Abstract
Rhus verniciflua Stokes (RVS) has been used as a traditional herbal medicine for its various biological activities including anti-adipogenic effects. Activity-guided separation led to the identification of the anti-adipogenic functions of butein. Butein, a novel anti-adipogenic compound, robustly suppressed lipid accumulation and inhibited expression of adipogenic markers. Molecular studies showed that activated transforming growth factor-β (TGF-β) and suppressed signal transducer and activator of transcription 3 (STAT3) signaling pathways were mediated by butein. Analysis of the temporal expression profiles suggests that TGF-β signaling precedes the STAT3 in the butein-mediated anti-adipogenic cascade. Small interfering RNA-mediated silencing of STAT3 or SMAD2/3 blunted the inhibitory effects of butein on adipogenesis indicating that an interaction between two signaling pathways is required for the action of butein. Upon butein treatments, stimulation of TGF-β signaling was still preserved in STAT3 silenced cells, whereas regulation of STAT3 signaling by butein was significantly impaired in SMAD2/3 silenced cells, further showing that TGF-β acts upstream of STAT3 in the butein-mediated anti-adipogenesis. Taken together, the present study shows that butein, a novel anti-adipogenic compound from RVS, inhibits adipocyte differentiation through the TGF-β pathway followed by STAT3 and peroxisome proliferator-activated receptor γ signaling, further implicating potential roles of butein in TGF-β- and STAT3-dysregulated diseases.
Keywords: adipocyte differentiation, peroxisome proliferator-activated receptor γ, butein, obesity, Rhus verniciflua Stokes, C3H10T1/2, 3T3-L1, resveratrol, genistein
Adipocyte differentiation is determined by a number of transcriptional cascades, including those of the peroxisome proliferator-activated receptor γ (PPARγ), CCAAT/enhancer binding protein (C/EBP) family, and adipocyte determination and differentiation-dependent transcription factors (1). Importantly, loss and gain of function studies have demonstrated that PPARγ is the master regulator of adipocyte differentiation (2). In addition to its roles in adipocyte differentiation, PPARγ also plays a role in glucose homeostasis as demonstrated by treatment of type 2 diabetes with thiazolidinediones, which are a class of PPARγ ligands (3). Therefore, proper regulation of PPARγ can provide new strategies against obesity and its related diseases, including diabetes (4).
Natural products to prevent obesity have been widely investigated (5). Bioactive compounds identified from natural products have also been extensively studied for their effects on obesity and its underlying molecular mechanisms (6). Mechanistic studies have indicated that resveratrol from red grapes activates sirtuin 1 (SIRT1) and PPARγ coactivator 1-α (7, 8). Epigallocatechin gallate inhibits adipocyte proliferation by decreasing levels of cdk2, cyclin D1 (CycD1), and activated Erk1/2 (9) and has also been shown to increase the activity of AMPK and fatty acid oxidation (10). The administration of anthocyanins from blueberries to mice decreases fat mass, serum triglycerides, cholesterol, and leptin levels (11). Curcumin from Curcuma longa modulates angiogenesis by decreasing angiogenic factors such as vascular endothelial growth factor and fibroblast growth factor and alters lipid metabolism by suppressing the adipogenic factors PPARγ and C/EBPα (12, 13). Therefore, identification of anti-adipogenic compounds from natural products can provide therapeutic and preventive strategies for the development of new applications.
Rhus verniciflua Stokes (RVS) has been used as a food additive and traditional medicine mainly in eastern Asia, and its extracts have been shown to have anti-cancer, anti-oxidant, anti-platelet, anti-inflammatory, anti-fibrogenic, anti-apoptotic, and anti-obese effects (14–18). Several bioactive compounds such as sulfuretin, fisetin, fustin, kaempferol, gallic acid, quercetin, protocatechuic acid, and butein have been isolated from RVS and may mediate numerous actions by RVS extracts (16, 19). Therefore, RVS extracts are potential sources from which to isolate anti-adipogenic bioactive compounds. This study isolated bioactive compounds from RVS and investigated their molecular effects on adipocyte differentiation and PPARγ expression.
MATERIALS AND METHODS
Extraction and isolation
Detoxified RVS extracts were purchased from Chamotnara (Wonju, Korea) and were filtered, dried, and extracted with methanol. The filtrate was evaporated in a rotary vacuum evaporator and freeze-dried in a Heto FD3 at −50°C to obtain the extract (10 g). The methanol extract was further fractionated with ethanol, n-hexane, dichloromethane (DCM), ethyl acetate, and n-butanol, in that order. The ethanol fractions were then separated by silica gel chromatography to produce six subfractions. Each subfraction was then tested for biological activity. Selected subfractions were further chromatographed on an RP-C18 silica gel (230–400 mesh, 200 g) column as described in (20) and eluted with methanol-water (2:3 → 1:0, gradient system) to yield six fractions (A–F). Fraction D (560 mg) was subjected to silica gel column chromatography (CC) (CHCl3-methanol, 17:1) to yield six subfractions (D1–D6). Subfraction D3 (87 mg) was purified by preparative reversed-phase high-performance liquid chromatography (HPLC) using a solvent system of 48% methanol to afford compound M1 (10 mg). Subfraction E (1.9 g) was subjected to CC (CHCl3-methanol, 15:1) to obtain five subfractions (E1–E5). Subfraction E1 (50 mg) was purified by preparative reversed-phase HPLC using a solvent system of 55% methanol to furnish compounds M2 (3 mg) and M3 (3 mg). Compounds M4 (25 mg) and M5 (6 mg) were obtained from subfraction E3 (300 mg) by separation on preparative reversed-phase HPLC using a solvent system of 60% methanol. Subfraction E5 (560 mg) was separated further on an RP-C18 silica gel (230–400 mesh, 100 g) column eluted with methanol-water (2:3 → 1:0, gradient system) to give four subfractions (E511–E514). Subfractions E511 (110 mg) and E514 (45 mg) were purified by preparative reversed-phase HPLC to afford compounds M6 (4 mg, 100% water) and M7 (25 mg, 60% methanol), respectively. The isolated compounds were identified based on 1H nuclear magnetic resonance (NMR) and 13C NMR spectra and by comparison with published literature. Butein was purchased from Sigma (St. Louis, MO) and Tokyo Chemical Industry (Tokyo, Japan).
Cell culture
Mouse C3H10T1/2 and 3T3-L1 cell lines were purchased from the American Type Culture Collection (Rockville, MD). Cells were cultured in Dulbecco's modified Eagle's medium (DMEM) (Hyclone, Logan, UT) supplemented with 10% fetal bovine serum (FBS) (Hyclone) and antibiotics (penicillin and streptomycin, Hyclone) and were incubated at 37°C under 5% CO2 as described previously (21). A subculture of mouse 3T3-L1 preadipocytes was supplemented with 10% fetal calf serum (Hyclone). Mouse embryonic fibroblasts (MEFs) were freshly isolated from E13.5-E14.5 embryos as previously described (22). For isolation of primary bone marrow cells, the tibia and femur were dissected from 6–8-week-old Sprague Dawley rats (Orient Co. Ltd., Young-In, Korea). Bone marrow cells were obtained by inserting syringes with 27 gauge needles into the end of the bone and flushing strongly with DMEM containing 15% FBS and 1× penicillin/streptomycin. The cells were then cultured at 37°C in a 5% CO2 atmosphere, and the medium was changed twice weekly. The cells were seeded in 6-well plates until reaching 70–80% confluence. After the cells reached confluence, differentiation was initiated. For adipocyte differentiation of 3T3-L1, cells were seeded at 5 × 104/ml in 6-well tissue culture plates, and then confluent cells were incubated for 2 days in DMEM supplemented with 10% FBS, 1 μM dexamethasone (Sigma, St. Louis, MO), 0.5 mM isobutyl-1-methylxanthine (Sigma), and 5 μg/ml insulin (Sigma). Cells were refreshed with DMEM containing 10% FBS and 5 μg/ml insulin every 2 days. Additionally, C3H10T1/2, MEFs, and bone marrow cells were treated with 20 nM GW7845 (a PPARγ ligand, kindly provided by the Tontonoz Lab) for adipocyte differentiation. At 6–8 days after differentiation, cells were fixed with 4% paraformaldehyde in PBS at room temperature for 4 h, then stained with 0.5% Oil Red O (Sigma) in a mixture of isopropanol and distilled water at a 3:2 ratio for 45 min. Cells were washed with water, then photographed under a microscope. To quantify intracellular triglyceride content, stained cells from at least two independent experiments were resolved with isopropanol and measured with a spectrophotometer at 520 nm.
