Abstract
Ginsenoside Rb1 (Rb1), a natural compound extracted from ginseng, exerts anti-obesity activity and improves insulin sensitivity in high-fat diet (HFD)-induced obese rats. The objective of the current study was to evaluate the protective effect of Rb1 on fatty liver in HFD-induced obese rats and to elucidate underlying mechanisms. After chronic intraperitoneal administration, Rb1 (10 mg/kg) significantly ameliorated hepatic fat accumulation in HFD-induced obese rats, as demonstrated by reduced liver weight, hepatic triglyceride content, and histological evaluation of liver sections by hematoxylin and eosin and Oil Red O staining. Using primary cultured rat hepatic cells, we found that the rate of fatty acid oxidation and the activity of carnitine palmitoyltransferase 1 (CPT1), a key enzyme in fatty acid β-oxidation, were significantly elevated in Rb1-treated hepatocytes compared with those of vehicle-treated cells. HPLC analysis revealed that Rb1 increased the cellular AMP/ATP ratio, which is associated with elevated activation of hepatic AMP-activated protein kinase (AMPK) and phosphorylated acetyl-CoA carboxylase. Consistent with the activation of AMPK, Rb1 stimulated the expression of genes encoding fatty acid oxidative enzymes and proteins, and suppressed the expression of genes encoding enzymes or proteins that function in lipogenesis, assessed by quantitative PCR. We conclude that Rb1 has a potent ability to reduce hepatic fat accumulation and might be useful as a therapeutic agent for fatty liver disorder.
Keywords: obesity, fatty liver, signaling pathway, ginseng
Fatty liver, the initial stage of nonalcoholic fatty liver disease (NAFLD), is a common metabolic symptom and is strongly associated with obesity and insulin resistance (1). Fatty liver is characterized by an increased content of hepatocellular lipids and is frequently associated with steatohepatitis and hepatocellular injury, which eventually may result in severe liver damage including bridging fibrosis and cirrhosis (2). Although the molecular mechanisms underlying fatty liver are not fully understood, dysregulation of hepatic lipid homeostasis caused by pathological conditions such as reduced fatty acid oxidation, enhanced de novo lipogenesis, elevated hepatic fatty acid influx, and/or increased systemic insulin resistance are thought to be important in the development of fatty liver (3). Indeed, therapies aimed at reducing body weight and/or alleviating insulin resistance reduce fatty liver (4).
AMP-activated protein kinase (AMPK) is an intracellular fuel sensor important in the regulation of lipid metabolism (5). In the liver, activation of AMPK leads to increased fatty acid oxidation and simultaneously to decreased lipid synthesis (6). Of interest, antidiabetic drugs, including metformin and the thiazolidinediones, alleviate fatty liver in humans and rodents by regulating lipid metabolism through AMPK activation (7). Thus AMPK represents an attractive target for therapeutic intervention in the treatment of hepatic disorders (8).
Ginsenoside Rb1 (Rb1), a natural compound extracted from ginseng root, has a glucose-lowering action in vitro (9), and we have demonstrated that Rb1 significantly reduces body weight, improves glucose tolerance, and enhances insulin action in high-fat diet (HFD)-induced obese rats (10). These findings prompted us to ask whether Rb1 could reduce hepatic fat accumulation associated with obesity; and if so, to determine the mechanisms responsible for the therapeutic effect of Rb1 on HFD-induced fatty liver.
MATERIALS AND METHODS
Animals
Adult male Long-Evans rats (Harlan, Indianapolis, IN) were individually housed in a temperature-controlled vivarium on a 12/12 h light/dark cycle (lights on at 0000 h, lights off at 1200 h). Diets and water were provided ad libitum except where noted. All animal procedures were approved by the Institutional Animal Care and Use Committee of the University of Cincinnati.
Materials
The high-fat diet containing 20 g fat (19 g butter oil and 1 g soybean oil)/100 g diet was prepared by Research Diets (Catalog #: D03082706; New Brunswick, NJ) (11). Rb1 purified by high-performance liquid chromatography (HPLC) was purchased from Jilin University in China (10). We performed HPLC (Shimadzu Corp., Kyoto, Japan) analysis and confirmed that the Rb1 had a purity of ≥98% using an Rb1 standard obtained from LKT Laboratories (St. Paul, MN). Radiochemical [9,10-3H(N9)]palmitic acid and l-[3H]carnitine were purchased from PerkinElmer (Boston, MA). HBSS, Williams’ E medium, and HepatoZYME-SFM medium were obtained from Life Technologies (Carlsbad, CA). Collagenase II was purchased from Worthington Biochemical Co. (Lakewood, NJ). The chemicals, including dexamethasone, palmitic acid, compound C, etc., were purchased from Sigma (St. Louis, MO). The antibodies for phosphorylated AMPK, AMPK, phosphorylated acetyl-CoA carboxylase (ACC), and ACC were from Cell Signaling Technology (Beverly, MA), and antibodies for LKB1 and actin from EMD Millipore (Billerica, MA).
HFD-induced obese rats
Thirty-six rats were fed the HFD for 13 weeks. The rats became obese (average body weight 596 g), and then were divided into three groups of comparable body weight. Groups 1 and 2 received ip saline or Rb1 (10 mg/kg/day), respectively, and continued to have free access to the same HFD. Because Rb1-treated rats had reduced food intake, a HFD pair-fed group was included as a control. Those rats were given the HFD but with an amount limited to the average daily consumption of the Rb1-treated rats (10). Body weight and food intake were recorded daily. At the end of the experiment, the rats were sacrificed after 6 h fasting, and several pieces of liver were excised and either stored at −80°C or placed in 10% formalin in phosphate buffer solution (Fisher Scientific, Pittsburgh, PA) for further analyses as detailed below.
Intracellular triglyceride measurement
About 1 g of frozen liver was homogenized, and lipids were extracted with a chloroform-methanol mixture (2:1, v/v) as described by Folch (12). The concentration of triglyceride (TG) in liver was determined using a commercial kit, Infinity™ Triglycerides Reagent (Thermo, Middletown, VA) (10).
Histopathological examination
Liver tissue samples fixed in 10% formalin were embedded with paraffin for histological analysis. Tissue sections (5 μm) were cut with a microtome (Leica, Germany) and mounted on microscope slides. They were then stained with hematoxylin and eosin (H and E) and photographed at 200× magnification. The degree of liver steatosis was scored by a pathologist who was unaware of the treatment that the animals had received. Steatosis was graded on a scale of 0–3; where 0 was <5%, 1 was 5–33%, 2 was 33–66%, and 3 was >66% of hepatocytes affected (13). This grading is recommended by the American Gastroenterological Association when assessing NAFLD (14). In addition, 10 μm sections were cut from frozen liver samples using a Leica cryostat (CM 3050, Germany) and stained with Oil Red O for confirming the fat deposition (15).
