Abstract
Electric detection using a nanocomponent may lead to platforms for rapid and simple biosensing. Sensors composed of nanotips or nanodots have been described for highly sensitive amperometry enabled by confined geometry. However, both fabrication and use of nanostructured sensors remain challenging. This paper describes a dendritic nanotip used as an amperometric biosensor for highly sensitive detection of target bacteria. A dendritic nanotip is structured by Si nanowires coated with single-walled carbon nanotubes (SWCNTs) for generation of a high electric field. For reliable measurement using the dendritic structure, Si nanowires were uniformly fabricated by ultraviolet (UV) lithography and etching. The dendritic structure effectively increased the electric current density near the terminal end of the nanotip according to numerical computation. The electrical characteristics of a dendritic nanotip with additional protein layers was studied by cyclic voltammetry and I–V measurement in deionized (DI) water. When the target bacteria dielectrophoretically captured onto a nanotip were bound with fluorescence antibodies, the electric current through DI water decreased. Measurement results were consistent with fluorescence- and electron microscopy. The sensitivity of the amperometry was 10 cfu/sample volume (103 cfu/mL), which was equivalent to the more laborious fluorescence measurement method. The simple configuration of a dendritic nanotip can potentially offer an electrolyte-free detection platform for sensitive and rapid biosensors.
1. Introduction
Over the past decade, various detection methods have been described for use in the rapid and sensitive identification of target biomarkers.1 Potential detection methods are nucleic acid amplification,2 magnetic particle-based methods,3, 4 microfluidic-based approaches,5–7 and nanotechnology-based techniques.8, 9 However, the DNA amplification methods are cumbersome, slow, and expensive with requirement of stable electric power. Magnetic-based approaches are cumbersome in preparation with a limited sensitivity. A microfluidic device handles a limited small volume, and viscous samples can be clogged in the channel. Nanotechnology-based devices can be sensitive, but scale-up manufacturing is still challenging. In addition, most methods require sophisticated instruments in stationary laboratories with demand on optics and skilled personnel. Therefore, a simple and portable detection method that functions independently of laboratory infrastructure without compromising sensor performance would greatly facilitate rapid diagnosis in nontraditional, point-of-care (POC) settings.10, 11
As detection elements in rapid diagnostic tools, various nanomaterials have been evaluated for their potentially rapid response and high sensitivity. Among nanomaterials, single walled carbon nanotubes (SWCNTs) could be useful due to their sensitive responses upon binding of targets.12 To detect a target in a liquid phase, electric current is measured through SWCNTs. A nanostructured tip is advantageous for electrical detection because the capacitance due to an electrical double layer can be minimized by the confined geometry.13–19 To reduce the capacitance with electric insulation, the nanotip area except for the terminal end has been sealed with polymer- or other dielectric layers.1, 19 However, insulated nanotips have proved challenging for fabrication in a reproducible fashion. Moreover, the concentration of target analytes onto the exposed terminal area of a nanotip remains a daunting task.
In our previous work, microtips and nanotips were fabricated to concentrate target analytes.17, 18, 20–25 Nanoparticles and DNA were effectively concentrated onto a dendritic naontip by an electric field, which were detected by a fluorescence microscope.20, 23–25 Previously, dendritic nanostructures have been studied to enlarge the surface area for increasing absorption sites of molecules. However, most dendritic nanostructures are materials not a device.26, 27 In our previous study, a dendritic nanotip concentrated as few as 10 copies in a sample volume of 2 μL due to a large surface area with a high intensity electric field. However, precise measurement with a fluorescence microscope was required for identification of DNA. In this paper, an alternative dendritic nanotip strategy was studied for electric detection of target bacteria. A dendritic nanotip coated with probe molecules was used for concentration of target bacteria, which were detected by electrical measurement. Immunocomplex formation between surface antigens of bacteria and fluorescein-labeled antibodies was measured by the change of electric current between a nanotip and a ring-shaped electrode.
