Abstract
Delayed clearance of triglyceride-rich lipoprotein (TRL) by white adipose tissue (WAT) promotes hypertriglyceridemia and elevated apoB-lipoproteins, which are primarily in the form of LDL. This study examines whether LDL promotes delayed clearance of TRL by WAT. Following the ingestion of a 13C-triolein-labeled high-fat meal, obese women with high plasma apoB (> median 0.93 g/l, N = 11, > 98% as IDL/LDL) had delayed clearance of postprandial 13C-triglyceride and 13C-NEFA over 6 h compared with controls. AUC6 h of plasma 13C-triglyceride and 13C-NEFA correlated with plasma apoB but not with LDL diameter or adipocyte area. There was no group difference in 13C-triolein oxidation rate, which suggests lower 13C-NEFA storage in peripheral tissue in women with high apoB. Ex vivo/in vitro plasma apoB correlated negatively with WAT 3H-lipid following a 4 h incubation of women's WAT with synthetic 3H-triolein-TRL. LDL-differentiated 3T3-L1 adipocytes had lower 3H-TRL hydrolysis and 3H-NEFA storage. Treatment of women's WAT with their own LDL decreased 3H-TRL hydrolysis and 3H-NEFA uptake. Finally, LDL, although not an LPL substrate, reduced LPL-mediated 3H-TRL hydrolysis as did VLDL and HDL. Exposure to LDL decreases TRL clearance by human WAT ex vivo. This may promote production of apoB-lipoproteins and hypertriglyceridemia through a positive-feedback mechanism in vivo.
Keywords: dyslipidemia, hyperapoB, apolipoprotein B48, apolipoprotein B100, dietary triglyceride clearance
Postprandial hypertriglyceridemia is an independent risk factor for cardiometabolic disease (1). Many factors have been implicated in the etiology of hyperlipidemia; among the most common is reduced triglyceride-rich lipoprotein (TRL) clearance by peripheral tissue. White adipose tissue (WAT) is a major regulator of TRL clearance, particularly in the postprandial state (2–6). Following a meal, dietary fat enters the circulation in the form of chylomicrons, TRL with apoB48. Efficient clearance of chylomicrons by WAT requires three sequential steps: i) the hydrolysis of chylomicrons by endothelial lipoprotein lipase (LPL); ii) the uptake of LPL-generated nonesterified fatty acid (NEFA) by underlying adipocytes; and iii) the utilization or storage of NEFA (3, 5). Dietary TRL remnants and NEFA that are not cleared by peripheral tissue are then taken up by the liver for utilization and resecretion as VLDL (TRL with apoB100).
Healthy WAT is able to respond promptly to postprandial signals, such as insulin increasing the hydrolysis of dietary TRL and the uptake and storage of generated NEFA, thus reestablishing the homeostasis in plasma lipids. The storage versus the release of TRL-generated NEFA in human subcutaneous WAT was reported to be almost absent in the fasting state, to increase to 100% 1 h after the ingestion of a meal, and to decrease to 10–30% 6 h after the meal (5). Accordingly, delayed plasma clearance of postprandial TRL by WAT is believed to increase the influx of dietary TRL remnants and NEFA into nonadipose peripheral tissues, including muscle, pancreas, and liver, inducing lipotoxicity and insulin resistance (6–8). In the liver, this also leads to increased synthesis and secretion of VLDL, which further reduces chylomicron clearance due to competitive binding to LPL (9–14). Altogether, this increases the plasma concentrations of apoB-lipoproteins, which is measured as plasma apoB and represents mostly LDL particles (>90%) (14–16). Dysfunctional WAT is thus closely associated with hypertriglyceridemia and hyperapoB in humans (6, 14, 17–19). Inherent abnormalities in WAT function are also believed to lead to the most common primary dyslipoproteinemia, familial combined hyperlipidemia (FCHL), which is characterized by hypertriglyceridemia, hypercholesterolemia, hyperapoB, small dense LDL, and insulin resistance (19, 20).
The mechanisms responsible for delayed plasma clearance of postprandial TRL by WAT are not fully understood. However, the uptake of LDL, albeit oxidized, by 3T3-L1 adipocytes was reported to increase cell proliferation and decrease cell differentiation (21–23). Moreover, multiple clinical studies have demonstrated that statin therapy, which reduces plasma concentrations of apoB-lipoproteins, also improves plasma clearance of triglyceride (TG) (24–27). Therefore, it is possible that elevated concentrations of apoB-lipoproteins not only are a consequence of dysfunctional WAT and hypertriglyceridemia but also play an active role in their pathology.
We thus examined the relation of elevated numbers of apoB-lipoproteins, specifically LDL, to postprandial TRL clearance and WAT function in healthy postmenopausal overweight and obese women. Our hypotheses were that elevated levels of apoB-lipoproteins in the form of LDL associates with reduced plasma clearance of dietary TRL and NEFA in vivo and directly reduces TRL clearance by women's WAT and 3T3-L1 adipocytes ex vivo and in vitro.
METHODS
Study design and population
Twenty-two postmenopausal women were studied. The inclusion criteria were the following: body mass index (BMI) ≥ 27 kg/m2; age = 45–74 years; nonsmoker; sedentary (<2 h of structured physical exercise/week); and low alcohol consumption (<2 drinks/day). The exclusion criteria were the following: elevated risk of cardiovascular disease (≥20% calculated Framingham Risk Score) (28); prior history of chronic disease (untreated thyroid disease, cardiovascular disease, diabetes); inflammatory disease or cancer within the last 3 years; claustrophobia; abnormal plasma values (Hb < 120 g/l, creatinine > 100 µmol/l, AST or ALT > 3 times normal limit); abnormal blood coagulation; concomitant medications [hormone replacement therapy (except thyroid hormone at a stable dose), lipid-lowering hypotensive agents, systemic corticosteroids, antipsychotic medication, psychoactive medication, anticoagulant treatment, weight-loss agents, or adrenergic agonist]; known substance abuse; and lack of time to participate in the full length of the study (five weeks). The 22 women were separated into two groups based on median plasma apoB, which represents an average of two values measured three weeks apart. All participants gave written informed consent prior to initiation of the study, which had been approved by the Ethics Board at IRCM.
Anthropometric and metabolic measurements
All analyses were conducted at the end of a four-week weight stabilization period (±2 kg) to eliminate the effects of weight fluctuation on the measured outcomes (29). Body composition was measured by dual energy X-ray absorptiometry (General Electric Lunar Corp. version 6.10.019) (30, 31). Resting metabolic rate (RMR) and substrates oxidation rates were measured over 20 min by indirect calorimetry (Vmax encore, Cardinal Health) (31–33). Serum lipids and apoB were measured by an automated analyzer and LDL-C was calculated by the Friedewald equation (34) (COBAS INTEGRA 400, Roche Diagnostic). Plasma apoB48 was measured by ELISA (BioVendor) (35), serum glucose was measured by an automated analyzer (YSI 2300 STAT Plus, Life Sciences), and insulin was measured by a human radioimmunoassay (Millipore Corp.).
Postprandial in vivo fat clearance
The clearance and oxidation of a 13C-labeled high-fat meal was assessed as previously published (33, 36). At T = −1 h, fasting subcutaneous WAT samples were obtained by the physicians of the study from the right hip by needle biopsy under local anesthesia (Xylocaine 20 mg/ml, AstraZeneca) (37, 38). Fasting RMR, breath, and blood samples were collected at T = 0 h, followed by the consumption of a high-fat meal that was labeled with 13C-triolein (glycerol tri(oleate-1-13C), 99 atom% 13C, Sigma-Aldrich, Canada) standardized to body surface area (600 kcal/m2, 0.017 g 13C-triolein/g fat, 68% fat, 18% carbohydrate). Serial measurements of respiration and collection of breath and blood samples were conducted at 1, 2, 4, and 6 h postprandially. The 13C enrichment in the breath 13CO2 and plasma 13C-TG and 13C-NEFA were analyzed by isotopic ratio mass spectrometer with a continuous flow module (Vario Micro CHNS Cube, Elementar Americas Inc.). The mass spectrometry was calibrated using two international L-glutamate standards (USGS40 and USGS41, International Atomic Energy Agency http://nucleus.iaea.org) with 13C-enrichment of −26.389 and +37.626 ppm. The 13C-enrichment of the samples was calculated as previously described (33):
| (Eq. 1) |
where δ13C ‰ t = i = delta at time = i h in ppm; RS t = i = 13C/12C of the sample at time = i h; and RVPDB = 13C/12C of the international standard VPDB = 0.0112372.