Isolation and culture of primary rat hepatocytes
Rat hepatocytes were cultured as described previously (16). Briefly, rats fasted for 4 h were anesthetized and the liver was surgically exposed. The livers were first perfused with HBSS, and this was followed by perfusing HBSS containing collagenase II (3.3 U/ml). After perfusion, the liver was minced in a Petri dish with a cell scraper, filtered through a 100 μm pore size mesh nylon filter, and washed three times in Williams’ E medium. The cells were then plated as 5 × 105 cells per well in 6-well plates and cultured at 37°C in a 5% CO2 incubator. Four hours later, the medium was replaced with HepatoZYME-SFM, a serum-free medium for the long-term culture of hepatocytes, and the cells were cultured for an additional 14 h.
Fatty acid oxidation in hepatocytes
The measurement of fatty acid oxidation in hepatocytes was conducted as described previously (17). Hepatocytes cultured for 18 h were washed twice with Dulbecco's PBS. The reaction was carried out in duplicate (250 μl per well), with each well containing vehicle (PBS), Rb1 (10 μM), and compound C (20 μM) (7), or compound C (20 μM) plus Rb1 (10 μM), and 22 μM [9,10(n)-3H]palmitate [32.4 Ci (1 Ci = 37 GBq)/mmol], which was prepared after complete removal of the solvent under a stream of air containing 5% CO2, and the residue was suspended in HBSS containing 10 mg/ml fatty acid-free BSA. The compound C was added into the medium 30 min prior to Rb1 treatment. After additional 6 h incubation, the reaction medium was transferred to centrifuge tubes and 2.5 ml of methanol-chloroform (2:1) and 1 ml of 2 M KCl/2 M HCl were added. After vigorously mixing the reactions, mixtures were centrifuged at 3,000 g for 5 min, and the aqueous phase (1 ml) containing 3H2O was transferred to a new tube, treated once more with the methanol-chloroform and KCl-HCl mixture and recovered, and radioactivity was measured by LS 6500 Scintillation Counter (Beckman Coulter, Fullerton, CA).
CPT1 activity in hepatocytes
Carnitine palmitoyltransferase 1 (CPT1) activity of hepatocytes was measured as described by Sleboda et al. (18) with some modifications. Hepatocytes were cultured for 18 h and then treated with PBS, Rb1 (10 μM), and compound C (20 μM), or compound C (20 μM) plus Rb1 (10 μM) for an additional 6 h. The compound C was added into medium 30 min prior to the Rb1 treatment. Then the cells were washed with PBS, and the medium was replaced with 0.7 ml of assay medium consisting of 50 mM imidazole, 70 mM KCl, 80 mM sucrose, 1 mM EGTA, 2 mM MgCl2, 1 mM DTT, 1 mM KCN, 1 mM ATP, 0.1% fatty acid-free BSA, 1 μCi of l-[3H]carnitine, and 40 μg of digitonin. After the cells were incubated for 6 min, the reaction was stopped by the addition of 0.5 ml of 4 M ice-cold perchloric acid. The mixture was centrifuged at 8,000 g for 10 min and the pellet was washed with 0.5 ml of 2 mM perchloric acid, resuspended in 800 μl of water, and extracted with 400 μl of n-butanol, and the radioactivity in 180 μl of n-butanol was determined by liquid scintillation counter.
Quantitative real-time PCR
Total RNA was extracted from the cultured primary rat hepatic cells or rat liver tissues with an RNAqueous-Micro Kit (Ambion, Austin, TX) and treated with DNase I before cDNA synthesis (19). The DNase I-treated total RNA (1 μg) was reverse-transcribed to first-strand cDNA following the manufacturer's instructions (GE Healthcare Bio-Sciences, Piscataway, NJ). Quantitative real-time PCR (qPCR) was performed in a 25 μl final reaction volume with an iCycler iQ Detection System using iQ SYBR Green Supermix (Bio-Rad Laboratories, Hercules, CA) as described previously (10). Real-time PCR conditions were as follows: 95°C for 3 min for one cycle followed by 38 cycles of 95°C for 30 s and 58°C for 30 s. No other products were amplified because melting curves revealed only one peak in each sample. Threshold cycle readings for each of the unknown samples were then used, and the results were transferred and analyzed using the ΔΔ-CT method described previously (10, 20). Cyclophilin mRNA levels from each sample were used as internal controls to normalize the mRNA levels. The sequences of the primers for rat sterol regulatory element-binding protein 1c (SREBP1c), fatty acid synthase (FAS), ACC, stearoyl-CoA desaturase-1 (SCD-1), peroxisome proliferator-activated receptor γ coactivator-1α (PGC-1α), peroxisome proliferator activated receptor (PPAR)α, carnitine palmitoyltransferase 1A (CPT1A), acyl-CoA oxidase 1 (Acox1), and cyclophilin are listed in Table 1.
TABLE 1.
