Abstract
Cytochrome P450 epoxygenase 2J2 (CYP2J2) metabolizes arachidonic acids to form epoxyeicosatrienoic acids (EETs), which possess various beneficial effects on the cardiovascular system. However, whether increasing EETs production by CYP2J2 overexpression in vivo could prevent abdominal aortic aneurysm (AAA) remains unknown. Here we investigated the effects of recombinant adeno-associated virus (rAAV)-mediated CYP2J2 overexpression on angiotensin (Ang) II-induced AAA in apoE-deficient mice. rAAV-CYP2J2 delivery led to an abundant aortic CYP2J2 expression and increased EETs generation. It was shown that CYP2J2 overexpression attenuated matrix metalloproteinase expression and activity, elastin degradation, and AAA formation, which was associated with reduced aortic inflammation and macrophage infiltration. In cultured vascular smooth muscle cells (VSMCs), rAAV-mediated CYP2J2 overexpression and EETs markedly suppressed Ang II-induced inflammatory cytokine expression. Moreover, overexpressed CYP2J2 and EETs inhibited Ang II-induced macrophage migration in a VSMC-macrophage coculture system. We further indicated that these protective effects were mediated by peroxisome proliferator-activated receptor (PPAR)γ activation. Taken together, these results provide evidence that rAAV-mediated CYP2J2 overexpression prevents AAA development which is likely via PPARγ activation and anti-inflammatory action, suggesting that increasing EETs levels could be considered as a potential strategy to prevent and treat AAA.
Keywords: epoxyeicosatrienoic acid, cytochrome P450 epoxygenase 2J2, abdominal aortic aneurysm, angiotensin II, inflammation
Abdominal aortic aneurysms (AAAs) mostly occur in humans over 65 years old (1, 2). The most dreaded complication of AAA is rupture, and it is the 13th leading cause of death in the United States (1). At the present time, there is no efficacious pharmacological therapy, and the surgical treatments carry a high mortality (2). In the past decades, many studies supported the view that inflammation played an essential role in the pathogenesis of the disease (2–4).
Cytochrome P450 epoxygenase 2J2 (CYP2J2), which is of human origin and mainly expressed in the cardiovascular system, metabolizes arachidonic acids to epoxyeicosatrienoic acids (EETs) (5). EETs possess diverse biological functions, and observations reveal that EETs exert protective effects on various cardiovascular diseases, including attenuation of heart injuries and anti-hypertension (6–13). Recently, Zhang et al. (5, 14) reported that administration of soluble epoxide hydrolase (s-EH) inhibitor, which prevents EET hydration, could prevent angiotensin (Ang) II-induced AAA in mice. However, the underlying mechanisms by which EETs exert the effect and whether increased circulation of EETs by CYP2J2 overexpression could prevent AAA formation remain unknown.
EETs are the ligands of peroxisome proliferator-activated receptor (PPAR)γ and exert anti-inflammatory effects (15). Jones et al. (16) recently reported that activation of PPARγ by rosiglitazone could reduce the progression and rupture of AAA in mice. Regarding the essential role of inflammation during AAA development (3, 4), we therefore hypothesized that increased EETs resulting from CYP2J2 overexpression in vivo may prevent the development of AAA in mice potentially via its anti-inflammatory effects through PPARγ activation.
In this study, we examined the beneficial effects of recombinant adeno-associated virus (rAAV)-mediated CYP2J2 overexpression on Ang II-induced AAA in apoE-deficient (ApoE−/−) mice. Our data strongly suggest that rAAV-mediated CYP2J2 overexpression is protective against AAA development, which is potentially mediated by PPARγ activation to reduce aortic inflammation.
MATERIALS AND METHODS
Construction and preparation of rAAV vectors
The rAAV vectors (type 2) containing CYP2J2 or green fluorescent protein (GFP) were produced by triple plasmid cotransfection in HEK293 cells as previously described (17–19). The vectors were purified, titered, and stored at −80°C before use.
Animals
ApoE−/− mice (C57BL/6 background, n = 32) were housed at the animal care facility of Tongji Medical College under specific pathogen-free conditions, and were fed with normal diet. All animal experimental protocols complied with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996) and the Public Health Service (PHS) Policy on Humane Care and Use of Laboratory Animals. The animal studies were approved by the Institutional Animal Research Committee of Tongji Medical College.
AAA model and gene delivery protocols
AAAs were induced in ApoE−/− mice by a 28 day continuous Ang II (1,000 ng/kg/min) (Sigma-Aldrich, St. Louis, MO) infusion by subcutaneous osmotic pump implantation as described previously (16, 20). Thirty-two 8-week-old ApoE−/− mice were randomly assigned into 4 groups: control group (n = 8), receiving saline; Ang II group (n = 8), receiving Ang II (1000 ng/kg/min); Ang II + rAAV-GFP group (n = 8), receiving Ang II (1,000 ng/kg/min) infusion supplemented with rAAV-GFP injection; and Ang II + rAAV-CYP2J2 group, receiving Ang II (1,000 ng/kg/min) infusion supplemented with rAAV-CYP2J2 injection. For animals in the Ang II + rAAV-GFP and Ang II + rAAV-CYP2J2 groups, corresponding rAAV-GFP and rAAV-CYP2J2 (1 × 1011 pfu) respectively, were injected via tail veins 4 weeks before Ang II infusion.
Analysis and quantification of AAA
Animals were sacrificed at the end of the interventions. For AAA quantification, the maximum width of the abdominal aorta was measured in each mouse by Image Pro Plus software (Media Cybernetics, Bethesda, MD). AAA in mice was defined as a 50% or greater increase in the external width of the suprarenal aorta compared with aortas from the controls (21).
Histological analysis
Abdominal aortic tissues were harvested, fixed in 4% paraformaldehyde in PBS, and embedded in paraffin for histological analysis. Some aortic tissues were obtained and kept frozen in liquid nitrogen immediately, and then stored at −80°C for Western blot and gelatin zymographic analysis. Three micron cross-sections were prepared and subsequently stained with hematoxylin and eosin, and van Gieson, respectively. Immunohistochemical staining was performed according to the manufacture's description (Zsbio, Beijing, China) as described (19, 22). The following antibodies were applied: matrix metalloproteinase (MMP)2, MMP9, CD68, and monocyte chemotactic protein-1 (MCP-1) from Santa Cruz Biotechnologies (Santa Cruz, CA). The immunohistochemical staining results were quantified by Image Pro Plus software as described previously (19). For quantifying elastin degradation, a standard for the grades of elastin degradation was applied as described previously (21). The grades were defined briefly as follows: grade 1, no degradation; grade 2, mild elastin degradation; grade 3, severe elastin degradation; and grade 4, aortic rupture (21).
