Abstract
ABCB4 is necessary for the secretion of phospholipids from hepatocytes into bile and for the protection of cell membranes against bile salts. Lipid rafts are plasma membrane microdomains containing high contents of cholesterol and sphingolipids, which are separated by Triton X-100 extraction or OptiPrep gradient centrifugation. In this study, we investigated the relationship between the function of ABCB4 and lipid rafts using mouse canalicular membranes and HEK293 cells stably expressing ABCB4. ABCB4 and ABCB1 were mainly distributed in nonraft membranes. The expression of ABCB4, but not ABCB1, led to significant increases in the phosphatidylcholine (PC), phosphatidylethanolamine (PE), and sphingomyelin (SM) contents in nonraft membranes and further enrichment of SM and cholesterol in raft membranes. The ABCB4-mediated efflux of PC, PE, and SM was significantly stimulated by taurocholate, while the efflux of PE and SM was much less than that of PC. This ABCB4-mediated efflux was completely abolished by BODIPY-verapamil, which hardly partitioned into raft membranes. In addition, ABCB1 and ABCB4 mediated the efflux of rhodamine 123 and rhodamine 6G from nonraft membranes, which was not affected by taurocholate. We conclude that ABCB4 located in nonrafts, but not in rafts, is predominantly involved in the efflux of phospholipids and other substrates.
Keywords: raft, phosphatidylcholine, phosphatidylethanolamine, sphingomyelin, cholesterol, ABC transporter, Triton X-100, BODIPY-verapamil, HEK293 cell, canalicular membrane
ABCB4, a member of the ABC transporter family, is present in the canalicular membranes of hepatocytes and plays an essential role in the secretion of phospholipids into bile (1, 2). The function of biliary phospholipid secretion is to protect the membranes of cells facing the biliary tree against bile salts. Biliary phospholipids also play a key role in solubilizing cholesterol (Chol). The secretion of both phospholipids and Chol into bile is almost completely impaired in Abcb4 knockout mice, although Abcg5 and Abcg8 are the main transporters that secrete biliary Chol (3–6). The complexation of bile salts with phospholipids and cholesterol into mixed micelles strongly reduces the cytotoxic detergent effect of bile salts. Mutations in the ABCB4 gene result in progressive familial intrahepatic cholestasis type 3 (7, 8), intrahepatic cholestasis of pregnancy, low-phospholipid-associated cholelithiasis, and primary biliary cirrhosis (1, 9). In vivo and cell culture studies have demonstrated that the excretion of phospholipids depends on both ABCB4 expression and bile salts (3, 10, 11). ABCB4 has been predicted to be a floppase that translocates phospholipids from the inner leaflet to the outer leaflet of the canalicular membrane (12–16), or a transporter that moves phospholipids for direct extraction by bile salts (11, 17). However, the molecular mechanism of ABCB4-mediated phospholipid efflux is poorly understood.
ABCB1 and ABCB4 are 76% identical and 86% similar in terms of their amino acid sequences. ABCB4 is mainly expressed in the liver, while ABCB1 is normally present in various tissues including the liver (18). ABCB1 extrudes a large number of structurally unrelated hydrophobic compounds. ABCB4 is unable to export most ABCB1 substrates efficiently (19), although ABCB1 and ABCB4 have a limited number of common substrates, such as short-chain phospholipids, digoxin, paclitaxel, vinblastine, and aureobasidin A (20, 21). Abcb4 knockout mice do not excrete any long-chain phospholipids into bile (3, 10), even though substantial amounts of Abcb1a and Abcb1b are expressed in the canalicular membranes of hepatocytes. In the presence of bile salts, ABCB4, but not ABCB1, mediates the cellular efflux of long-chain phospholipids, preferentially phosphatidylcholine (PC) (11). The inhibitors of ABCB1, verapamil and itraconazole, have been shown to completely abrogate the bile salt-dependent efflux of phospholipids mediated by ABCB4 (11, 22). The functional differences between ABCB1 and ABCB4 have been suggested to be mainly due to differences in the transmembrane region (23, 24), and ABCB4 binds a subset of ABCB1 substrates or inhibitors (11, 20, 21).
Lipid rafts are small (10–200 nm) plasma membrane domains containing high levels of sphingolipids, mainly sphingomyelin (SM) and Chol, which are characterized physicochemically by tight packing and reduced fluidity leading to a liquid-ordered phase surrounded by bulk liquid-disordered membranes (25–27). Membrane raft domains are resistant to extraction in cold nonionic detergent, such as Triton X-100. Lipid rafts have been implicated in a number of cellular processes, including trafficking and signal transduction. Several lines of evidence have suggested that ABCB1 is at least partly located in lipid rafts (25, 26). On the other hand, the relationship between the function of ABCB4 and lipid rafts has not been previously reported. In the current study, we examined the distribution of ABCB4 between Triton X-100-soluble (TXS) and -insoluble (TXI) membranes in HEK293 cells and the effect of ABCB4 expression on the lipid compositions of raft and nonraft membranes compared with ABCB1 expression. We also attempted to clarify whether the bile salt-dependent efflux of phospholipids was mediated by ABCB4 located in raft membranes or nonraft membranes using BODIPY®-verapamil, an inhibitor exclusively partitioning into nonraft regions. Furthermore, the efflux of nonphospholipid substrates mediated by ABCB1 or ABCB4 was investigated.
MATERIALS AND METHODS
Materials
Sodium taurocholate (NaTC) was obtained from Nacalai Tesque (Kyoto, Japan). Rhodamine 123 and rhodamine 6G were purchased from Sigma-Aldrich (St. Louis, MO). BODIPY®-verapamil was purchased from Molecular Probes (Eugene, OR). Mouse Mdr1a (Abcb1a) membranes and Mdr1b (Abcb1b) membranes prepared from baculovirus-infected High Five insect cells were obtained from Gentest (Woburn, MA). Mouse Abcb4 recombinant protein (amino acids 352–708) produced in yeast was purchased from MyBioSource (San Diego, CA). OptiPrep was purchased from Axis-Shield (Oslo, Norway). All other chemicals used were of the highest reagent grade.
Isolation of mouse canalicular liver plasma membranes
Experiments were performed with male FVB mice at 7 weeks of age (25–29 g). Mice were fed standard chow and water ad libitum. Mouse canalicular and basolateral liver plasma membranes were isolated from the homogenate of livers from six mice according to the method for isolating rat canalicular and basolateral membranes (28, 29). All animal experiments were conducted with the approval of the Research Center for Animal Life Science at Shiga University of Medical Science.
Recombinant plasmid construction
The human ABCB1 gene was inserted into the EcoR I and Xba I sites of pcDNA3.1(+) (Invitrogen, Carlsbad, CA) to make an expression vector, pcDNA3.1(+)/ABCB1. The human ABCB4 gene was inserted into the Nhe I and Sma I sites of the pIREShyg3 mammalian expression vector (Clontech, Mountain View, CA) to generate the plasmid, pIREShyg3/ABCB4. pIREShyg3 contains an internal ribosome entry site, which permits the translation of two open reading frames from one mRNA. This expression system facilitates the establishment of pools of stably transfected cell lines whereby nearly all cells surviving in selective media express the gene of interest, because the hygromycin B phosphotransferase gene is expressed under the control of the same promoter (30).
Cell culture
HEK293 cells were grown in DMEM supplemented with 10% heat-inactivated FBS in a humidified incubator (5% CO2) at 37°C.
Establishment of stable transformants of ABCB1 and ABCB4
HEK293 cells were transfected with pcDNA3.1(+)/ABCB1 or pIREShyg3/ABCB4 using Lipofectamine Reagent and PLUS Reagent (Invitrogen). Cells transfected with pcDNA3.1(+)/ABCB1 were selected with 100 nM vinblastine, and a large number of vinblastine-resistant clones were pooled in one dish. Cells transfected with pIREShyg3/ABCB4 were selected with 400 μg/ml hygromycin, and a large number of hygromycin-resistant clones were pooled in one dish. The expression of ABCB1 or ABCB4 was examined by immunoblotting. To prepare a whole cell lysate, cells were lysed with PBS containing 1% Triton X-100 and protease inhibitors (100 μg/ml p-APMSF, 10 μg/ml leupeptin, and 2 μg/ml aprotinin) and sonicated.
Preparation of nonraft and raft membrane fractions
Triton X-100 insolubility assay with HEK293 cells was performed as previously described (31). Briefly, cells were harvested, suspended in buffer A (protease inhibitors, 150 mM NaCl, 5 mM EDTA, and 20 mM HEPES; pH 7.4), disrupted in a Dounce homogenizer on ice and centrifuged for 10 min at 1,000 g. The supernatant was centrifuged for 1 h at 75,000 g. The high-speed pellet was resuspended in buffer A supplemented with 1% Triton X-100 for 15 min on ice, and centrifuged for 1 h at 75,000 g. After removing the supernatant (TXS fraction), the pellet (TXI fraction) was resuspended in an equal volume of buffer A containing 1% Triton X-100 and sonicated. Equal volumes of each fraction were used for immunoblot analysis. PC, phosphatidylethanolamine (PE), SM, and Chol contents in the fractions were measured by enzymatic fluorometric assays (32–34). Protein contents were determined using a BCA protein assay kit (Thermo Fisher Scientific, Rockford, IL).