Real-time PCR primer sequence for genes associated with hepatic fatty acid synthesis and metabolism in liver
| Gene Name | Accession # | Forward (from 5′′ to 3′) | Reverse (from 5′ to 3′) | Product Size (bp) |
| SREBP1c | XM_213329 | CAGGCTGAGAAAGGATGCTC | TCAGTGCCAGGTTAGAAGCA | 125 |
| FAS | NM_017332 | GGACATGGTCACAGACGATGAC | GTCGAACTTGGACAGATCCTTCA | 94 |
| ACC | J03808 | GCCTCTTCCTGACAAACGAG | TCCATACGCCTGAAACATGA | 101 |
| SCD-1 | NM_139192 | GGGCGAGTCCTGATAAAACA | GTCTGCAGGAAAACCTCTGC | 125 |
| PGC-1α | NM_031347 | CGATGACCCTCCTCACACCA | TTGGCTTGAGCATGTTGCG | 111 |
| PPARα | NM_013196 | CTCCCTCCTTACCCTTGGAG | GCCTCTGATCACCACCATTT | 124 |
| CPT1A | NM_031559 | AACATCCCTAAGCCCACAAG | GATCAAGCCTTTGCCGAAAG | 137 |
| Acox1 | NM_017340 | CCAAGATTCAAGACAAAGCCG | TCCGAGCGTTTACTTGTGAC | 144 |
| Cyclophilin | M19533 | ATTCATGTGCCAGGGTGGTGAC | TCAGTCTTGGCAGTGCAGAT | 182 |
Western blot analysis
Total proteins were extracted from the cultured primary hepatic cells or from the livers of the rats using the method described previously (20). Twenty micrograms of the proteins were separated by 4–15% SDS-PAGE gradient gels (Bio-Rad Life Science) and then electrotransferred to polyvinylidene fluoride membranes (Millipore, Bedford, MA). After incubating in blocking buffer, membranes were incubated with an anti-phospho AMPKα (Thr 172) or anti-phospho ACC antibodies (1:1000 dilution) overnight at 4°C with gentle shaking. After washing with PBS, the membranes were incubated with horseradish peroxidase-conjugated secondary antibody (1:5,000 dilution; Dako, Carpinteria, CA). The amount of immune complexes was quantitated using an enhanced chemiluminescence detection system (Millipore). The reacted membranes were exposed to X-ray film (Kodak Scientific, Rochester, NY). Membranes were then stripped and reblotted with anti-AMPKα or anti-ACC antibodies (1:1,000 dilution) to verify equal loading of protein in each lane. A similar procedure was used for the measurements of LKB1 and actin levels, where the anti-LKB1 antibody was diluted at 1:1,000 and anti-actin antibody was diluted at 1:10,000. Film density, measured as transmittance, was expressed as volume-adjusted optical density. The amount of each protein was normalized to the respective individual density values reflecting protein levels of AMPK and ACC, respectively, and was expressed as a ratio. Actin protein was used as internal control for LKB1 to normalize protein loading levels.
Adenine nucleotide contents assay by HPLC
After treatment with either PBS or Rb1 for 6 h, the cultured hepatocytes from each well of a 6-well dish (5 × 105/well) were washed twice with cold PBS, immediately scraped into a microcentrifuge tube, and stored at −80°C until processing. Each pellet was suspended on ice in 0.4 M perchloric acid and briefly sonicated (5 to 10 times of a 1 s burst) until cells were clearly disrupted. Samples were then neutralized with 5 M KOH, stored on ice for ∼30 min and then centrifuged to remove the precipitate. After adjusting the final pH to 6–8, supernatants were aliquoted and frozen. HPLC analysis was performed within 24 h of preparation. ATP, ADP, and AMP were resolved by reversed-phase HPLC essentially as described by (21) using a 25 cm, 4.6 micron, Restek Ultra II C18 column (Restek Corp., Bellefonte, PA) at 25°C attached to an Agilent 1200 ChemStation system. Mobile phases were: A) 35 mM NaPO4 pH 6.8 and B) 98% acetonitrile, 1.9% water, 0.1% trifluoroacetic acid. Running time was 16 min at 1.2 ml/min starting at 100% A, to 5% B at 2 min, 20% B at 4 min, 25% B at 5.5 min, and 100% A at 10 min. Column output was monitored at 254 nm and peaks were identified by comparison to standards purchased from Sigma. Under the conditions we used, the analytes of interest were well separated from other material with absorbance at 254 nm.
Statistical analysis
All data are presented as mean ± standard error (SE). In vitro experiments were carried out in triplicate and performed on three to five separate occasions. Differences among more than two groups were determined using one-way ANOVA followed by Student-Newman-Keuls test for comparison between treatments or by Dunnett's test for comparison of all groups as a control. Student's t-test was used for comparison of effects between Rb1 and vehicle treatments. P values less than 0.05 were considered statistically significant.
RESULTS
Rb1 reduces fat accumulation in the liver of HFD-induced obese rats
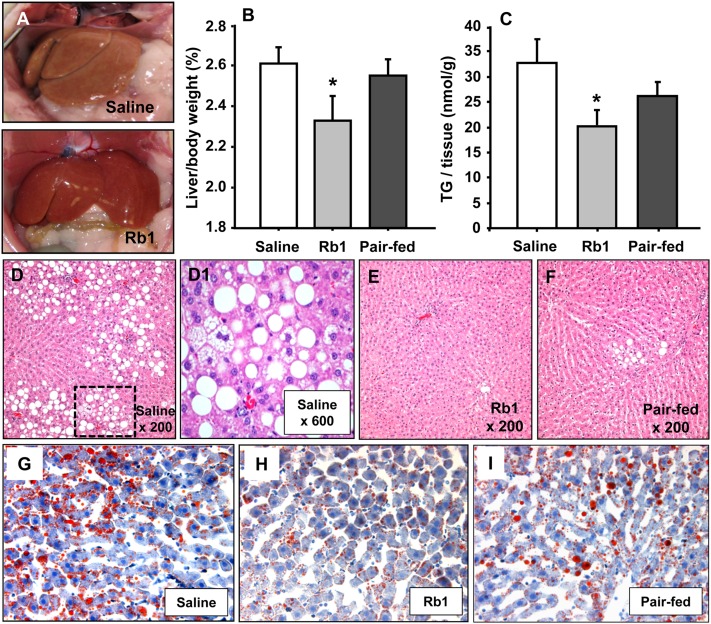
In our previous report, Rb1 treatment (10 mg/kg/day, ip) for 4 weeks significantly reduced average daily food intake (23.1 ± 0.98 g vs. 18.0 ± 1.88 g), body weight gain (51.7 ± 3.66 g vs. 12.7 ± 3.26 g), and fasting blood glucose (168.3 ± 8.69 mg/dl vs. 125.4 ± 2.67 mg/dl) and improved glucose tolerance in HFD-induced obese rats, compared with vehicle treatment (10). In the present study, we asked whether Rb1 treatment could ameliorate fatty liver in obese animals. As depicted in Fig. 1A, liver morphology was considerably improved in Rb1-treated obese rats (bottom) versus vehicle (saline)-treated controls (top). The percentage of liver weight to body weight (g/g) and the hepatic TG content in Rb1-treated obese rats was significantly reduced compared with saline controls (Fig. 1B, C). Caloric restriction alone also reduced liver weight and hepatic TG content, but no significant difference was found for these parameters between pair-fed rats and saline controls (Fig. 1B, C) as determined by one-way ANOVA. Although there was no significant difference between pair-fed rats and Rb1-treated rats, the apparent differences in liver weight and hepatic TG content between Rb1-treated and pair-fed rats suggest that Rb1 has additional effects on lipid metabolism in the liver, which led us to pursue the following studies.
Fig. 1.