Determination of MMPS activity
The evaluation of MMPS activity was performed as described previously (23). Twenty micrograms of protein in tissue homogenates was electrophoresed in SDS-PAGE gels containing 1 mg/ml gelatin. Gels were washed in 2.5% Triton X-100 for 30 min and incubated overnight in zymography developing buffer at 37°C. Gels were stained with Coomassie brilliant blue.
Evaluation of serum and urine EETs and dihydroxyeicosatrienoic acids
Serum and urine samples from all mice were collected. ELISA kits (Detroit R and D, Detroit, MI) were used to determine concentrations of the 11,12- and 14,15-EETs, and their stable metabolites 11,12- and 14,15-dihydroxyeicosatrienoic acids (DHETs) in serum and urine as described previously (24).
Measurement of serum lipid profiles
Serum concentrations of total cholesterol, triglyceride, LDL, and HDL were measured with the indicated kits (Biosino, Beijing, China) following the manufacturer's instructions.
Cell culture and treatments
Vascular smooth muscle cells (VSMCs) were harvested from the aortas of wild-type C57BL/6 mice as previously described (21, 25). VSMCs and RAW264.7 cells (ATCC, VA), a mouse macrophage cell line, were cultured in 10% FBS (Gibco, Grand Island, NY) containing DMEM (Gibco) under 37°C and 5% CO2 conditions. After confluence, VSMCs were incubated with 10 μmol/l Ang II (Sigma-Aldrich) for 12 h. If needed, 100 nmol/l 8,9-, 11,12-, or 14,15-EET (Sigma-Aldrich), 10 μmol/l compound 26 (C26) (specific CYP2J2 inhibitor synthesized in our laboratory), 1 μmol/l GW9662 (Sigma-Aldrich), and 10 μmol/l BAY 11-7082 (Sigma-Aldrich) were added 1 h before Ang II treatment. For rAAV-CYP2J2 or rAAV-GFP transfection, VSMCs were plated in 6-well plates, and after 60% confluence, viral solutions of rAAV-CYP2J2 or rAAV-GFP were added respectively (26), and incubated for 7 days.
Luciferase assays
The PPAR and nuclear factor κB (NF-κB) reporter kits were purchased from SABiosciences (Valencia, CA). For PPAR activation assay, HEK293 cells were transfected in 24-well plates with pcDNA-PPARγ, PPAR-reporter, or negative control by Lipofectamine 2000 reagents (Invitrogen, Carlsbad, CA) for 24 h. Cells were then treated with or without 100 nmol/l 11,12-EET, DMSO, or 1 μmol/l GW9662. For NF-κB activation assay, HEK293 cells were transfected with NF-κB reporter or negative control for 24 h, and then dosed with 10 μmol/l Ang II, 100 nmol/l 11,12-EET, or 1 μmol/l GW9662 accordingly. Luciferase activity was determined 6 h after incubation using the Dual-Luciferase Reporter Assay System (Promega, Madison, WI) as described previously (27).
ELISA analysis of inflammatory cytokines
The levels of interleukin (IL)-1β, IL-6, and MCP-1 in mouse serum and in VSMC cultured media were assayed using ELISA kits from R&D Systems (Minneapolis, MN) following the manufacturer's instructions.
Western blot analysis
Tissue samples and cells were harvested and homogenized, and Western blots were performed using the antibodies against MMP2, MMP9, MCP-1, PPARγ, NF-κB p65, CYP2J2, GFP, β-actin, lamin B1 (Santa Cruz Biotechnologies), and inhibitor of nuclear factor κB α (IκBα) (Cell Signaling Technology, Danvers, MA) respectively. β-actin and lamin B1 were used as internal references for total protein or cytosolic protein determination and nuclear protein determination, respectively. Bands were quantified by densitometry using Quantity One software (Bio-Rad, Hercules, CA).
Macrophage migration determination
Macrophage chemotaxis assay was performed as described previously (25). Macrophages (2 × 105; RAW264.7, a monocyte/macrophage cell line) were placed in the upper chamber of Costar 24-well transwell plates with 5 μm pore filters (Corning, Inc., Corning, NY), while the lower chamber was plated with confluent VSMCs. Ang II (10 μmol/l) was added to stimulate macrophage migration. If needed, 100 nmol/l 11,12-EET, 10 μmol/l C26, 1 μmol/l GW9662, and 10 μg/ml anti-MCP-1 antibody (Novus Biologicals, Littleton, CO) or mouse IgG (Novus Biologicals) were added before Ang II incubation. After incubating for 6 h at 37°C, migrated cells on the bottom of the filters were stained with DAPI (Sigma-Aldrich) and counted.
Statistical analysis
All data are presented as mean ± SD. After confirming the normal distribution using the Kolmogorov-Smirnov test, statistical differences were evaluated by ANOVA followed by Bonferroni's multiple comparison test (28). P < 0.05 was accepted as statistically significant.
RESULTS
Delivery of rAAV-CYP2J2-induced overexpression of aortic CYP2J2 and increased circulating EETs levels in ApoE−/− mice significantly
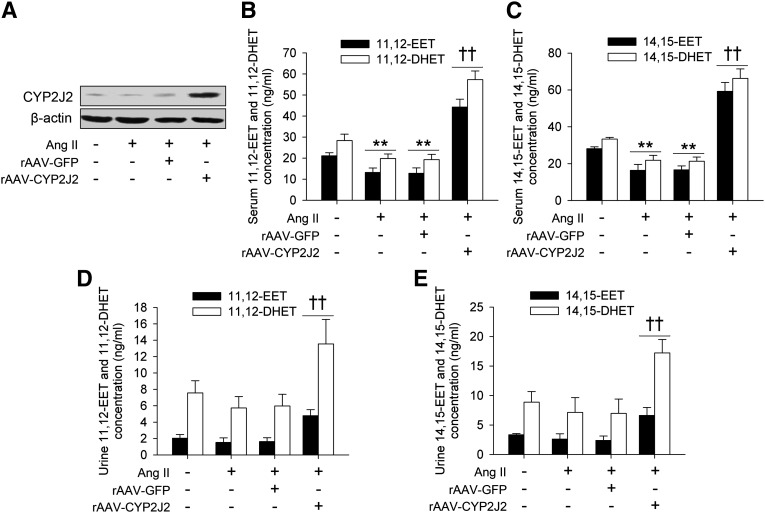
Eight weeks after rAAV-CYP2J2 injection, CYP2J2 expression in the abdominal aortic tissue was abundant as evaluated by Western blot (Fig. 1A). CYP2J2 metabolizes arachidonic acids to form EETs, and EETs can be quickly hydrolyzed to their corresponding DHETs with much lower biological activity. We therefore determined the levels of 11,12- and 14,15-EETs, and their corresponding 11,12- and 14,15-DHETs in serum and urine, respectively. As depicted in Fig. 1B–E, rAAV-CYP2J2 injection caused a significant elevation in both serum and urine levels of 11,12- and 14,15-EETs, as well as their corresponding 11,12- and 14,15-DHETs. Interestingly, we also found that the 11,12- and 14,15-EET levels in Ang II-infused mice were lower than the controls (Fig. 1B–E). These results suggest that the overexpressed CYP2J2 induces production of EETs in vivo.