The canalicular membranes isolated from mouse livers were resuspended in buffer A supplemented with 1% Triton X-100 for 15 min on ice, and were centrifuged for 1 h at 75,000 g. After removing the supernatant (TXS fraction), the pellet (TXI fraction) was resuspended in an equal volume of buffer A containing 1% Triton X-100 and sonicated. All steps in the preparation of canalicular TXS and TXI fractions from mouse livers were carried out within a day.
Detergent-free rafts were prepared using the OptiPrep gradient method of Macdonald and Pike (35).
Immunoblotting
Samples were separated by SDS-PAGE on a 7%, 10%, or 12% polyacrylamide gel calibrated with Precision Plus Protein WesternC standards (Bio-Rad Laboratories, Hercules, CA), transferred to polyvinylidene difluoride membranes and immunoblotted with monoclonal anti-P-glycoprotein antibody C219 (Merck Millipore, Billerica, MA) (1:400 dilution), monoclonal anti-MDR3 P-glycoprotein antibody P3II-26 (Merck Millipore) (1:100 dilution), monoclonal anti-Na+/K+-ATPase α-1 antibody C464.6 (Merck Millipore) (1:10,000 dilution), monoclonal anti-transferrin receptor H68.4 (Invitrogen) (1:500 dilution), or rabbit anti-flotillin-1 antibody (Sigma-Aldrich) (1:1,000 dilution). Protein-antibody complexes were detected by enhanced chemiluminescence using horseradish peroxidase-conjugated goat anti-mouse IgG (Invitrogen) or goat anti-rabbit IgG (Merck Millipore), and exposed to X-ray films. The film was scanned and the integrated optical densities of the bands were measured using ImageJ software (National Institutes of Health).
Cellular lipid efflux assay
Cells were subcultured in poly-d-Lys-coated 6-well plates at a density of 2.0 × 106 cells in DMEM supplemented with 10% FBS. After incubation for 48 h, the cells were washed with fresh medium and incubated with DMEM containing 0.02% BSA in the presence or absence of NaTC or BODIPY-verapamil for 24 h at 37°C. The medium was centrifuged to remove cellular debris, and lipids in the medium were extracted as described previously (11, 36). The lipid extracts were dissolved in 1% Triton X-100, and the amounts of PC, PE, SM, and Chol were quantified by enzymatic fluorometric assays (32–34). DMEM containing 0.02% BSA contained no detectable amount of PC, PE, SM, or Chol. The cells were dissolved in 1% Triton X-100 and sonicated, and the cell protein concentration was measured using a BCA protein assay kit.
Cellular accumulation assay
Cells were subcultured in poly-d-Lys-coated 12-well plates at a density of 4.0 × 105 cells in DMEM supplemented with 10% FBS. After incubation for 48 h, the cells were washed with HEPES buffer (137 mM NaCl, 5.4 mM KCl, 0.6 mM MgCl2, 1.1 mM CaCl2, 6.1 mM d-glucose, and 10 mM HEPES; pH 7.4) and incubated with HEPES buffer containing BODIPY-verapamil, rhodamine 123, or rhodamine 6G for 2 h at 37°C. The cells were chilled on ice, washed twice with cold HEPES buffer, dissolved in 1% Triton X-100 and sonicated. The fluorescence intensities of BODIPY-verapamil (excitation 485 nm, emission 538 nm), rhodamine 123 (excitation 485 nm, emission 538 nm), and rhodamine 6G (excitation 544 nm, emission 590 nm) were measured using a fluorescence microplate reader (Fluoroskan Ascent FL, Thermo Fisher Scientific), and the concentration of cellular protein was measured using a BCA protein assay kit.
Statistical analysis
The statistical significance of differences between mean values was analyzed using the nonpaired t-test. Multiple comparisons were performed using the Bonferroni test following ANOVA. Differences were considered significant at P < 0.05. Unless indicated otherwise, results are given as the mean ± SE (n = 3).
RESULTS
Distribution of ABCB4 between raft and nonraft membranes
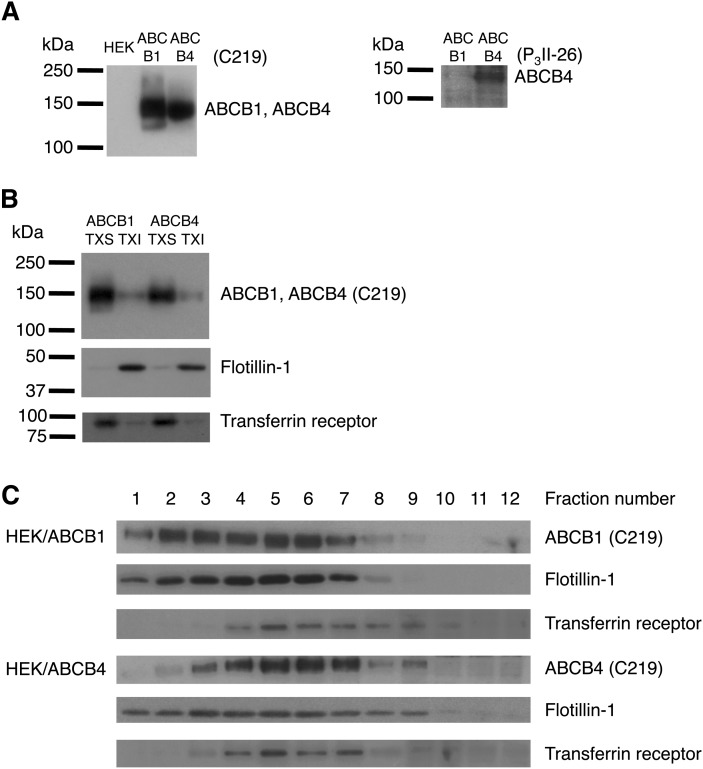
We questioned whether mouse Abcb4 is associated with the lipid raft domains of canalicular liver plasma membranes. Mouse Abcb4 has an amino acid sequence with 85% and 83% similarity to those of mouse Abcb1a and Abcb1b, respectively. The monoclonal antibody C219 recognizes human ABCB1, human ABCB4, mouse Abcb1a, mouse Abcb1b, and mouse Abcb4 (37). Although the monoclonal antibody P3II-26 has been reported to react with human ABCB4 but not human ABCB1 (38), the reactivity of P3II-26 against mouse Abcb1a, Abcb1b, or Abcb4 has not been determined. First, we tested the specificity of the antibody P3II-26 by Western blotting using mouse Abcb1a membranes, mouse Abcb1b membranes, and mouse Abcb4 recombinant fragment (amino acids 352–708). The results depicted in Fig. 1A show that C219 cross-reacted with mouse Abcb1a, Abcb1b, and Abcb4 as expected, and that P3II-26 specifically detected mouse Abcb4.
Fig. 1.
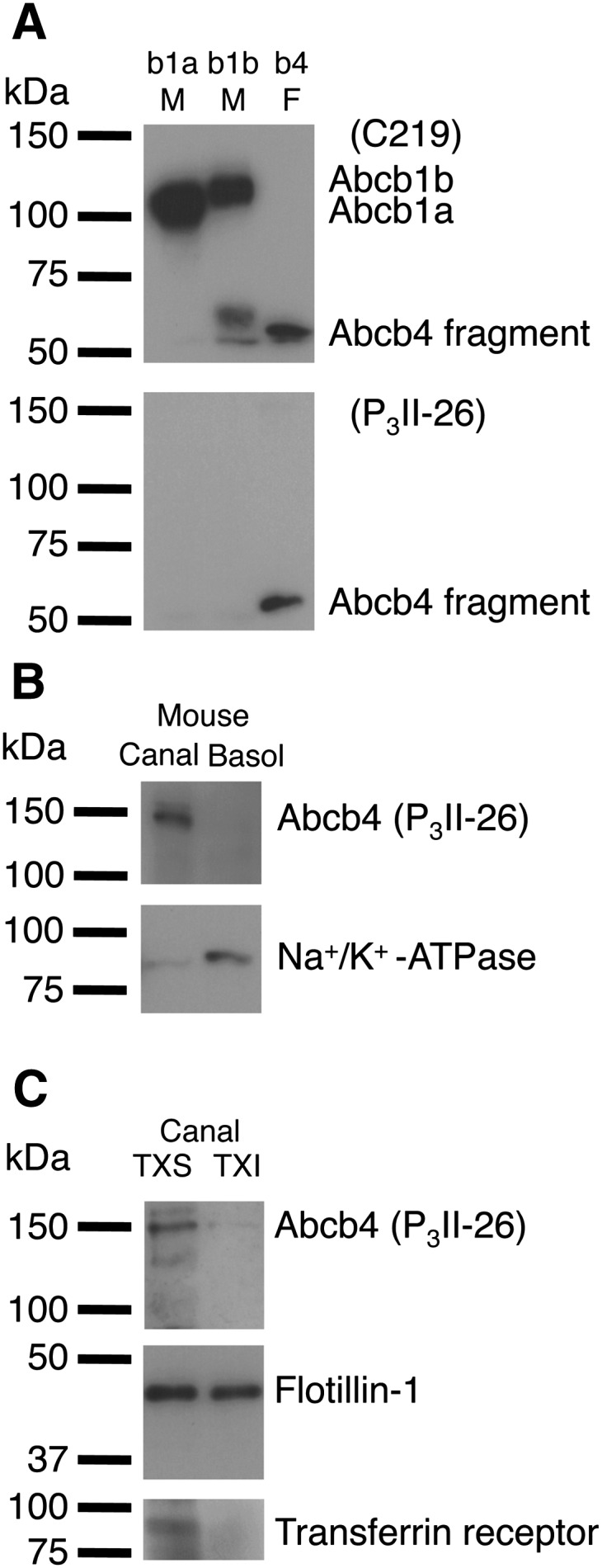
Distribution of Abcb4 between canalicular nonraft and raft membranes in mouse hepatocytes. A: Immunoreactivity of mouse Abcb1a, Abcb1b, and Abcb4 with C219 and P3II-26 monoclonal antibodies. Mouse Abcb1a membranes (b1a M) (2 μg of protein), mouse Abcb1b membranes (b1b M) (2 μg of protein), and mouse Abcb4 recombinant fragment (amino acids 352–708) (b4 F) (27.2 ng of protein) were separated by 10% SDS/PAGE. B: Mouse canalicular liver plasma membranes (Canal) and basolateral liver plasma membranes (Basol) (37.4 μg of protein) were separated by 10% SDS/PAGE. C: TXS and TXI fractions of mouse canalicular liver plasma membranes (equal volumes) were separated by 10% or 12% SDS/PAGE. Abcb4, Na+/K+-ATPase (basolateral marker), flotillin-1 (raft marker), and transferrin receptor (nonraft marker) were detected with specific antibodies.