Liver characteristics following Rb1 or saline treatment in HFD-induced obese rats. A: Morphology of livers from saline- (top) and Rb1-treated (bottom) obese rats. B: Liver weight expressed as a percent of body weight. C: Liver TG content in rats. Data are means ± S.E., n = 10, *P < 0.05 compared with saline controls. No significant differences were found either between pair-fed rats and saline controls or between pair-fed and Rb1-treated rats. H and E stained sections of livers from saline-treated (D), Rb1-treated (E), and pair-fed obese rats (F). D1: An amplified image from the box in (D). Liver sections stained with Oil Red O from saline-treated (G), Rb1-treated (H), and pair-fed obese rats (I) were photographed at ×500 magnification.
Evaluation of liver histology
Severe steatosis and ballooning were observed in saline-treated obese rats (Fig. 1D, D1). The livers from Rb1-treated obese rats had a marked reduction in the degree of steatosis and ballooning (Fig. 1E). Ballooning was also present in pair-fed rats (Fig. 1F). The histological scores are detailed in Table 2. No histologic evidence of hepatotoxicity, such as necrosis or cholestasis, was observed in Rb1- or saline-treated obese rats. Using Oil Red O staining for neutral lipids, the difference between the livers of Rb1- and saline-treated rats was apparent. There were only scattered and smaller lipid drops in Rb1-treated animals compared with numerous large lipid drops in saline-treated rats (Fig. 1G–I). Rb1 reduced the degree of steatosis and neutral lipids to a greater extent than pair-feeding, indicating that Rb1’s effects on the reduction of fatty liver in HFD-induced obese animals are beyond that of the simply caloric restriction.
TABLE 2.
Hepatic steatosis grading in HFD-induced obese rats with different treatments
| Steatosis Grade | Score | Saline-control Rats | Rb1-treated Rats | Pair-fed Rats |
| <5% | 0 | 1 | 8 | 4 |
| 6–33% | 1 | 2 | 0 | 4 |
| 33–66% | 2 | 1 | 0 | 0 |
| >66% | 3 | 4 | 0 | 0 |
| Average | 2 | 0**,# | 0.5* |
n = 8; **P < 0.01; *P < 0.05 versus saline-controls; #P < 0.05 versus pair-fed, determined by Kruskal-Wallis one-way ANOVA.
Rb1 increases fatty acid oxidation
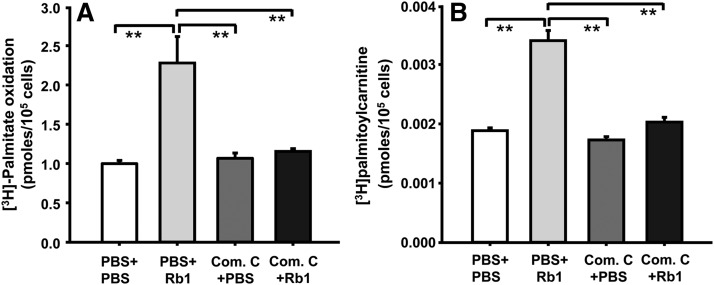
One of the potential mechanisms by which Rb1 reduces fatty liver is to enhance the oxidation of fatty acids within the liver, precluding excess hepatic fat accumulation. Consistent with this possibility, the amount of palmitate oxidized by Rb1-treated hepatocytes was significantly higher than that oxidized by PBS (vehicle)-treated hepatocytes, and this effect was significantly attenuated by pretreatment with compound C, an inhibitor of AMPK signaling pathway (Fig. 2A).
Fig. 2.
Rb1 increased fatty acid oxidation and CPT1 activity in cultured hepatocytes, and this was significantly attenuated by pretreatment with compound C, an inhibitor of AMPK pathway. Hepatocytes were cultured in HepatoZYME-SFM for 18 h. Fatty acid oxidation (A) was determined by incubating hepatocytes with Rb1 or vehicle (PBS) and 0.2 mM [3H]palmitate for an additional 6 h. CPT1 activity (B) was determined by formation of [3H]palmitoylcarnitine from l-[3H]carnitine after incubating hepatocytes with Rb1 or PBS for an additional 6 h. Compound C (Com. C) was added into medium 30 min before Rb1 treatment. Data are means ± S.E. (n = 4–7), **P < 0.01 compared with the other three groups.
Rb1 increases CPT1 activity
To determine whether the higher palmitate oxidation in Rb1-treated hepatocytes is due to reduced inhibition of CPT1, CPT1 activity was assayed. As depicted in Fig. 2B, CPT1 activity was almost 2-fold higher in Rb1-treated hepatocytes compared with PBS-treated cells, and pretreatment with compound C almost completely blocked Rb1’s effect (Fig. 2B).
Rb1 regulated gene expression encoding enzymes and proteins that function in lipogenesis or lipolysis in liver
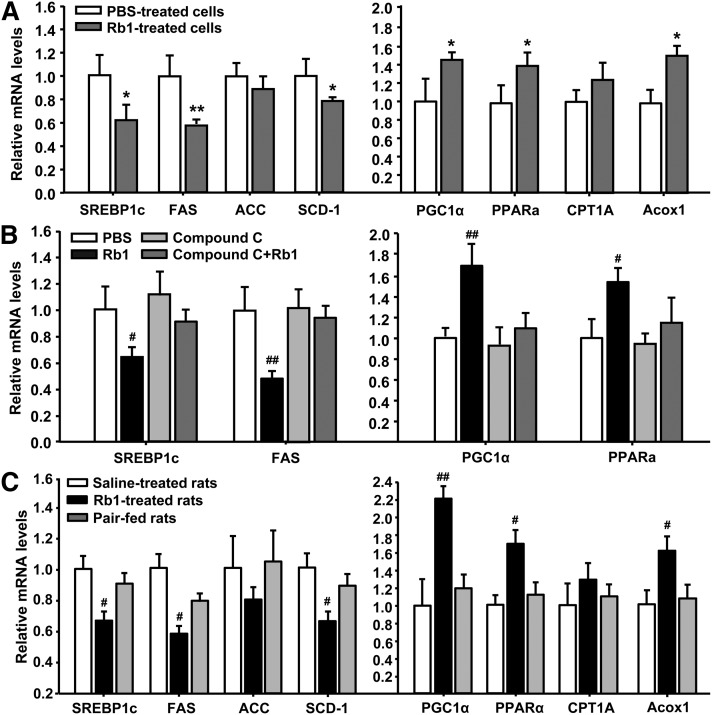
We hypothesized that Rb1-induced reduction of cellular lipid accumulation in the liver could also result from suppressed expression of genes encoding enzymes or proteins that function in lipogenesis and/or increased expression of genes encoding fatty acid oxidative enzymes and proteins. Consistent with this hypothesis, we found that the mRNA levels of the transcription factor SREBP1c and of the key enzymes FAS and SCD-1, but not ACC, were significantly reduced in Rb1-treated hepatocytes compared with those in PBS-treated cells (Fig. 3A). In contrast, Rb1 significantly increased the expression of genes encoding proteins with roles in fatty acid oxidation, including PGC-1α, PPARα, and Acox1, but not CPT1A, in the cultured hepatocytes, compared with that in PBS-treatment (Fig. 3A). These observations were further confirmed in the liver from Rb1- or saline-treated rats (Fig. 3C). To determine if the effects of Rb1 on the gene expression require the activation of AMPK, compound C, an inhibitor of the AMPK signaling pathway, was added into the medium 30 min prior of Rb1 treatment. Compound C significantly attenuated Rb1’s effects on the expression of several key genes, including SREBP1c, FAS, PGC1α, and PPARα (Fig. 3B).
