubMed] [Google Scholar]
- 23.Shadish WR, Cook TD, Campbell DT. Experimental and quasi-experimental designs for generalized causal inference. New York: Houghton Mifflin; 2002. [Google Scholar]
- 24.Rutter M. Proceeding from observed correlation to causal inference: The use of natural experiments. Perspectives on Psychological Science. 2007;2:377–395. doi: 10.1111/j.1745-6916.2007.00050.x. [DOI] [PubMed] [Google Scholar]
- 25.Academy of Medical Sciences Working Group. Identifying the Environmental Causes of Disease: How Should We Decide What to Believe and When to Take Action? London: Academy of Medical Sciences; 2007. [Google Scholar]
- 26.D’Onofrio BM, Van Hulle CA, Waldman ID, Rodgers JL, Harden KP, Rathouz PJ, Lahey BB. Smoking during pregnancy and offspring externalizing problems: An exploration of genetic and environmental confounds. Development and Psychopathology. 2008;20:139–164. doi: 10.1017/S0954579408000072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gilman SE, Gardener H, Buka SL. Maternal smoking during pregnancy and children’s cognitive and physical development: A causal risk factor? Am J Epidemiol. 2008;168:522–531. doi: 10.1093/aje/kwn175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rice F, Harold GT, Boivin J, Hay DF, Van den Bree M, Thapar A. Disentangling prenatal and inherited influences in humans with an experimental design. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0808798106. Early Edition Online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Obel C, Olsen J, Henriksen TB, Rodriguez A, Jarvelin MR, Oilanen MI, Parner E, Linnet KM, Taanila A, Ebeling H, Heiervang E, Gissler M. Is maternal smoking during pregnancy a risk factor for Hyperkinetic disorder?—findings from a sibling design. International Journal of Epidemiology. 2011;40(2):338–345. doi: 10.1093/ije/dyq185. [DOI] [PubMed] [Google Scholar]
- 30.Lindblad F, Hjern A. ADHD after fetal exposure to maternal smoking. Nicotine & Tobacco Research. 2010;12(4):408–415. doi: 10.1093/ntr/ntq017. [DOI] [PubMed] [Google Scholar]
- 31.Lundberg F, Cnattingius S, D’Onofrio B, Altman D, Lambe M, Hultman C, Iliadou A. Maternal smoking during pregnancy and intellectual performance in young adult Swedish male offspring. Pediatric and Perinatal Epidemiology. 2010;24:79–87. doi: 10.1111/j.1365-3016.2009.01073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.D’Onofrio BM, Singh AL, Iliadou A, Lambe M, Hultman C, Neiderhiser JM, Langstrom N, Lichtenstein P. A quasi-experimental study of maternal smoking during pregnancy and offspring academic achievement. Child Development. 2010;81:80–100. doi: 10.1111/j.1467-8624.2009.01382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lambe M, Hultman C, Torrang A, MacCabe J, Cnattingius S. Maternal smoking during pregnancy and school performance at age 15. Epidemiology. 2006;17:524–530. doi: 10.1097/01.ede.0000231561.49208.be. [DOI] [PubMed] [Google Scholar]
- 34.D’Onofrio BM, Singh AL, Iliadou A, Lambe M, Hultman C, Grann M, Neiderhiser JM, Langstrom N, Lichtenstein P. Familial confounding of the association between maternal smoking during pregnancy and offspring criminality: A population-based study in Sweden. Archives of General Psychiatry. 2010;67:529–538. doi: 10.1001/archgenpsychiatry.2010.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.D’Onofrio BM, Van Hulle CA, Goodnight JA, Rathouz PJ, Lahey BB. Is maternal smoking during pregnancy a causal environmental risk factor for adolescent antisocial behavior? Testing etiological theories and assumptions. Psychological Medicine. doi: 10.1017/S0033291711002443. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Talati A, Weissman MM. In utero smoking exposure warrants further investigation. Archives of General Psychiatry. 2010;67:1094. doi: 10.1001/archgenpsychiatry.2010.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hingson RW, Heeren T, Winter MR. Age at drinking onset and alcohol dependence: Age at onset, duration, and severity. Archives of Pediatric & Adolescent Medicine. 2006;160(7):739–746. doi: 10.1001/archpedi.160.7.739. [DOI] [PubMed] [Google Scholar]