The purity of the canalicular membrane subfraction was confirmed using Western blotting for Abcb4 and the basolateral marker Na+/K+-ATPase (Fig. 1B). Triton X-100 insolubility has been widely used as a tool to isolate lipid rafts and associated proteins (31, 39–42). As shown in Fig. 1C, Abcb4 in the canalicular membranes was preferentially solubilized by Triton X-100. The ratio of TXI/TXS for Abcb4 was 0.0995, although the TXI/TXS ratio for canalicular membrane proteins was 0.217. Flotillin-1 has been shown to be a major lipid raft marker and also present in cellular compartments other than lipid rafts (35, 43), and that the transferrin receptor is associated with membranes distinct from raft domains (35, 44). The canalicular TXI fraction was associated with flotillin-1, but not with the transferrin receptor. These results suggest the exclusive localization of Abcb4 in canalicular nonraft membranes.
To study the relationships between the functions of ABCB4 and lipid rafts, we established HEK293 cell lines stably expressing ABCB1 (HEK/ABCB1) and ABCB4 (HEK/ABCB4). Both ABCB1 and ABCB4 were expressed as proteins of ∼140 kDa (Fig. 2A). Neither ABCB1 nor ABCB4 was detected in the host HEK293 cells.
Fig. 2.
Distribution of ABCB4 between nonraft and raft membranes in HEK293 cells. A: Whole cell lysates (25.9 μg of protein) from HEK293, HEK/ABCB1, and HEK/ABCB4 cells were separated by 7% SDS/PAGE. B: TXS and TXI fractions (equal volumes) were separated by 7%, 10%, or 12% SDS/PAGE. C: OptiPrep gradient fractions (equal volumes) were separated by 10% or 12% SDS/PAGE. ABCB1, ABCB4, flotillin-1 (raft marker), and transferrin receptor (nonraft marker) were detected with specific antibodies.
We examined the distributions of ABCB1, ABCB4, and domain-specific membrane proteins between the TXS and TXI fractions in HEK293 cells. Results shown in Fig. 2B demonstrate that ABCB1 and ABCB4 were predominantly distributed into TXS membrane fractions. Quantitatively, the ratios of TXI/TXS for ABCB1 and ABCB4 were 0.301 and 0.204, respectively, whereas the TXI/TXS ratios for whole membrane proteins were 1.07 and 1.03 in HEK/ABCB1 and HEK/ABCB4 cells, respectively. Flotillin-1 was exclusively recovered from TXI membranes. In contrast, the majority of transferrin receptors were present in the TXS membrane fractions.
Concerns have been raised that the extraction of cells with detergent may yield clusters of raft lipids and proteins that do not exist in intact cells (35). To avoid artifacts caused by the use of detergents, we also used the OptiPrep gradient method for the isolation of detergent-free lipid rafts. In both HEK/ABCB1 and HEK/ABCB4 cells, a significant portion of the raft marker flotillin-1 was found in the lightest fractions of the gradient, although flotillin-1 was broadly distributed in the gradient (Fig. 2C), in agreement with previous results (35). The nonraft marker, the transferrin receptor was recovered primarily in fractions 4–8, indicating that fractions 1–3 corresponded to the raft domains. ABCB4 was found mainly in fractions 3–9, which was denser than ABCB1 found in fractions 1–7. The distribution ratios of raft/nonraft for ABCB1 and ABCB4 were 0.624 and 0.158, respectively. Taken together, these results suggest that ABCB1 and ABCB4 are preferentially distributed in the nonraft membranes irrespective of the use of detergents.
Effects of ABCB4 expression on lipid compositions of raft and nonraft membranes
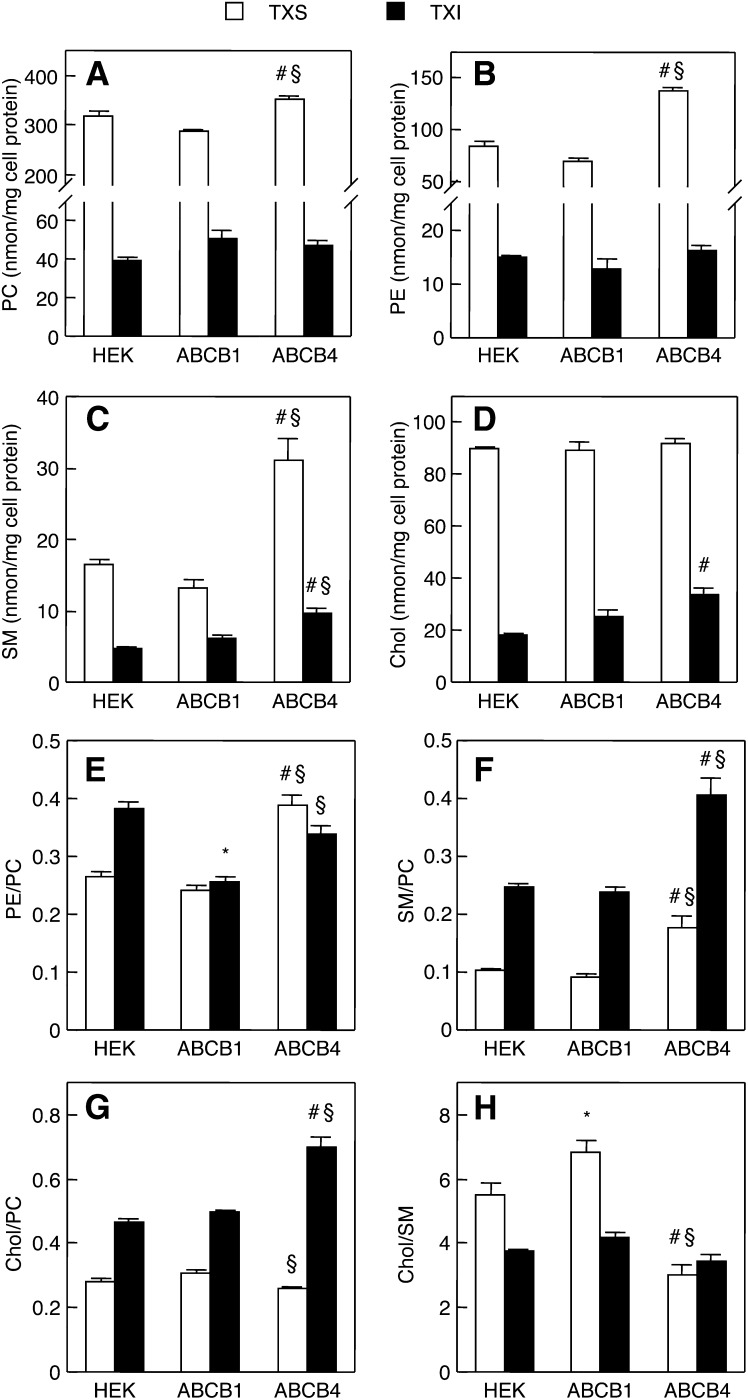
We hypothesized that ABCB4 expression affects the lipid compositions of raft and nonraft membranes. To test this, we quantified PC, PE, SM, and Chol contents in the TXS and TXI membrane fractions of HEK293, HEK/ABCB1, and HEK/ABCB4 cells in the absence of bile salts. As shown in Fig. 3A, the PC content in the TXS fraction of HEK/ABCB4 cells was significantly higher than that in the TXS fraction of HEK293 or HEK/ABCB1 cells. On the other hand, there was no significant difference in the PC contents in the TXI fractions of HEK293, HEK/ABCB1, and HEK/ABCB4 cells. Interestingly, HEK/ABCB4 cells also exhibited higher PE content in the TXS fraction, but not in the TXI fraction, than HEK293 and HEK/ABCB1 cells (Fig. 3B). The expression of ABCB4 resulted in increased SM content in both TXS and TXI fractions (Fig. 3C). Although the content of Chol in the TXS fractions was similar between the three cell lines, the TXI fraction of HEK/ABCB4 cells contained more Chol than those of HEK293 and HEK/ABCB1 cells (Fig. 3D). Unlike ABCB4, ABCB1 expression induced no significant changes in the lipid contents of both membrane fractions. The ratios of PE/PC, SM/PC, and Chol/PC were determined as indices of the concentrations of these lipids in the TXS and TXI membrane fractions because PC is the most abundant lipid in these fractions. In the TXS fractions, the ratios of PE/PC and SM/PC, but not Chol/PC, were significantly elevated by the expression of ABCB4 (Fig. 3E–G). The ratios of SM/PC and Chol/PC, but not PE/PC, in the TXI fractions were markedly higher in HEK/ABCB4 cells than in HEK293 and HEK/ABCB1 cells, whereas ABCB1 expression led to a decrease in the PE/PC ratio in the TXI fractions. We also assessed the Chol/SM ratio in both membrane fractions. The Chol/SM ratio in the TXS fraction was significantly reduced in HEK/ABCB4 cells, but increased in HEK/ABCB1 cells (Fig. 3H). However, the Chol/SM ratio in the TXI fraction was not altered by the expression of ABCB1 or ABCB4.