Fig. 3.
Rb1 treatment regulates gene expression in cultured hepatic cells (A, B) and rat liver tissues (C). In in vitro study, hepatocytes were cultured in HepatoZYME-SFM for 18 h, and the expression of genes encoding enzymes and proteins that function in lipogenesis or lipolysis was determined by qPCR following an additional 6 h incubation with vehicle (PBS), Rb1 (10 μM), compound C (20 μM), or compound C (20 μM) plus Rb1 (10 μM) for an additional 6 h. The compound C was added into the medium 30 min prior to Rb1 treatment. The genes encoding enzymes/proteins that function in lipogenesis included SREBP1c, FAS, ACC, and SCD-1; and the genes encoding enzymes/proteins that function in lipolysis included PGC-1α, PPAR, CPT1A, and Acox1. Data are means ± S.E. (n = 3–5). *P < 0.05 and **P < 0.01, Rb1-treated cells versus PBS-treated cells. #P < 0.05 and ##P < 0.01, compared with all other groups.
Rb1 increases phosphorylation of AMPK in both cultured hepatocytes and obese rats
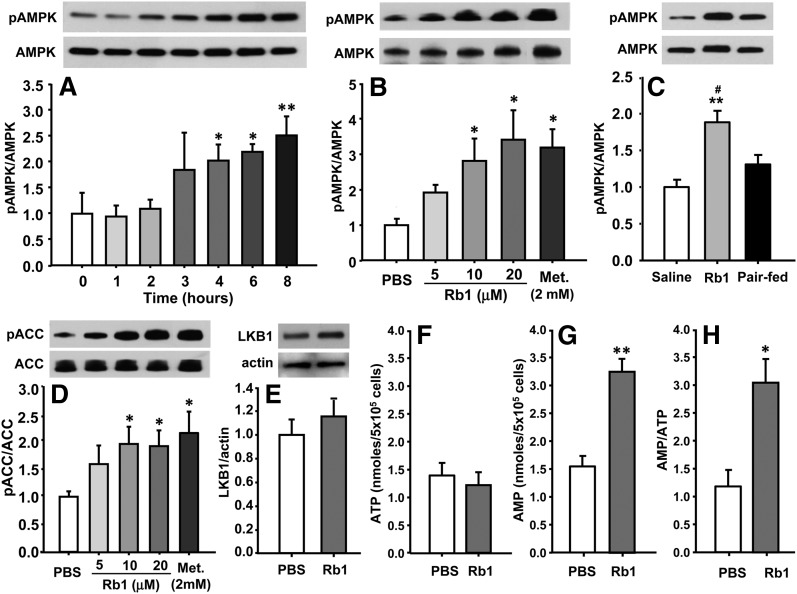
To test the hypothesis that Rb1 signaling in the liver involves the activation of the AMPK cascade, we assayed Rb1-associated AMPK activity in cultured primary hepatocytes and in obese rats. Rb1 increased phosphorylation of hepatic AMPK in time-dependent (Fig. 4A) and dose-dependent (Fig. 4B) manners in cultured hepatocytes, whereas the total amount of AMPK protein was not significantly changed. The minimal time for Rb1, at a dose of 10 μM, to induce AMPK phosphorylation is 3 h after treatment; and the minimal effective dose is 10 μM in the cultured hepatocytes. These changes in the activation of AMPK were confirmed in Rb1-treated HFD-induced obese rats, when compared with saline controls (Fig. 4C). Because it is well established that peripheral AMPK activation promotes fatty acid oxidation by inactivating ACC (5), we determined whether Rb1 induces ACC phosphorylation in the cultured hepatic cells. Consistent with the increased AMPK phosphorylation, Rb1 dose dependently elevated the level of phosphorylated ACC, which is the inactive form of this enzyme (Fig. 4D). Metformin (2.0 mM) also increased the phosphorylation of AMPK and ACC, consistent with previous reports (Fig. 4B, D) (7). It has been reported that LKB1 acts as a master upstream kinase, directly phosphorylating and activating AMPK (22), so we measured LKB1 protein expression in Rb1- or PBS-treated cells. Although Rb1 treatment elevated LKB1 expression relative to PBS treatment, the difference did not reach significance statistically (Fig. 4E).
Fig. 4.
Rb1 time-dependently and dose-dependently increased the phosphorylation of AMPK (A, B) in cultured primary hepatic cells. In the time course study, overnight-cultured hepatic cells were treated with Rb1 (10 μM) for 0, 1, 2, 3, 4, 6, or 8 h respectively. In the dose-effect study, the cells were treated with Rb1 at 5, 10, and 20 μM, metformin at 2 mM, or PBS for an additional 6 h. The increased phosphorylation of AMPK was confirmed in Rb1- or saline-treated HFD-induced obese rats (C). Rb1 also dose-dependently increased the phosphorylation of ACC (D). These measurements were conducted by Western blot. Top panels are representative immunoblots and bottom panels are quantitative analyses (A–E). To investigate the mechanisms mediating Rb1’s effects on the activation of AMPK, cultured hepatocytes were treated with Rb1 (10 μM) or PBS for 6 h. LKB1 protein levels were determined by Western blot and cellular AMP and ATP levels were measured by HLPC. Data are means ± SE (n = 3∼4 in in vitro study, n = 6 in in vivo study). *P < 0.05; **P < 0.01 versus vehicle control; and #P < 0.05 versus pair-fed animals.