The percentage of 13C-recovered in breath 13CO2 per hour was calculated as:
| (Eq. 2) |
where mM excess 13C/mM CO2 t = i = (δ13C ‰ t = i − δ13C ‰ t = 0) × RVPDB × 10−3
| (Eq. 3) |
| (Eq. 4) |
where mg 13C-triolein = weight of administered 13C-triolein; M = molecular weight of 13C-triolein or 885.4 g/mol; P = 13C-isotope purity or 99%; n = number of labeled carbon position or 3; 1.3 = correction factor to adjust for the uptake of label into the HCO3 pool with bolus feeding (33, 39–41); and 22.4 is the volume in liters of 1 mol of CO2.
The concentrations of 13C-TG and 13C-NEFA in the plasma pools at each time point were calculated as follows (33, 39–41);
| (Eq. 5) |
where RS t = i = 13C/12C of the sample at time = i, and 13C APE represents percentage increase in the 13C-enrichment in plasma samples taken after the ingestion of the 13C-labeled meal compared with fasting background 13C-enrichment.
Lipoprotein profiling and LDL isolation
LDL diameter was measured by an automated polyacrylamide gel electrophoresis system that separates apoB-lipoproteins based on size into one TRL, three IDL, and seven LDL fractions (FDA-approved, Lipoprint system, Quantimetrix) (42). Fresh plasma lipoproteins were also fractioned by size using fast protein liquid chromatography (FPLC, Sepharose 6 column) (36). As a quality control, we measured and compared the concentrations of cholesterol in the fasting TRL and IDL/LDL fractions as separated by the FPLC and by the Lipoprint. The cholesterol concentrations measured by these two techniques were highly correlated (TRL, r = 0.82; IDL/LDL, r = 0.92; P < 0.0001), indicating the reliability of the FPLC fractionation of the lipoproteins. ApoB and apoE concentrations in 80 FPLC fractions were quantified by in-house ELISA using polyclonal antibodies against human apolipoproteins (Academy Biomedical) as published (36, 43). LDL was isolated from fresh fasting plasma by sequential ultracentrifugation using KBr density solution (1.019–1.063 g/ml, 0.01% EDTA) and Beckman Ti-50 rotor (45,000 rpm, 32 h, 5°C), after removal of TRL and IDL (density < 1.019 g/ml). LDL solutions were desalted 3× with 12 ml saline (0.01% EDTA), then sterile-filtered (0.2 μm filters) and stored at 4°C for later use in the ex vivo and in vitro experiments within two to three weeks of isolation. LDL was not contaminated with HDL since the concentration of apoA-I in isolated samples was under detectable limits (<0.058 g/l by COBAS).
Women's WAT function
WAT function was assessed as in situ LPL activity. In situ LPL activity refers to the hydrolysis of synthetic 3H-triolein-labeled TRL (3H-TRL) and the uptake and storage of LPL-released 3H-NEFA as previously described (44, 45). Fresh WAT samples (5–10 mg) were cleaned, blotted dry, weighed, and collected in warm HBSS buffer for 30 min, then transferred to fresh 24-well plates containing 500 µl 3H-TRL (95% TG, 1.27 mM TG, 0.54 M Tris-HCl, pH 7.2, in DMEM/F12, 5.1% BSA, and 7.5% fasting serum) (44, 45) and incubated on a shaking plate for 4 h. WAT and medium 3H-lipid and 3H-TG were extracted, separated by TLC, and directly counted (44, 45). (The WAT 3H-lipid of five women was unavailable for analysis.) The amount of 3H-TRL hydrolyzed was calculated by subtracting medium 3H-TG remaining after the 4 h incubation from the added 3H-TRL dose, both expressed per milligram of WAT. The accumulation of 3H-NEFA was measured as medium 3H-NEFA remaining after the 4 h incubation expressed per milligram of WAT. Of note, we previously reported that addition of the LPL inhibitor tetrahydrolipstatin to the 3H-TRL substrate reduces intracellular 3H-lipid in 3T3-L1 adipocytes, indicating that the accumulation of intracellular 3H-lipid is dependent on LPL activity (44).
In addition, to assess WAT function independent of LPL activity, a second set of fresh WAT samples was incubated with 500 µl 3H-oleate-labeled NEFA bound to BSA (1 mM oleate:0.167 mM BSA in DMEM/F12) (45). WAT and medium 3H-lipid and 3H-TG were extracted, separated by TLC, and counted (44, 45). The amount of 3H-NEFA uptake was calculated by subtracting medium 3H-NEFA remaining after the 4 h incubation from the added 3H-NEFA:BSA dose, both expressed per milligram of WAT. The average of 3–6 WAT samples/woman/condition is reported.
Chronic effect of LDL on 3T3-L1 adipocyte function
3T3-L1 preadipocytes were differentiated for seven days, as previously published (44), in the presence or absence of women's LDL (1.4 g apoB/l) with medium change every two days. On the day of experiments, medium was removed and adipocytes were washed and incubated with the 3H-TRL substrate (0.56 mM TG) for 4 h, and in situ LPL activity was measured as above (44). Cell viability was determined by measuring lactate dehydrogenase (LDH) release in the medium using a commercial assay (CytoTox 96 assay, Promega) (44). The average of N = 6 experiments from different LDL women-donors is presented.
Acute effect of LDL on women's WAT function and LPL activity
The direct effect of LDL on WAT in situ LPL activity and NEFA:BSA uptake over 4 h was assessed as described above but in the presence of each woman's own LDL (1.2 g apoB/l). The average of three to six WAT samples/woman/condition is reported for each set of experiments.
To examine the direct effect of LDL on LPL activity, adipocyte-associated LPL was collected following the differentiation of 3T3-L1-adipocytes for seven days and their incubation with 100 µg/ml heparin for 45 min (control). Heparin-releasable LPL activity was assessed using the 3H-TRL (1.41 mM TG) as previously published (44) in the presence or absence of physiological concentrations of lipoproteins. The LDL concentrations used (0.6, 1.2, and 1.8 g apoB/l) represented the range of concentrations measured in women's fasting plasma samples, HDL concentrations were equal to those of apoB expressed in apoA-I units (1.2 and 1.8 g apoA-I/l), and VLDL concentrations were set as ∼10% of LDL (0.12 and 0.15 g apoB/l). Of note, HDL and VLDL were enough to generate only two experimental doses per woman; thus, the highest doses possible were used. The activity of LPL dimers naturally released into the medium over 45 min was also measured as background. Moreover, the activity of a standard curve of purified LPL (Sigma-Aldrich, Canada) was measured over 45 min using the 3H-TRL substrate (44) in the presence or absence of similar concentrations of the same woman's lipoproteins as above. A representative experiment of N = 4 is presented.
Adipocyte area
WAT samples obtained by the needle biopsy were immediately fixed overnight at 4°C in 4% paraformaldehyde, embedded in paraffin, and cut into 4 μm slices. Adipocyte area was measured in a blinded fashion by digital-imaging analysis. The average surface area of 1,111 ± 96 adipocytes in six fields of view in three WAT slides is reported per woman. The three slides were at least 48 µm apart to avoid multilayered images of one cell. The adipocyte area represents the area of the pixels within intact cell membranes measured using MATLAB software (MathWorks) at the microscopy core at IRCM. Images were obtained using Leica DMRB microscope (Leica, Montreal, Canada) and a Retiga EXi camera (Q-Imaging, Burnaby, Canada) on a 0.67× mount over a 10×/NA 0.3 phase contrast objective.
Statistical analysis
Data are presented as mean ± SEM. Group differences over the 6 h time curves were analyzed by repeated-measures two-way ANOVA with interaction. When interaction was significant, intrasubject differences were analyzed by paired t-test, whereas intersubject differences were analyzed by unpaired t-test. Nonparametric Wilcoxon-rank sum test was used when equal variance, measured by Mauchly's sphericity test, failed (plasma 13C-TG and 13C-NEFA data). Spearman correlation (two-tailed) was used to examine the association between variables. Statistical analysis was performed using SPSS V13, and significance was set at P ≤ 0.05.