Fig. 1.
rAAV-CYP2J2 delivery led to aortic CYP2J2 overexpression and increased EET synthesis in vivo. A: CYP2J2 was overexpressed in aortic tissues after rAAV-CYP2J2 injection. rAAV-CYP2J2 injection increased the serum concentrations of 11,12-EET and corresponding 11,12-DHET (B), as well as 14,15-EET and 14,15-DHET (C). In addition, Ang II infusion led to a marked reduction of serum 11,12- and 14,15-EETs, and 11,12- and 14,15-DHETs (B, C). rAAV-CYP2J2 delivery also markedly elevated the urine concentrations of 11,12-EET and 11,12-DHET (D), as well as 14,15-EET and 14,15-DHET (E) (n = 8 for each group; **P < 0.01 vs. control; ††P < 0.01 vs. Ang II + rAAV-GFP).
The effects of rAAV-CYP2J2 delivery on circulating lipid profiles in Ang II-infused ApoE−/− mice
ApoE−/− mice develop hypercholesterolemia spontaneously. As shown in Table 1, there were no significant differences in lipid profiles between Ang II-infused mice and the controls. Treatment with rAAV-CYP2J2 lowered the total cholesterol level. However, CYP2J2 overexpression had no significant effects on triglyceride, LDL, and HDL levels among groups, although CYP2J2 had a LDL-lowering trend.
TABLE 1.
Serum lipid profiles in ApoE−/− mice with different interventions
| Control | Ang II | Ang II + rAAV-GFP | Ang II + rAAV-CYP2J2 | |
| Total cholesterol, mmol/l | 16.72 ± 2.31 | 17.53 ± 2.96 | 17.22 ± 2.85 | 15.66 ± 1.23*††‡‡ |
| Triglyceride, mmol/l | 1.78 ± 0.17 | 1.86 ± 0.44 | 1.83 ± 0.38 | 1.69 ± 0.23 |
| LDL, mmol/l | 15.35 ± 1.43 | 16.74 ± 3.02 | 16.18 ± 2.59 | 14.89 ± 1.67 |
| HDL, mmol/l | 0.43 ± 0.05 | 0.37 ± 0.09 | 0.36 ± 0.10 | 0.39 ± 0.06 |
n = 8 for each group;
P < 0.05 versus control;
P < 0.01 versus Ang II;
P < 0.01 versus Ang II + rAAV-GFP.
CYP2J2 overexpression suppressed Ang II-induced AAA formation in ApoE−/− mice
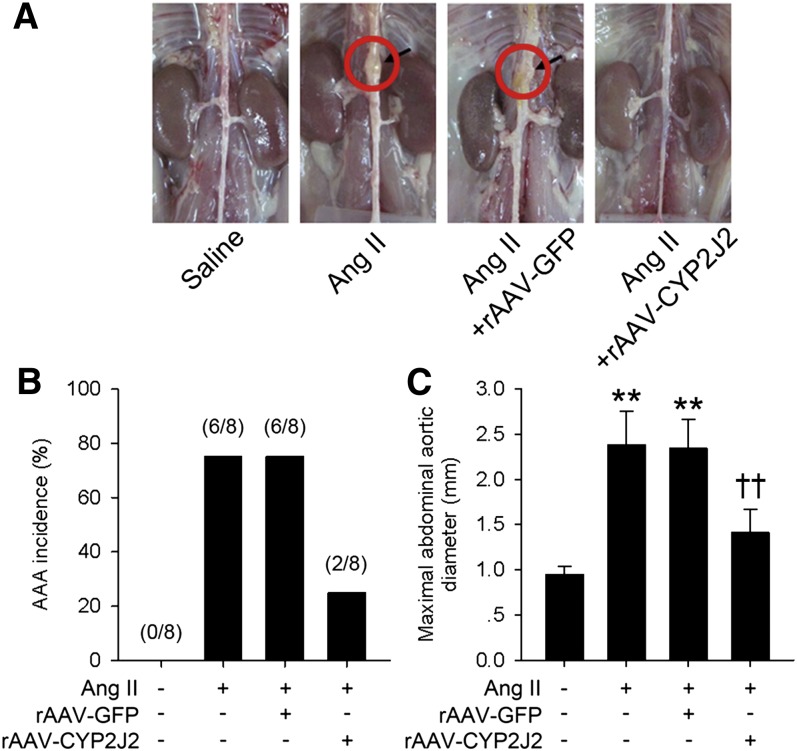
We next assessed the effects of CYP2J2 overexpression on Ang II-induced AAA progression. After 4 weeks, Ang II infusion significantly increased the incidence of AAA formation (75%, 6 of 8) and maximal aortic diameters in ApoE−/− mice (Fig. 2). However, rAAV-CYP2J2 treatment markedly lowered the incidence of AAA (25%, 2 of 8) and decreased the maximal aortic diameters (Fig. 2).
Fig. 2.
rAAV-mediated CYP2J2 overexpression attenuated Ang II-induced AAA development in ApoE−/− mice. A: Representative images of aortas isolated from mice with indicated interventions. rAAV-mediated CYP2J2 overexpression significantly reduced AAA incidence (B) and attenuated maximal abdominal aortic diameter enlargement (C) in Ang II infused mice (n = 8 for each group; **P < 0.01 vs. control; ††P < 0.01 vs. Ang II + rAAV-GFP).
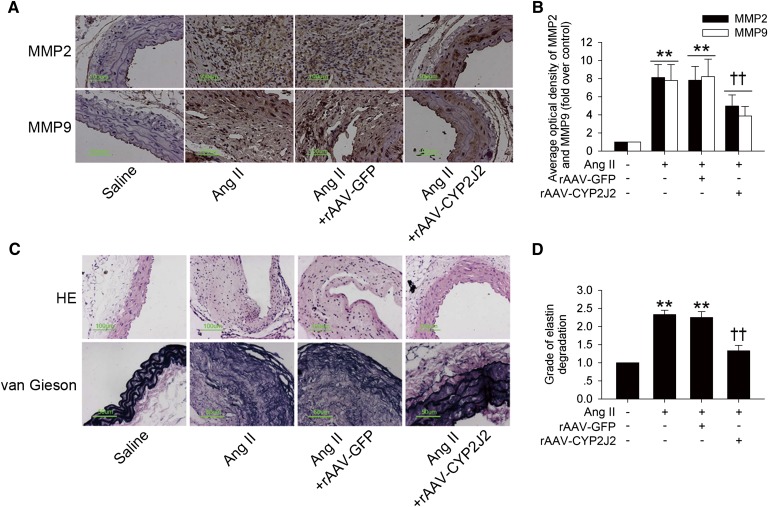
MMPs, especially MMP2 and MMP9, are responsible for aortic elastin and collagen degradation, and thus play a key role in the initiation and development of AAA (21, 29). Ang II infusion led to a marked increase in MMP2 and MMP9 expression (Fig. 3A, B and supplementary Fig. I), as well as their activity in abdominal aortas assayed by gelatin zymography (supplementary Fig. II), while rAAV-CYP2J2 delivery greatly prevented these effects in abdominal aortic tissue, in contrast. Moreover, CYP2J2 overexpression also markedly inhibited aortic elastin degradation induced by Ang II (Fig. 3C, D). Taken together, these results indicated that CYP2J2 overexpression protected ApoE−/− mice against Ang II-induced AAA development.