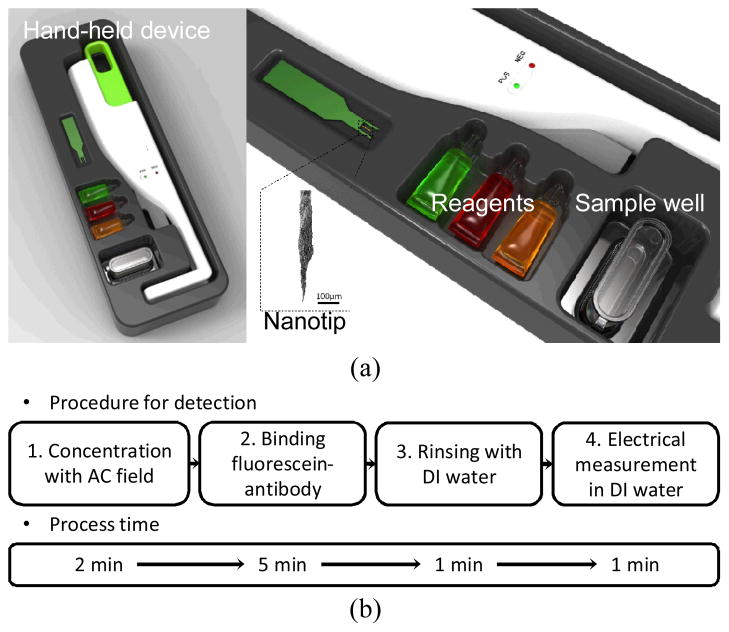
The electric measurement was conducted within a medium of deionized (DI) water in order to reduce chemical reactions and an electrical double layer effect, which enabled a reference-free detection. The electrical detection results were validated by fluorescence- and scanning electron microscopy (SEM). Ultimately, we aim to develop a POC diagnostic tool for infectious diseases. A nanotip sensor is integrated into a hand-held devce as illustrated in Fig. 1. With the simple detection step, the assay can be completed within 10 minutes at low cost.
Fig. 1.
(a) Conceptual design; a point-of-care (POC) diagnostic tool for infectious diseases. (b) Flow chart of the diagnostic step using a dendritic nanotip with the process time.
2. Electric measurement using a dendritic nanotip
Fig. 2(a) shows an experimental configuration of a dendritic nanotip for electric measurement. It is composed of Si nanowires coated with SWCNTs. The Si nanowires are interconnected with SWCNTs for electric conduction. When a target analyte binds to the nanotip, the electric current between the nanotip and a ring-shaped electrode is changed due to changes in the resistance of SWCNTs and the contact resistance at the SWCNTs-liquid interface. The magnitude of the change depends on the charge carrier density of SWCNTs, which is comparable to the surface charge density of molecules.28 When bulk SWCNTs were utilized, the charge transport through SWCNTs was averaged over metallic and semiconducting tubes.29 The electron transfer characteristics through the radial direction of SWCNTs were similar for metallic and semiconducting tubes,30 which was advantageous for reproducible measurement regardless of chirality of SWCNTs.
Fig. 2.

(a) Configuration for electrical measurement using a dendritic nanotip. The nanotip is composed of Si nanowires wrapped with SWCNTs. (b) Equivalent circuit for the electrical measurement. RSWCNT, RDI, and Rring are the resistances of SWCNTs on a nanotip, DI water, and a ring-shaped electrode, respectively. CSWCNT and Cring are the double layer capacitances on SWCNTs of a nanotip and a ring-shaped electrode, respectively. DC potential is applied for current measurement. An internal potential is generated due to oxidation of a ring-shaped electrode made of aluminum (Al).