RESULTS
Twenty-two healthy postmenopausal overweight and obese women were separated based on plasma apoB median (0.93 g/l) into two groups of low and high apoB. Women with high apoB had higher fasting plasma TG, total cholesterol (TC), LDL-C, and apoB48 and smaller LDL diameter (Table 1). There were no other group differences in the anthropometric and metabolic parameters measured.
TABLE 1.
Baseline characteristics of the postmenopausal women examined (N = 11/group)
| Characteristic | Low ApoB | High ApoB |
| ApoB (g/l) | 0.73 ± 0.16 | 1.20 ± 0.27** |
| Age (years) | 59.1 ± 1.2 | 58.8 ± 1.9 |
| Weight (kg) | 79.2 ± 3.0 | 71.8 ± 3.1 |
| BMI (kg/m2) | 31.9 ± 1.2 | 30.2 ± 1.1 |
| BSA (m2) | 1.80 ± 0.04 | 1.69 ± 0.04 |
| Waist (cm) | 102.6 ± 3.2 | 101.6 ± 2.2 |
| LBM (kg) | 40.0 ± 1.0 | 36.9 ± 1.1 |
| Fat mass (kg) | 36.1 ± 2.7 | 32.0 ± 2.2 |
| Android fat (kg) | 3.4 ± 0.3 | 3.1 ± 0.2 |
| Gynoid fat (kg) | 6.6 ± 0.5 | 5.8 ± 0.5 |
| Adipocyte area (μm2) | 3,145 ± 153 | 3,086 ± 287 |
| BMR (kcal/day) | 1,292 ± 40 | 1,205 ± 33 |
| SBP (mmHg) | 113 ± 5 | 120 ± 5 |
| DBP (mmHg) | 76 ± 3 | 77 ± 2 |
| Plasma glucose (mM) | 4.9 ± 0.1 | 5.1 ± 0.2 |
| Plasma insulin (µU/ml) | 14.5 ± 1.7 | 12.9 ± 1.8 |
| Plasma TC (mM) | 5.0 ± 0.1 | 6.8 ± 0.3** |
| Plasma LDL-C (mM) | 2.8 ± 0.1 | 4.5 ± 0.2** |
| Plasma HDL-C (mM) | 1.8 ± 0.2 | 1.4 ± 0.1 |
| Plasma TG (mM) | 1.0 ± 0.1 | 1.9 ± 0.3* |
| Plasma NEFA (mM) | 0.62 ± 0.04 | 0.56 ± 0.05 |
| Plasma apoB48 (mg/l) | 3.56 ± 0.42 | 8.34 ± 1.47** |
| LDL diameter (Å) | 271 ± 1 | 267 ± 2* |
*P < 0.05 and **P < 0.001 for group difference by unpaired t-test. Bold typeface indicates significant differences.
Postprandial plasma clearance of dietary TG and NEFA
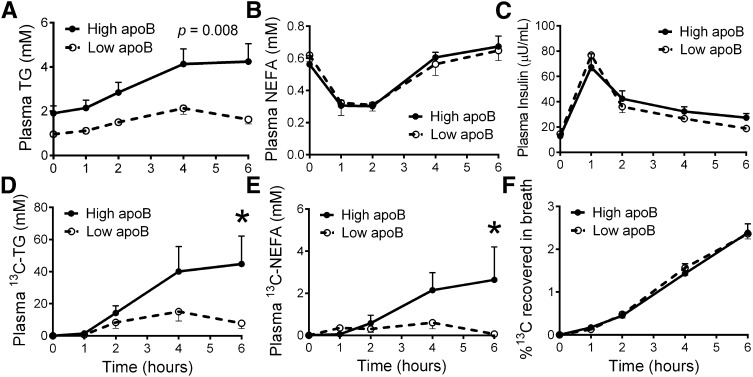
The oxidation versus circulation of the 13C high-fat meal was measured in all women over 6 h. Women with high apoB had delayed plasma clearance of total TG (Fig. 1A), and higher increment increase in the area under the 6 h curve of plasma TG above baseline (iAUC6 h) (high versus low: 8.46 ± 1.43 versus 4.00 ± 0.69 mM, P = 0.011). The two groups had identical concentrations of NEFA with similar postprandial drop at 1 and 2 h, suggesting equivalent insulin-induced inhibition of WAT lipolysis (Fig. 1B). There were no significant group differences in postprandial plasma insulin (Fig. 1C) or glucose (data not shown).
Fig. 1.
The postprandial changes in plasma TG (A), plasma NEFA (B), plasma insulin (C), plasma 13C-TG (D), plasma 13C-NEFA (E), and percentage of 13C-dose recovered in breath CO2 (F) following the ingestion of 13C-triolein-labeled high-fat meal in women with high and low plasma apoB (N = 11/group). Group differences were analyzed by repeated-measures two-way ANOVA, *P < 0.01.
Specific to TRL of dietary origins, women with high apoB also had delayed plasma clearance of 13C-TG at 6 h (high versus low: 44.8 ± 17.4 versus 7.81 ± 3.33 µM, P = 0.039, Fig. 1D) and greater AUC6 h of plasma 13C-TG (high versus low: 147.8 ± 54.5 versus 51.4 ± 19.8 µM, P = 0.045). Plasma 13C-NEFA remained elevated at the end of the 6 h in the women with high apoB, whereas it returned to fasting, nonenriched levels in women with low apoB (2.64 ± 1.56 versus 0.06 ± 0.22 µM, P = 0.014, Fig. 1E). As there was no group difference in the rate of 13C-NEFA oxidation (Fig. 1F), this suggests less uptake of LPL-released 13C-NEFA for storage by peripheral tissue in women with high apoB.
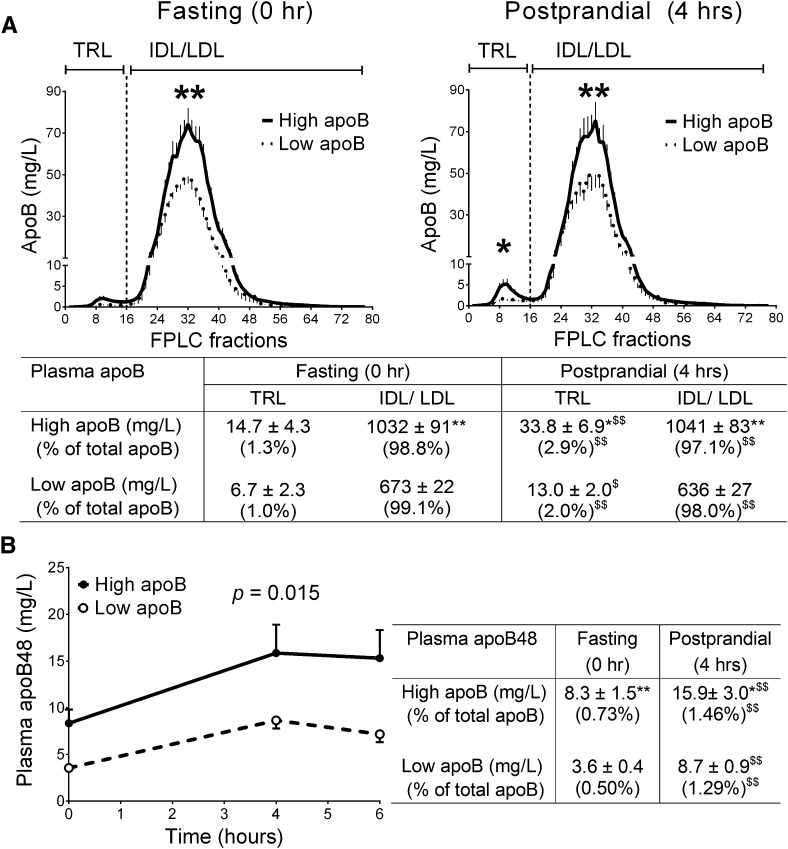
The types of apoB-lipoproteins were further characterized and compared in the two groups of women (Fig. 2). In the fasting state, ∼1.0% of total plasma apoB (apoB48 and apoB100) was recovered in the TRL fractions (chylomicron and VLDL), with no significant group differences in TRL concentrations or percentage TRL of total apoB (Fig. 2A). The group difference in plasma apoB was secondary to differences in IDL/LDL (P < 0.0001), which represented ∼99% of plasma apoB in both groups. Women with high apoB had higher fasting apoB48 (chylomicron remnants), which represented an equivalent minor percentage of total apoB in both groups (∼0.6%, Fig. 2B, table insert). In line with the 13C-TG data, both TRL and apoB48-lipoprotein particle numbers increased in the postprandial state and to a higher extent in women with high apoB.