Fig. 3.
Cellular contents of PC, PE, SM, and Chol in TXS and TXI. HEK293, HEK/ABCB1, and HEK/ABCB4 cells were cultured in DMEM containing 10% FBS at 37°C. The contents of PC (A), PE (B), SM (C), and Chol (D) and the ratios of PE/PC (E), SM/PC (F), Chol/PC (G), and Chol/SM (H) in TXS (open bars) and TXI (filled bars) fractions were determined by enzymatic measurements of PC, PE, SM, and Chol and protein assay. Each bar represents the mean ± S.E. of three measurements. The absence of an error bar signifies an S.E. value smaller than the graphic symbol. *P < 0.05, significant difference between HEK293 and HEK/ABCB1 cells. #P < 0.05, significant difference between HEK293 and HEK/ABCB4 cells. §P < 0.05, significant difference between HEK/ABCB1 and HEK/ABCB4 cells.
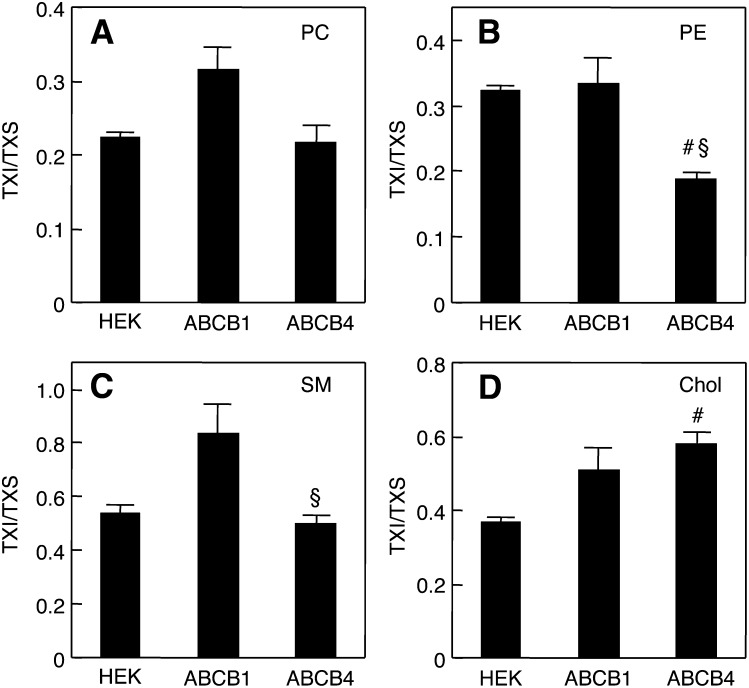
Moreover, the partition coefficients of PC, PE, SM, and Chol between the TXS and TXI membranes were compared in HEK293, HEK/ABCB1, and HEK/ABCB4 cells. The expression of ABCB4 did not affect the TXI/TXS ratio for PC (Fig. 4A), but resulted in a decreased TXI/TXS ratio for PE (Fig. 4B). Although the TXI/TXS ratio for SM was not altered by ABCB4 expression (Fig. 4C), the TXI/TXS ratio for Chol was markedly higher in HEK/ABCB4 cells than in HEK293 cells (Fig. 4D). On the other hand, ABCB1 expression had no significant effect on the TXI/TXS ratios for PC, PE, SM, and Chol. Collectively, these results confirmed that ABCB4 expression leads to alterations in the contents and distributions of membrane lipids between raft and nonraft regions.
Fig. 4.
Partition coefficients of PC, PE, SM, and Chol between TXS and TXI fractions. HEK293, HEK/ABCB1, and HEK/ABCB4 cells were cultured in DMEM containing 10% FBS at 37°C. TXI/TXS ratios of PC (A), PE (B), SM (C), and Chol (D) were determined by enzymatic measurements of PC, PE, SM, and Chol. Each bar represents the mean ± S.E. of three measurements. #P < 0.05, significant difference between HEK293 and HEK/ABCB4 cells. §P < 0.05, significant difference between HEK/ABCB1 and HEK/ABCB4 cells.
Effect of BODIPY-verapamil on ABCB4-mediated phospholipid efflux stimulated by NaTC
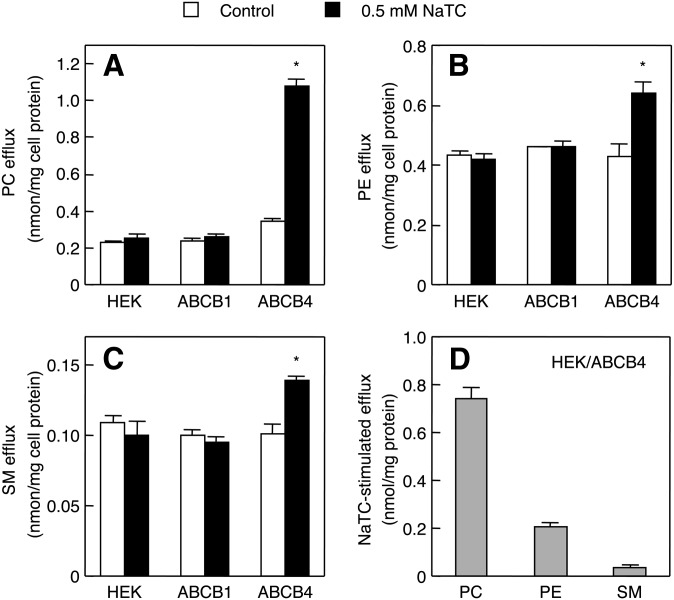
It has been previously shown that the addition of NaTC remarkably increases the efflux of choline-containing phospholipids from HEK293 cells expressing ABCB4 (11). We also recently developed enzyme-based fluorometric methods to quantify PC, PE, and SM, which are simple, rapid, sensitive, and high throughput (33, 34). Next, we analyzed the phospholipids secreted from HEK/ABCB4 cells in the presence of NaTC, using new enzymatic fluorometric assays. Strikingly, the PC efflux from HEK/ABCB4 cells was enhanced by NaTC (Fig. 5A). The addition of NaTC also significantly increased the efflux of PE and SM from HEK/ABCB4 cells (Fig. 5B, C), although the enhancement in PE or SM efflux by NaTC was less marked than that of PC efflux (Fig. 5D). The PE/PC and SM/PC ratios for NaTC-stimulated efflux from HEK/ABCB4 cells were 0.287 ± 0.042 and 0.053 ± 0.014, respectively. In contrast, the efflux of PC, PE, and SM from HEK293 or HEK/ABCB1 cells was not influenced by NaTC.
Fig. 5.
Effects of NaTC on efflux of cellular lipids. HEK293, HEK/ABCB1, and HEK/ABCB4 cells were incubated with DMEM containing 0.02% BSA in the absence (control; open bars) or presence of 0.5 mM NaTC (filled bars) for 24 h at 37°C. The efflux of PC (A), PE (B), and SM (C) from the cells was determined by enzymatic measurements of PC, PE, and SM and protein assay. The NaTC-stimulated efflux of lipids from HEK/ABCB4 cells (D) was calculated by subtracting the efflux in the absence of NaTC from that in the presence of NaTC. Each bar represents the mean ± S.E. of three measurements. The absence of an error bar signifies an S.E. value smaller than the graphic symbol. *P < 0.05, significantly different from the control.