Expression analysis
Total RNA was isolated from 3T3-L1 and C3H10T1/2 cells using TRIzol reagent (Invitrogen, Carlsbad, CA). First-strand cDNA (cDNA) was synthesized from 0.5 μg of total RNA using the AMV Reverse Transcription System kit (Promega, Madison, WI) with random primers. After cDNA synthesis, the final 25 μl volume of the amplification mixture containing Power SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA), primers, and cDNA was subjected to 40 amplification cycles of polymerase chain reaction (PCR) using a Thermal Cycler Dice (Takara, Shiga, Japan) according to the manufacturer's instructions. Expression was normalized to 36B4. All real-time PCRs were performed at least twice. The oligonucleotide primer (Integrated DNA Technologies, San Diego, CA) sequences used for PCR were as follows: peroxisome proliferator-activated receptor γ (PPARγ), PPARγ F, 5′-CCATTCTGGCCCACCAAC-3′ and PPARγ R, 5′-AATGCGAGTGGTCTTCCATCA-3′; adipocyte binding protein 2 (aP2), aP2 F, 5′-CACCGCAGACGACAGGAAG-3′ and aP2 R, 5′-GCACCTGCACCAGGGC-3′; cluster of differentiation 36 (CD36), CD36 F, 5′-GGCCAAGCTATTGCGACAT-3′ and CD36 R, 5′-CAGATCCGAACACAGCGTAGA-3′ lipoprotein lipase (LPL), LPL F, 5′-GTGGCCGAGAGCGAGAAC-3′ and LPL R, 5′-AAGAAGGAGTAGGTTTTATTTGTGGAA-3′; acidic ribosomal phosphoprotein P0 (36B4), 36B4 F, 5′-AGATGCAGCAGATCCGCAT-3′ and 36B4 R, 5′-GTTCTTGCCCATCAGCACC-3′. Δcycle threshold (CT) was used to calculate the differences between the target CT value and the control (36B4) for each sample: ΔCT = CT (target) − CT (control). The relative expression level was calculated using 2−ΔCT.
Western blotting was performed as previously described (23). Cells were harvested in 200 μl of sample buffer and heated at 100°C for 10 min. The proteins were separated by 10% SDS-PAGE and electrophoretically transferred to nitrocellulose membranes (Amersham Biosciences, Piscataway, NJ). The membranes were blocked with blocking buffer (5% skim milk in TBS, 0.1% Tween 20) for 30 min at room temperature and treated with PPARγ (sc-7196; Santa Cruz Biotechnology, Santa Cruz, CA) or β-actin antibodies (A5316; Sigma), followed by incubation with horseradish peroxidase-conjugated secondary antibody (Zymed Laboratories, San Francisco, CA). Antibody binding was detected on X-ray film using Enhanced Chemiluminescence Western Blotting Detection Reagent (Amersham Biosciences).
Knockdown studies
Scramble control, signal transducer and activator of transcription 3 (STAT3)-specific, and SMAD2/3-specific oligos were synthesized by Genolution Pharmaceuticals, Inc. (Seoul, Korea). Two independent small interfering RNAs (siRNAs) were used to silence STAT3, SMAD2, and SMAD3 expression. Sense sequences of STAT3-specific siRNA were as follows: Stat3 #1: 5′-GAGUUGAAUUAUCAGCUUAUU-3′; Stat3 #2: 5′-CAUCAAUCCUGUGGUAUAAUU-3′. Sense sequences of SMAD2-specific siRNA were as follows: SMAD2 #1: 5′-GUUCAAUCGCAUACUAUGAUU-3′; SMAD2 #2: 5′-CGAAUGUGCACCAUAAGAAUU-3′. Sense sequences of SMAD3-specific siRNA were as follows: SMAD3 #1: 5′-GGAUGAAGUGUGUGUAAAUUU-3′; SMAD3 #2: 5′-GUGAAGAAGCUCAAGAAGAUU-3′. The sense sequence of control nonspecific scramble RNA was 5′-CCUCGUGCCGUUCCAUCAGGUAGUU-3′. Cells plated at a density of 1 × 105 cells per well in a 6-well plate were transfected with 30 pmol of scramble RNA, STAT3-specific siRNA, or SMAD2/3 specific siRNAs (two independent combinations of SMAD2 #1 and SMAD3 #1 or SMAD2 #2 and SMAD3 #2) using RNAiMAX (Invitrogen), as previously described (22). Cells were treated with siRNA for 6 h, and then the medium was exchanged. After 48 h, cells were processed using differentiation protocols. Transfection was carried out in duplicated wells and repeated three times.
Statistical analysis
Data are presented as the mean ± SEM. Differences in gene expression and lipid accumulation were analyzed using a two-tailed unpaired Student's t-test. Statistical significance was defined as P < 0.05. All computations were performed using statistical analysis software (PASW Statistics 17).
RESULTS
RVS ethanol-fractionated extracts inhibit adipogenesis in C3H10T1/2 and 3T3-L1 cells
Previous studies showed that RVS extracts prevented diet-induced obesity in mice (18). To identify the bioactive compounds responsible for anti-adipogenic effects, C3H10T1/2 cells were differentiated into adipocytes and treated with 10, 25, and 50 μg/ml of the extracts. The extracts dose dependently inhibited lipid accumulation in C3H10T1/2 cells (supplementary Fig. I). The extracts were further separated successively into ethanol, hexane, DCM, ethyl acetate, n-butanol, and water fractions to enrich the bioactive compounds potentially mediating the anti-adipogenic effects of RVS (supplementary Fig. IC). Ethanol fractions were the most potent inhibitors of lipid accumulation in C3H10T1/2 cells. In addition, ethanol fractions consistently suppressed adipocyte differentiation of preadipocyte 3T3-L1 cells (supplementary Fig. ID). Thus, this study focused on the ethanol fractions to identify bioactive compounds that mediate the anti-adipogenic actions of RVS.
Isolation of single compounds from activity-guided fractionation of the ethanol-fractionated extracts
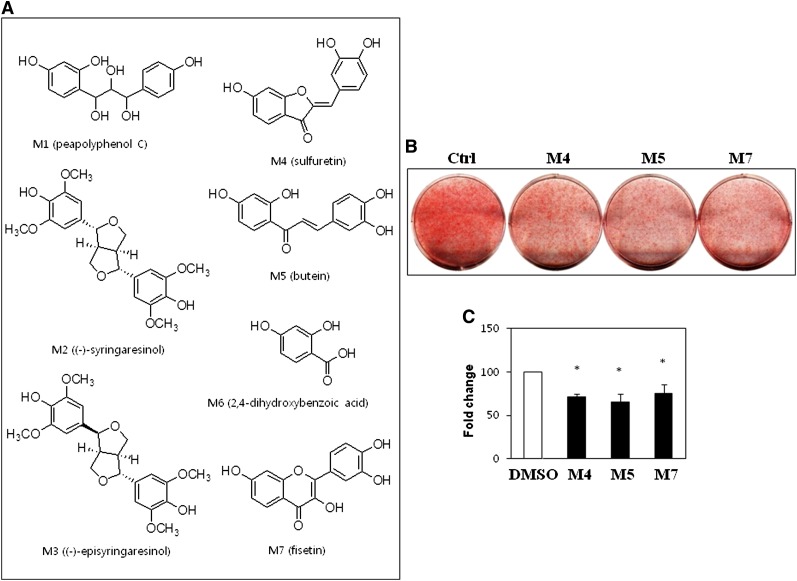
The ethanol fractions were further divided by silica gel chromatography to produce six subfractions, and active subfraction was further separated by reverse-phase HPLC to produce the seven compounds as shown in Fig. 1A. Some of these compounds, such as sulfuretin (M4), butein (M5), and fisetin (M7), had been previously identified from RVS (15), whereas others, such as peapolyphenol C (M1), syringaresinol (M2), and dihydroxybenzoic acid (M6), had not been previously isolated. Fisetin has been reported to have lipolytic activity in adipocytes (24). In particular, a recent report suggested that sulfuretin and fisetin possess anti-adipogenic activity in 3T3-L1 cells (25). Therefore, successful isolation of the anti-adipogenic compounds sulfuretin and fisetin from RVS validates this approach to identify anti-adipogenic compounds.
Fig. 1.
Structures and anti-adipogenic activities of single compounds isolated from RVS. A: Structures of single compounds isolated from ethanol-fractionated extracts of RVS. M1, peapolyphenol C; M2, syringaresinol; M3, episyringaresinol; M4, sulfuretin; M5, butein; M6, 2,4-dihydroxybenzoic acid; M7, fisetin. B: Identification of anti-adipogenic compounds. C3H10T1/2 cells were treated with 10 μM of the compounds, and adipocytes were induced to differentiate for 7 days followed by Oil Red O staining. C: The effects of single compounds on lipid accumulation in C3H10T1/2 cells. Lipid accumulation was quantified by measuring the extracted dye at 520 nm. Data shown represent the mean ± SEM of three independent experiments. Statistical significance was determined relative to a control by the Student's t-test (*P < 0.05). Ctrl, control.