Fig. 3.
rAAV-CYP2J2 delivery reduced aortic MMPs synthesis and elastin degradation induced by Ang II infusion in ApoE−/− mice. A: Representative MMP2 and MMP9 immunohistochemical staining images of abdominal aortas with indicated interventions. B: CYP2J2 overexpression markedly attenuated Ang II infusion-induced aortic MMPs expression. C: Representative images of hematoxylin and eosin (HE) and elastin van Gieson staining of abdominal aortas with different interventions. D: rAAV-mediated CYP2J2 overexpression significantly suppressed Ang II-induced aortic elastin degradation (n = 8 for each group; **P < 0.01 vs. control; ††P < 0.01 vs. Ang II + rAAV-GFP).
CYP2J2 overexpression reduced aortic inflammation and restored aortic PPARγ expression induced by Ang II infusion
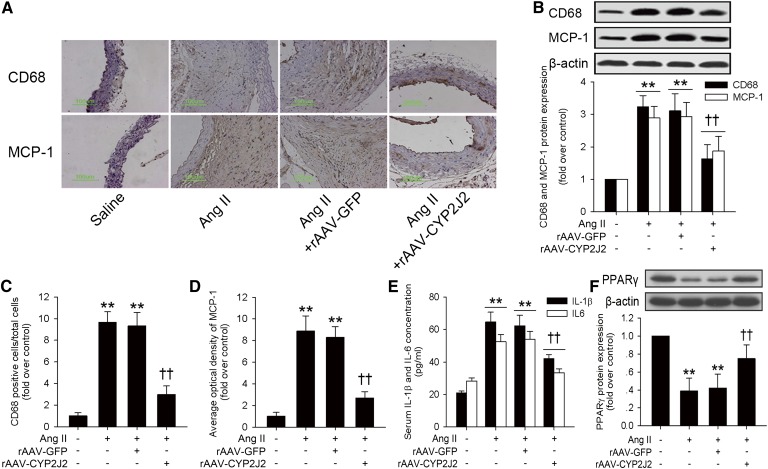
Inflammation plays a central role throughout the progression of AAA (3, 4). We found that Ang II infusion substantially increased aortic macrophage infiltration and MCP-1 expression as evaluated by both immunohistochemical staining and Western blot (Fig. 4A–D). Moreover, serum concentrations of inflammatory cytokines were also elevated in Ang II-infused mice, while CYP2J2 overexpression markedly suppressed these effects (Fig. 4E). Furthermore, Ang II infusion reduced the aortic expression of PPARγ, while CYP2J2 overexpression significantly attenuated this effect (Fig. 4F). These results suggest that CYP2J2 overexpression is associated with reduced aortic inflammation, which might possibly be mediated by PPARγ activation.
Fig. 4.
rAAV-mediated CYP2J2 overexpression reduced Ang II-induced aortic inflammation and restored aortic PPARγ expression in ApoE−/− mice. A: Representative immunohistochemical staining images for macrophage marker CD68 and MCP-1 in abdominal aortas with corresponding interventions. CYP2J2 overexpression attenuated Ang II-induced aortic macrophage infiltration and MCP-1 expression as evaluated by Western blot (B) and immunohistochemical staining quantification (C, D). ELISA analysis showed that rAAV-CYP2J2 delivery also reduced concentrations of inflammatory cytokines IL-1β and IL-6 induced by Ang II infusion. E, F: Ang II infusion significantly reduced aortic PPARγ expression in ApoE−/− mice. However, rAAV-CYP2J2 injection markedly prevented this effect (n = 8 for each group; **P < 0.01 vs. control; ††P < 0.01 vs. Ang II + rAAV-GFP).
CYP2J2 overexpression and EETs reduced Ang II-induced inflammatory response in VSMCs
VSMCs are the major cellular component in the aorta (21). We examined the effects of CYP2J2 treatment on inflammatory cytokine expression in VSMCs. rAAV-CYP2J2 transfection led to a substantial expression of CYP2J2 in VSMCs (supplementary Fig. III). Moreover, ELISA analysis showed that CYP2J2 overexpression markedly suppressed the expression of inflammatory cytokines including IL-6 and MCP-1 induced by Ang II (supplementary Fig. IV). These effects could be markedly suppressed by C26, a selective CYP2J2 epoxygenase inhibitor (supplementary Fig. V) (30).
We next assayed the effects of CYP2J2 metabolites EETs on inflammatory cytokine expression in VSMCs. As depicted in supplementary Fig. VI, 8,9-, 11,12-, and 14,15-EETs all markedly reduced IL-6 and MCP-1 expression induced by Ang II in VSMCs, and 11,12-EET exhibited the most significant effects. Therefore, we used 11,12-EET as the representative EET in the following studies.
The anti-inflammatory effects of 11,12-EET are through the PPARγ-mediated NF-κB pathway
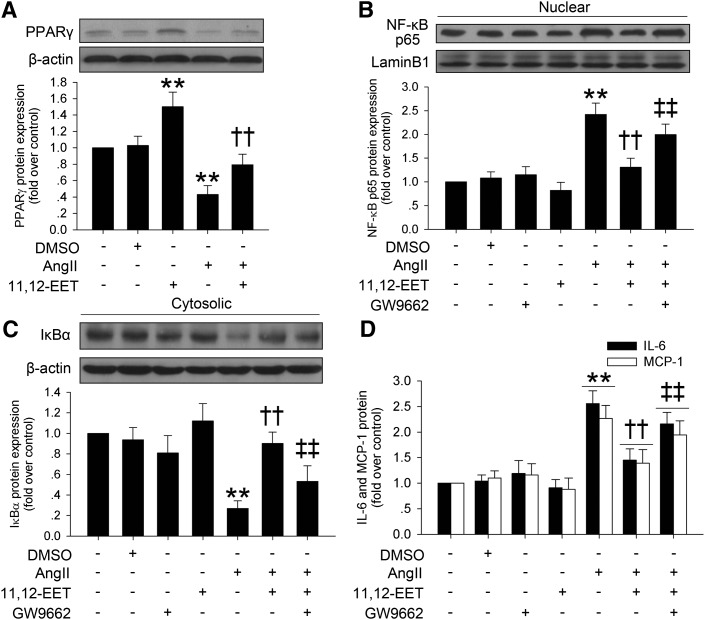
It has been documented that PPARγ is an effector of EETs (15), we therefore investigated whether the anti-inflammatory effects of EETs were mediated by PPARγ activation in VSMCs. Consistent with observations in the in vivo AAA model, Ang II incubation significantly reduced PPARγ expression in VSMCs, and 11,12-EET markedly restrained this effect (Fig. 5A). We further found that 11,12-EET markedly induced PPARγ activity in HEK293 cells (supplementary Fig. VII).