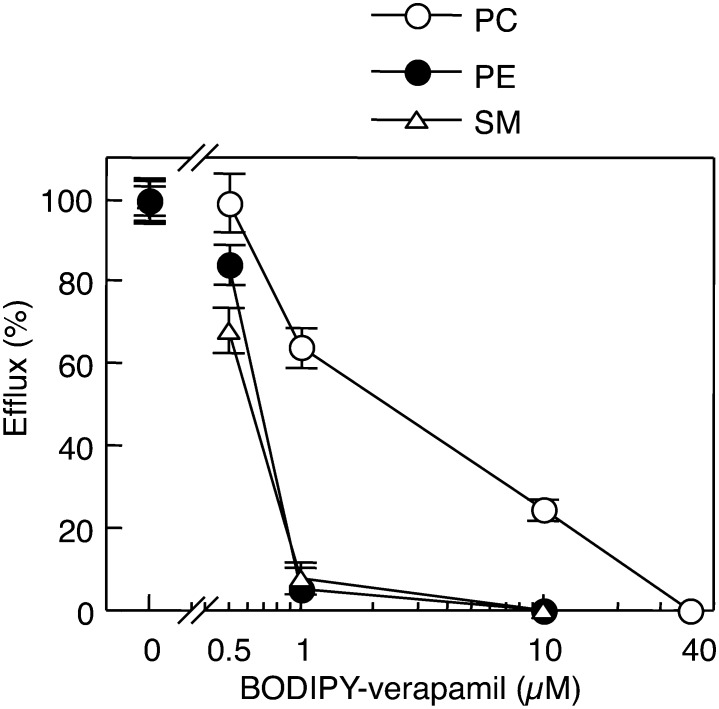
BODIPY-verapamil has been shown to be a transport substrate for ABCB1 (45). We tested the effect of BODIPY-verapamil on the ABCB4-mediated efflux of PC, PE, and SM stimulated by NaTC. The efflux of PC, PE, and SM from HEK/ABCB4 cells was decreased with increasing concentrations of BODIPY-verapamil in the presence of 0.5 mM NaTC (Fig. 6). The addition of 40 μM BODIPY-verapamil completely inhibited the efflux of PC. Subsequently, we investigated the partitioning of BODIPY-verapamil between the TXS and TXI fractions of HEK293 cells. The ratio of TXI/TXS for BODIPY-verapamil was very low (0.021 ± 0.003), indicating that BODIPY-verapamil partitions almost exclusively into the TXS membranes. Therefore, ABCB4 localized in nonraft membranes may play major roles in the efflux of phospholipids.
Fig. 6.
Effects of BODIPY-verapamil on ABCB4-mediated efflux of cellular lipids in the presence of NaTC. HEK/ABCB4 cells were incubated with DMEM containing 0.02% BSA and 0.5 mM NaTC in the absence (control) or presence of the indicated concentrations of BODIPY-verapamil for 24 h at 37°C. PC efflux (open circles), PE efflux (filled circles), and SM efflux (open triangles) from HEK/ABCB4 cells were determined by enzymatic measurements of PC, PE, and SM and protein assay. The efflux of each lipid in the absence of BODIPY-verapamil (control) was taken as 100%. Each point represents the mean ± S.E. of three measurements.
Efflux of nonphospholipid substrates mediated by ABCB4
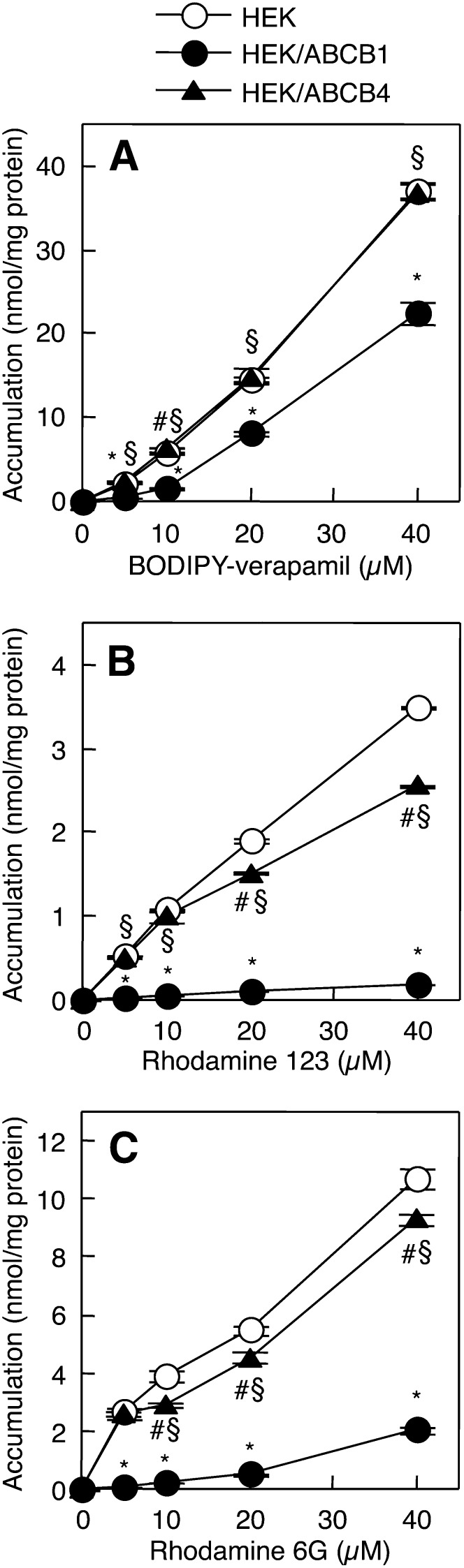
We next examined the relationships between lipid rafts and the efflux of nonphospholipid compounds, BODIPY-verapamil, rhodamine 123, and rhodamine 6G, which are known substrates for ABCB1 (45–47). The TXI/TXS ratios for rhodamine 123 and rhodamine 6G were 0.019 ± 0.001 and 0.018 ± 0.0004, respectively, indicating that these two compounds, in addition to BODIPY-verapamil, hardly partition into the raft membranes. Drug accumulation assays are the standard methods for evaluating the export activity of ABCB1, and a reduction in the intracellular accumulation of the drug corresponds to increased export activity (43, 45, 47, 48). As expected, the accumulation of BODIPY-verapamil, rhodamine 123, and rhodamine 6G was much less in HEK/ABCB1 cells than in HEK293 cells (Fig. 7A–C). The accumulation of BODIPY-verapamil was similar between HEK293 and HEK/ABCB4 cells, while HEK/ABCB4 cells accumulated significantly less rhodamine 123 and rhodamine 6G than HEK293 cells. Reductions in the accumulation of rhodamine 123 and rhodamine 6G were more marked in HEK/ABCB1 cells than in HEK/ABCB4 cells. These results suggest that ABCB4 as well as ABCB1 can mediate the efflux of nonphospholipid substrates partitioning into the nonraft membranes.
Fig. 7.
Cellular accumulation of BODIPY-verapamil, rhodamine 123, and rhodamine 6G. HEK293 (open circles), HEK/ABCB1 (filled circles), and HEK/ABCB4 (filled triangles) cells were incubated with HEPES buffer containing the indicated concentrations of BODIPY-verapamil (A), rhodamine 123 (B), or rhodamine 6G (C) for 2 h at 37°C. Each point represents the mean ± S.E. of three measurements. *P < 0.05, significant difference between HEK293 and HEK/ABCB1 cells. #P < 0.05, significant difference between HEK293 and HEK/ABCB4 cells. §P < 0.05, significant difference between HEK/ABCB1 and HEK/ABCB4 cells.
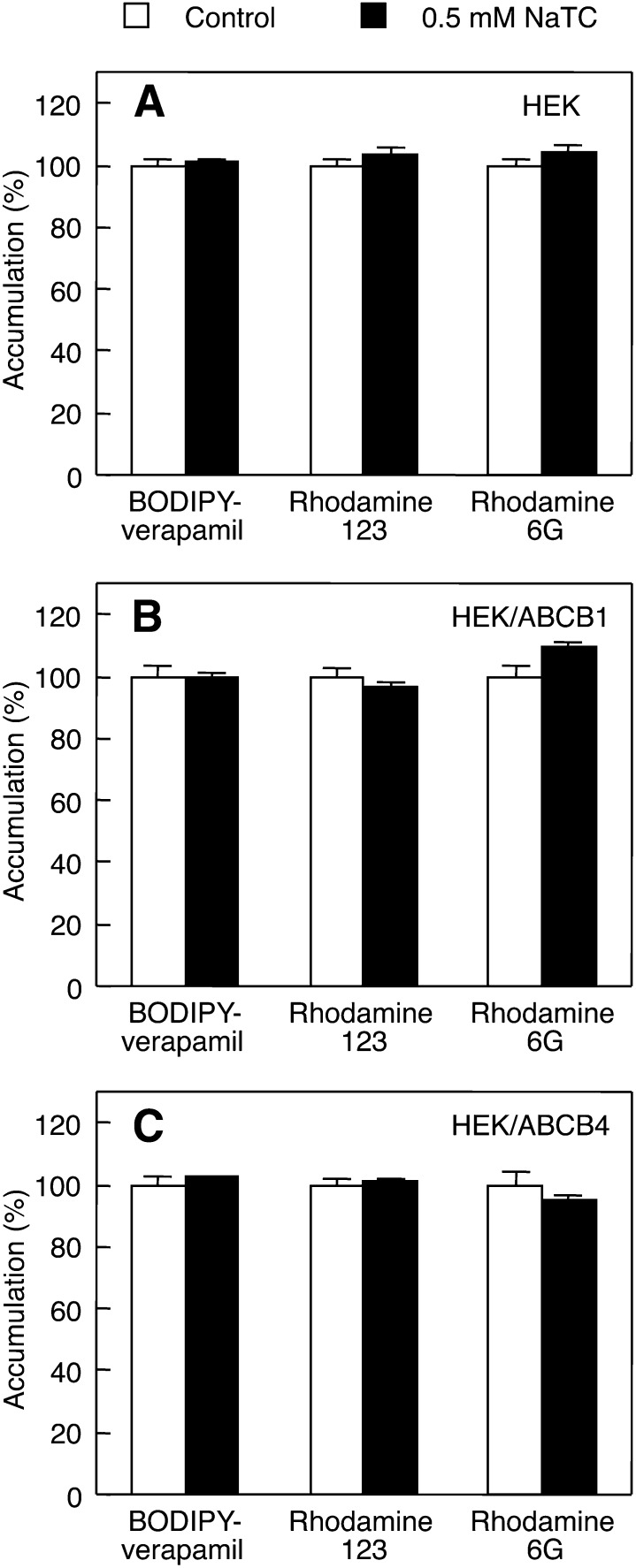
Taurocholate has been reported to have no effect on the ABCB1-mediated efflux of rhodamine 123 (49). Additionally, we investigated whether or not the ABCB4-mediated efflux of nonphospholipid substrates is further facilitated by NaTC. As shown in Fig. 8A–C, the addition of NaTC caused no changes in the accumulation of BODIPY-verapamil, rhodamine 123, and rhodamine 6G in HEK293, HEK/ABCB1, and HEK/ABCB4 cells, suggesting that NaTC has no effect on the efflux of the three compounds mediated by ABCB1 or ABCB4, or on their passive diffusion into HEK293 cells.