Anti-adipogenic actions of the compounds isolated from ethanol-fractionated RVS extracts
Isolation of the previously known anti-adipogenic compounds sulfuretin and fisetin prompted an investigation of other compounds isolated from RVS on adipocyte differentiation. C3H10T1/2 cells were treated with an adipogenic cocktail and 10 μM of each compound to evaluate anti-adipogenic activity. In line with a previous report, both M4 (sulfuretin) and M7 (fisetin) suppressed lipid accumulation. Interestingly, another compound M5 (butein) also suppressed lipid accumulation and morphological changes during adipocyte differentiation (Fig. 1B, C). Lipid accumulation and expression of the adipogenic master regulator PPARγ decreased gradually following treatment with 10–40 μM of M4, M5, and M7 (supplementary Fig. II). These results suggest that the combination of these anti-adipogenic compounds, sulfuretin, fisetin, and butein, could mediate the anti-adipogenic actions of RVS.
Identification of butein as a novel anti-adipogenic compound
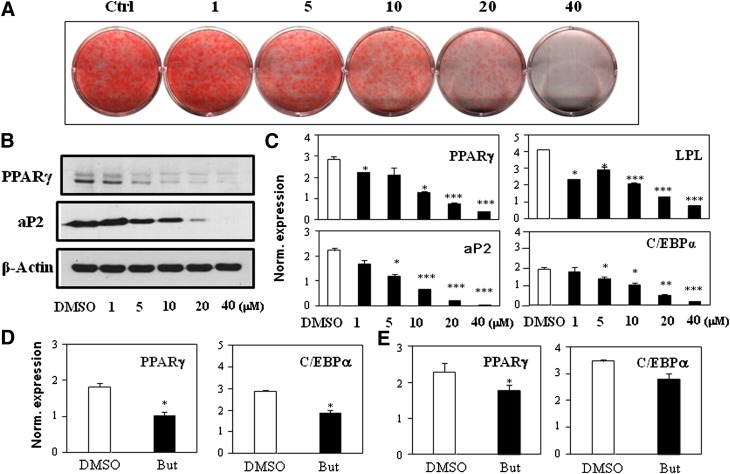
Interestingly, butein has not been reported as an anti-adipogenic compound. Therefore, to further verify the effects of butein in adipogenesis, commercially available butein was also tested. Similar to the observed effects of M5 (butein) isolated from RVS, commercial butein consistently inhibited adipogenesis in C3H10T12 cells (supplementary Fig. III). In addition, butein from another company had very similar anti-adipogenic effects. To further verify anti-adipogenic activity, preadipocyte 3T3-L1 cells were differentiated into adipocytes in the presence of butein. Butein consistently inhibited adipocyte differentiation and lipid accumulation in 3T3-L1 cells (Fig. 2A). Protein expression of the adipogenic master regulator PPARγ and its target aP2 was also dose dependently inhibited by butein (Fig. 2B). Furthermore, mRNA expression of PPARγ and its target genes, including LPL, aP2, and C/EBPα, was also suppressed by butein (Fig. 2C). Freshly isolated MEFs or rat primary bone marrow cells were also differentiated into adipocytes. Consistent inhibitory effects of butein were observed in the MEFs and bone marrow cells as assessed by adipocyte marker expression (Fig. 2D, E). Taken together, lipid accumulation and expression analysis for adipocyte markers in multiple cells demonstrated the inhibitory activity of butein and further verified the chemical identity of isolated butein.
Fig. 2.
Butein inhibits adipogenesis in 3T3-L1 cells and MEFs. A: Commercially available butein suppressed lipid accumulation in 3T3-L1 cells in a dose-dependent manner. B: 3T3-L1 cells were differentiated and treated with butein for 7 days, and protein expression of PPARγ, aP2, and β-actin was measured by Western blot analysis. C: 3T3-L1 cells were differentiated and treated with various doses of butein, and mRNA expression of PPARγ, LPL, aP2, and C/EBPα was measured by real-time PCR. D, E: Butein inhibited adipogenesis of freshly isolated MEFs and primary bone marrow cells. MEFs (D) or bone marrow cells (E) were differentiated and treated with 10 μM of butein for 7 days and mRNA expression of PPARγ and C/EBPα was measured by real-time PCR. Data shown represent the mean ± SEM from three independent experiments. Statistical significance was determined relative to a control by the Student's t-test (*P < 0.05; **P < 0.005; ***P < 0.0005). Ctrl, control; Norm., normalized; But, butein.
Butein is a potent anti-adipogenic compound
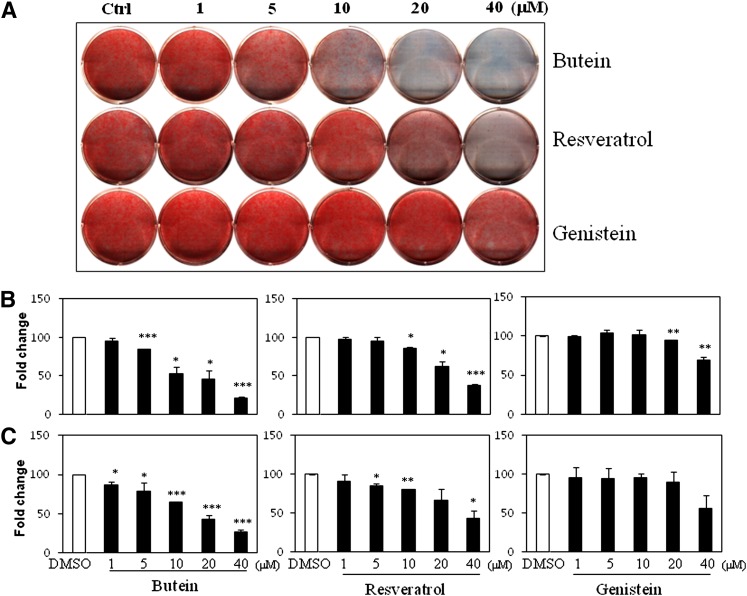
Butein was further compared with the well-known anti-adipogenic compounds genistein and resveratrol (6–8, 10). As expected, both resveratrol and genistein dose dependently inhibited lipid accumulation in C3H10T1/2 cells. Resveratrol at >20 μM and genistein at 40 μM clearly suppressed lipid accumulation (Fig. 3A, B). Interestingly, butein was more effective than genistein and resveratrol at controlling adipogenesis. Butein (10 μM) strongly inhibited lipid accumulation with stronger activity at higher doses, whereas 10 μM of resveratrol and genistein did not suppress lipid accumulation, which was similar to the findings of a previous report (26). 3T3-L1 cells also differentiated in the presence of an adipogenic cocktail with various doses of butein, resveratrol, or genistein. The consistent potent anti-adipogenic effects of butein were also observed in 3T3-L1 cells (Fig. 3C). Furthermore, adipocyte marker expression profiles consistently confirmed the effects of butein during adipogenesis in C3H10T1/2 cells (supplementary Fig. IV). PPARγ, aP2, and LPL mRNA expression decreased by approximately 50% following treatment with 5 μM of butein, and was almost completely suppressed at 20 μM. In contrast, resveratrol and genistein suppressed adipocyte marker expression by 50% at 20 and 40 μM, respectively. These data demonstrate that butein is a novel and potent anti-adipogenic compound that warrants further testing for its effects on adipogenesis.
Fig. 3.
Butein is a potent novel anti-adipogenic compound. A: C3H10T1/2 cells were differentiated in the presence of various concentrations (1, 5, 10, 20, and 40 μM) of butein, genistein, and resveratrol for 7 days. B: Differentiated cells were stained with Oil Red O, and lipid accumulation was quantified by measuring the extracted dye at 520 nm. C: 3T3-L1 cells were differentiated with DMI and various doses of butein, resveratrol, and genistein for 7 days, and lipid accumulation was quantified. Data shown represent the mean ± SEM from three or more independent experiments. Statistical significance was determined relative to a control by the Student's t-test (*P < 0.05; **P < 0.005; ***P < 0.0005). Ctrl, control; DMI, dexamethasone, IBMX, insulin.