When a target analyte binds onto a dendritic nanotip wrapped with SWCNTs, the equivalent circuit for the measurement is illustrated in Fig. 2(b). For the electric potentials in the circuit, an external voltage (0~1V) is applied for current measurement between a nanotip and a counter electrode. Upon introduction of liquid, a redox potential is generated between the electrodes. When the counter electrode is made of aluminum (Al), the Al electrode is oxidized with generation of electrons. To reduce the redox potential (i.e. internal potential) due to the oxidation, DI water is used as the medium. The reactions in DI water are:
The electric current due to the redox potential is measured at 0V to observe the dominancy in the measured current. The limitation of ions in DI water significantly decreases the capacitances (CSWCNT and Cring) on the electrodes. As a result, the change of the electric current is dominated by the resistance at the interface between SWCNTs (RSWCNT) and DI water.
To study the geometrical dependence of a dendritic nanotip on the electric current, the current density of a dendritic nanotip was compared with those of single nanotips with and without an insulation layer. In previous works, when a single nanotip was insulated by a polymer layer except at the terminal end, the electrical double layer effect could be suppressed for sensitive measurement.13, 15 Using a numerical model, the current densities for a single-, an insulated-, and a dendritic nanotip were compared to study the path of electric current. A finite element method software (COMSOL Multiphysics®, COMSOL, Inc., Palo Alto, CA) was used to calculate the electric field and the current density near the nanotip.
Fig. 3(a) shows a 3-dimensional computational model with boundary conditions. The model consisted of a coil (outside diameter: 2.1 mm and inside diameter: 1.9 mm), a spherical droplet (diameter: 2.0 mm) and a nanotip (the terminal end diameter: 1 μm and length: 250 μm). The nanotip was connected to a microwire (diameter: 50 μm and length: 350 μm). In Fig. 3(a), the ground condition (V=0V) and the electric potential (V0=1V) were applied to the coil and the microwire, respectively. Electric insulation condition (−n·J=0, J is current density) is given at the surface of the droplet. In the model, three kinds of nanotips were made as a single-, an insulated- and a dendritic nanotip. The single nanotip was modeled as conical in shape with a terminal end diameter of 1 μm. The insulated nanotip had the same geometry but was covered with 2 μm-thick dielectric layer except at the terminal end. The dendritic nanotip was modeled to be composed of 1 nanotip and 6 nanowires. Each nanowire was 1 μm in diameter and 10 μm in length. Each model was composed of tetrahedron elements (1,824,875~2,718,187 elements). An 8-core processor (clock speed: 2.67GHz) was used for computation.
Fig. 3.
(a) Numerical model showing two electrodes composed of a nanotip and a ring-shaped electrode (top). The distribution of an electric field is shown for a single-, an insulated-, and a dendritic nanotip (bottom images). See the main text for the specification of each tip. (b) Comparison of electric current densities on a single-, an insulated-, and a dendritic nanotip.
According to the numerical results [Fig. 3(b)], the current density is highest at the insulated nanotip followed by the dendritic nanotip. The single nanotip showed the lowest current density at the terminal end. Since the electric current flows throughout the nanotip surface, the background noise of a single nanotip should be greater than those of insulated- and a dendritic nanotips.