Fig. 8.
Effects of NaTC on cellular accumulation of BODIPY-verapamil, rhodamine 123, and rhodamine 6G. HEK293 (A), HEK/ABCB1 (B), and HEK/ABCB4 (C) cells were incubated with HEPES buffer containing 20 μM BODIPY-verapamil, 20 μM rhodamine 123, or 20 μM rhodamine 6G in the absence (control; open bars) or presence of 0.5 mM NaTC (filled bars) for 2 h at 37°C. Each bar represents the mean ± S.E. of three measurements. The absence of an error bar signifies an S.E. value smaller than the graphic symbol.
DISCUSSION
Although intrinsic membrane proteins are largely excluded from lipid rafts, a variety of proteins are selectively enriched in rafts (27, 35). It has been shown that ABCA1 and ABCC1 are not associated with membrane raft domains (39, 40, 50, 51), while ABCG2 is located in membrane rafts (44). Although ABCB1 has been reported to exist in rafts and nonraft membrane domains, depending on the cell type, experimental conditions, and methods, it is generally accepted that ABCB1 is marginally associated with TXI membranes (26). We showed that ABCB1 was localized mainly in the nonraft membranes of HEK293 cells (Fig. 2B, C). In addition, ABCB1 mediated the cellular efflux of BODIPY-verapamil, rhodamine 123, and rhodamine 6G, which were partitioned almost exclusively into the nonraft membranes (Fig. 7A–C). Most of the ABCB1 substrates are unlikely to be incorporated into the tightly packed membrane rafts owing to their bulky structures, and none of the ABCB1 substrates have been previously reported to partition into raft fractions. Thus, it is conceivable that ABCB1 in nonraft membranes exports the substrates more efficiently than ABCB1 in raft membranes.
We demonstrated that a large proportion of ABCB4 was distributed in the nonraft membranes of HEK293 cells (Fig. 2B, C). The expression of ABCB4 resulted in an increase in the PC content of nonraft fractions, but not of raft fractions (Fig. 3A). It is likely that PC molecules are recruited to nonraft membranes by the floppase activity of ABCB4 in the absence of bile salts. ABCB4 expression was also accompanied by increases in the contents of PE and SM in the nonraft membranes (Fig. 3B, C), and conversely, the ratios of PE/PC and SM/PC were elevated in the nonrafts by ABCB4 expression (Fig. 3E, F), implying that ABCB4 may not induce the formation of PC-enriched membranes. However, we cannot exclude the possibility that the local concentration of PC may become higher at the outer leaflet around ABCB4 protein molecules. Both SM and Chol contents in the TXI raft membranes were elevated by ABCB4 expression (Fig. 3C, D), but interestingly, the Chol/SM ratios were constant in the rafts (Fig. 3H), suggesting that the Chol/SM ratio is an important factor for the formation of membrane rafts. Consequently, ABCB4 expression may bring about further enrichment of SM and Chol in raft regions. In contrast, the Chol/PC ratios were maintained in the TXS nonraft membranes (Fig. 3G). However, at present, it is largely undetermined how ABCB4 expression leads to alterations in the membrane organization of raft and nonraft regions in the absence of bile salts.
The hepatocyte plasma membrane is functionally divided into the canalicular region adjacent to the lumen of the bile canaliculus and the basolateral region in close contact with sinusoidal blood. We also showed that mouse Abcb4 was mostly localized in the nonraft domains of the hepatocyte canalicular membranes (Fig. 1C). The contents of SM and Chol are higher in the canalicular membranes than in the sinusoidal membranes, which lead to decreased fluidity and increased resistance against the detergent effects of bile salts (52). Although SM-containing vesicles without Chol are very sensitive to micellar solubilization upon NaTC addition, the incorporation of Chol rendered SM-containing vesicles highly resistant to the detergent effects of NaTC (53). Elevations in the contents of SM and Chol in raft domains by ABCB4 expression (Fig. 3C, D) may help to protect the canalicular membranes from the micellizing effects of bile salts.
The predominant (90–95%) biliary phospholipid is PC, while SM is only present in trace amounts (54). In this study, NaTC promoted the ABCB4-mediated efflux of PC, PE, and SM (Fig. 5A–D). ABCB4 preferentially mediated the efflux of PC over that of SM, which agrees with previous results from mass spectrometry analysis of the phospholipids secreted from HEK293 cells expressing ABCB4 (11). The ABCB4-mediated efflux of PC, PE, and SM stimulated by NaTC were completely abolished by BODIPY-verapamil, which hardly partitioned into the TXI raft membranes (Fig. 6), although a small proportion of ABCB4 existed in the TXI raft membranes (Fig. 2B, C). These findings reveal that ABCB4-mediated phospholipid efflux occurs mostly in the nonraft membranes. The PE/PC ratio for NaTC-stimulated efflux (0.287 ± 0.042) was lower than the PE/PC ratio in the TXS nonraft membranes of HEK/ABCB4 cells (0.388 ± 0.018) (Fig. 3E), although PE is localized mainly on the inner leaflet of plasma membrane (55), suggesting that the efflux activity of ABCB4 is more specific for PC than for PE. Furthermore, NaTC induced only a slight increase in the efflux of SM from HEK/ABCB4 cells (Fig. 5C, D), which may be caused by the distribution of SM predominantly on the outer leaflet of the plasma membrane and/or the low specificity of ABCB4 for SM. ABCB4-mediated release from epithelial cells into the apical albumin-containing medium has been shown to be selective for fluorescent-labeled PC, but not for fluorescent-labeled PE or fluorescent-labeled SM (14). However, we cannot exclude the possibility that NaTC extracts PC preferentially over PE and SM. Our results also indicated that the efflux of rhodamine 123 and rhodamine 6G were mediated by ABCB4 in nonrafts (Fig. 7B, C), and that BODIPY-verapamil is not a transport substrate of ABCB4, but an inhibitor of ABCB4-mediated phospholipid efflux (Figs. 6, 7A). Nonphospholipid compounds may compete with PC for binding to ABCB4. Interestingly, unlike the phospholipid efflux, NaTC did not stimulate the ABCB4-mediated efflux of nonphospholipid substrates (Fig. 8C). We attribute this to the sufficient solubility of the substrates in the aqueous medium. Because the critical micelle concentration of NaTC is 2.5 mM in the medium (11), NaTC monomers (0.5 mM) can solubilize phospholipids in the aqueous medium. The ABCB4-mediated phospholipid efflux stimulated by NaTC is greater than that stimulated by the more hydrophobic bile salt, sodium cholate (11). It is noteworthy that apolipoprotein A-I or HDL cannot enhance ABCB4-mediated phospholipid efflux, despite their ability to accept phospholipids from ABCA1-expressing cells (56, 57), whereas NaTC also stimulates the phospholipid efflux mediated by ABCA1 (57). Crawford et al. (15) have observed abundant unilamellar vesicles in the bile canaliculi of Abcb4 (+/+) mice but not Abcb4 (−/−) mice, and proposed a model in which biliary phospholipids are secreted as vesicles. In this model, vesiculation may be induced by ABCB4-mediated translocation of PC to the outer leaflet of the canalicular membrane and by destabilization of the membrane by luminal bile salts interacting preferentially with the Chol- and SM-poor fluid membrane microdomains composed of PC. Our results do not contradict this model.
In conclusion, we showed that ABCB4 was localized mainly to the nonraft membranes. We also demonstrated that the ABCB4-mediated efflux of PC was much greater than that of PE or SM in the presence of NaTC, and that this phospholipid efflux was completely inhibited by BODIPY-verapamil, which hardly partitions into the raft membranes, suggesting that the NaTC-stimulated phospholipid efflux is mediated exclusively by ABCB4 located in the nonraft membranes. Our findings may provide clues to understanding the cellular and molecular processes involved in the lipid efflux mediated by ABCB4 or other ABC transporters.