Effects of Rb1 on adenine nucleotide contents in hepatocytes
The activity of AMPK is also regulated by the AMP/ATP ratio (23). To determine whether Rb1 changes cellular adenine nucleotide content in cells, ATP and AMP levels were examined in cultured hepatocytes after Rb1 (10 μM) or vehicle treatment for 6 h. We found that ATP was essentially the same (Fig. 4F), but AMP was significantly increased (Fig. 4G) by Rb1. Thus, the ratio of AMP to ATP was elevated by 2.5-fold (Fig. 4H). These data indicate that the increased ratio of AMP to ATP in cultured hepatocytes may stimulate the activation of AMPK.
DISCUSSION
In mammals, the liver plays a pivotal role in the metabolism of fatty acids. Using a HFD-induced obese rat model, we demonstrated that long-term (17 weeks) feeding of HFD to rats induced significant hepatic cellular fat accumulation and steatosis (10). Rb1, after being administered ip for four weeks, significantly reduced the lipid accumulation and attenuated hepatic steatosis in this animal model, and this change was associated with the activation of AMPK induced by Rb1 in the liver. Consistent with this, Rb1 attenuated ACC activation by increasing its phosphorylation and enhanced activity of CPT1, leading to an increased rate of fatty acid oxidation. Additionally, Rb1 stimulated fatty acid oxidative gene expression and suppressed the expression of genes encoding enzymes or proteins that function in lipogenesis. Therefore, our results are the first to suggest that Rb1 might serve as a therapeutic agent to prevent hepatic steatosis by targeting the hepatic AMPK system.
The Long-Evans rat strain has been used for studies of obesity and diabetes because of its susceptibility to these diseases when fed a high-fat diet (11). In the present study, we further demonstrated that these rats also developed fatty liver after chronic HFD feeding. The rats on a HFD had significantly increased hepatic TG content, which was confirmed by Oil Red O staining. The photomicrographs of liver sections stained with H and E revealed that the obese rats had developed steatosis and numerous lipid droplets (Fig. 1). The fatty liver in those obese animals was ameliorated by chronic treatment with Rb1. Rb1 significantly reduced the percentage of liver weight to body weight as well as the hepatic TG content. A marked reduction in the degree of steatosis and neutral lipids was noted in the livers from Rb1-treated obese rats versus saline-treated controls (Fig. 1). Caloric restriction alone also improved fatty liver, but the difference in all of these parameters (e.g., liver weight, hepatic TG content, and the degree of steatosis and neutral lipids) between the saline and pair-fed group was not as prominent as between the saline and Rb1 groups (Fig. 1), indicating that Rb1’s effects on the reduction of fatty liver in HFD-induced obese animals are beyond the simply restricted energy intake.
Lipid accumulation in liver may be caused by reduced lipid catabolism and/or enhanced de novo lipogenesis. We therefore assessed whether Rb1 reduces fatty liver by increasing fatty acid oxidation in the liver, and found that the level of oxidized palmitate in Rb1-treated hepatocytes was significantly higher than that in PBS-treated cells. To confirm that the higher palmitate oxidation induced by Rb1 is due to reduced inhibition of CPT1, we also assayed the activity of CPT1 and found that it was 2-fold higher in Rb1-treated hepatocytes compared with levels in PBS-treated cells (Fig. 2).
AMPK is a key sensor of cellular energy status and has been implicated in the control of hepatic lipid homeostasis (5). AMPK consists of a heterotrimeric complex containing a catalytic subunit α and two regulatory subunits β and γ. The α subunit contains a threonine residue (Thr 172) which could be phosphorylated by upstream kinases such as the serine/threonine-protein kinase LKB1 (22). Activation of AMPK by phosphorylating its α-subunit at site Thr172 leads to the phosphorylation and regulation of a number of downstream targets involved in lipid metabolism (24). Among these, ACC has been well identified. ACC is the rate-limiting enzyme in the synthesis of malonyl-CoA, which is not only a critical precursor for the biosynthesis of fatty acids, but also a potent inhibitor of mitochondrial fatty acid oxidation through its inhibitory action on CPT-1, a transporter of long-chain fatty acyl groups into the mitochondria to undergo β-oxidation (25). The point is that AMPK phosphorylates/inactivates ACC, leading to a fall in malonyl-CoA content, as well as a subsequent increase in fatty acid translocation and oxidation in the mitochondria.
We hypothesized that Rb1-induced improvement of fatty liver results from increased hepatic AMPK activity. To test this, we determined the phosphorylation levels of AMPK in Rb1-treated obese rats. Concomitant with substantially attenuated fat accumulation in the liver, chronic treatment with Rb1 significantly enhanced AMPK activation (Fig. 4C), suggesting that targeting the hepatic AMPK system plays an important role in Rb1’s antisteatotic function. This in vivo observation was further supported by our in vitro experiments using primary cultured rat hepatocytes, in which Rb1 treatment led to increased phosphorylation of AMPK and ACC in time- and dose-dependent manners (Fig. 4A, B).
AMPK is a Ser/Thr protein kinase, and the activity of AMPK is regulated by upstream activating kinases including the LKB1, a serine/threonine kinase (22), and by the AMP/ATP ratio (23). To determine the mechanisms mediating Rb1’s effect on AMPK, cultured hepatocytes were treated with Rb1 (10 μM) or vehicle for 6 h. LKB1 protein expression was measured by Western blot, and AMP and ATP contents were determined by HPLC analyses. LKB1 expression was not significantly increased in Rb1-treated cells (Fig. 4E) compared with PBS-treated cells. However, we found that Rb1 significantly increased the AMP levels compared with vehicle-treatment, while ATP levels were similar, thus leading to 2.5-fold increase in the ratio of AMP to ATP (Fig. 4F–H). These data indicate that the increased ratio of AMP to ATP induced by Rb1 in cultured hepatocytes may stimulate activation of AMPK.
As a major regulator of energy expenditure, AMPK has been found to coordinate metabolic programs that increase energy expenditure and decrease energy storage by modulating the activities of the key transcriptional regulators such as SREBP1c and PGC-1α (7). SREBP1c is well known as the transcription factor regulating the expression of genes encoding enzymes or proteins that function in lipogenesis in the liver including FAS, a key enzyme in de novo fatty acid synthesis in mammals (26), and SCD-1, a microsomal rate-limiting enzyme in the biosynthesis of monounsaturated fatty acids, which are necessary for the biosynthesis of TG in the liver (27). In the present study, expressions of SREBP1c, FAS, and SCD-1 were significantly downregulated by Rb1 treatment, indicating that Rb1-induced reduction of lipid accumulation is associated with decreased expression of SREBP1c and its downstream genes.