Fig. 2.
FPLC-fractioned apoB-lipoproteins at fasting and postprandial states (graphs) and the sum of apoB concentrations in each faction (table) (A). Concentrations of apoB48 at fasting and postprandial states (graph and table) in women with high and low apoB (N = 11/ group) (B). *P < 0.05, **P < 0.01 for group differences; $P < 0.01 for intragroup differences.
As apoE plays a major role in the hepatic clearance of TRL and IDL, we measured the concentration of apoE in TRL and IDL/LDL fractions. Women with high apoB had higher TRL and IDL/LDL apoE in both fasting (TRL = 0.27 ± 0.08 versus 0.09 ± 0.03 µM, IDL/LDL = 0.78 ± 0.06 versus 0.50 ± 0.05 µM, P < 0.05) and postprandial states (TRL = 0.72 ± 0.11 versus 0.28 ± 0.06 µM, IDL/LDL = 0.76 ± 0.07 versus 0.49 ± 0.04 µM, P < 0.01). However, correcting for total number of TRL (i.e., TRL apoB) eliminated the group differences in the fasting (high = 238.0 ± 35.8 versus low = 202.6 ± 27.8 µM apoE/µM apoB, not significant) and the postprandial state (high = 145.7 ± 17.1 versus low = 86.7 ± 24.4 µM apoE/µM apoB, not significant). The increase in the enrichment of fasting TRL with apoE in the postprandial state was equally significant in both groups. (Analysis of the IDL/LDL enrichment with apoE was not conducted, as most of apoB is bound to LDL, which is poor in apoE.)
As presented in Table 2, in the whole group, both total apoB and all forms of apoB measured correlated with iAUC6 h of plasma apoB48, TG, 13C-TG, and 13C-NEFA; however, fasting IDL/IDL showed stronger associations with these parameters than TRL (except 13C-NEFA). Despite correction for baseline levels, fasting plasma apoB48 remained strongly correlated to iAUC6 h of plasma apoB48 and fasting plasma TG to iAUC6 h of plasma TG. This, indicating that the inhibitory effect of elevated baseline remnants and TG on postprandial TRL clearance cannot be totally accounted for by a mathematical correction. Plasma IDL/LDL correlated best with the iAUC6 h of plasma TG and eliminated the association of LDL size with iAUC6 h of plasma TG once corrected for in regression models. Taken together, this data suggest that women with high apoB, mainly in the form of IDL/LDL, have delayed hydrolysis and clearance of postprandial dietary TRL by peripheral tissue and/or reduced uptake of TRL remnants by the liver, resulting in increased postprandial plasma TG and TRL particle number.
TABLE 2.
Spearman correlations between measured parameters in postmenopausal overweight and obese women (N = 22)
| Fasting Plasma Measure | iAUC6 h of Plasma Parameters above Baseline Values | |||
| ApoB48 | TG | 13C-TG | 13C-NEFA | |
| ApoB48 | 0.847** | 0.628** | 0.571** | 0.306 |
| Total apoB | 0.600** | 0.697** | 0.571** | 0.504* |
| TRL apoB | 0.604** | 0.563* | 0.412 | 0.295 |
| IDL/LDL apoB | 0.616** | 0.821** | 0.519* | 0.317 |
| LDL diameter | −0.366 | - 0.644** | −0.354 | −0.163 |
| TG | 0.693** | 0.695** | 0.686** | 0.515* |
| NEFA | −0.179 | −0.299 | −0.164 | 0.068 |
*P < 0.05 and **P < 0.01 (two-tailed); all of the presented significant correlations (bolded values) with Spearman were also significant with Pearson correlation analysis.
Effect of chronic exposure to apoB-lipoproteins on women's WAT and 3T3-L1 adipocyte function
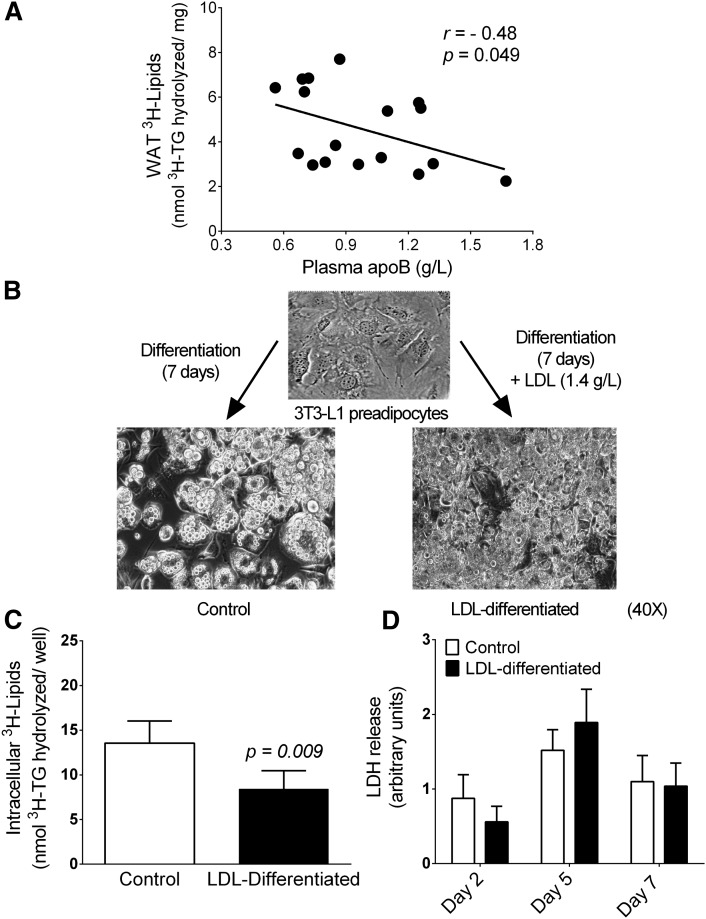
To examine whether WAT dysfunction may contribute to reduced dietary TRL clearance in women with high apoB, WAT samples were incubated for 4 h with synthetic 3H-TRL, and tissue 3H-lipid was measured. As presented in Fig. 3A, plasma apoB correlated negatively with WAT 3H-lipid (r = −0.48, P = 0.049), indicating lower hydrolysis of 3H-TRL and uptake and storage of generated 3H-NEFA with increased plasma apoB. However, the correlation of WAT 3H-lipid was with IDL/LDL apoB (r = −0.54; P = 0.048), not with TRL apoB. As a control, we measured background medium 3H-NEFA after incubating the 3H-TRL substrate for 4 h in the absence of WAT. The negligible amount measured (0.69 ± 0.01%) indicated that medium 3H-NEFA was the result of 3H-TRL hydrolysis. Most of the WAT 3H-lipid was in the form of WAT 3H-TG (83.2 ± 6.5% of 3H-lipid). This suggests that chronic exposure to a high concentration of IDL/LDL has a negative association with WAT-mediated TRL clearance and TG storage.
Fig. 3.
Chronic effect of apoB-lipoproteins/LDL. The correlation of plasma apoB with WAT 3H-lipids in 17 women (A), the morphology of 3T3-L1 adipocytes differentiated ± LDL (1.4 g apoB/l) (B), intracellular 3H-lipid following 4 h incubation with 3H-TRL in 3T3-L1 adipocytes differentiated ± LDL (C), and lactate dehydrogenase released during differentiation ± LDL (D).
To explore that directly in adipocytes, we differentiated 3T3-L1 preadipocytes in the presence or absence of elevated yet physiological concentrations of LDL (1.4 g apoB/l). Differentiation with LDL led to morphological changes, as LDL-differentiated adipocytes appeared overconfluent compared with control adipocytes (Fig. 3B). Following adipocyte incubation with 3H-TRL for 4 h, LDL-differentiated adipocytes had lower hydrolysis of 3H-TRL and uptake and storage of 3H-NEFA measured as lower intracellular 3H-lipid (Fig. 3C and in percentage, −38.3 ± 19.9%, P < 0.05). The inhibitory effect of LDL was not secondary to the reduction in cell viability as LDH released from the adipocytes was not affected by LDL treatment over the seven days of differentiation (Fig. 3D).