Early stage adipocyte differentiation is critical for the inhibitory actions of butein
3T3-L1 cells were stimulated with an adipogenic cocktail and treated with 10 μM of butein at various time points to elucidate the critical time frame during differentiation processes affected by butein. 3T3-L1 cells treated with butein from days 0–2, 2–4, 0–4, 4–6, and 0–6 of adipocyte differentiation were compared with controls (supplementary Fig. V). Cells treated with butein from days 0–2, 0–4, and 0–6 showed significant inhibition of adipocyte differentiation compared with controls. Treatment of butein from days 0–2 and days 0–6 in C3H10T1/2 cells also exhibited similar inhibitory effects (supplementary Fig. V). However, cells exposed to butein from days 2–4 and days 4–6, at the later stages of adipocyte differentiation, showed little inhibition similar to vehicle control (DMSO) treatments in both cell types. These results indicate that butein acts mostly at the early stage (days 0–2) of adipocyte differentiation.
STAT3 mediates the anti-adipogenic effects of butein
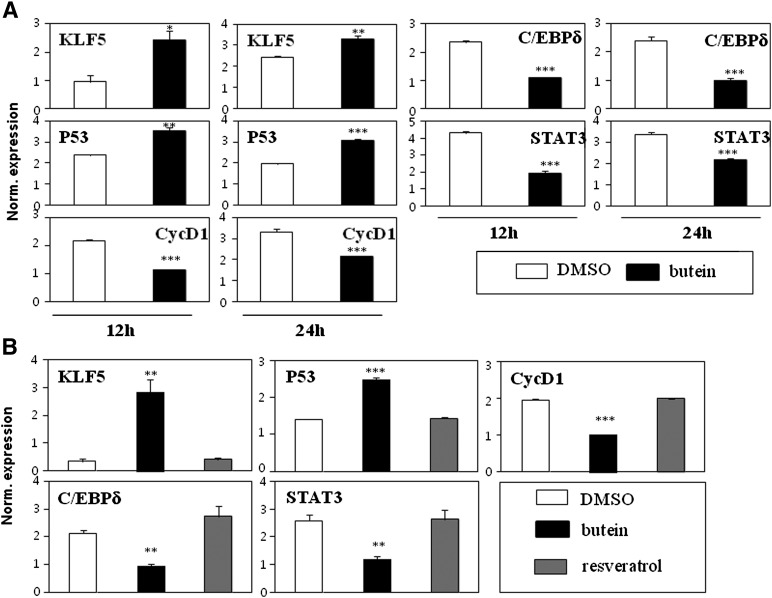
Adipocyte differentiation is dictated by transcriptional cascades involving PPARγ (1). Numerous signaling pathways play important roles in the regulation of PPARγ, thereby regulating critical adipocyte differentiation events. Recent studies performed using cancer cells showed that butein inhibits cancer growth through the suppression of STAT3 activation in vitro and in vivo (27, 28). Intriguingly, STAT3 knockdown by siRNA demonstrated that STAT3 is required for adipocyte differentiation in 3T3-L1 cells (29). Therefore, we investigated the possibility that STAT3 would mediate the anti-adipogenic effects of butein at the early stages of adipocyte differentiation. 3T3-L1 cells were treated with butein for 12 and 24 h, and the expression of several known STAT3-regulated genes was analyzed. STAT3 is known to regulate several target genes, including KLF5, P53, CycD1, C/EBPδ, and STAT3 (30). The known STAT3-downregulated genes KLF5 and P53 were induced by butein. Conversely, STAT3-induced genes, including CycD1, C/EBPδ, and STAT3 were suppressed by butein (Fig. 4A). STAT3 target genes were also similarly regulated by butein in C3H10T1/2 cells, but the expression of these genes was not affected by resveratrol, further suggesting that the specific action of butein differs from that of resveratrol in preadipocytes (Fig. 4B). These data agree with the hypothesis that butein is a regulator of STAT3 at the early stages of adipocyte differentiation.
Fig. 4.
STAT3 is selectively regulated by butein in adipocyte differentiation. A: Expression of known STAT3 target genes was regulated by butein in 3T3-L1 cells. 3T3-L1 cells were treated with butein for 12 and 24 h, and the expression of STAT3 target genes was assessed. Expression of KLF5 and P53, which are genes known to be downregulated by STAT3, were induced by butein. C/EBPδ, CycD1, and STAT3, which are known STAT3-induced genes, were suppressed by treatment with butein. B: Expression of STAT3 target genes was regulated by treatment with butein, but not by resveratrol, in C3H10T1/2 cells. C3H10T1/2 cells were treated with butein or resveratrol for 12 h and the expression of STAT3 target genes was assessed. STAT3 target genes were regulated by butein, whereas STAT3 target genes were not affected by resveratrol treatment. Data shown represent the mean ± SEM from three or more independent experiments. Statistical significance was determined relative to a control by the Student's t-test (*P < 0.05; **P < 0.005; ***P < 0.0005). Norm., normalized.
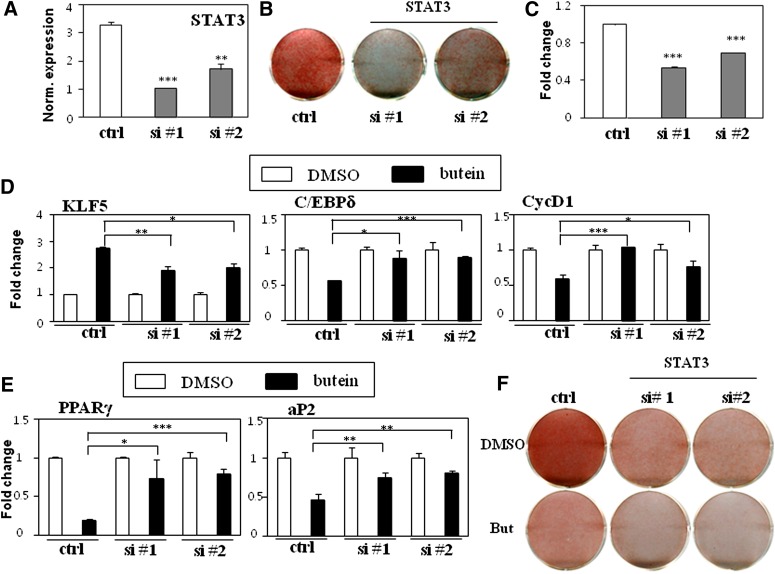
To further evaluate the role of STAT3 and the inhibitory effects of butein on adipocyte differentiation, siRNA-mediated STAT3 silencing was performed. Two independent and validated STAT3 siRNAs that have been shown to successively suppress STAT3 expression were transiently transfected into 3T3-L1 cells (29). Consistent with the results of previous studies, adipocyte differentiation was significantly impaired by STAT3 inhibition when the two independent STAT3 siRNAs were introduced as compared with the control nonspecific scramble siRNA (Fig. 5AndashC). STAT3-specific siRNA transfected cells compared with control siRNA transfected cells blunted the effects of butein on the expression of STAT3 target genes such as KLF5, C/EBPδ, and CycD1 (Fig. 5D). Treatment with butein consistently reduced PPARγ and aP2 expression in control siRNA transfected cells, whereas almost no additional butein effects were observed in STAT3 siRNA transfected cells (Fig. 5E and supplementary Fig. VI). Furthermore, STAT3-specific siRNA transfected cells compromised the inhibitory effects of butein on lipid accumulation (Fig. 5F). Taken together, these data demonstrate that butein exerts its anti-adipogenic effects at least in part by suppressing STAT3 activation.
Fig. 5.
Butein inhibits adipocyte differentiation through STAT3 signaling. A: Transient transfection of 3T3-L1 cells with two independent STAT3 siRNAs (si #1 or si #2) reduced STAT3 expression. B, C: Silencing the expression of STAT3 impaired adipocyte differentiation. Transient transfection of 3T3-L1 cells with two independent STAT3 siRNAs inhibited adipocyte differentiation as assessed by Oil Red O staining after 6 days (B) and quantification of lipid accumulation by measuring the extracted dye at 520 nm (C). D: Knockdown of STAT3 attenuated the effects on expression of STAT3 target genes by butein. Expression of STAT3 target genes KLF5, C/EBPδ, and CycD1 in control and siRNA-transfected cells treated with DMSO or butein (10 μM) for 12 h was measured by real-time PCR. E, F: Silencing expression of STAT3 blunted butein's inhibitory effects on adipocyte differentiation in 3T3-L1 cells. E: Knockdown of STAT3 attenuated the effects of butein on expression of adipocyte markers. Expression of PPARγ and its target gene aP2 in control nonspecific siRNA- and specific siRNA-transfected cells treated with DMSO or butein (10 μM) for 6 days was measured by real-time PCR. F: Silencing STAT3 compromised butein's inhibitory effects on lipid accumulation in 3T3-L1 cells. Control and siRNA-transfected cells were treated with DMSO or butein (10 μM) and then differentiated for 6 days. Differentiated cells were stained with Oil Red O. Data shown represent the mean ± SEM from three independent experiments. Statistical significance was determined relative to a control by the Student's t-test (*P < 0.05; **P < 0.005; ***P < 0.0005). Ctrl, control; Norm., normailzed; But, butein.