Although the dendritic nanotip is computed to be somewhat less sensitive than the insulated nanotip, the sensitivity of the actual dendritic nanotip can approach that of the insulated nanotip because the actual dendritic nanotip has a much larger number of nanowires than that of the numerical model. To validate the possible improvement of the sensitivity with a larger number of nanowires, additional simulation was conducted (ESI†). The computational results predicted that more nanowires with a more densely packaged form could enhance the senstivitiy of a nanotip. In addition, the fabrication of a dendritic nanotip is much more straightforward than the insulated nanotip. The larger surface area of the dendritic nanotip can concentrate targets more efficiently than an insulated nanotip.23
3. Experimental Section
3.1 Dendritic nanotip fabrication
A dendritic nanotip was made of a mixture of Si nanowires and SWCNTs. A procedure to fabricate a dendritic nanotip made of SiC nanowires and SWCNTs was developed previously.31 To achieve more uniform shape and thus more consistent performance, non-uniform shaped SiC nanowires were replaced by microfabricated Si nanowires. Si nanowires were fabricated on a 100mm-diameter Si wafer by using conventional photolithography and deep reactive ion etching (DRIE) technique (Fig. 4a). The collected Si nanowires were dispersed in dimethylformamide (DMF). SWCNTs (Unidym™,HiPco® SWCNTs) were dispersed in DMF by sonication for 10 hours. The concentration of the SWCNT solution was 125 mg/L while the concentration of Si nanowire solution was 1 g/L. Subsequently, 4 mL of a pure SWCNT solution and 1 mL of a pure Si nanowire solution were mixed and sonicated in a vial. A gold-plated tungsten (W)-wire having 50 μm in diameter (Sylvania, Towanda, PA) was positioned on an xyz stage for manipulation. As a counter electrode, a silver-plated metal coil (OK industries, Tuckahoe, NY) was attached on a platform (Fig. 4b). An AC potential (20 Vpp, peak-to-peak voltage) at 5 MHz was applied to the W-wire and the ring-shaped electrode that held a 2 μL solution drop. The W-wire was positioned perpendicularly to the metal ring and immersed in the drop. After 1 minute of immersion time for attraction of Si nanowires and SWCNTs, the W-wire was gently withdrawn from the drop. The fabricated nanotip had a conical shape but a dendritic structure was formed because of protruded Si nanowires (Fig. 4c). The nanowires were wrapped with SWCNTs as a sensing element (Fig. 4d). The average diameter of the terminal end of a nanotip was 0.7~1.2 μm.
Fig. 4.
(a) SEM image for fabricated Si nanowires on a wafer (b) Experimental setup for the concentration of bacteria cells (c) SEM images for fabricated dendritic nanotip (d) SEM image for Si nanowire wrapped with SWCNTs.
3.2 Characterization of dendritic nanotip by cyclic voltammetry
Cyclic voltammetry (CV) was used to characterize the dendritic nanotip. 10μL DI water was loaded on an aluminum coil, and a dendritic nanotip was immersed in the solution. When the cyclic voltage was swept from −1V to 1V with a ramp function (Agilent 33220A, Santa Clara, CA), the current was measured using picoammeter (Keithley, 6487). The sweeping rates were 100, 200, 500, and 1,000 mV/s.
For electric measurement, DI water was used as the medium throughout this paper, instead of a conventional electrochemical buffer15. The electrochemical buffers are convenient media for electric measurement because electrochemical reactions are well-characterized with the assistance of a third, reference electrode. However, precise control of the gap size among three nanoelectrodes including two electrodes plus a reference electrode is challenging in nanofabrication. In addition, ionic concentration can continuously change due to temperature and humidity, which requires calibration by a reference electrode. In fact, excessive ionic concentrations of a medium in electric measurement can interfere the electrochemical measurement of analytes due to unwanted reactions.32 Therefore, this study evaluated the use of DI water as a medium to detect targets without a reference electrode.
3.3 Characterization of functionalized nanotips
For specific binding, the dendiritic nanotip was functionalized with streptavidin and anitbodies. Streptavidin (1mg/mL in 1xPBS, Sigma) was nonspecifically immobilized on the surface followed by the binding of biotinylated polyclonal IgY antibodies (5 mg/ml biotin-Antibody in PBS). The antibodies were raised against acetone-fixed M. bovis BCG cells as described previously.22 To study the electric response of the functionalized layers, electrical measurement was conducted for both streptavidin and antibody layers. After the coating, the dendritic nanotip was immersed into 10 μL DI water which was hung on the aluminum ring for the electric measurement (Fig. 2a). Both the dendritic nanotip and the Al coil were then connected for current measurement. A picoammeter (Keithley, 6487) was used for voltage input (0~1V) and current measurement. To study the electric response of the functionalized layers, electrical measurement was conducted individually for each layer.