- 38.McGue M, Iacono WG, Legrand LN, Elkins I. Origins and consequences of age at first drink. II. Familial risk and heritability. Alcoholism: Clinical and Experimental Research. 2001;25(7):1166–1173. [PubMed] [Google Scholar]
- 39.Kerr DCR, Owen LD, Capaldi DM. The timing of smoking onset, prolonged abstinence, and relapse in men: A prospective study from ages 18 to 32 years. Addiction. doi: 10.1111/j.1360-0443.2011.03500.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ehlers CL, Slutske WS, Gilder DA, Lau P. Age of first marijuana use and the occurrence of marijuana use disorders in Southwest California Indians. Pharmacology Biochemistry and Behavior. 2007;86(2):290–296. doi: 10.1016/j.pbb.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 41.Lahey BB, D’Onofrio BM. All in the family: Comparing siblings to test causal hypotheses regarding environmental influences on behavior. Current Directions in Psychological Science. 2010;19:319–323. doi: 10.1177/0963721410383977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Donovan SJ, Susser E. Commentary: Advent of sibling designs. International Journal of Epidemiology. 2011;40(2):345–349. doi: 10.1093/ije/dyr057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baker P, Mott FL. NLSY child handbook. Columbus, OH: Center for Human Resource Research; 1989. [Google Scholar]
- 44.Chase-Lansdale PL, Mott FL, Brooks-Gunn J, Phillips DA. Children of the National Longitudinal Survey of Youth: A unique research opportunity. Developmental Psychology. 1991;27:918–931. [Google Scholar]
- 45.D’Onofrio BM, Van Hulle CA, Waldman ID, Rodgers JL, Rathouz PJ, Lahey BB. Causal inferences regarding prenatal alcohol exposure and childhood externalizing problems. Archives of General Psychiatry. 2007;64:1296–1304. doi: 10.1001/archpsyc.64.11.1296. [DOI] [PubMed] [Google Scholar]
- 46.D’Onofrio BM, Goodnight JA, Van hulle CA, Waldman ID, Rodgers JL, Rathouz PJ, Lahey BB. A quasi-experimental analysis of the association between family income and offspring conduct problems. Journal of Abnormal Child Psychology. 2009;37:415–429. doi: 10.1007/s10802-008-9280-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rodgers JL, Rowe DC, Li C. Beyond nature vs. nurture: DF analyses of nonshared influences on problem behaviors. Developmental Psychology. 1994;30:374–384. [Google Scholar]
- 48.Centers for Disease Control and Prevention. Youth Risk Behavior Surveillance - United States, 2009. MMWR CDC Surveill Summ. 2010;59(SS-5) [Google Scholar]
- 49.Centre for Epidemiology. The Swedish Medical Birth Register - A Summary of Content and Quality. [Google Scholar]
- 50.Cnattingius S, Ericson A, Gunnarskog J, Kallen B. A quality study of a medical birth registry. Scand J Soc Med Jun. 1990;18(2):143–148. doi: 10.1177/140349489001800209. [DOI] [PubMed] [Google Scholar]
- 51.Statistics Sweden. Multi-generation register 2005 – A description of contents and quality. Vol. 2006. Örebro: Statistics Sweden; 2006. p. 6. [Google Scholar]
- 52.Fazel S, Grann M. The population impact of severe mental illness on violent crime. Am J Psychiatry Aug. 2006;163(8):1397–1403. doi: 10.1176/ajp.2006.163.8.1397. [DOI] [PubMed] [Google Scholar]
- 53.Centre for Epidemiology. The Swedish Hospital Discharge Register. http://www.sos.se/epc/english/ParEng.htm#Publications.
- 54.Statistics Sweden. Educational attainment of the population. http://www.scb.se/templates/Product____9577.asp.