Decreased mitochondrial fatty acid oxidation has long been considered to be a major mechanism underlying disturbances in lipid accumulation in liver. PGC-1α is a crucial factor in the transcriptional regulation of mitochondrial biogenesis and fatty acid oxidation (28). PGC-1α was initially discovered as a coactivator for PPARγ. It has subsequently been found to serve as a coactivator of PPARα and is therefore involved in the transcriptional control of the genes encoding enzymes of fatty acid oxidation (29). PPARα, which is highly expressed in the liver, coordinates transcriptional activation of peroxisomal fatty Acox1 (30) and CPT1A (31, 32). In the current study, Rb1 significantly increased the activity of CPT1 (Fig. 2B) and gene expression of PGC1α, PPARα, and Acox1 (Fig. 3), consistent with upregulated fatty acid β-oxidation in both mitochondria and peroxisomes of hepatic cells. All of the regulatory changes in gene expression observed in cultured hepatocytes given Rb1 were further confirmed in liver tissues from Rb1- or vehicle-treated obese rats (Fig. 3C). Interestingly, the effects of Rb1 on the expression of several key genes, including SREBP1c, FAS, PGC1α, and PPARα, were significantly attenuated by compound C, an inhibitor of AMPK signaling pathway (Fig. 3B), indicating that such effects of Rb1 require the activation of AMPK pathway.
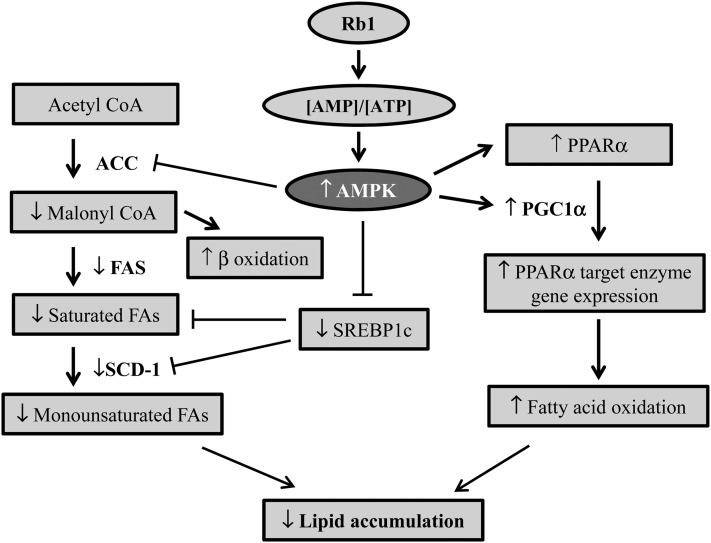
In conclusion, our data demonstrate that Rb1 significantly reduces cellular lipid accumulation in the liver of HFD-induced obese animals. As depicted in Fig. 5, this effect is likely mediated by increasing the AMP/ATP ratio, leading to the activation of the AMPK signaling pathway. Consistent with this, Rb1 treatment increased the rate of fatty acid oxidation, stimulated the expression of fatty acid oxidative genes, and suppressed the expression of genes encoding enzymes or proteins that function in lipogenesis. These data contribute new understanding as to how Rb1 affects lipid metabolism, and provide strong evidence for further evaluation of the potential therapeutic role of Rb1 in NAFLD.
Fig. 5.
Schematic diagram of the potential mechanisms that underlie the protective action of Rb1 against fatty liver. Rb1 significantly increased the cellular ratio of AMP to ATP, which then activates AMPK and phosphorylates and inhibits the activity of ACC, resulting in reduced malonyl-CoA formation and increased fatty acid oxidation. Additionally, the activated AMPK suppresses the expression of genes encoding enzymes or proteins that function in lipogenesis, including SREBP1c, FAS, and SCD-1, leading to reduced fatty acid synthesis. Rb1 also significantly increased the expression of PGC-1α, PPARα, and Acox1, which enhances fatty acid β-oxidation in hepatic cells.
Acknowledgments
The authors acknowledge Dr. Jeffrey Welge for statistical consultation.
Footnotes
Abbreviations:
- ACC
- acetyl-CoA carboxylase
- Acox1
- acyl-CoA oxidase 1
- AMPK
- AMP-activated protein kinase
- CPT
- carnitine palmitoyltransferase
- FAS
- fatty acid synthase
- H and E
- hematoxylin and eosin
- HFD
- high-fat diet
- NAFLD
- nonalcoholic fatty liver disease
- PGC-1α
- proliferator-activated receptor γ coactivator-1α
- PPAR
- peroxisome proliferator-activated receptor
- qPCR
- quantitative real-time PCR
- Rb1
- ginsenoside Rb1
- SCD-1
- stearoyl-CoA desaturase-1
- SREBP1c
- sterol regulatory element-binding protein 1c
- TG
- triglyceride
This work was supported by the National Center for Research Resources and the National Institute of Diabetes and Digestive and Kidney Diseases through grant numbers DK95440, DK92779, DK70992, and DK77170.