3.4 Concentration and immunocomplex formation of target bacteria
The experimental setup for concentration of bacterial cells was composed of a dendritic nanotip, a solution drop in an Al coil, and a signal generator (Fig. 4b). The fabricated nanotip was placed on an xyz stage, and the axial motion of the nanotip was controlled to maintain the same distance from the terminal end of a nanotip to the metal coil for all the experiments. A liquid drop (10 μL) containing target bacteria was placed on an aluminum coil. All the experiments were conducted on the xyz stage under an optical microscope with an illuminator (MI-150, Dolan-Jenner, Lawrence, MA). A function generator (Agilent 33220A, Santa Clara, CA) was used to apply an AC electric potential (100 kHz, 18 Vpp). To study the sensitivity of a dendritic nanotip, various concentrations of BCG cells (103~105 cfu/mL) were suspended in PBS buffer. Note that the concentrations of BCG were chosen on the basis of the clinical significance.2, 33 A 10 μL solution of prepared bacteria sample was loaded onto the metal coil. The functionalized nanotip was immersed in the 10 μL droplet for 2 minutes with an applied AC field (18 Vpp at 100 kHz). The nanotip was withdrawn from the solution and then immersed in a prepared fluorescein-conjugated antibody solution (2 μL, 125μg/mL) for 5 minutes. The nanotip was then rinsed with DI water (300 μL) for 10 seconds. For concentration, the sample volume of 10 μL was chosen to enhance the sensitivity. Due to the mass of the droplet, 10 μL was the largest volume which could be held on the coil by surface tension. For detection, the small sample volume (2μL) was chosen because the volume was large enough to cover the nanotip surface for 5 minutes. To control the sample volume precisely, a pipette (Eppendorf Reference® adjustable 2~20μL) was utilized to transfer the exact volume of the solution to the metal coil for all the experiments. The experiment was repeated 3 times for each concentration. For the control experiment, 1×PBS buffer without bacterial cells was used.
3.5 Electrical and fluorescence measurement
Both electrical and fluorescence measurement methods were used to detect concentrated target bacteria on the dendritic nanotip. For fluorescence measurement, the rinsed nanotip was observed under an epi-fluorescence microscope (Olympus BX-41, OlympusAmerica, Melville, NY, USA). The fluorescence intensity was measured by using software, ImageJ (NIH). After fluorescence measurement, the same nanotip was immersed into 10 μL DI water which was hung on the Al coil for electrical measurement. A picoammeter (Keithley, 6487) was used to measure the electric current between a nanotip and a ring-shaped Al coil. The current was measured at 1V.
4. Results and discussion
Fig. 5a shows the CV characteristics measured for a dendritic nanotip composed of SWCNTs and Si nanowires. The sweeping rates were 100, 200, 500, and 1,000 mV/s for current measurement. In the graph, the capacitance is the accumulated charge on tip surface, which is concealed within the inner region of CV for one cycle. With the increase of a sweeping rate, both the capacitance and the current increased along with the noise. According to the measurement, the sweeping rates of 100 and 200 mV/s showed reproducible signals with low noise. Thus 200 mV/s was chosen for the rest of the measurement.
Fig. 5.
(a) Cyclic voltammetry for a dendritic nanotip made of Si nanowires and SWCNTs. The sweeping rates vary from 100 mV/sec to 1 V/sec (b) I–V response for a dendritic nanotip without coating, with coating of streptavidin, and streptavidin + biotinylated IgY at a sweeping rate of 200 mV/sec (N=3).
When the dendritic nanotips were used to measure the electric current for the functionalized layers of streptavidin and antibodies, the average currents at 1V (scan rate: 200mV/second) were 0.24, 0.35, and 0.3 μA for nanotips without coating, streptavidin-coated nanotip, and additional antibody-coated nanotip, respectively (Fig. 5b). Either increase or decrease of the electric current depended on the protein layers on SWCNTs. When streptavidin was coated on SWCNTs, the electric current at 1V increased. However, the current was reduced when the biotinylated IgY antibody was bound onto the streptavidin layer. The measurement results showed a potential as a reference-free amperometry sensor.