Acknowledgments
The authors thank Dr. Kazumitsu Ueda (Institute for Integrated Cell-Material Sciences, Kyoto University, Kyoto, Japan) for invaluable discussions.
Footnotes
Abbreviations:
- Chol
- cholesterol
- NaTC
- sodium taurocholate
- PC
- phosphatidylcholine
- PE
- phosphatidylethanolamine
- TXI
- Triton X-100-insoluble
- TXS
- Triton X-100-soluble
This work was supported in part by a Grant-in-Aid for Young Scientists (B) from the Japanese Ministry of Education, Culture, Sports, Science, and Technology (22790053) and by a grant from Takeda Science Foundation.
REFERENCES
- 1.Oude Elferink R. P., Paulusma C. C. 2007. Function and pathophysiological importance of ABCB4 (MDR3 P-glycoprotein). Pflugers Arch. 453: 601–610 [DOI] [PubMed] [Google Scholar]
- 2.Borst P., Zelcer N., van Helvoort A. 2000. ABC transporters in lipid transport. Biochim. Biophys. Acta. 1486: 128–144 [DOI] [PubMed] [Google Scholar]
- 3.Smit J. J., Schinkel A. H., Oude Elferink R. P., Groen A. K., Wagenaar E., van Deemter L., Mol C. A., Ottenhoff R., van der Lugt N. M., van Roon M. A., et al. 1993. Homozygous disruption of the murine mdr2 P-glycoprotein gene leads to a complete absence of phospholipid from bile and to liver disease. Cell. 75: 451–462 [DOI] [PubMed] [Google Scholar]
- 4.Oude Elferink R. P., Ottenhoff R., van Wijland M., Frijters C. M., van Nieuwkerk C., Groen A. K. 1996. Uncoupling of biliary phospholipid and cholesterol secretion in mice with reduced expression of mdr2 P-glycoprotein. J. Lipid Res. 37: 1065–1075 [PubMed] [Google Scholar]
- 5.Smith A. J., de Vree J. M., Ottenhoff R., Oude Elferink R. P., Schinkel A. H., Borst P. 1998. Hepatocyte-specific expression of the human MDR3 P-glycoprotein gene restores the biliary phosphatidylcholine excretion absent in Mdr2 (−/−) mice. Hepatology. 28: 530–536 [DOI] [PubMed] [Google Scholar]
- 6.Langheim S., Yu L., von Bergmann K., Lutjohann D., Xu F., Hobbs H. H., Cohen J. C. 2005. ABCG5 and ABCG8 require MDR2 for secretion of cholesterol into bile. J. Lipid Res. 46: 1732–1738 [DOI] [PubMed] [Google Scholar]
- 7.Deleuze J. F., Jacquemin E., Dubuisson C., Cresteil D., Dumont M., Erlinger S., Bernard O., Hadchouel M. 1996. Defect of multidrug-resistance 3 gene expression in a subtype of progressive familial intrahepatic cholestasis. Hepatology. 23: 904–908 [DOI] [PubMed] [Google Scholar]
- 8.de Vree J. M., Jacquemin E., Sturm E., Cresteil D., Bosma P. J., Aten J., Deleuze J. F., Desrochers M., Burdelski M., Bernard O., et al. 1998. Mutations in the MDR3 gene cause progressive familial intrahepatic cholestasis. Proc. Natl. Acad. Sci. USA. 95: 282–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lucena J. F., Herrero J. I., Quiroga J., Sangro B., Garcia-Foncillas J., Zabalegui N., Sola J., Herraiz M., Medina J. F., Prieto J. 2003. A multidrug resistance 3 gene mutation causing cholelithiasis, cholestasis of pregnancy, and adulthood biliary cirrhosis. Gastroenterology. 124: 1037–1042 [DOI] [PubMed] [Google Scholar]
- 10.Oude Elferink R. P., Ottenhoff R., van Wijland M., Smit J. J., Schinkel A. H., Groen A. K. 1995. Regulation of biliary lipid secretion by mdr2 P-glycoprotein in the mouse. J. Clin. Invest. 95: 31–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morita S. Y., Kobayashi A., Takanezawa Y., Kioka N., Handa T., Arai H., Matsuo M., Ueda K. 2007. Bile salt-dependent efflux of cellular phospholipids mediated by ATP binding cassette protein B4. Hepatology. 46: 188–199 [DOI] [PubMed] [Google Scholar]
- 12.Ruetz S., Gros P. 1994. Phosphatidylcholine translocase: a physiological role for the mdr2 gene. Cell. 77: 1071–1081 [DOI] [PubMed] [Google Scholar]
- 13.Smith A. J., Timmermans-Hereijgers J. L., Roelofsen B., Wirtz K. W., van Blitterswijk W. J., Smit J. J., Schinkel A. H., Borst P. 1994. The human MDR3 P-glycoprotein promotes translocation of phosphatidylcholine through the plasma membrane of fibroblasts from transgenic mice. FEBS Lett. 354: 263–266 [DOI] [PubMed] [Google Scholar]
- 14.van Helvoort A., Smith A. J., Sprong H., Fritzsche I., Schinkel A. H., Borst P., van Meer G. 1996. MDR1 P-glycoprotein is a lipid translocase of broad specificity, while MDR3 P-glycoprotein specifically translocates phosphatidylcholine. Cell. 87: 507–517 [DOI] [PubMed] [Google Scholar]
- 15.Crawford A. R., Smith A. J., Hatch V. C., Oude Elferink R. P., Borst P., Crawford J. M. 1997. Hepatic secretion of phospholipid vesicles in the mouse critically depends on mdr2 or MDR3 P-glycoprotein expression. Visualization by electron microscopy. J. Clin. Invest. 100: 2562–2567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Groen A., Romero M. R., Kunne C., Hoosdally S. J., Dixon P. H., Wooding C., Williamson C., Seppen J., Van den Oever K., Mok K. S., et al. 2011. Complementary functions of the flippase ATP8B1 and the floppase ABCB4 in maintaining canalicular membrane integrity. Gastroenterology. 141: 1927–1937 [DOI] [PubMed] [Google Scholar]
- 17.Ruetz S., Gros P. 1995. Enhancement of Mdr2-mediated phosphatidylcholine translocation by the bile salt taurocholate. Implications for hepatic bile formation. J. Biol. Chem. 270: 25388–25395 [DOI] [PubMed] [Google Scholar]
- 18.Gottesman M. M., Fojo T., Bates S. E. 2002. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat. Rev. Cancer. 2: 48–58 [DOI] [PubMed] [Google Scholar]
- 19.Schinkel A. H., Roelofs E. M., Borst P. 1991. Characterization of the human MDR3 P-glycoprotein and its recognition by P-glycoprotein-specific monoclonal antibodies. Cancer Res. 51: 2628–2635 [PubMed] [Google Scholar]
- 20.Smith A. J., van Helvoort A., van Meer G., Szabo K., Welker E., Szakacs G., Varadi A., Sarkadi B., Borst P. 2000. MDR3 P-glycoprotein, a phosphatidylcholine translocase, transports several cytotoxic drugs and directly interacts with drugs as judged by interference with nucleotide trapping. J. Biol. Chem. 275: 23530–23539 [DOI] [PubMed] [Google Scholar]
- 21.Kino K., Taguchi Y., Yamada K., Komano T., Ueda K. 1996. Aureobasidin A, an antifungal cyclic depsipeptide antibiotic, is a substrate for both human MDR1 and MDR2/P-glycoproteins. FEBS Lett. 399: 29–32 [DOI] [PubMed] [Google Scholar]
- 22.Yoshikado T., Takada T., Yamamoto T., Yamaji H., Ito K., Santa T., Yokota H., Yatomi Y., Yoshida H., Goto J., et al. 2011. Itraconazole-induced cholestasis: involvement of the inhibition of bile canalicular phospholipid translocator MDR3/ABCB4. Mol. Pharmacol. 79: 241–250 [DOI] [PubMed] [Google Scholar]
- 23.Zhou Y., Gottesman M. M., Pastan I. 1999. Studies of human MDR1-MDR2 chimeras demonstrate the functional exchangeability of a major transmembrane segment of the multidrug transporter and phosphatidylcholine flippase. Mol. Cell. Biol. 19: 1450–1459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou Y., Gottesman M. M., Pastan I. 1999. Domain exchangeability between the multidrug transporter (MDR1) and phosphatidylcholine flippase (MDR2). Mol. Pharmacol. 56: 997–1004 [DOI] [PubMed] [Google Scholar]
- 25.Orlowski S., Martin S., Escargueil A. 2006. P-glycoprotein and ‘lipid rafts’: some ambiguous mutual relationships (floating on them, building them or meeting them by chance?). Cell. Mol. Life Sci. 63: 1038–1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klappe K., Hummel I., Hoekstra D., Kok J. W. 2009. Lipid dependence of ABC transporter localization and function. Chem. Phys. Lipids. 161: 57–64 [DOI] [PubMed] [Google Scholar]
- 27.Quinn P. J. 2010. A lipid matrix model of membrane raft structure. Prog. Lipid Res. 49: 390–406 [DOI] [PubMed] [Google Scholar]