Acute effect of LDL on women's WAT function
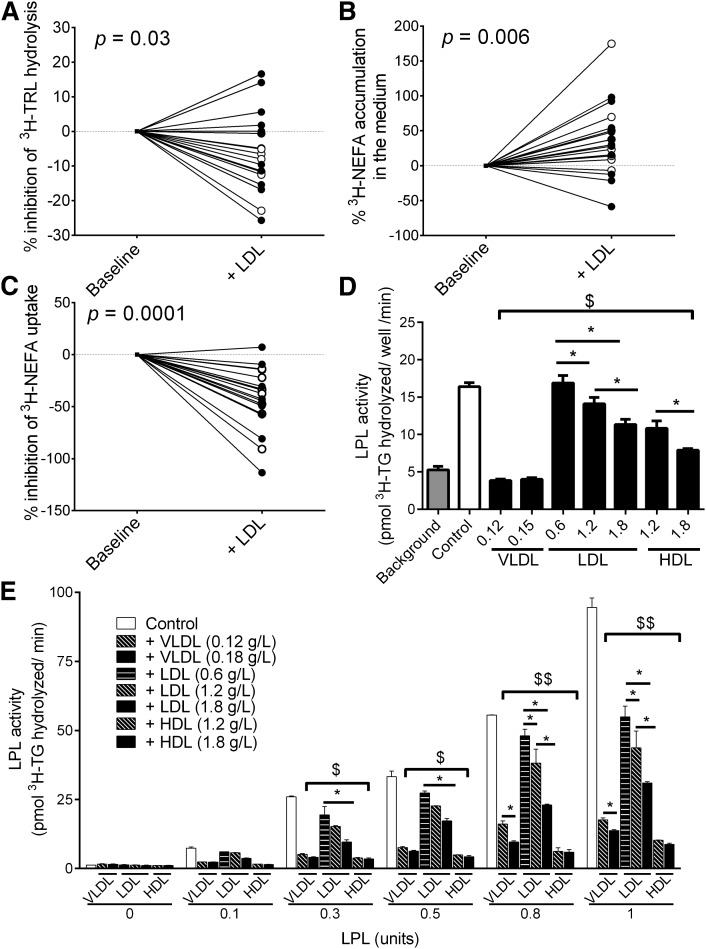
To explore whether LDL may also have an acute inhibitory effect on WAT function, WAT samples were incubated for 4 h with synthetic 3H-TRL ± each woman's own LDL (1.2 g apoB/l). Of importance, LDL alone was used instead of IDL/LDL, because unlike IDL, LDL is not an LPL substrate since it is poor in apoC-II (an LPL activator) (46). There were no group differences in the response of WAT to LDL treatment; thus, the two groups were pooled for analysis of LDL effects on WAT (Fig. 4A–C). LDL treatment increased medium accumulation of 3H-TRL, indicating reduced 3H-TRL hydrolysis by WAT, whether examined in absolute values (baseline versus LDL: 81.7 ± 5.1 versus 75.6 ± 5.0 nmol TG/mg WAT, P = 0.006) or percentage changes (P = 0.03, Fig. 4A). Moreover, LDL treatment increased medium accumulation of released 3H-NEFA, whether examined in absolute values (baseline versus LDL: 0.84 ± 0.20 versus 1.16 ± 0.26 nmol FA/mg WAT, P = 0.015) or percentage changes (P = 0.006, Fig. 4B). On the other hand, despite evidence of reduced 3H-TRL hydrolysis and 3H-NEFA uptake with LDL treatment, LDL treatment did not affect WAT 3H-lipid or its association with plasma apoB that was presented in Fig. 3A (data not shown).
Fig. 4.
Acute effects of LDL. Percentage of LDL-mediated inhibition of 3H-TRL hydrolysis (A), accumulation of LPL-released 3H-NEFA following WAT-incubation of with 3H-TRL (B), and percentage LDL inhibition of 3H-NEFA uptake following WAT incubation with 3H-NEFA:BSA (C) in women with low (open circles) and high (filled circles) apoB (N = 11/group, LDL = 1.2 g apoB/l). Lipoprotein-mediated inhibition of heparin-releasable LPL activity from 3T3-L1 adipocytes (D) and LPL standard-curve activity (E). *P < 0.05 for intralipoprotein-type differences, $P < 0.001 for differences from control for all lipoproteins except LDL at 0.6 g apoB/l, $$P < 0.001 for differences from control for all lipoproteins.
To explore whether the inhibitory effects of LDL on 3H-TRL hydrolysis were LPL-independent, we incubated another set of WAT with 3H-NEFA:BSA in the presence or absence of women's own LDL (1.2 g apoB/l). Four-hour LDL treatment reduced the uptake of 3H-NEFA by WAT, whether examined as absolute values (baseline versus LDL: 65.6 ± 4.8 versus 39.0 ± 5.9 nmol FA/mg WAT, P < 0.0001) or percentage change (P = 0.0001, Fig. 4C). Similar to the 3H-TRL data, there was no significant effect of LDL treatment on WAT 3H-lipid (baseline versus LDL: 2.6 ± 0.4 versus 2.3 ± 0.3 nmol FA/mg WAT, P = 0.062). Of note, there was no association of adipocyte size with WAT 3H-lipid, 3H-TRL hydrolysis, or 3H-NEFA uptake when the WAT samples were incubated with 3H-TRL or 3H-NEFA:BSA.
Finally, LDL is known to bind to LPL (47, 48), and phosphatidylcholine-containing liposomes were reported to compete with LDL for binding to LPL (48). This suggests that LDL may directly compete with 3H-TRL binding to LPL; however, this effect may not be LDL-specific as other lipoprotein contains phospholipids. Thus, we measured LPL activity in the presence or absence of women's LDL, VLDL, and HDL. As presented in Fig. 4D, all lipoproteins, except for 0.6 g/l LDL, inhibited LPL activity compared with control (i.e., adipocyte-associated LPL released by heparin). This effect was dose-dependent for HDL and LDL but not VLDL, where both doses of VLDL blocked LPL activity, reaching similar levels as background. Similar to the LPL standard curve (Fig. 4E), all lipoproteins inhibited LPL activity at all doses used (except at 0.6 g/l LDL with 0.3 and 0.5 units of LPL). Lipoprotein-induced inhibition of LPL activity increased as a function of LPL concentrations, reaching a maximum inhibition of −85.5%, −67.2%, and −90.7% at 0.18 g apoB/l VLDL, 1.8 g apoB/l LDL, and 1.8 g apoA-I/l HDL, respectively, P < 0.001. There was a dose-dependent inhibition of LPL activity for all VLDL and LDL doses at LPL concentrations of 0.8 and 1.0 units.
DISCUSSION
Data in this study demonstrate that plasma clearance of dietary TG and NEFA is delayed in postmenopausal obese women with high plasma apoB without any difference in the oxidation rate of NEFA, which points to reduced TG storage in peripheral tissue. Given the role of WAT in postprandial TRL clearance, we further examined the effects of apoB-lipoproteins on WAT function. A chronic inhibitory effect of apoB-lipoproteins/LDL was revealed ex vivo and in vitro as i) plasma apoB was inversely related to in situ LPL activity and NEFA storage in women's WAT and ii) LDL-differentiated 3T3-L1 adipocytes had decreased in situ LPL activity and NEFA storage. In addition, LDL had a direct acute inhibitory effect on TRL clearance as i) LDL treatment of WAT for 4 h reduced the hydrolysis of synthetic TRL and increased the accumulation of LPL-derived NEFA, ii) LDL treatment of WAT for 4 h reduced the uptake of albumin-bound NEFA, and finally, iii) LDL directly inhibited LPL activity.