In comparison to the CV measurement, the electric current of the dendritic nanotip at 1V of the I–V measurement was reduced because of a different measurement setup. The CV was measured using a function generator and a current meter while the I–V was measured using a current meter with its own voltage source. To investigate the effect of the redox potential in the current measurement, the redox potential in DI water was measured as 0.22 V in an open circuit. The electric current due to the redox potential in DI water was smaller than 0.03 μA in the closed circuit at the external voltage of 0V (Fig. 5b). The current was also smaller than the current at 1 V by an order of magnitude. Thus effect of the redox potential could be neglected in the measurement at 1 V.
For the sensitivity test, various concentrations of BCG cells were captured and detected. The sensitivity was 10 cfu in 10 μL buffer solution for both fluorescence (Fig. 6a) and electrical measurement (Fig. 6b). Thus, the sensitivity of electric measurement using the dendridic nanotip approached that of the more laborious fluorescence measurement method. As the cell concentration increased, the fluorescence intensity increased because of more binding events of fluorescence antibodies. In the electrical measurement, the electric current was reduced with increase of the cell concentration. The charge transfer on SWCNT surface was decreased with the binding of cells and antibodies, which was detected by the measurement of electric current. The captured cells on the nanotip were also visible by SEM and fluorescence microscopy (Fig. 7).
Fig. 6.

Sensitivity test for BCG cells in PBS buffer by using (a) fluorescence measurement (b) corresponding electrical measurement (error bars are the standard deviation; N=3).
Fig. 7.

Dendritic nanotip for capturing BCG (a) optical image of a dendritic nanotip, (b) optical image of a magnified nanotip, (c) SEM image of a dendritic nanotip (d) corresponding fluorescence image. Bright spots are BCG cells stained by fluorescent antibodies. The circles in (c) and (d) are the same spots indicating BCG cells.
For comparison, the current ratio (−ΔI/Io) was compared with the fluorescence intensity at each concentration of BCG (Fig. 8). The current ratio increased proportionally with fluorescence intensity. The deviation of both signals might be caused by different characteristics of fluorescence- and electrical measurement. The fluorescence measurement was strongly dependent on the size of the captured cell clumps because more binding spots were generated by the surface antigen on cell surface. The electrical measurement was strongly dependent on the location and size of bound cell clumps because a higher current density was created toward the terminal end of a dendritic nanotip as computed in Fig. 3(b).
Fig. 8.
Comparison between the current ratio and fluorescence intensity at various concentrations of BCG in PBS buffer.
To improve the sensitivity of the device, the required sample volume should be increased over 100 μL. Considering the DEP force effective within 1 mm range, the combination with circulation flow can enhance the concentration efficiency for a large volume sample.22 Therefore, with a larger sample volume, a higher sensitivity can be achieved by combining of circulation flow with an electric field for concentration. The electrolyte-free and reference-free configuration potentially offers a rapid and simple platform for rapid disease diagnosis as illustrated in Fig. 1.
Conclusions
In summary, a rapid and simple amperometric immunosensor was developed by using a dendritic nanotip composed of Si nanowires coated with SWCNTs. Due to the confined current paths of the dendritic nanotip, a capacitance effect by an electrical double layer could be suppressed while the current change on SWCNT surface could be sensitively measured upon binding of target cells bound with fluorescence-antibodies. The electric measurement was conducted through a medium of DI water, which reduced the double-layer capacitance effect and chemical reactions. The electric measurement could be conducted without a reference electrode. The detection results were validated by fluorescence and SEM studies. Using the electrical measurement setup, the sensitivity was 10 cfu/sample volume (i.e. 10 μL), comparable to a more laborious fluorescence staining method and among the most sensitive amperometric methods reported to date. The simple measurement configuration will offer a rapid diagnostic platform for diseases.