REFERENCES
- 1.Postic C., Girard J. 2008. Contribution of de novo fatty acid synthesis to hepatic steatosis and insulin resistance: lessons from genetically engineered mice. J. Clin. Invest. 118: 829–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neuschwander-Tetri B. A., Caldwell S. H. 2003. Nonalcoholic steatohepatitis: summary of an AASLD Single Topic Conference. Hepatology. 37: 1202–1219 [DOI] [PubMed] [Google Scholar]
- 3.Parekh S., Anania F. A. 2007. Abnormal lipid and glucose metabolism in obesity: implications for nonalcoholic fatty liver disease. Gastroenterology. 132: 2191–2207 [DOI] [PubMed] [Google Scholar]
- 4.Adams L. A., Angulo P. 2006. Treatment of non-alcoholic fatty liver disease. Postgrad. Med. J. 82: 315–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kahn B. B., Alquier T., Carling D., Hardie D. G. 2005. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 1: 15–25 [DOI] [PubMed] [Google Scholar]
- 6.Lin C. L., Huang H. C., Lin J. K. 2007. Theaflavins attenuate hepatic lipid accumulation through activating AMPK in human HepG2 cells. J. Lipid Res. 48: 2334–2343 [DOI] [PubMed] [Google Scholar]
- 7.Zhou G., Myers R., Li Y., Chen Y., Shen X., Fenyk-Melody J., Wu M., Ventre J., Doebber T., Fujii N., et al. 2001. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Invest. 108: 1167–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Viollet B., Foretz M., Guigas B., Horman S., Dentin R., Bertrand L., Hue L., Andreelli F. 2006. Activation of AMP-activated protein kinase in the liver: a new strategy for the management of metabolic hepatic disorders. J. Physiol. 574: 41–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park S., Ahn I. S., Kwon D. Y., Ko B. S., Jun W. K. 2008. Ginsenosides Rb1 and Rg1 suppress triglyceride accumulation in 3T3–L1 adipocytes and enhance beta-cell insulin secretion and viability in Min6 cells via PKA-dependent pathways. Biosci. Biotechnol. Biochem. 72: 2815–2823 [DOI] [PubMed] [Google Scholar]
- 10.Xiong Y., Shen L., Liu K. J., Tso P., Xiong Y., Wang G., Woods S. C., Liu M. 2010. Antiobesity and antihyperglycemic effects of ginsenoside Rb1 in rats. Diabetes. 59: 2505–2512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Woods S. C., Seeley R. J., Rushing P. A., D'Alessio D., Tso P. 2003. A controlled high-fat diet induces an obese syndrome in rats. J. Nutr. 133: 1081–1087 [DOI] [PubMed] [Google Scholar]
- 12.Folch J., Lees M., Sloane Stanley G. H. 1957. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 226: 497–509 [PubMed] [Google Scholar]
- 13.Brunt E. M., Janney C. G., Di Bisceglie A. M., Neuschwander-Tetri B. A., Bacon B. R. 1999. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am. J. Gastroenterol. 94: 2467–2474 [DOI] [PubMed] [Google Scholar]
- 14.Sanyal A. J. 2002. AGA technical review on nonalcoholic fatty liver disease. Gastroenterology. 123: 1705–1725 [DOI] [PubMed] [Google Scholar]
- 15.Rector R. S., Thyfault L. P., Morris R. T., Laye M. J., Borengasser S. J., Booth F. W., Ibdah J. A. 2008. Daily exercise increases hepatic fatty acid oxidation and prevents steatosis in Otsuka Long-Evans Tokushima Fatty rats. Am. J. Physiol. Gastrointest. Liver Physiol. 294: G619–G626 [DOI] [PubMed] [Google Scholar]
- 16.Shen L., Hillebrand A., Wang D. Q., Liu M. 2012. Isolation and primary culture of rat hepatic cells. J. Vis. Exp. (64): 3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moon A., Rhead W. J. 1987. Complementation analysis of fatty acid oxidation disorders. J. Clin. Invest. 79: 59–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sleboda J., Risan K. A., Spydevold O., Bremer J. 1999. Short-term regulation of carnitine palmitoyltransferase I in cultured rat hepatocytes: spontaneous inactivation and reactivation by fatty acids. Biochim. Biophys. Acta. 1436: 541–549 [DOI] [PubMed] [Google Scholar]
- 19.Shen L., Wang D. Q., Lo C. M., Tso P., Davidson W. S., Woods S. C., Liu M. 2010. Estradiol increases the anorectic effect of central apolipoprotein A-IV. Endocrinology. 151: 3163–3168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shen L., Tso P., Woods S. C., Sakai R. R., Davidson W. S., Liu M. 2007. Hypothalamic apolipoprotein A-IV is regulated by leptin. Endocrinology. 148: 2681–2689 [DOI] [PubMed] [Google Scholar]
- 21.Liu H., Jiang Y. M., Luo Y. B., Jiang W. B. 2006. A simple and rapid determination of ATP, ADP and AMP concentrations in pericarp tissue of litchi fruit by high performance liquid chromatography. Food Technol. Biotechnol. 44: 531–534 [Google Scholar]
- 22.Woods A., Johnstone S. R., Dickerson K., Leiper F. C., Fryer L. G., Neumann D., Schlattner U., Wallimann T., Carlson M., Carling D. 2003. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr. Biol. 13: 2004–2008 [DOI] [PubMed] [Google Scholar]
- 23.Suter M., Riek U., Tuerk R., Schlattner U., Wallimann T., Neumann D. 2006. Dissecting the role of 5′-AMP for allosteric stimulation, activation, and deactivation of AMP-activated protein kinase. J. Biol. Chem. 281: 32207–32216 [DOI] [PubMed] [Google Scholar]
- 24.Tessari P., Coracina A., Cosma A., Tiengo A. 2009. Hepatic lipid metabolism and non-alcoholic fatty liver disease. Nutr. Metab. Cardiovasc. Dis. 19: 291–302 [DOI] [PubMed] [Google Scholar]
- 25.Munday M. R. 2002. Regulation of mammalian acetyl-CoA carboxylase. Biochem. Soc. Trans. 30: 1059–1064 [DOI] [PubMed] [Google Scholar]
- 26.Shimano H. 2000. Sterol regulatory element-binding protein-1 as a dominant transcription factor for gene regulation of lipogenic enzymes in the liver. Trends Cardiovasc. Med. 10: 275–278 [DOI] [PubMed] [Google Scholar]
- 27.Ntambi J. M. 1995. The regulation of stearoyl-CoA desaturase (SCD). Prog. Lipid Res. 34: 139–150 [DOI] [PubMed] [Google Scholar]
- 28.Finck B. N., Kelly D. P. 2006. PGC-1 coactivators: inducible regulators of energy metabolism in health and disease. J. Clin. Invest. 116: 615–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vega R. B., Huss J. M., Kelly D. P. 2000. The coactivator PGC-1 cooperates with peroxisome proliferator-activated receptor alpha in transcriptional control of nuclear genes encoding mitochondrial fatty acid oxidation enzymes. Mol. Cell. Biol. 20: 1868–1876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee S. S., Pineau T., Drago J., Lee E. J., Owens J. W., Kroetz D. L., Fernandez-Salguero P. M., Westphal H., Gonzalez F. J. 1995. Targeted disruption of the alpha isoform of the peroxisome proliferator-activated receptor gene in mice results in abolishment of the pleiotropic effects of peroxisome proliferators. Mol. Cell. Biol. 15: 3012–3022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim Y. I., Hirai S., Goto T., Ohyane C., Takahashi H., Tsugane T., Konishi C., Fujii T., Inai S., Iijima Y., et al. 2012. Potent PPARalpha activator derived from tomato juice, 13-oxo-9,11-octadecadienoic acid, decreases plasma and hepatic triglyceride in obese diabetic mice. PLoS ONE. 7: e31317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rakhshandehroo M., Hooiveld G., Muller M., Kersten S. 2009. Comparative analysis of gene regulation by the transcription factor PPARalpha between mouse and human. PLoS ONE. 4: e6796. [DOI] [PMC free article] [PubMed] [Google Scholar]