Fig. 5.
The protective effects of 11,12-EET on Ang II-induced inflammatory responses is mediated by PPARγ activation in VSMCs. A: 11,12-EET (100 nmol/l) incubation markedly increased PPARγ expression and restored Ang II-induced (10 μmol/l) PPARγ reduction in VSMCs. B, C: 11,12-EET reduced Ang II-induced nuclear NF-κB p65 expression and cytosolic IκBα degradation, while PPARγ antagonist GW9662 (1 μmol/l) markedly inhibited these protective effects. D: ELISA analysis showed that 11,12-EET prevented Ang II-induced IL-6 and MCP-1 expression, but GW9662 (1 μmol/l) significantly blocked this effect (n = 3 for each experiment; **P < 0.01 vs. control; ††P < 0.01 vs. Ang II; ‡‡P < 0.01 vs. Ang II + 11,12-EET).
The activation of PPARγ has been shown to inhibit the activation of NF-κB. As shown in Fig. 5B, C, Ang II incubation reduced the expression of cytosolic IκBα and led to a significant elevation in NF-κB p65 nuclear translocation. As expected, pretreatment with 11,12-EET markedly inhibited Ang II-induced NF-κB activation, which could be significantly suppressed by addition of GW9662, a selective PPARγ inhibitor (Fig. 5B, C, supplementary Fig. VIII).
We assayed the effects of the EET/PPARγ/NF-κB pathway in Ang II-induced inflammatory cytokine expression in VSMCs. Results showed that 11,12-EET preincubation reduced the expression of IL-6 and MCP-1 induced by Ang II in VSMCs, and GW9662 significantly inhibited these effects of 11,12-EET (Fig. 5D). The pro-inflammatory role of NF-κB in VSMCs was further supported by the effects of the NF-κB inhibitor BAY 11-7082, which suppressed the Ang II-induced expression of IL-6 and MCP-1 (supplementary Fig. IX).
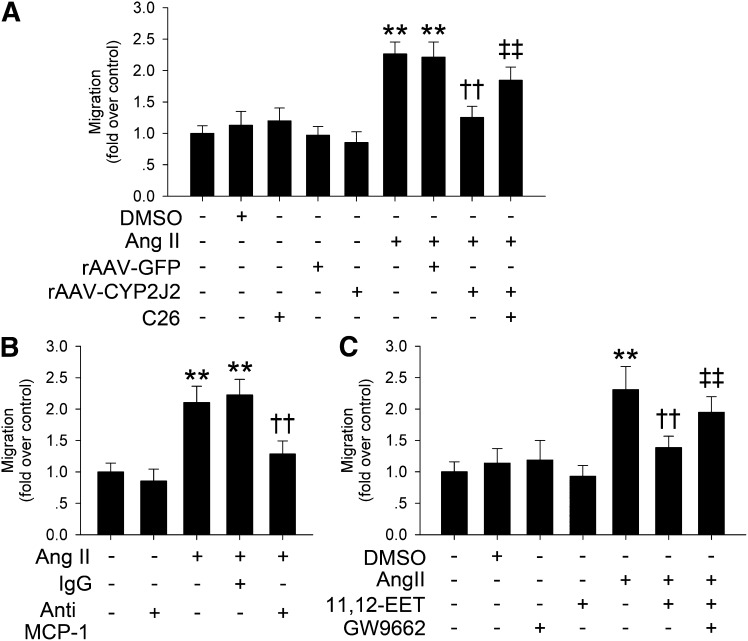
CYP2J2 overexpression and 11,12-EET inhibited macrophage migration via PPARγ activation
Infiltrated macrophages are thought to be the major source of elastase activity in aneurismal tissues (25). We applied a macrophage-VSMC coculture system to investigate the effects of CYP2J2 overexpression and 11,12-EET on Ang II-induced macrophage migration. Results showed that Ang II markedly prompted RAW264.7 cells to migrate through a porous membrane, and CYP2J2 overexpression in VSMCs markedly inhibited this chemotactic effect induced by Ang II. CYP2J2 epoxygenase inhibitor C26 significantly blocked this protective effect of CYP2J2 overexpression (Fig. 6A). Because Ang II induced a significant expression of MCP-1 in VSMCs, and CYP2J2 overexpression or its metabolites, EETs, could prevent MCP-1 expression (supplementary Fig. II), we hypothesized that the released MCP-1 from Ang II-incubated VSMCs is responsible for macrophage migration. As expected, preincubation with a neutralizing antibody to MCP-1 effectively blocked macrophage migration induced by Ang II in the macrophage-VSMC coculture system (Fig. 6B). We further examined the effect of the EET/PPARγ pathway on Ang II-induced macrophage migration in our coculture system. Consistently, 11,12-EET significantly blocked the chemotactic effect of Ang II. However, this effect was markedly suppressed by the addition of PPARγ inhibitor GW9662 (Fig. 6C).
Fig. 6.
CYP2J2 overexpression and 11,12-EET suppressed Ang II-induced macrophage migration is through reducing MCP-1 release via PPARγ activation. A: rAAV-CYP2J2 transfection reduced Ang II-induced (10 μmol/l) macrophage migration, and CYP2J2 inhibitor C26 (10 μmol/l) prevented this effect. B: Neutralizing antibody against MCP-1 (10 μg/ml) suppressed the chemotactic effect of Ang II on macrophage. C: PPARγ antagonist GW9662 (1 μmol/l) blocked the antichemotactic effect of 11,12-EET on Ang II-induced macrophage migration [n = 3 for each experiment; **P < 0.01 vs. control; ††P < 0.01 vs. Ang II + rAAV-GFP in (A), Ang II + IgG in (B), or Ang II in (C); ‡‡P < 0.01 vs. Ang II + rAAV-CYP2J2 in (A) or Ang II + 11,12-EET in (C)].
Taken together, these results suggest that the suppressive effect of CYP2J2 overexpression and 11,12-EET on Ang II-induced macrophage migration is mediated by PPARγ activation.