- 28.Prpić V., Green K. C., Blackmore P. F., Exton J. H. 1984. Vasopressin-, angiotensin II-, and alpha 1-adrenergic-induced inhibition of Ca2+ transport by rat liver plasma membrane vesicles. J. Biol. Chem. 259: 1382–1385 [PubMed] [Google Scholar]
- 29.Kobayashi K., Sogame Y., Hara H., Hayashi K. 1990. Mechanism of glutathione S-conjugate transport in canalicular and basolateral rat liver plasma membranes. J. Biol. Chem. 265: 7737–7741 [PubMed] [Google Scholar]
- 30.Newman E. A., Muh S. J., Hovhannisyan R. H., Warzecha C. C., Jones R. B., McKeehan W. L., Carstens R. P. 2006. Identification of RNA-binding proteins that regulate FGFR2 splicing through the use of sensitive and specific dual color fluorescence minigene assays. RNA. 12: 1129–1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zaks-Makhina E., Li H., Grishin A., Salvador-Recatala V., Levitan E. S. 2009. Specific and slow inhibition of the kir2.1 K+ channel by gambogic acid. J. Biol. Chem. 284: 15432–15438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Amundson D. M., Zhou M. 1999. Fluorometric method for the enzymatic determination of cholesterol. J. Biochem. Biophys. Methods. 38: 43–52 [DOI] [PubMed] [Google Scholar]
- 33.Morita S. Y., Takeuchi A., Kitagawa S. 2010. Functional analysis of two isoforms of phosphatidylethanolamine N-methyltransferase. Biochem. J. 432: 387–398 [DOI] [PubMed] [Google Scholar]
- 34.Morita S. Y., Soda K., Teraoka R., Kitagawa S., Terada T. 2012. Specific and sensitive enzymatic measurement of sphingomyelin in cultured cells. Chem. Phys. Lipids. 165: 571–576 [DOI] [PubMed] [Google Scholar]
- 35.Macdonald J. L., Pike L. J. 2005. A simplified method for the preparation of detergent-free lipid rafts. J. Lipid Res. 46: 1061–1067 [DOI] [PubMed] [Google Scholar]
- 36.Abe-Dohmae S., Suzuki S., Wada Y., Aburatani H., Vance D. E., Yokoyama S. 2000. Characterization of apolipoprotein-mediated HDL generation induced by cAMP in a murine macrophage cell line. Biochemistry. 39: 11092–11099 [DOI] [PubMed] [Google Scholar]
- 37.Georges E., Bradley G., Gariepy J., Ling V. 1990. Detection of P-glycoprotein isoforms by gene-specific monoclonal antibodies. Proc. Natl. Acad. Sci. USA. 87: 152–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scheffer G. L., Kool M., Heijn M., de Haas M., Pijnenborg A. C., Wijnholds J., van Helvoort A., de Jong M. C., Hooijberg J. H., Mol C. A., et al. 2000. Specific detection of multidrug resistance proteins MRP1, MRP2, MRP3, MRP5, and MDR3 P-glycoprotein with a panel of monoclonal antibodies. Cancer Res. 60: 5269–5277 [PubMed] [Google Scholar]
- 39.Nagao K., Takahashi K., Hanada K., Kioka N., Matsuo M., Ueda K. 2007. Enhanced apoA-I-dependent cholesterol efflux by ABCA1 from sphingomyelin-deficient Chinese hamster ovary cells. J. Biol. Chem. 282: 14868–14874 [DOI] [PubMed] [Google Scholar]
- 40.Mendez A. J., Lin G., Wade D. P., Lawn R. M., Oram J. F. 2001. Membrane lipid domains distinct from cholesterol/sphingomyelin-rich rafts are involved in the ABCA1-mediated lipid secretory pathway. J. Biol. Chem. 276: 3158–3166 [DOI] [PubMed] [Google Scholar]
- 41.Brown D. A., Rose J. K. 1992. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 68: 533–544 [DOI] [PubMed] [Google Scholar]
- 42.Romanenko V. G., Fang Y., Byfield F., Travis A. J., Vandenberg C. A., Rothblat G. H., Levitan I. 2004. Cholesterol sensitivity and lipid raft targeting of Kir2.1 channels. Biophys. J. 87: 3850–3861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meyer dos Santos S., Weber C. C., Franke C., Muller W. E., Eckert G. P. 2007. Cholesterol: Coupling between membrane microenvironment and ABC transporter activity. Biochem. Biophys. Res. Commun. 354: 216–221 [Erratum. 2011. Biochem. Biophys. Res. Commun. 408: 193.] [DOI] [PubMed] [Google Scholar]
- 44.Storch C. H., Ehehalt R., Haefeli W. E., Weiss J. 2007. Localization of the human breast cancer resistance protein (BCRP/ABCG2) in lipid rafts/caveolae and modulation of its activity by cholesterol in vitro. J. Pharmacol. Exp. Ther. 323: 257–264 [DOI] [PubMed] [Google Scholar]
- 45.Troost J., Lindenmaier H., Haefeli W. E., Weiss J. 2004. Modulation of cellular cholesterol alters P-glycoprotein activity in multidrug-resistant cells. Mol. Pharmacol. 66: 1332–1339 [DOI] [PubMed] [Google Scholar]
- 46.Kimura Y., Kioka N., Kato H., Matsuo M., Ueda K. 2007. Modulation of drug-stimulated ATPase activity of human MDR1/P-glycoprotein by cholesterol. Biochem. J. 401: 597–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zastre J. A., Jackson J. K., Wong W., Burt H. M. 2008. P-glycoprotein efflux inhibition by amphiphilic diblock copolymers: relationship between copolymer concentration and substrate hydrophobicity. Mol. Pharm. 5: 643–653 [DOI] [PubMed] [Google Scholar]
- 48.Luker G. D., Pica C. M., Kumar A. S., Covey D. F., Piwnica-Worms D. 2000. Effects of cholesterol and enantiomeric cholesterol on P-glycoprotein localization and function in low-density membrane domains. Biochemistry. 39: 7651–7661 [DOI] [PubMed] [Google Scholar]
- 49.Mazzanti R., Fantappie O., Kamimoto Y., Gatmaitan Z., Gentilini P., Arias I. M. 1994. Bile acid inhibition of P-glycoprotein-mediated transport in multidrug-resistant cells and rat liver canalicular membrane vesicles. Hepatology. 20: 170–176 [DOI] [PubMed] [Google Scholar]
- 50.Landry Y. D., Denis M., Nandi S., Bell S., Vaughan A. M., Zha X. 2006. ATP-binding cassette transporter A1 expression disrupts raft membrane microdomains through its ATPase-related functions. J. Biol. Chem. 281: 36091–36101 [DOI] [PubMed] [Google Scholar]
- 51.Cerf E., Gasper R., Belani J. D., Rychnovsky S., Chang X. B., Buyse F., Ruysschaert J. M. 2007. Multidrug resistance protein 1 is not associated to detergent-resistant membranes. Biochem. Biophys. Res. Commun. 355: 1025–1030 [Erratum. 2008. Biochem. Biophys. Res. Commun. 366: 605.] [DOI] [PubMed] [Google Scholar]
- 52.Kremmer T., Wisher M. H., Evans W. H. 1976. The lipid composition of plasma membrane subfractions originating from the three major functional domains of the rat hepatocyte cell surface. Biochim. Biophys. Acta. 455: 655–664 [DOI] [PubMed] [Google Scholar]
- 53.Eckhardt E. R., Moschetta A., Renooij W., Goerdayal S. S., van Berge-Henegouwen G. P., van Erpecum K. J. 1999. Asymmetric distribution of phosphatidylcholine and sphingomyelin between micellar and vesicular phases. Potential implications for canalicular bile formation. J. Lipid Res. 40: 2022–2033 [PubMed] [Google Scholar]
- 54.Alvaro D., Cantafora A., Attili A. F., Ginanni Corradini S., De Luca C., Minervini G., Di Biase A., Angelico M. 1986. Relationships between bile salts hydrophilicity and phospholipid composition in bile of various animal species. Comp. Biochem. Physiol. B. 83: 551–554 [DOI] [PubMed] [Google Scholar]
- 55.Vance J. E. 2008. Phosphatidylserine and phosphatidylethanolamine in mammalian cells: two metabolically related aminophospholipids. J. Lipid Res. 49: 1377–1387 [DOI] [PubMed] [Google Scholar]
- 56.Pennings M., Hildebrand R. B., Ye D., Kunne C., Van Berkel T. J., Groen A. K., Van Eck M. 2007. Bone marrow-derived multidrug resistance protein ABCB4 protects against atherosclerotic lesion development in LDL receptor knockout mice. Cardiovasc. Res. 76: 175–183 [DOI] [PubMed] [Google Scholar]
- 57.Nagao K., Zhao Y., Takahashi K., Kimura Y., Ueda K. 2009. Sodium taurocholate-dependent lipid efflux by ABCA1: effects of W590S mutation on lipid translocation and apolipoprotein A-I dissociation. J. Lipid Res. 50: 1165–1172 [DOI] [PMC free article] [PubMed] [Google Scholar]