- 55.Lindqvist R, Lendahlsm L, Tollbom O, Aberg H, Hakansson A. Smoking during pregnancy: Comparison of self-reports and cotinine levels in 496 women. Acta Obstet Gynecol Scand. 2002;81:240–244. doi: 10.1034/j.1600-0412.2002.810309.x. [DOI] [PubMed] [Google Scholar]
- 56.Allison PD. Fixed effects regression models. Washington DC: Sage; 2009. [Google Scholar]
- 57.D’Onofrio BM, Turkheimer E, Eaves LJ, Corey LA, Berg K, Solaas MH, Emery RE. The role of the Children of Twins design in elucidating causal relations between parent characteristics and child outcomes. Journal of Child Psychology & Psychiatry. 2003;44:1130–1144. doi: 10.1111/1469-7610.00196. [DOI] [PubMed] [Google Scholar]
- 58.McGue M, Osler M, Christensen K. Causal inference and observational research: The utility of twins. Perspectives on Psychological Science. 2010;5:546–556. doi: 10.1177/1745691610383511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jacobson SW, Chiodo LM, Sokol RJ, Jacobson JL. Validity of maternal report of prenatal alcohol, cocaine, and smoking in relation to neurobehavioral outcome. Pediatrics. 2002;109(5):815–815. doi: 10.1542/peds.109.5.815. [DOI] [PubMed] [Google Scholar]
- 60.Pickett KE, Kasza K, Biesecker G, Wright RJ, Wakschlag LS. Women who remember, women who do not: A methodological study of maternal recall of smoking in pregnancy. Nicotine & Tobacco Research. 2009;11(10):1166–1174. doi: 10.1093/ntr/ntp117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cnattingius S, Haglund B. Decreasing smoking prevalence during pregnancy in Sweden: the effect on small-for-gestational-age births. American Journal of Public Health. 1997;87:410–413. doi: 10.2105/ajph.87.3.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hatziandreu EJ, Pierce JP, Fiore MC, Grise V, Novotny TE, Davis RM. The reliability of self- reported cigarette consumption in the United States. American Journal of Public Health. 1989;79:1020–1023. doi: 10.2105/ajph.79.8.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Knopik VS, Heath AC, Jacob T, Slutske WS, Bucholz KK, Madden PAF, Waldron M, Martin NG. Maternal alcoholism and offspring ADHD: Disentangling genetic and environmental effects using a children-of-twins design. Psychological Medicine. 2006;2006:1461–1471. doi: 10.1017/S0033291706007884. [DOI] [PubMed] [Google Scholar]
- 64.Leve LD, Neiderhiser JM, Scaramella LV, Reiss D. The early growth and development study: Using the prospective adoption design to examine genotype-environment interplay. Behavior Genetics. 2010;40:306–314. doi: 10.1007/s10519-010-9353-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Maughan B, Taylor C, Taylor A, Butler N, Bynner J. Pregnancy smoking and childhood conduct problems: A causal association? Journal of Child Psychology and Psychiatry. 2001;42(8):1021–1028. doi: 10.1111/1469-7610.00800. [DOI] [PubMed] [Google Scholar]
- 66.Cnattingius S, Svensson T, Granath F, Iliadou A. Maternal smoking during pregnancy and risks of suicidal acts in young offspring. Eur J Epidemiol. 2011;26:485–492. doi: 10.1007/s10654-011-9556-7. [DOI] [PubMed] [Google Scholar]
- 67.D’Onofrio BM, Rathouz PJ, Lahey BB. The importance of understanding gene-environment correlations in the development of antisocial behavior. In: Kendler KS, Jaffee SR, Romer D, editors. The Dynamic Genome and Mental Health: The Role of Genes and Environments in Youth Development. New York: Oxford University Press; 2011. [Google Scholar]
- 68.Nestler EJ, Hyman SE. Animal models of neuropsychiatric disorders. Nat Neurosci. 2010;13:1161–1169. doi: 10.1038/nn.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fernando ABP, Robbins TW. Animal models of neuropsychiatrics disorders. Annual Review of Clinical Psychology. 2011;7:39–61. doi: 10.1146/annurev-clinpsy-032210-104454. [DOI] [PubMed] [Google Scholar]
- 70.Mitchell BF, Taggert MJ. Are animal models relevant to key aspects of human parturition? American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2009;297:R525–R545. doi: 10.1152/ajpregu.00153.2009. [DOI] [PubMed] [Google Scholar]
- 71.Moffitt TE, Caspi A, Rutter M. Strategy for investigating interactions between measured genes and measured environments. Archives of General Psychiatry. 2005;62:473–481. doi: 10.1001/archpsyc.62.5.473. [DOI] [PubMed] [Google Scholar]
- 72.Johansson ALV, Dickman PW, Kramer MS, Cnattingius S. Maternal smoking and infant mortality: Does quitting smoking reduce the risk of infant death? Epidemiology. 2009;20:1–8. doi: 10.1097/EDE.0b013e31819dcc6a. [DOI] [PubMed] [Google Scholar]
- 73.Olds DL. Prenatal and infancy home visiting by nurses: From randomized trials to community replication. Prevention Science Sep. 2002;3(3):153–172. doi: 10.1023/a:1019990432161. [DOI] [PubMed] [Google Scholar]