Butein stimulates transforming growth factor-β pathway
To gain further insight into the mechanism of butein's effects in the regulation of STAT3 activity, previously known signaling pathways involved in adipogenesis including Wnt, Notch, BMP, and transforming growth factor-β (TGF-β) were examined. 3T3-L1 cells were treated with butein and the expression patterns of Wnt, TGF-β, Notch, and BMP target genes were investigated. Butein did not consistently affect expression of target genes for Wnt, Notch, and BMP pathways, whereas TGF-β regulated target genes SMAD7, PAI-1, and ID1 were upregulated by treatment with butein (Fig. 6A). This finding is similar to a previous study that the anti-inflammatory cytokine TGF-β1 inhibits the effects of IL-6 by suppressing STAT3 activity in prostate epithelial cells (31).
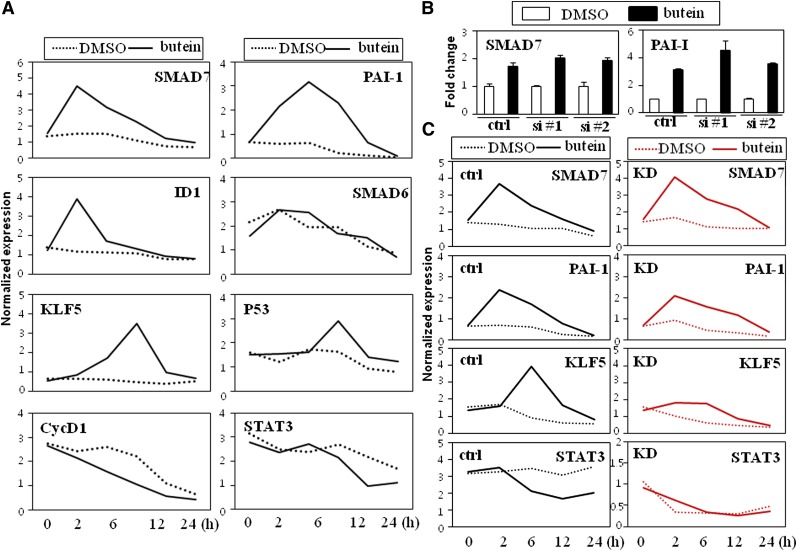
Fig. 6.
Stimulatory effects on the TGF-β signaling pathway precede the regulation of STAT3 activity in the butein-mediated anti-adipogenic cascade. A: Expression profiles of STAT3 and TGF-β target genes regulated by butein in 3T3-L1 cells. Cells were treated with butein for the various time points indicated and expression of TGF-β target genes (SMAD7, PAI-1, and ID1) and STAT3 target genes (KLF5, P53, CycD1, and STAT3) were measured by real-time PCR. Expression of SMAD6 is shown to demonstrate the specificity of butein. B, C: Knockdown of STAT3 attenuated the expression of STAT3 target genes regulated by butein but not TGF-β target genes. B: Silencing STAT3 (si #1 or si #2) maintained the stimulatory effects of butein on TGF-β target genes SMAD7 and PAI-1. C: Nonspecific control (ctrl) and STAT3 knockdown (KD, si #1) cells were cultured in the presence of DMSO or butein (10 μM) for the various time points indicated and expression profiles of TGF-β and STAT3 target genes were measured. Knockdown of STAT3 blunted butein's inhibitory effects on expression of STAT3 target genes (KLF5 and STAT3) but preserved the stimulatory effects on expression of TGF-β target genes (SMAD7 and PAI-1). Expression of TGF-β and STAT3 target genes was measured by real-time PCR. Data shown represent the mean ± SEM from three independent experiments. Statistical significance was determined relative to a control by the Student's t-test (*P < 0.05; **P < 0.005; ***P < 0.0005). Ctrl, control; si, small interfering.
To delineate the sequential expression of butein response genes, temporal expression profiles of TGF-β and STAT3 target genes were investigated. Butein stimulated expression of SMAD7 and ID1 as early as 1 h with a stronger response at 2 h. Stimulatory effects on expression of SMAD7 by butein seemed to be specific since expression of SMAD6 was not affected by butein. Another TGF-β regulated gene, PAI-1, was upregulated at 2 h with peak expression at 4 h. In an identical experimental setting, expression profiles of STAT3 target genes were also compared with the temporal regulation of TGF-β by butein. STAT3 target genes such as KLF5, P53, Cyclin D1, and STAT3 were not affected by butein at 2 h but regulated only after 6 h with a peak at 8–12 h (Fig. 6A). These expression profiles show that butein affects TGF-β signaling and further suggest that the stimulatory effects on the TGF-β pathway precede the regulation of STAT3 signaling by butein.
The TGF-β pathway may act upstream of STAT3 activity upon treatment of preadipocytes with butein
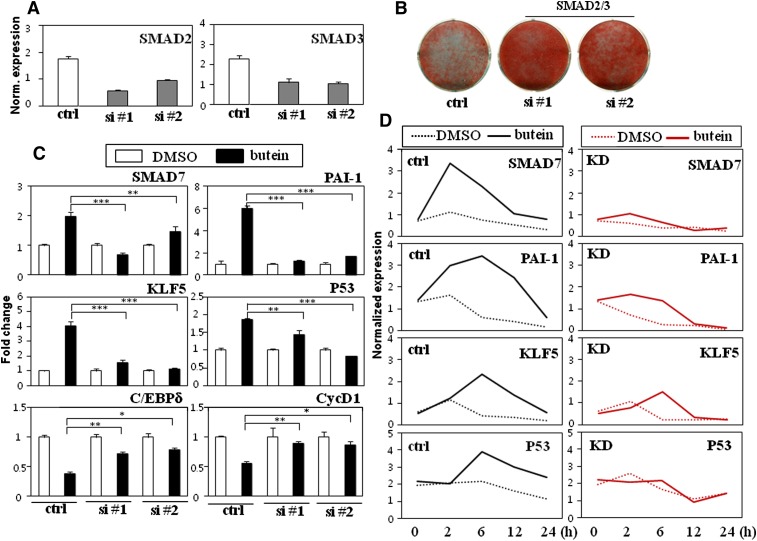
Temporal regulation of TGF-β and STAT3 target genes by butein suggest that the anti-adipogenic effects of butein are due to TGF-β acting upstream of STAT3. To further validate this hypothesis, STAT3-specific siRNA and control nonspecific siRNA were transfected into 3T3-L1 cells and treated with butein followed by expression analysis of TGF-β target genes. As expected, expression of STAT3 target genes by butein was impaired in STAT3 silenced cells (Fig. 6C). In contrast, induced expression of TGF-β target genes was not prevented in either control or STAT3 siRNA transfected cells in the presence of butein (Fig. 6B, C), suggesting that TGF-β may act earlier than STAT3 in the butein-mediated anti-adipogenic cascade. To further test this, the TGF-β pathway was suppressed by silencing both SMAD2 and SMAD3 transcription factors. SMAD2/3s are directly activated by TGF-β and are the core of the TGF-β pathway in mediating the TGF-β response (32). Accordingly, silencing of both SMAD2 and SMAD3 was previously shown to successfully block the TGF-β pathway (33). In line with previous studies, expression of two independent sets of siRNA against SMAD2 and SMAD3 (SMAD2/3) impaired lipid accumulation showing the critical roles of TGF-β signaling in adipocyte differentiation (Fig. 7A, B). 3T3-L1 cells transfected with nonspecific siRNA or SMAD2/3 specific siRNAs were treated with butein followed by analysis of TGF-β and STAT3 target gene expression. In accordance with the effects of butein in TGF-β signaling, SMAD7 and PA1-I were induced by treatment of control cells with butein, whereas stimulatory effects on the expression of these TGF-β target genes by butein were markedly prevented in SMAD2/3 siRNA transfected cells (Fig. 7C, D). Interestingly, reduction of STAT3 target genes of KLF5, P53, C/EBPδ, and CycD1 by butein was also blunted in SMAD2/3 siRNA transfected cells compared with control cells (Fig. 7C, D). Taken together, temporal regulation of TGF-β and STAT3 target genes by butein, STAT3, and SMAD2/3 knockdown studies indicate that TGF-β acts upstream of STAT3 signaling in the anti-adipogenic cascade mediated by butein in preadipocytes.
Fig. 7.