Before further discussion of the significance of this data, it is important to highlight certain strengths and limitations of our study. The use of the gold-standard stable-isotopes technique to trace dietary fat represented a major strength as it increased the sensitivity to detect group differences in dietary NEFA clearance. These differences would have been missed had the analysis been dependent solely on total plasma NEFA, which is a pool of both endogenous and exogenous NEFA with variable intersubject contributions. Although the correlative nature of our in vivo findings does not allow causal relations to be established between elevated apoB-lipoproteins and delayed TRL clearance, it provided the first evidence that this relationship exists in humans. Furthermore, it is not possible to dissect whether elevated postprandial TG in vivo was secondary to reduced LPL-induced TRL hydrolysis by peripheral tissue, reduced hepatic uptake of TRL remnants, or both. Whereas the in vitro and ex vivo models support an inhibitory effect of LDL on WAT function, an effect of LDL on other tissue cannot be excluded. Particularly in relation to the liver, hepatic clearance of TRL and IDL is facilitated by the binding of their content of apoE to hepatic LDL receptors (49). Although there were no group differences in the enrichment of TRL particles with apoE, elevated concentrations of LDL per se may have hindered hepatic TRL/IDL clearance by competitive binding to the LDL receptors. This is indeed supported by elevated fasting plasma apoB48 in women with high apoB.
Both chronic and acute effects of LDL examined in this study support an inhibitory role of LDL on TRL clearance by human WAT. This role of LDL in healthy subjects is in line with that published on patients with hyperapoB due to FCHL, in which adipocytes taken from these patients had reduced efficiency of exogenous NEFA esterification and TG synthesis (50). More recently, elegant carbon dating studies by Arner et al. demonstrated that WAT of nonobese subjects with FCHL also have reduced exogenous TG clearance and storage (51). Although the underlying mechanisms remain to be explored, our findings in LDL-differentiated 3T3-L1 adipocytes suggest that LDL may negatively impact adipocyte differentiation. This helps explain both reduced TRL clearance in women with high apoB and their reduced WAT function following WAT extraction from the body. Of note, oxidized LDL were shown to bind and be internalized by CD36 in mouse 3T3-L1 cells, causing the upregulation of preadipocyte factor-1 (Pref-1), whose suppression is key for the expression of peroxisome proliferator-activated receptor γ and adipocyte differentiation (21–23). Although higher oxidized LDL levels are likely more abundant in women with high apoB and their effects on adipocytes function cannot be excluded; our data demonstrate that LDL taken from women with both low and high apoB induced equivalent negative effects on WAT function (Fig. 4). Thus, the inhibitory effect of LDL on adipocyte function is likely mediated by native LDL per se. Like oxidized LDL however, the effect of native LDL may entail particle uptake and internalization. Supporting this is that adipocytes (3T3-L1 and human) interact with apoB-lipoproteins via several mechanisms, including VLDL receptor, LDL receptor-related protein, and cell surface proteoglycans, the expression of all of which increases with differentiation (52, 53). Moreover, the absence of hypertriglyceridemia in patients with familial hypercholesterolemia (54) suggests that LDL receptor in particular may be involved in the effects of LDL on WAT.
Although exposure of WAT to LDL for 4 h decreased TRL hydrolysis and NEFA uptake, this was not translated into decreased 3H-lipid in WAT. Although this may seem contradictory, it should be taken into account that plasma apoB was reported to be negatively associated with noradrenaline-induced lipolysis ex vivo in subcutaneous adipocytes of healthy obese men (55). Moreover, carbon dating data showed that WAT from nonobese FCHL patients had not only reduced exogenous TG clearance and storage but also reduced endogenous TG turnover and increased TG age (51). Thus LDL-incubated WAT may have reduced lipolysis of both exogenous TRL and endogenous TG, with a reverse direction in control WAT. Reduced exogenous 3H-TRL clearance and storage in LDL-incubated WAT will be offset by increased endogenous 3H-TG lipolysis in control WAT, resulting in no net group differences in WAT 3H-lipid, despite the accumulation of the 3H-TRL in the medium of LDL-incubated WAT. Moreover, large variability in the fate of 3H-NEFA once taken up by WAT between oxidation and storage may have existed. Although there was no group difference in the percentage of ingested 13C recovered in breath CO2, energy expenditure in vivo is better reflected by substrate oxidation in nonadipose than in adipose tissue. For example, it was reported that energy expenditure per gram of tissue in obese women was 98-fold higher in the heart and kidneys and 2.9-fold higher in skeletal muscles than in adipose tissue (56). However, endogenous TG lipolysis and NEFA oxidation were not assessed in the present study given WAT sample size limitations and should be investigated in future new studies.
Finally, there are a couple of mechanisms by which LDL may hinder TRL clearance by WAT. The work presented here demonstrates that LDL inhibits LPL activity via direct or indirect mechanisms. One particle of LDL is documented to bind up to 15 LPL dimers, which is believed to be the mechanism responsible for the retention of LDL by macrophages in an atherosclerotic lesion (47, 48). Moreover, phosphatidylcholine-containing liposomes were reported to bind and compete with LDL binding to LPL (48); thus, all lipoproteins may potentially compete with 3H-TRL binding to LPL. This indeed was the case as both VLDL and HDL inhibited LPL activity to a greater extent than did LDL. However, the higher inhibition of LPL activity by VLDL and HDL may be secondary to HDL, and particularly TG-rich VLDL, being an LPL substrate as they contain apoC-II. Although it cannot be determined in vivo which lipoprotein subclass provided a higher competitor for 13C-TRL binding to LPL, the fasting plasma concentration that distinguished women with high apoB was higher IDL/LDL particle number, not higher HDL or TRL apoB.
Alternatively, LDL may indirectly inhibit LPL activity by favoring the accumulation of NEFA in the vicinity of LPL. LDL is reported to associate with albumin-bound NEFA in a concentration-dependent manner (0.25–2 mM) following incubation for 4 h (57–59), which is similar to conditions used in our experiments. Binding of LDL to NEFA in vivo is believed to be responsible for the generation of electronegative or minimally modified/oxidized LDL that characterizes subjects with hypertriglyceridemia, type 2 diabetes, and coronary heart disease (60). Binding of LDL to NEFA may not only reduce its availability for uptake by WAT, resulting in its medium accumulation, but may also hinder TRL clearance. This is because NEFA are well documented to inhibit LPL activity by several mechanisms, including displacing LPL from the endothelial surface and interfering with LPL binding to apoC-II and to its anchors on the cell membrane (61–67). However, it should be underscored that delayed clearance of dietary TRL and remnants in women with high apoB may also have contributed to elevated plasma NEFA pool due to prolonged spillover from TG hydrolysis. This, however, cannot explain LDL-induced reduction in albumin-bound-NEFA uptake ex vivo into WAT in the absence of TRL.
In conclusion, LDL promotes delayed plasma clearance of TRL and NEFA in subcutaneous WAT of postmenopausal obese women, which may favor the postprandial hypertriglyceridemia observed in this group. We hypothesize that targeted reduction of plasma apoB may ameliorate WAT function, TRL clearance, and associated cardiometabolic risks in humans.
Acknowledgments
The authors acknowledge the invaluable work of Dr. Remi Rabasa-Lhoret in subject screening, recruitment, and medical follow-up. The authors thank Miguel Chagnon (Statistics Department, Université de Montréal) for his advice on the statistical analysis of the data.
Footnotes
Abbreviations:
- AUC
- area under the curve
- BMI
- body mass index
- 3H-TRL
- 3H-triolein-labeled TRL
- FCHL
- familial combined hyperlipidemia
- RMR
- resting metabolic rate
- TC
- total cholesterol
- TG
- triglyceride
- TRL
- triglyceride-rich lipoprotein
- WAT
- white adipose tissue
This work was supported by Canadian Institutes of Health Research (CIHR) Grant 93581 and New Investigator Award (to M.F.); by Canadian Foundation for Innovation (CFI) Leader's Opportunity Fund (to M.F.); and by CIHR Frederick Banting and Charles Best Canada Master's Awards (to H.S. and N.S-P.).