DISCUSSION
The current study investigated the effects of rAAV-mediated CYP2J2 overexpression on Ang II-induced AAA formation in ApoE−/− mice. Here, we showed that rAAV-mediated CYP2J2 gene delivery led to an abundant aortic expression of CYP2J2 and elevated the levels of circulating EETs. rAAV-CYP2J2 delivery reduced the total serum cholesterol level, prevented Ang II-induced aortic MMPs expression and activity, elastin degradation, and retarded AAA formation and development. These effects were associated with upregulation of PPARγ and reduction of aortic inflammation. Our cellular observations further showed that CYP2J2 overexpression and its metabolic products, EETs, particularly 11,12-EET, suppressed production of Ang II-induced inflammatory cytokines in VSMCs and prevented Ang II-induced macrophage migration. Moreover, we demonstrated that these protective effects were mediated by the EET/PPARγ/NF-κB pathway.
CYP2J2 predominantly expresses in the human cardiovascular system (5, 31). Our previous studies have already reported that using rAAV vectors, CYP2J2 could be successfully overexpressed in rodent tissues and achieve its biological activities (11, 13, 19, 32). Indeed, in this study, rAAV-CYP2J2 injection efficiently overexpressed CYP2J2 in aortic tissue and elevated the serum and urine EET and DHET levels.
The pathophysiology of AAA is complex and involves multiple factors, including age, sex, hypertension, dyslipidemia, elastin and collagen degradation, and inflammation, etc. (2, 33). Strategies to elevate circulating EETs have been well documented to be protective against various cardiovascular diseases, including hypertension, heart failure, and atherosclerosis etc. (6–10, 12, 34). Although without detailed mechanisms, a recent study of s-EH inhibitor AR9276 showed that elevation of the circulating EETs levels was also protective against Ang II-induced AAA in ApoE−/− mice (14). We here overexpressed CYP2J2 to increase the circulating EETs levels, and reconfirmed the protective effects of EETs on Ang II-induced AAA development. The effect of CYP2J2 may involve various mechanisms.
Although Ang II clearly elevated blood pressure in this AAA model, and overexpression of CYP2J2 or s-EH inhibitor administration have been reported to lower blood pressure (7, 8, 11), it is very unlikely for CYP2J2 overexpression to suppress Ang II-induced AAA. A recent study by Cassis et al. (20) demonstrated that Ang II infusion-promoted AAA formation was independent of increases in blood pressure. Some other agents, like vitamin E and rosiglitazone, exhibited inhibitory effects on Ang II-induced AAA formation without altering the blood pressure (16, 35). Moreover, the s-EH inhibitor AR9276 did not reduce blood pressure in this AAA model (14).
The lipid profiles of rAAV-CYP2J2-treated animals did not change significantly in this study, except for lowered total cholesterol. However, we also do not consider it as the major underlying mechanism of CYP2J2 overexpression to attenuate Ang II-induced AAA progression. Research of statins in the Ang II-induced AAA model led to contradictory results (36–38), but it is sure that the protective effects of statins on AAA are independent of lipid lowering (36). In addition, HDL and LDL levels are more sensitive predictors of AAA than total cholesterol level (39), whereas rAAV-CYP2J2 treatment had no significant influence on these parameters in the present study.
Aortic inflammation is a crucial event during AAA development. Recent studies have demonstrated that EETs suppress the inflammatory response in vitro and in vivo (5, 40, 41), and we recently reported that CYP2J2 overexpression reduced inflammatory responses and protected against heart and renal injury (12, 19). Consistent with a previous study of the s-EH inhibitor (14), our work demonstrates that CYP2J2 overexpression has a substantial inhibitory effect on vascular inflammation, which is considered to be the major mechanism of Ang II-induced AAA development (4, 20). Our in vitro observations further confirmed the anti-inflammatory effects of rAAV-CYP2J2 transfection and its metabolites, EETs, in Ang II-incubated VSMCs.
It is crucial that VSMCs and infiltrated macrophages synthesize MMPs for AAA development, and the infiltrated macrophages in the vessel wall present a major source of proteolytic enzymes that weaken the aortic wall (42, 43). Indeed, paralleled with reduced aortic macrophage infiltration, we found CYP2J2 overexpression significantly attenuated vascular MMP expression and activation, as well as elastin degradation in the Ang II-induced AAA model. It has been reported that Ang II induced VSMCs to secrete MCP-1 to stimulate macrophage migration (25). Our macrophage-VSMC coculture system showed that CYP2J2 and EETs suppressed Ang II-induced macrophage migration, which further supports our in vivo observation.
EETs activate PPARγ (15), and PPARγ activation is associated with AAA development (16, 44). Studies showed that VSMC PPARγ deletion promotes AAA (44), and the administration of the PPARγ agonist, rosiglitazone, prevents AAA progression (16). Our cellular experiments suggested an important role of the PPARγ pathway in mediating the anti-inflammatory effects of CYP2J2 overexpression and EETs. This notion was supported by the addition of the PPARγ antagonist GW9662, which significantly suppressed the protective effects of EETs on NF-κB activation and inflammatory responses in VSMCs, and inhibited macrophage migration induced by Ang II.
In summary, our results demonstrate that rAAV-mediated CYP2J2 overexpression increases EETs levels and can remarkably reduce Ang II-induced AAA development in ApoE−/− mice, which is associated with reduced aortic inflammation and macrophage recruitment. The anti-inflammatory effects of CYP2J2 overexpression and its metabolites, EETs, are possibly mediated by PPARγ activation. Our data provide evidence that rAAV-CYP2J2 delivery, or other strategies which increase plasma EETs levels, could be novel approaches for the prevention or treatment of AAA development.
Supplementary Material
Acknowledgments
The authors acknowledge Dr. Jia Wei for the great graphic assistance.
Footnotes
Abbreviations:
- AAA
- abdominal aortic aneurysm
- Ang
- angiotensin
- ApoE−/−
- apoE-deficient
- C26
- compound 26
- CYP2J2
- cytochrome P450 epoxygenase 2J2
- DHET
- dihydroxyeicosatrienoic acid
- EET
- epoxyeicosatrienoic acid
- GFP
- green fluorescent protein
- IκBα
- inhibitor of nuclear factor κB α
- IL
- interleukin
- MCP-1
- monocyte chemotactic protein-1
- MMP
- matrix metalloproteinase
- NF-κB
- nuclear factor κB
- PPAR
- peroxisome proliferator-activated receptor
- rAAV
- recombinant adeno-associated virus
- s-EH
- soluble epoxide hydrolase
- VSMC
- vascular smooth muscle cell
This work was supported by grants from “973” Programs (2012CB518004 and 2012CB517801) and National Science Foundation of China Project (31130031 and 81170111). The authors declare no conflict of interest.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of eight figures.