TGF-β acts upstream of STAT3 in the butein mediated anti-adipogenesis. A: Transient transfection of 3T3-L1 cells with two independent combinations of SMAD2 #1 and SMAD3 #1 (15 μM of each, si #1) or SMAD2 #2 and SMAD3 #2 (15 μM of each, si #2) siRNAs reduced SMAD2 and SMAD3 expression. B: Silencing TGF-β signaling increased adipocyte differentiation. Transient transfection of 3T3-L1 cells with two independent combinations of SMAD2/3 siRNAs (si #1 or si #2) promoted lipid accumulation as assessed by Oil Red O staining. C: Knockdown of TGF-β signaling attenuated butein's inhibitory effects on expression of STAT3 target genes (KLF5, P53, C/EBPδ, and CycD1) and butein's stimulatory effects on expression of TGF-β target genes (SMAD7 and PAI-1). Expression of STAT3 and TGF-β target genes in nonspecific control and SMAD2/3 siRNA-transfected cells treated with DMSO or butein (10 μM) was measured by real-time PCR. D: Control (ctrl) and SMAD2/3 knockdown (KD, si #1) cells were cultured in the presence of DMSO or butein (10 μM) for the various time points indicated and expression profiles of TGF-β and STAT3 target genes were measured. Data shown represent the mean ± SEM from three independent experiments. Statistical significance was determined relative to a control by the Student's t-test (*P < 0.05; **P < 0.005; ***P < 0.0005). Norm, normalized.
The TGF-β pathway mediates the anti-adipogenic effects of butein
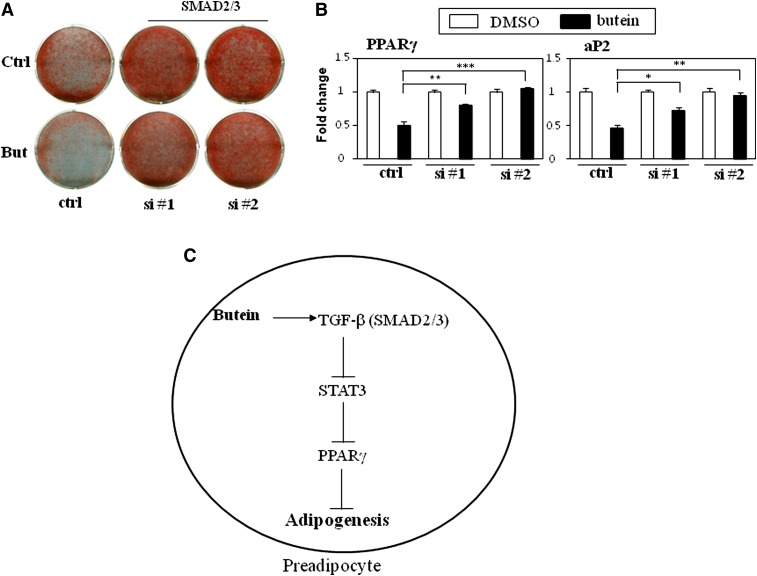
Our data show that butein acts on TGF-β and this leads to inhibition of STAT3 and PPARγ signaling. However, it is not clear yet whether the upstream action of TGF-β on STAT3 is necessary for the anti-adipogenic effects of butein. To assess the critical role of TGF-β signaling on the anti-adipogenic effects of butein, two sets of siRNAs were transiently transfected into 3T3-L1 cells and then the cells were treated with butein during adipogenesis. Silencing SMAD2 and SMAD3 with two independent sets of siRNAs induced adipocyte differentiation and lipid accumulation in 3T3-L1 cells. Remarkably, the anti-lipogenic effects of butein were almost abrogated in cells with defects in TGF-β signaling while consistent anti-lipogenic effects of butein were observed in control siRNA transfected control cells (Fig. 8A). Furthermore, inhibition of PPARγ and aP2 mRNA expression was blunted by butein in SMAD2/3 siRNA transfected cells compared with the significant reduction of these genes in control siRNA transfected cells (Fig. 8B). Thus, these data combined with the STAT3 siRNA results indicate that activation of the TGF-β pathway followed by inhibition of STAT3 signaling is required for the anti-adipogenic effects of butein during adipocyte differentiation (Fig. 8C).
Fig. 8.
TGF-β signaling is required for the inhibitory effects of butein on adipocyte differentiation. A, B: Silencing TGF-β signaling blunted butein's inhibitory effects on adipocyte differentiation in 3T3-L1 cells. A: Differentiated cells were stained with Oil Red O. B: Knockdown of TGF-β signaling attenuated butein's effects on expression of adipocyte markers. Expression of PPARγ and its target gene aP2 in control (ctrl) and siRNA-transfected cells (si #1, si #2) treated with DMSO or butein (10 μM) was measured by real-time PCR. Data shown represent the mean ± SEM from three independent experiments. Statistical significance was determined relative to a control by the Student's t-test (*P < 0.05; **P < 0.005; ***P < 0.0005). C: Schematic diagram showing the mechanism by which butein regulates adipocyte differentiation.
DISCUSSION
In this study, we isolated three anti-adipogenic compounds from RVS and verified their actions on adipocyte differentiation in multiple adipogenic cell lines. First, activity-guided fractionation was used to identify anti-adipogenic compounds in the ethanol-fractionated extracts. Second, fisetin, sulfuretin, and butein were found to inhibit lipid accumulation in preadipocytes. Third, the analysis of adipocyte markers by real-time PCR or Western blot analyses further confirmed the anti-adipogenic actions of these compounds.
Fisetin, sulfuretin, and butein have been shown to exert various biological activities (34). Sulfuretin has anti-platelet, anti-mutagenic, and anti-inflammatory effects. Recent studies have demonstrated that sulfuretin blocks the inflammatory response in type 1 diabetes and allergic airway inflammation models by suppressing the nuclear factor-κB pathway (35, 36). Fisetin is common in various vegetables and fruits, including RVS. It also exhibits a variety of biological effects, including anti-inflammatory, anti-platelet, anti-oxidant, anti-cancer, and anti-angiogenic properties (34). Several studies have demonstrated that butein possesses anti-inflammatory, anti-cancer, anti-fibrogenic, and anti-osteoclastic activities (34). Most notably, a recent investigation identified sulfuretin and fisetin isolated from RVS as putative inhibitors of adipocyte differentiation in 3T3-L1 preadipocyte cells (25). However, the role of butein in adipocyte differentiation has not been reported. This study showed that both butein (M5) isolated from RVS and commercially available butein inhibited lipid accumulation and the expression of adipocyte markers in C3H10T1/2, 3T3-L1, and primary cells, providing new insights into the actions of butein as an anti-adipogenic compound.
The well-known anti-adipogenic compounds genistein and resveratrol were also compared with butein. Butein was more effective than resveratrol and genistein at inhibiting PPARγ expression and lipid accumulation. The 50% inhibitory concentration of PPARγ expression was 5 μM for butein, 20 μM for resveratrol, and 40 μM for genistein in C3H10T1/2 cells. However, the potent effects of butein were not due to cytotoxicity, as butein showed similar, if not less, cytotoxic activity in the 3-(4,5-Dimethylthiazol-2-Yl)-2,5-Diphenyltetrazolium Bromide assay (data not shown). Further pharmacological evaluations should be explored in future studies.
Butein has been previously identified in cashews (Semecarpus anacardium), Dalbergia odorifera, and Caragana jubata (28). Similar to resveratrol, butein activates SIRT1, but to a lesser extent than resveratrol (37). Although it is impossible to exclude the possibility completely, it is unlikely that SIRT1-related activity mediates anti-adipogenic actions, as butein is more potent than resveratrol in terms of inhibiting adipogenesis and STAT3 activation. Other studies have shown that butein inhibits aldo-keto reductase 1B family members, resulting in less prostaglandin F2α production (38, 39). However, knockdown and pharmacological inhibition of aldo-keto reductase induces adipogenesis and PPARγ expression (40). Thus, possible inhibition of aldo-keto reductase by butein may not account for its effects on adipocyte differentiation. Butein has also been shown to inhibit proliferation of hepatocellular carcinoma and mesothelioma cells by suppressing STAT3 signaling (27, 28). In line with this, the present study identified STAT3 as a critical signaling molecule regulated by butein for the control of lipid accumulation and expression of adipocyte markers. Additionally, we established that TGF-β signaling acts upstream of STAT3 and PPARγ in the butein-mediated anti-differentiation cascade. Temporal expression profiles of genes regulated by TGF-β and STAT3 signaling showed that butein exerts its effects earlier on TGF-β than STAT3. Introduction of SMAD2/3 specific siRNAs abrogated the regulatory effects of butein on both TGF-β and STAT3 signaling. However, knockdown of STAT3 impaired STAT3 and PPARγ signaling, while TGF-β signaling was unaffected in butein-treated preadipocytes. Together, these data support the notion that stimulation of TGF-β by butein occurs upstream of inhibition of STAT3, PPARγ, and adipogenesis (Fig. 8C). TGF-β and STAT3 signaling are often perturbed in numerous diseases such as cancer, atherosclerosis, and inflammation (41, 42), therefore our studies further show the potential use of butein for the modulation of these two signaling pathways linked to disease states.