REFERENCES
- 1.Nordestgaard B. G., Benn M., Schnohr P., Tybjaerg-Hansen A. 2007. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA. 298: 299–308 [DOI] [PubMed] [Google Scholar]
- 2.Samra J. S., Clark M. L., Humphreys S. M., Macdonald I. A., Frayn K. N. 1996. Regulation of lipid metabolism in adipose tissue during early starvation. Am. J. Physiol. 271: E541–E546 [DOI] [PubMed] [Google Scholar]
- 3.Frayn K. N., Coppack S. W., Fielding B. A., Humphreys S. M. 1995. Coordinated regulation of hormone-sensitive lipase and lipoprotein lipase in human adipose tissue in vivo: implications for the control of fat storage and fat mobilization. Adv. Enzyme Regul. 35: 163–178 [DOI] [PubMed] [Google Scholar]
- 4.Bickerton A. S. T., Roberts R., Fielding B. A., Hodson L., Blaak E. E., Wagenmakers A. J. M., Gilbert M., Karpe F., Frayn K. N. 2007. Preferential uptake of dietary fatty acids in adipose tissue and muscle in the postprandial period. Diabetes. 56: 168–176 [DOI] [PubMed] [Google Scholar]
- 5.Evans K., Burdge G. C., Wootton S. A., Clark M. L., Frayn K. N. 2002. Regulation of dietary fatty acid entrapment in subcutaneous adipose tissue and skeletal muscle. Diabetes. 51: 2684–2690 [DOI] [PubMed] [Google Scholar]
- 6.Faraj M., Lu H. L., Cianflone K. 2004. Diabetes, lipids, and adipocyte secretagogues. Biochem. Cell Biol. 82: 170–190 [DOI] [PubMed] [Google Scholar]
- 7.Carpentier A., Mittelman S. D., Bergman R. N., Giacca A., Lewis G. F. 1999. Acute enhancement of insulin secretion by FFA in humans is lost with prolonged FFA elevation. Am. J. Physiol. 276: E1055–E1066 [DOI] [PubMed] [Google Scholar]
- 8.Lewis G. F., Carpentier A., Adeli K., Giacca A. 2002. Disordered fat storage and mobilization in the pathogenesis of insulin resistance and type 2 diabetes. Endocr. Rev. 23: 201–229 [DOI] [PubMed] [Google Scholar]
- 9.Sniderman A. D., Cianflone K., Frayn K. 1997. The pathogenetic role of impaired fatty acid trapping by adipocytes in generating the pleiotropic features of hyperapoB. Diabetologia. 40: S152–S154 [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y. L., Hernandez-Ono A., Ko C., Yasunaga K., Huang L. S., Ginsberg H. N. 2004. Regulation of hepatic apolipoprotein B-lipoprotein assembly and secretion by the availability of fatty acids. I. Differential response to the delivery of fatty acids via albumin or remnant-like emulsion particles. J. Biol. Chem. 279: 19362–19374 [DOI] [PubMed] [Google Scholar]
- 11.Lewis G. F. 1997. Fatty acid regulation of very low density lipoprotein production. Curr. Opin. Lipidol. 8: 146–153 [DOI] [PubMed] [Google Scholar]
- 12.Lewis G. F., Uffelman K. D., Szeto L. W., Steiner G. 1993. Effects of acute hyperinsulinemia on VLDL triglyceride and VLDL apoB production in normal weight and obese individuals. Diabetes. 42: 833–842 [DOI] [PubMed] [Google Scholar]
- 13.Malmström R., Packard C. J., Caslake M., Bedford D., Stewart P., Yki-Jarvinen H., Shepherd J., Taskinen M. R. 1997. Defective regulation of triglyceride metabolism by insulin in the liver in NIDDM. Diabetologia. 40: 454–462 [DOI] [PubMed] [Google Scholar]
- 14.Ginsberg H. N., Zhang Y., Hernandez-Ono A. 2005. Regulation of plasma triglycerides in insulin resistance and diabetes. Arch. Med. Res. 36: 232–240 [DOI] [PubMed] [Google Scholar]
- 15.Sniderman A., Shapiro S., Marpole D., Skinner B., Teng B., Kwiterovich P. O., Jr 1980. Association of coronary atherosclerosis with hyperapobetalipoproteinemia [increased protein but normal cholesterol levels in human plasma low density (beta) lipoproteins]. Proc. Natl. Acad. Sci. USA. 77: 604–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sacks F. M. 2006. The apolipoprotein story. Atheroscler. Suppl. 7: 23–27 [DOI] [PubMed] [Google Scholar]
- 17.Yost T. J., Froyd K. K., Jensen D. R., Eckel R. H. 1995. Change in skeletal muscle lipoprotein lipase activity in response to insulin/glucose in non-insulin-dependent diabetes mellitus. Metabolism. 44: 786–790 [DOI] [PubMed] [Google Scholar]
- 18.Frayn K. N., Humphreys S. M., Coppack S. W. 1996. Net carbon flux across subcutaneous adipose tissue after a standard meal in normal-weight and insulin-resistant obese subjects. Int. J. Obes. Relat. Metab. Disord. 20: 795–800 [PubMed] [Google Scholar]
- 19.de Graaf J., Veerkamp M., Stalenhoef A. F. H. 2002. Metabolic pathogenesis of familial combined hyperlipidaemia with emphasis on insulin resistance, adipose tissue metabolism and free fatty acids. J. R. Soc. Med. 95: 46–53 [PMC free article] [PubMed] [Google Scholar]
- 20.Arner P. 1997. Is familial combined hyperlipidaemia a genetic disorder of adipose tissue? Curr. Opin. Lipidol. 8: 89–94 [DOI] [PubMed] [Google Scholar]
- 21.Kuniyasu A., Hayashi S., Nakayama H. 2002. Adipocytes recognize and degrade oxidized low density lipoprotein through CD36. Biochem. Biophys. Res. Commun. 295: 319–323 [DOI] [PubMed] [Google Scholar]
- 22.Masella R., Vari R., D'Archivio M., Santangelo C., Scazzocchio B., Maggiorella M. T., Sernicola L., Titti F., Sanchez M., Di Mario U., et al. 2006. Oxidised LDL modulate adipogenesis in 3T3-L1 preadipocytes by affecting the balance between cell proliferation and differentiation. FEBS Lett. 580: 2421–2429 [DOI] [PubMed] [Google Scholar]
- 23.D'Archivio M., Scazzocchio B., Filesi C., Vari R., Maggiorella M. T., Sernicola L., Santangelo C., Giovannini C., Masella R. 2008. Oxidised LDL up-regulate CD36 expression by the Nrf2 pathway in 3T3–L1 preadipocytes. FEBS Lett. 582: 2291–2298 [DOI] [PubMed] [Google Scholar]
- 24.Cianflone K., Bilodeau M., Davignon J., Sniderman A. D. 1990. Modulation of chylomicron remnant metabolism by an HMG CoA reductase inhibitor. Metabolism. 39: 274–280 [DOI] [PubMed] [Google Scholar]
- 25.Cabezas M. C., de Bruin T. W., Jansen H., Kock L. A., Kortlandt W., Erkelens D. W. 1993. Impaired chylomicron remnant clearance in familial combined hyperlipidemia. Arterioscler. Thromb. 13: 804–814 [DOI] [PubMed] [Google Scholar]
- 26.Bredie S. J., de Bruin T. W., Demacker P. N., Kastelein J. J., Stalenhoef A. F. 1995. Comparison of gemfibrozil versus simvastatin in familial combined hyperlipidemia and effects on apolipoprotein-B- containing lipoproteins, low-density lipoprotein subfraction profile, and low-density lipoprotein oxidizability. Am. J. Cardiol. 75: 348–353 [DOI] [PubMed] [Google Scholar]
- 27.Castro Cabezas M., Verseyden C., Meijssen S., Jansen H., Erkelens D. W. 2004. Effects of atorvastatin on the clearance of triglyceride-rich lipoproteins in familial combined hyperlipidemia. J. Clin. Endocrinol. Metab. 89: 5972–5980 [DOI] [PubMed] [Google Scholar]
- 28.Genest J., McPherson R., Frohlich J., Anderson T., Campbell N., Carpentier A., Couture P., Dufour R., Fodor G., Francis G., et al. 2009. 2009 Canadian Cardiovascular Society/Canadian guidelines for the diagnosis and treatment of dyslipidemia and prevention of cardiovascular disease in the adult - 2009 recommendations. Can. J. Cardiol. 25: 567–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weinsier R. L., Nagy T. R., Hunter G. R., Darnell B. E., Hensrud D. D., Weiss H. L. 2000. Do adaptive changes in metabolic rate favor weight regain in weight-reduced individuals? An examination of the set-point theory. Am. J. Clin. Nutr. 72: 1088–1094 [DOI] [PubMed] [Google Scholar]