Supplementary Material
Acknowledgments
We gratefully acknowledge funding for this work provided by NSF Career (ECCS-0846454) and NIH NIAID (R01AI093418). We appreciate the Center for Nanotechnology and the Microfabrication Facility of University of Washington for the fabrication of Si nanowires. We thank Prof. M.P. Anantram for helpful discussions. We appreciate Professor Sang-gyeun Ahn at Department of Industrial Design, University of Washington for the drawing of a conceptual device in Fig. 1
Footnotes
Electronic supplementary information (ESI) available: current density comparison with various numbers of nanowires on nanotip, Fig. S1 is provided. See DOI: 10.1039/b000000x/
References
- 1.McNerney R, Daley P. Nat Rev Microbiol. 2011;9:204–213. doi: 10.1038/nrmicro2521. [DOI] [PubMed] [Google Scholar]
- 2.Blakemore R, Story E, Helb D, Kop J, Banada P, Owens MR, Chakravorty S, Jones M, Alland D. J Clin Microbiol. 2010;48:2495–2501. doi: 10.1128/JCM.00128-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee H, Sun E, Ham D, Weissleder R. Nat Med. 2008;14:869–874. doi: 10.1038/nm.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Albert H, Ademun PJ, Lukyamuzi G, Nyesiga B, Manabe Y, Joloba M, Wilson S, Perkins MD. Bmc Infect Dis. 2011:11. doi: 10.1186/1471-2334-11-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weigl B, Domingo G, LaBarre P, Gerlach J. Lab Chip. 2008;8:1999–2014. doi: 10.1039/b811314a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chin CD, Laksanasopin T, Cheung YK, Steinmiller D, Linder V, Parsa H, Wang J, Moore H, Rouse R, Umviligihozo G, Karita E, Mwambarangwe L, Braunstein SL, van de Wijgert J, Sahabo R, Justman JE, El-Sadr W, Sia SK. Nat Med. 2011;17:1015–U1138. doi: 10.1038/nm.2408. [DOI] [PubMed] [Google Scholar]
- 7.Yager P, Edwards T, Fu E, Helton K, Nelson K, Tam MR, Weigl BH. Nature. 2006;442:412–418. doi: 10.1038/nature05064. [DOI] [PubMed] [Google Scholar]
- 8.Allen BL, Kichambare PD, Star A. Adv Mater. 2007;19:1439–1451. [Google Scholar]
- 9.Gruner G. Anal Bioanal Chem. 2006;384:322–335. doi: 10.1007/s00216-005-3400-4. [DOI] [PubMed] [Google Scholar]
- 10.Griffiths D, Hall G. Trends Biotechnol. 1993;11:122–130. doi: 10.1016/0167-7799(93)90086-O. [DOI] [PubMed] [Google Scholar]
- 11.Owen VM. Biosens Bioelectron. 1994;9:xxix–xxxv. doi: 10.1016/0956-5663(94)90024-8. [DOI] [PubMed] [Google Scholar]
- 12.Maroto A, Balasubramanian K, Burghard M, Kern K. Chemphyschem. 2007;8:220–223. doi: 10.1002/cphc.200600498. [DOI] [PubMed] [Google Scholar]
- 13.Boo H, Jeong RA, Park S, Kim KS, An KH, Lee YH, Han JH, Kim HC, Chung TD. Anal Chem. 2006;78:617–620. doi: 10.1021/ac0508595. [DOI] [PubMed] [Google Scholar]
- 14.Jouzi M, Kerby MB, Tripathi A, Xu J. Langmuir. 2008;24:10786–10790. doi: 10.1021/la703630a. [DOI] [PubMed] [Google Scholar]
- 15.Krapf D, Wu MY, Smeets RMM, Zandbergen HW, Dekker C, Lemay SG. Nano Lett. 2006;6:105–109. doi: 10.1021/nl052163x. [DOI] [PubMed] [Google Scholar]