TGF-β and its signaling components are strong inhibitors of adipocyte differentiation. SMAD3, a critical signaling mediator of TGF-β interacts with C/EBP and represses transactivation function of C/EBP (43). TGF-β and SMAD signaling also play roles in differentiation processes of other cell types. SMAD3 represses MyoD function in myogenesis and inhibits CBAF1 during osteoblastic differentiation (44, 45). TGF-β also promotes chondrogenesis and hematopoiesis (46). As TGF-β and its related proteins play an important role in regulating growth, development, and cell fate commitment of different cell types, it will be interesting to test the effects of butein in various cell types. TGF-β can also antagonize mitogenic actions of epithelial growth factor (EGF), fibroblast growth factor (FGF), and platelet derived growth factor (PDGF) (46). Similarly, a potent inhibitory effect of TGF-β in adipogenesis suggests that another molecular pathway might be modulated by TGF-β and SMAD signaling in preadipocytes. In this study, we determined that SMAD2/3 is necessary for the inhibitory effects on STAT3 in a butein-mediated anti-adipogenic cascade. These results are in accordance with the TGF-β inhibitory actions on IL-6 induced target gene expression and STAT3 activity in epithelial cells (31). Therefore, our data also bring new insights into the interaction between STAT3 and TGF-β signaling pathways during adipogenesis and further exemplify the utility of small molecules in dissecting adipocyte biology.
Supplementary Material
Footnotes
Abbreviations:
- aP2
- adipocyte fatty acid binding protein 2
- CC
- column chromatography
- CD36
- cluster of differentiation 36
- C/EBP
- CCAAT/enhancer binding protein
- CycD1
- cyclin D1
- DCM
- dichloromethane
- MEF
- mouse embryonic fibroblast
- PPARγ
- peroxisome proliferator-activated receptor γ
- RVS
- Rhus verniciflua Stokes
- siRNA
- small interfering RNA
- SIRT1
- sirtuin 1
- STAT3
- signal transducer and activator of transcription 3
- TGF-β
- transforming growth factor-β
This study was supported by a grant from the AGENDA program (project No. PJ008447), Rural Development Administration, Republic of Korea. This research was also supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (No. 2011-0014302). The authors declare no conflicts of interest.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of six figures.
REFERENCES
- 1.Tontonoz P., Spiegelman B. M. 2008. Fat and beyond: the diverse biology of PPARgamma. Annu. Rev. Biochem. 77: 289–312 [DOI] [PubMed] [Google Scholar]
- 2.Rosen E. D., Sarraf P., Troy A. E., Bradwin G., Moore K., Milstone D. S., Spiegelman B. M., Mortensen R. M. 1999. PPAR gamma is required for the differentiation of adipose tissue in vivo and in vitro. Mol. Cell. 4: 611–617 [DOI] [PubMed] [Google Scholar]
- 3.Lehmann J. M., Moore L. B., Smith-Oliver T. A., Wilkison W. O., Willson T. M., Kliewer S. A. 1995. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor gamma (PPAR gamma). J. Biol. Chem. 270: 12953–12956 [DOI] [PubMed] [Google Scholar]
- 4.Waki H., Park K. W., Mitro N., Pei L., Damoiseaux R., Wilpitz D. C., Reue K., Saez E., Tontonoz P. 2007. The small molecule harmine is an antidiabetic cell-type-specific regulator of PPARgamma expression. Cell Metab. 5: 357–370 [DOI] [PubMed] [Google Scholar]
- 5.Hasani-Ranjbar S., Nayebi N., Larijani B., Abdollahi M. 2009. A systematic review of the efficacy and safety of herbal medicines used in the treatment of obesity. World J. Gastroenterol. 15: 3073–3085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jung S. R., Kim Y. J., Gwon A. R., Lee J., Jo D. G., Jeon T. J., Hong J. W., Park K. M., Park K. W. 2011. Genistein mediates the anti-adipogenic actions of Sophora japonica L. extracts. J. Med. Food. 14: 360–368 [DOI] [PubMed] [Google Scholar]
- 7.Baur J. A., Pearson K. J., Price N. L., Jamieson H. A., Lerin C., Kalra A., Prabhu V. V., Allard J. S., Lopez-Lluch G., Lewis K., et al. 2006. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 444: 337–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feige J. N., Lagouge M., Canto C., Strehle A., Houten S. M., Milne J. C., Lambert P. D., Mataki C., Elliott P. J., Auwerx J. 2008. Specific SIRT1 activation mimics low energy levels and protects against diet-induced metabolic disorders by enhancing fat oxidation. Cell Metab. 8: 347–358 [DOI] [PubMed] [Google Scholar]
- 9.Hung P. F., Wu B. T., Chen H. C., Chen Y. H., Chen C. L., Wu M. H., Liu H. C., Lee M. J., Kao Y. H. 2005. Antimitogenic effect of green tea (-)-epigallocatechin gallate on 3T3–L1 preadipocytes depends on the ERK and Cdk2 pathways. Am. J. Physiol. Cell Physiol. 288: C1094–C1108 [DOI] [PubMed] [Google Scholar]
- 10.Hwang J. T., Park I. J., Shin J. I., Lee Y. K., Lee S. K., Baik H. W., Ha J., Park O. J. 2005. Genistein, EGCG, and capsaicin inhibit adipocyte differentiation process via activating AMP-activated protein kinase. Biochem. Biophys. Res. Commun. 338: 694–699 [DOI] [PubMed] [Google Scholar]
- 11.Prior R. L., Wu X., Gu L., Hager T., Hager A., Wilkes S., Howard L. 2009. Purified berry anthocyanins but not whole berries normalize lipid parameters in mice fed an obesogenic high fat diet. Mol. Nutr. Food Res. 53: 1406–1418 [DOI] [PubMed] [Google Scholar]
- 12.Shao Z. M., Shen Z. Z., Liu C. H., Sartippour M. R., Go V. L., Heber D., Nguyen M. 2002. Curcumin exerts multiple suppressive effects on human breast carcinoma cells. Int. J. Cancer. 98: 234–240 [DOI] [PubMed] [Google Scholar]
- 13.Ejaz A., Wu D., Kwan P., Meydani M. 2009. Curcumin inhibits adipogenesis in 3T3–L1 adipocytes and angiogenesis and obesity in C57/BL mice. J. Nutr. 139: 919–925 [DOI] [PubMed] [Google Scholar]
- 14.Jung C. H., Kim J. H., Hong M. H., Seog H. M., Oh S. H., Lee P. J., Kim G. J., Kim H. M., Um J. Y., Ko S. G. 2007. Phenolic-rich fraction from Rhus verniciflua Stokes (RVS) suppress inflammatory response via NF-kappaB and JNK pathway in lipopolysaccharide-induced RAW 264.7 macrophages. J. Ethnopharmacol. 110: 490–497 [DOI] [PubMed] [Google Scholar]
- 15.Lee J. C., Lim K. T., Jang Y. S. 2002. Identification of Rhus verniciflua Stokes compounds that exhibit free radical scavenging and anti-apoptotic properties. Biochim. Biophys. Acta. 1570: 181–191 [DOI] [PubMed] [Google Scholar]
- 16.Jeon W. K., Lee J. H., Kim H. K., Lee A. Y., Lee S. O., Kim Y. S., Ryu S. Y., Kim S. Y., Lee Y. J., Ko B. S. 2006. Anti-platelet effects of bioactive compounds isolated from the bark of Rhus verniciflua Stokes. J. Ethnopharmacol. 106: 62–69 [DOI] [PubMed] [Google Scholar]
- 17.Lee S. H., Nan J. X., Zhao Y. Z., Woo S. W., Park E. J., Kang T. H., Seo G. S., Kim Y. C., Sohn D. H. 2003. The chalcone butein from Rhus verniciflua shows antifibrogenic activity. Planta Med. 69: 990–994 [DOI] [PubMed] [Google Scholar]
- 18.Jeon W. K., Kim J. H., Lee H. W., Ko B. S., Kim H. K. 2003. Effects of Rhus verniciflua Stokes (RVS) extract on diet-induced obesity in C57BL/6 mouse. Kor. J. Pharmacognosy. 34: 339–343 [Google Scholar]
- 19.Lee J. C., Lee K. Y., Kim J., Na C. S., Jung N. C., Chung G. H., Jang Y. S. 2004. Extract from Rhus verniciflua Stokes is capable of inhibiting the growth of human lymphoma cells. Food Chem. Toxicol. 42: 1383–1388 [DOI] [PubMed] [Google Scholar]