REFERENCES
- 1.Cowan J. A., Jr, Dimick J. B., Henke P. K., Rectenwald J., Stanley J. C., Upchurch G. R., Jr 2006. Epidemiology of aortic aneurysm repair in the United States from 1993 to 2003. Ann. N. Y. Acad. Sci. 1085: 1–10 [DOI] [PubMed] [Google Scholar]
- 2.Sakalihasan N., Limet R., Defawe O. D. 2005. Abdominal aortic aneurysm. Lancet. 365: 1577–1589 [DOI] [PubMed] [Google Scholar]
- 3.Shah P. K. 1997. Inflammation, metalloproteinases, and increased proteolysis: an emerging pathophysiological paradigm in aortic aneurysm. Circulation. 96: 2115–2117 [DOI] [PubMed] [Google Scholar]
- 4.Shimizu K., Mitchell R. N., Libby P. 2006. Inflammation and cellular immune responses in abdominal aortic aneurysms. Arterioscler. Thromb. Vasc. Biol. 26: 987–994 [DOI] [PubMed] [Google Scholar]
- 5.Xu X., Zhang X. A., Wang D. W. 2011. The roles of CYP450 epoxygenases and metabolites, epoxyeicosatrienoic acids, in cardiovascular and malignant diseases. Adv. Drug Deliv. Rev. 63: 597–609 [DOI] [PubMed] [Google Scholar]
- 6.Edin M. L., Wang Z., Bradbury J. A., Graves J. P., Lih F. B., DeGraff L. M., Foley J. F., Torphy R., Ronnekleiv O. K., Tomer K. B., et al. 2011. Endothelial expression of human cytochrome P450 epoxygenase CYP2C8 increases susceptibility to ischemia-reperfusion injury in isolated mouse heart. FASEB J. 25: 3436–3447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jung O., Brandes R. P., Kim I. H., Schweda F., Schmidt R., Hammock B. D., Busse R., Fleming I. 2005. Soluble epoxide hydrolase is a main effector of angiotensin II-induced hypertension. Hypertension. 45: 759–765 [DOI] [PubMed] [Google Scholar]
- 8.Lee C. R., Imig J. D., Edin M. L., Foley J., DeGraff L. M., Bradbury J. A., Graves J. P., Lih F. B., Clark J., Myers P., et al. 2010. Endothelial expression of human cytochrome P450 epoxygenases lowers blood pressure and attenuates hypertension-induced renal injury in mice. FASEB J. 24: 3770–3781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Merabet N., Bellien J., Glevarec E., Nicol L., Lucas D., Remy-Jouet I., Bounoure F., Dreano Y., Wecker D., Thuillez C., et al. 2012. Soluble epoxide hydrolase inhibition improves myocardial perfusion and function in experimental heart failure. J. Mol. Cell. Cardiol. 52: 660–666 [DOI] [PubMed] [Google Scholar]
- 10.Ulu A., Davis B. B., Tsai H. J., Kim I. H., Morisseau C., Inceoglu B., Fiehn O., Hammock B. D., Weiss R. H. 2008. Soluble epoxide hydrolase inhibitors reduce the development of atherosclerosis in apolipoprotein e-knockout mouse model. J. Cardiovasc. Pharmacol. 52: 314–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xiao B., Li X., Yan J., Yu X., Yang G., Xiao X., Voltz J. W., Zeldin D. C., Wang D. W. 2010. Overexpression of cytochrome P450 epoxygenases prevents development of hypertension in spontaneously hypertensive rats by enhancing atrial natriuretic peptide. J. Pharmacol. Exp. Ther. 334: 784–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao G., Wang J., Xu X., Jing Y., Tu L., Li X., Chen C., Cianflone K., Wang P., Dackor R. T., et al. 2012. Epoxyeicosatrienoic acids protect rat hearts against tumor necrosis factor-alpha-induced injury. J. Lipid Res. 53: 456–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zheng C., Wang L., Li R., Ma B., Tu L., Xu X., Dackor R. T., Zeldin D. C., Wang D. W. 2010. Gene delivery of cytochrome p450 epoxygenase ameliorates monocrotaline-induced pulmonary artery hypertension in rats. Am. J. Respir. Cell Mol. Biol. 43: 740–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang L. N., Vincelette J., Cheng Y., Mehra U., Chen D., Anandan S. K., Gless R., Webb H. K., Wang Y. X. 2009. Inhibition of soluble epoxide hydrolase attenuated atherosclerosis, abdominal aortic aneurysm formation, and dyslipidemia. Arterioscler. Thromb. Vasc. Biol. 29: 1265–1270 [DOI] [PubMed] [Google Scholar]
- 15.Liu Y., Zhang Y., Schmelzer K., Lee T. S., Fang X., Zhu Y., Spector A. A., Gill S., Morisseau C., Hammock B. D., et al. 2005. The antiinflammatory effect of laminar flow: the role of PPARgamma, epoxyeicosatrienoic acids, and soluble epoxide hydrolase. Proc. Natl. Acad. Sci. USA. 102: 16747–16752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones A., Deb R., Torsney E., Howe F., Dunkley M., Gnaneswaran Y., Gaze D., Nasr H., Loftus I. M., Thompson M. M., et al. 2009. Rosiglitazone reduces the development and rupture of experimental aortic aneurysms. Circulation. 119: 3125–3132 [DOI] [PubMed] [Google Scholar]
- 17.Jiang J. G., Ning Y. G., Chen C., Ma D., Liu Z. J., Yang S., Zhou J., Xiao X., Zhang X. A., Edin M. L., et al. 2007. Cytochrome p450 epoxygenase promotes human cancer metastasis. Cancer Res. 67: 6665–6674 [DOI] [PubMed] [Google Scholar]
- 18.Tu L., Xu X., Wan H., Zhou C., Deng J., Xu G., Xiao X., Chen Y., Edin M. L., Voltz J. W., et al. 2008. Delivery of recombinant adeno-associated virus-mediated human tissue kallikrein for therapy of chronic renal failure in rats. Hum. Gene Ther. 19: 318–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao G., Tu L., Li X., Yang S., Chen C., Xu X., Wang P., Wang D. W. 2012. Delivery of AAV2–CYP2J2 protects remnant kidney in the 5/6-nephrectomized rat via inhibition of apoptosis and fibrosis. Hum Gene Ther. 23: 688–699 [DOI] [PubMed] [Google Scholar]
- 20.Cassis L. A., Gupte M., Thayer S., Zhang X., Charnigo R., Howatt D. A., Rateri D. L., Daugherty A. 2009. ANG II infusion promotes abdominal aortic aneurysms independent of increased blood pressure in hypercholesterolemic mice. Am. J. Physiol. Heart Circ. Physiol. 296: H1660–H1665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang S., Zhang C., Zhang M., Liang B., Zhu H., Lee J., Viollet B., Xia L., Zhang Y., Zou M. H. 2012. Activation of AMP-activated protein kinase α2 by nicotine instigates formation of abdominal aortic aneurysms in mice in vivo. Nat. Med. 18: 902–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li F., Cai Z., Chen F., Shi X., Zhang Q., Chen S., Shi J., Wang D. W., Dong N. 2012. Pioglitazone attenuates progression of aortic valve calcification via down-regulating receptor for advanced glycation end products. Basic Res. Cardiol. 107: 306. [DOI] [PubMed] [Google Scholar]