- 16.Lin YH, Lu F, Tu Y, Ren ZF. Nano Lett. 2004;4:191–195. [Google Scholar]
- 17.Yeo WH, Chou FL, Fotouhi G, Oh K, Stevens BT, Tseng HY, Gao DY, Shen AQ, Chung JH, Lee KH. Lab Chip. 2010;10:3178–3181. doi: 10.1039/c0lc00077a. [DOI] [PubMed] [Google Scholar]
- 18.Yeo WH, Liu S, Chung JH, Liu YL, Lee KH. Anal Bioanal Chem. 2009;393:1593–1600. doi: 10.1007/s00216-008-2591-x. [DOI] [PubMed] [Google Scholar]
- 19.Yum K, Wang N, Yu MF. Nanoscale. 2010;2:363–372. doi: 10.1039/b9nr00231f. [DOI] [PubMed] [Google Scholar]
- 20.Yeo WH, Chung JH, Liu YL, Lee KH. J Phys Chem B. 2009;113:10849–10858. doi: 10.1021/jp900618t. [DOI] [PubMed] [Google Scholar]
- 21.Kim S, Lu L, Chung JH, Lee K, Li Y, Jun S. Innov Food Sci Emerg Technol. 2011;12:617–622. [Google Scholar]
- 22.Kim JH, Yeo WH, Shu ZQ, Soelberg SD, Inoue S, Kalyanasundaram D, Ludwig J, Furlong CE, Riley JJ, Weigel KM, Cangelosi GA, Oh K, Lee KH, Gao DY, Chung JH. Lab Chip. 2012;12:1437–1440. doi: 10.1039/c2lc21131a. [DOI] [PubMed] [Google Scholar]
- 23.Kopacz AM, Yeo WH, Chung JH, Liu WK. Nanoscale. 2012;4:5189–5194. doi: 10.1039/c2nr31279d. [DOI] [PubMed] [Google Scholar]
- 24.Kalyanasundaram D, Inoue S, Kim JH, Lee HB, Kawabata Z, Yeo WH, Cangelosi GA, Oh K, Gao D, Lee KH, Chung JH. Microfluid Nanofluid. 2012:1–9. [Google Scholar]
- 25.Yeo WH, Kopacz AM, Kim JH, Chen X, Wu J, Gao DY, Lee KH, Liu WK, Chung JH. Nanotechnology. 2012 doi: 10.1088/0957-4484/23/48/485707. [DOI] [PubMed] [Google Scholar]
- 26.Li F, Han XP, Liu SF. Biosensors & Bioelectronics. 2011;26:2619–2625. doi: 10.1016/j.bios.2010.11.020. [DOI] [PubMed] [Google Scholar]
- 27.Lu WS, Lin L, Jiang L. Biosensors & Bioelectronics. 2007;22:1101–1105. doi: 10.1016/j.bios.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 28.Heller I, Kong J, Williams KA, Dekker C, Lemay SG. J Am Chem Soc. 2006;128:7353–7359. doi: 10.1021/ja061212k. [DOI] [PubMed] [Google Scholar]
- 29.Kong J, Franklin NR, Zhou CW, Chapline MG, Peng S, Cho KJ, Dai HJ. Science. 2000;287:622–625. doi: 10.1126/science.287.5453.622. [DOI] [PubMed] [Google Scholar]
- 30.Heller I, Kong J, Heering HA, Williams KA, Lemay SG, Dekker C. Nano Lett. 2005;5:137–142. doi: 10.1021/nl048200m. [DOI] [PubMed] [Google Scholar]
- 31.Yeo WH, Chou FL, Oh K, Chung JH, Lee KH. J Nanosci Nanotechno. 2009;9:7288–7292. doi: 10.1166/jnn.2009.1773. [DOI] [PubMed] [Google Scholar]
- 32.Ciszkowska M, Stojek Z. Anal Chem. 2000;72:754A–760A. doi: 10.1021/ac003000q. [DOI] [PubMed] [Google Scholar]
- 33.Boehme CC, Nabeta P, Hillemann D, Nicol MP, Shenai S, Krapp F, Allen J, Tahirli R, Blakemore R, Rustomjee R, Milovic A, Jones M, O’Brien SM, Persing DH, Ruesch-Gerdes S, Gotuzzo E, Rodrigues C, Alland D, Perkins MD. New Engl J Med. 2010;363:1005–1015. doi: 10.1056/NEJMoa0907847. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.