- 20.Kim K. H., Lee I. K., Piao C. J., Choi S. U., Lee J. H., Kim Y. S., Lee K. R. 2010. Benzylisoquinoline alkaloids from the tubers of Corydalis ternata and their cytotoxicity. Bioorg. Med. Chem. Lett. 20: 4487–4490 [DOI] [PubMed] [Google Scholar]
- 21.Park K. W., Waki H., Choi S. P., Park K. M., Tontonoz P. 2010. The small molecule phenamil is a modulator of adipocyte differentiation and PPARgamma expression. J. Lipid Res. 51: 2775–2784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park K. W., Waki H., Villanueva C. J., Monticelli L. A., Hong C., Kang S., MacDougald O. A., Goldrath A. W., Tontonoz P. 2008. Inhibitor of DNA binding 2 is a small molecule-inducible modulator of peroxisome proliferator-activated receptor-gamma expression and adipocyte differentiation. Mol. Endocrinol. 22: 2038–2048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jung S. R., Song N. J., Hwang H. S., An J. J., Cho Y. J., Kweon H. Y., Kang S. W., Lee K. G., Yoon K., Kim B. J., et al. 2011. Silk peptides inhibit adipocyte differentiation through modulation of the Notch pathway in C3H10T1/2 cells. Nutr. Res. 31: 723–730 [DOI] [PubMed] [Google Scholar]
- 24.Kuppusamy U. R., Das N. P. 1994. Potentiation of beta-adrenoceptor agonist-mediated lipolysis by quercetin and fisetin in isolated rat adipocytes. Biochem. Pharmacol. 47: 521–529 [DOI] [PubMed] [Google Scholar]
- 25.Kim S-G., Rhyu D.-Y., Kim D-K., Ko D-H., Kim Y-K., Lee Y-M., Jung H-J. 2010. Inhibitory effect of heartwood of Rhus verniciflua Stokes on lipid accumulation in 3T3–L1 cells. Kor. J. Pharmacognosy. 41: 21–25 [Google Scholar]
- 26.Rayalam S., Della-Fera M. A., Yang J. Y., Park H. J., Ambati S., Baile C. A. 2007. Resveratrol potentiates genistein's antiadipogenic and proapoptotic effects in 3T3–L1 adipocytes. J. Nutr. 137: 2668–2673 [DOI] [PubMed] [Google Scholar]
- 27.Rajendran P., Ong T. H., Chen L., Li F., Shanmugam M. K., Vali S., Abbasi T., Kapoor S., Sharma A., Kumar A. P., et al. 2011. Suppression of signal transducer and activator of transcription 3 activation by butein inhibits growth of human hepatocellular carcinoma in vivo. Clin. Cancer Res. 17: 1425–1439 [DOI] [PubMed] [Google Scholar]
- 28.Pandey M. K., Sung B., Ahn K. S., Aggarwal B. B. 2009. Butein suppresses constitutive and inducible signal transducer and activator of transcription (STAT) 3 activation and STAT3-regulated gene products through the induction of a protein tyrosine phosphatase SHP-1. Mol. Pharmacol. 75: 525–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang K., Guo W., Yang Y., Wu J. 2011. JAK2/STAT3 pathway is involved in the early stage of adipogenesis through regulating C/EBPbeta transcription. J. Cell. Biochem. 112: 488–497 [DOI] [PubMed] [Google Scholar]
- 30.Clarkson R. W., Boland M. P., Kritikou E. A., Lee J. M., Freeman T. C., Tiffen P. G., Watson C. J. 2006. The genes induced by signal transducer and activators of transcription (STAT)3 and STAT5 in mammary epithelial cells define the roles of these STATs in mammary development. Mol. Endocrinol. 20: 675–685 [DOI] [PubMed] [Google Scholar]
- 31.Starsichová A., Lincová E., Pernicová Z., Kozubik A., Soucek K. 2010. TGF-beta1 suppresses IL-6-induced STAT3 activation through regulation of Jak2 expression in prostate epithelial cells. Cell. Signal. 22: 1734–1744 [DOI] [PubMed] [Google Scholar]
- 32.Nakao A., Imamura T., Souchelnytskyi S., Kawabata M., Ishisaki A., Oeda E., Tamaki K., Hanai J., Heldin C. H., Miyazono K., et al. 1997. TGF-beta receptor-mediated signalling through Smad2, Smad3 and Smad4. EMBO J. 16: 5353–5362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choy L., Skillington J., Derynck R. 2000. Roles of autocrine TGF-beta receptor and Smad signaling in adipocyte differentiation. J. Cell Biol. 149: 667–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gupta S. C., Kim J. H., Prasad S., Aggarwal B. B. 2010. Regulation of survival, proliferation, invasion, angiogenesis, and metastasis of tumor cells through modulation of inflammatory pathways by nutraceuticals. Cancer Metastasis Rev. 29: 405–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Song M. Y., Jeong G. S., Kwon K. B., Ka S. O., Jang H. Y., Park J. W., Kim Y. C., Park B. H. 2010. Sulfuretin protects against cytokine-induced beta-cell damage and prevents streptozotocin-induced diabetes. Exp. Mol. Med. 42: 628–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Song M. Y., Jeong G. S., Lee H. S., Kwon K. S., Lee S. M., Park J. W., Kim Y. C., Park B. H. 2010. Sulfuretin attenuates allergic airway inflammation in mice. Biochem. Biophys. Res. Commun. 400: 83–88 [DOI] [PubMed] [Google Scholar]
- 37.Howitz K. T., Bitterman K. J., Cohen H. Y., Lamming D. W., Lavu S., Wood J. G., Zipkin R. E., Chung P., Kisielewski A., Zhang L. L., et al. 2003. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 425: 191–196 [DOI] [PubMed] [Google Scholar]
- 38.Lee E. H., Song D. G., Lee J. Y., Pan C. H., Um B. H., Jung S. H. 2008. Inhibitory effect of the compounds isolated from Rhus verniciflua on aldose reductase and advanced glycation endproducts. Biol. Pharm. Bull. 31: 1626–1630 [DOI] [PubMed] [Google Scholar]
- 39.Kabututu Z., Manin M., Pointud J. C., Maruyama T., Nagata N., Lambert S., Lefrancois-Martinez A. M., Martinez A., Urade Y. 2009. Prostaglandin F2alpha synthase activities of aldo-keto reductase 1B1, 1B3 and 1B7. J. Biochem. 145: 161–168 [DOI] [PubMed] [Google Scholar]
- 40.Fujimori K., Ueno T., Nagata N., Kashiwagi K., Aritake K., Amano F., Urade Y. 2010. Suppression of adipocyte differentiation by aldo-keto reductase 1B3 acting as prostaglandin F2alpha synthase. J. Biol. Chem. 285: 8880–8886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Debnath B., Xu S., Neamati N. 2012. Small molecule inhibitors of signal transducer and activator of transcription 3 (Stat3) protein. J. Med. Chem. 55: 6645–6668 [DOI] [PubMed] [Google Scholar]
- 42.Blobe G. C., Schiemann W. P., Lodish H. F. 2000. Role of transforming growth factor beta in human disease. N. Engl. J. Med. 342: 1350–1358 [DOI] [PubMed] [Google Scholar]
- 43.Choy L., Derynck R. 2003. Transforming growth factor-beta inhibits adipocyte differentiation by Smad3 interacting with CCAAT/enhancer-binding protein (C/EBP) and repressing C/EBP transactivation function. J. Biol. Chem. 278: 9609–9619 [DOI] [PubMed] [Google Scholar]
- 44.Alliston T., Choy L., Ducy P., Karsenty G., Derynck R. 2001. TGF-beta-induced repression of CBFA1 by Smad3 decreases cbfa1 and osteocalcin expression and inhibits osteoblast differentiation. EMBO J. 20: 2254–2272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu D., Black B. L., Derynck R. 2001. TGF-beta inhibits muscle differentiation through functional repression of myogenic transcription factors by Smad3. Genes Dev. 15: 2950–2966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Patil A. S., Sable R. B., Kothari R. M. 2011. An update on transforming growth factor-beta (TGF-beta): sources, types, functions and clinical applicability for cartilage/bone healing. J. Cell. Physiol. 226: 3094–3103 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.