- 23.Toth M., Sohail A., Fridman R. 2012. Assessment of gelatinases (MMP-2 and MMP-9) by gelatin zymography. Methods Mol. Biol. 878: 121–135 [DOI] [PubMed] [Google Scholar]
- 24.Xu X., Zhao C. X., Wang L., Tu L., Fang X., Zheng C., Edin M. L., Zeldin D. C., Wang D. W. 2010. Increased CYP2J3 expression reduces insulin resistance in fructose-treated rats and db/db mice. Diabetes. 59: 997–1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamanouchi D., Morgan S., Kato K., Lengfeld J., Zhang F., Liu B. 2010. Effects of caspase inhibitor on angiotensin II-induced abdominal aortic aneurysm in apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol. 30: 702–707 [DOI] [PubMed] [Google Scholar]
- 26.Wang Y., Wei X., Xiao X., Hui R., Card J. W., Carey M. A., Wang D. W., Zeldin D. C. 2005. Arachidonic acid epoxygenase metabolites stimulate endothelial cell growth and angiogenesis via mitogen-activated protein kinase and phosphatidylinositol 3-kinase/Akt signaling pathways. J. Pharmacol. Exp. Ther. 314: 522–532 [DOI] [PubMed] [Google Scholar]
- 27.Chen F., Chen C., Yang S., Gong W., Wang Y., Cianflone K., Tang J., Wang D. W. 2012. Let-7b inhibits human cancer phenotype by targeting cytochrome P450 epoxygenase 2J2. PLoS ONE. 7: e39197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ni L., Zhou C., Duan Q., Lv J., Fu X., Xia Y., Wang D. W. 2011. beta-AR blockers suppresses ER stress in cardiac hypertrophy and heart failure. PLoS ONE. 6: e27294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aziz F., Kuivaniemi H. 2007. Role of matrix metalloproteinase inhibitors in preventing abdominal aortic aneurysm. Ann. Vasc. Surg. 21: 392–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen C., Li G., Liao W., Wu J., Liu L., Ma D., Zhou J., Elbekai R. H., Edin M. L., Zeldin D. C., et al. 2009. Selective inhibitors of CYP2J2 related to terfenadine exhibit strong activity against human cancers in vitro and in vivo. J. Pharmacol. Exp. Ther. 329: 908–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rendic S., Guengerich F. P. 2010. Update information on drug metabolism systems–2009, part II: summary of information on the effects of diseases and environmental factors on human cytochrome P450 (CYP) enzymes and transporters. Curr. Drug Metab. 11: 4–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen G., Wang P., Zhao G., Xu G., Gruzdev A., Zeldin D. C., Wang D. W. 2011. Cytochrome P450 epoxygenase CYP2J2 attenuates nephropathy in streptozotocin-induced diabetic mice. Prostaglandins Other Lipid Mediat. 96: 63–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iribarren C., Darbinian J. A., Go A. S., Fireman B. H., Lee C. D., Grey D. P. 2007. Traditional and novel risk factors for clinically diagnosed abdominal aortic aneurysm: the Kaiser multiphasic health checkup cohort study. Ann. Epidemiol. 17: 669–678 [DOI] [PubMed] [Google Scholar]
- 34.Imig J. D., Zhao X., Zaharis C. Z., Olearczyk J. J., Pollock D. M., Newman J. W., Kim I. H., Watanabe T., Hammock B. D. 2005. An orally active epoxide hydrolase inhibitor lowers blood pressure and provides renal protection in salt-sensitive hypertension. Hypertension. 46: 975–981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gavrila D., Li W. G., McCormick M. L., Thomas M., Daugherty A., Cassis L. A., Miller F. J., Jr, Oberley L. W., Dellsperger K. C., Weintraub N. L. 2005. Vitamin E inhibits abdominal aortic aneurysm formation in angiotensin II-infused apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol. 25: 1671–1677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Y., Naggar J. C., Welzig C. M., Beasley D., Moulton K. S., Park H. J., Galper J. B. 2009. Simvastatin inhibits angiotensin II-induced abdominal aortic aneurysm formation in apolipoprotein E-knockout mice: possible role of ERK. Arterioscler. Thromb. Vasc. Biol. 29: 1764–1771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Golledge J., Cullen B., Moran C., Rush C. 2010. Efficacy of simvastatin in reducing aortic dilatation in mouse models of abdominal aortic aneurysm. Cardiovasc. Drugs Ther. 24: 373–378 [DOI] [PubMed] [Google Scholar]
- 38.Wang J. A., Chen W. A., Wang Y., Zhang S., Bi H., Hong B., Luo Y., Daugherty A., Xie X. 2011. Statins exert differential effects on angiotensin II-induced atherosclerosis, but no benefit for abdominal aortic aneurysms. Atherosclerosis. 217: 90–96 [DOI] [PubMed] [Google Scholar]
- 39.Takagi H., Manabe H., Kawai N., Goto S. N., Umemoto T. 2010. Serum high-density and low-density lipoprotein cholesterol is associated with abdominal aortic aneurysm presence: a systematic review and meta-analysis. Int. Angiol. 29: 371–375 [PubMed] [Google Scholar]
- 40.Bystrom J., Wray J. A., Sugden M. C., Holness M. J., Swales K. E., Warner T. D., Edin M. L., Zeldin D. C., Gilroy D. W., Bishop-Bailey D. 2011. Endogenous epoxygenases are modulators of monocyte/macrophage activity. PLoS ONE. 6: e26591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Deng Y., Edin M. L., Theken K. N., Schuck R. N., Flake G. P., Kannon M. A., DeGraff L. M., Lih F. B., Foley J., Bradbury J. A., et al. 2011. Endothelial CYP epoxygenase overexpression and soluble epoxide hydrolase disruption attenuate acute vascular inflammatory responses in mice. FASEB J. 25: 703–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Allaire E., Forough R., Clowes M., Starcher B., Clowes A. W. 1998. Local overexpression of TIMP-1 prevents aortic aneurysm degeneration and rupture in a rat model. J. Clin. Invest. 102: 1413–1420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McCormick M. L., Gavrila D., Weintraub N. L. 2007. Role of oxidative stress in the pathogenesis of abdominal aortic aneurysms. Arterioscler. Thromb. Vasc. Biol. 27: 461–469 [DOI] [PubMed] [Google Scholar]
- 44.Hamblin M., Chang L., Zhang H., Yang K., Zhang J., Chen Y. E. 2010. Vascular smooth muscle cell peroxisome proliferator-activated receptor-gamma deletion promotes abdominal aortic aneurysms. J. Vasc. Surg. 52: 984–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.