- 30.Faraj M., Messier L., Bastard J. P., Tardif A., Godbout A., Prud'homme D., Rabasa-Lhoret R. 2006. Apolipoprotein B: a predictor of inflammatory status in postmenopausal overweight and obese women. Diabetologia. 49: 1637–1646 [DOI] [PubMed] [Google Scholar]
- 31.Faraj M., Lavoie M-E., Messier L., Bastard J. P., Prud'homme D. 2010. Reduction in serum apoB is associated with reduced inflammation and insulin resistance in post-menopausal women: a MONET study. Atherosclerosis. 211: 682–688 [DOI] [PubMed] [Google Scholar]
- 32.Cunningham J. J. 1990. Calculation of energy expenditure from indirect calorimetry: assessment of the Weir equation. Nutrition. 6: 222–223 [PubMed] [Google Scholar]
- 33.Faraj M., Jones P., Sniderman A. D., Cianflone K. 2001. Enhanced dietary fat clearance in post-obese women. J. Lipid Res. 42: 571–580 [PubMed] [Google Scholar]
- 34.Schectman G., Patsches M., Sasse E. A. 1996. Variability in cholesterol measurements: comparison of calculated and direct LDL cholesterol determinations. Clin. Chem. 42: 732–737 [PubMed] [Google Scholar]
- 35.Reyes-Soffer G., Holleran S., Karmally W., Ngai C. I., Chen N. T., Torres M., Ramakrishnan R., Blaner W. S., Berglund L., Ginsberg H. N., et al. 2009. Measures of postprandial lipoproteins are not associated with coronary artery disease in patients with type 2 diabetes mellitus. J. Lipid Res. 50: 1901–1909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wassef H., Salem H., Bissonnette S., Baass A., Dufour R., Davignon J., Faraj M. 2012. White adipose tissue-apoC-I secretion; relation to delayed plasma clearance of dietary fat in humans. Arterioscler. Thromb. Vasc. Biol. 32: 2785–2793 [DOI] [PubMed] [Google Scholar]
- 37.Faraj M., Beauregard G., Loizon E., Moldes M., Clément K., Tahiri Y., Cianflone K., Vidal H., Rabasa-Lhoret R. 2006. Insulin regulation of gene expression and concentrations of white adipose tissue-derived proteins in vivo in healthy men: relation to adiponutrin. J. Endocrinol. 191: 427–435 [DOI] [PubMed] [Google Scholar]
- 38.Moldes M., Beauregard G., Faraj M., Peretti N., Ducluzeau P. H., Laville M., Rabasa-Lhoret R., Vidal H., Clément K. 2006. Adiponutrin gene is regulated by insulin and glucose in human adipose tissue. Eur. J. Endocrinol. 2006: 461–468 [DOI] [PubMed] [Google Scholar]
- 39.Binnert C., Laville M., Pachiaudi C., Rigalleau V., Beylot M. 1995. Use of gas chromatography/isotope ratio-mass spectrometry to study triglyceride metabolism in humans. Lipids. 30: 869–873 [DOI] [PubMed] [Google Scholar]
- 40.Binnert C., Pachiaudi C., Beylot M., Croset M., Cohen R., Riou J. P., Laville M. 1996. Metabolic fate of an oral long-chain triglyceride load in humans. Am. J. Physiol. 270: E445–E450 [DOI] [PubMed] [Google Scholar]
- 41.Knuth N. D., Remias D. B., Horowitz J. F. 2008. Adding carbohydrate to a high-fat meal blunts postprandial lipemia in women and reduces meal-derived fatty acids in systemic circulation. Appl. Physiol. Nutr. Metab. 33: 315–325 [DOI] [PubMed] [Google Scholar]
- 42.Vandermeersch A., Ameye S., Puype D., Petitjean D., De Buyzere M., Langlois M. R. 2010. Estimation of the low-density lipoprotein (LDL) subclass phenotype using a direct, automated assay of small dense LDL-cholesterol without sample pretreatment. Clin. Chim. Acta. 411: 1361–1366 [DOI] [PubMed] [Google Scholar]
- 43.Wassef H., Bernier L., Davignon J., Cohn J. S. 2004. Synthesis and secretion of apoC-I and apoE during maturation of human SW872 liposarcoma cells. J. Nutr. 134: 2935–2941 [DOI] [PubMed] [Google Scholar]
- 44.Faraj M., Sniderman A., Cianflone K. 2004. ASP enhances in situ lipoprotein lipase activity by increasing fatty acid trapping in adipocytes. J. Lipid Res. 45: 657–666 [DOI] [PubMed] [Google Scholar]
- 45.Faraj M., Cianflone K. 2004. Differential regulation of fatty acid trapping in mouse adipose tissue and muscle by ASP. Am. J. Physiol. Endocrinol. Metab. 287: E150–E159 [DOI] [PubMed] [Google Scholar]
- 46.Ginsberg H. N. 1998. Lipoprotein physiology. Endocrinol. Metab. Clin. North Am. 27: 503–519 [DOI] [PubMed] [Google Scholar]
- 47.Gustafsson M., Levin M., Skalen K., Perman J., Friden V., Jirholt P., Olofsson S. O., Fazio S., Linton M. F., Semenkovich C. F., et al. 2007. Retention of low-density lipoprotein in atherosclerotic lesions of the mouse: evidence for a role of lipoprotein lipase. Circ. Res. 101: 777–783 [DOI] [PubMed] [Google Scholar]
- 48.Boren J., Lookene A., Makoveichuk E., Xiang S., Gustafsson M., Liu H., Talmud P., Olivecrona G. 2001. Binding of low density lipoproteins to lipoprotein lipase is dependent on lipids but not on apolipoprotein B. J. Biol. Chem. 276: 26916–26922 [DOI] [PubMed] [Google Scholar]
- 49.Williams K. J., Chen K. 2010. Recent insights into factors affecting remnant lipoprotein uptake. Curr. Opin. Lipidol. 21: 218–228 [DOI] [PubMed] [Google Scholar]
- 50.Teng B., Forse A., Rodriguez M. A., Sniderman A. D. 1988. Adipose tissue glyceride synthesis in patients with hyperapobetalipoproteinemia. Can. J. Physiol. Pharmacol. 66: 239–242 [DOI] [PubMed] [Google Scholar]
- 51.Arner P., Bernard S., Salehpour M., Possnert G., Liebl J., Steier P., Buchholz B. A., Eriksson M., Arner E., Hauner H., et al. 2011. Dynamics of human adipose lipid turnover in health and metabolic disease. Nature. 478: 110–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wilsie L. C/, Chanchani S., Navaratna D., Orlando R. A. 2005. Cell surface heparan sulfate proteoglycans contribute to intracellular lipid accumulation in adipocytes. Lipids Health Dis. 4: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stanton L. A., van de Venter M., Oelofsen W. 1999. Interaction of plasma lipoprotein subfractions with differentiating 3T3–L1 and human mammary preadipocytes in culture. J. Cell. Biochem. 74: 181–193 [PubMed] [Google Scholar]
- 54.Fredrickson D. S., Lee R. 1965. A system for phenotyping hyperlipoproteinemia. Circulation. 31: 321–327 [DOI] [PubMed] [Google Scholar]
- 55.Skogsberg J., Dicker A., Ryden M., Astrom G., Nilsson R., Bhuiyan H., Vitols S., Mairal A., Langin D., Alberts P., et al. 2008. ApoB100-LDL acts as a metabolic signal from liver to peripheral fat causing inhibition of lipolysis in adipocytes. PLoS ONE. 3: e3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang Z., Ying Z., Bosy-Westphal A., Zhang J., Heller M., Later W., Heymsfield S. B., Muller M. J. 2012. Evaluation of specific metabolic rates of major organs and tissues: comparison between nonobese and obese women. Obesity (Silver Spring). 20: 95–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Benítez S., Sanchez-Quesada J. L., Lucero L., Arcelus R., Ribas V., Jorba O., Castellvi A., Alonso E., Blanco-Vaca F., Ordonez-Llanos J. 2002. Changes in low-density lipoprotein electronegativity and oxidizability after aerobic exercise are related to the increase in associated non-esterified fatty acids. Atherosclerosis. 160: 223–232 [DOI] [PubMed] [Google Scholar]
- 58.Benítez S., Villegas V., Bancells C., Jorba O., Gonzalez-Sastre F., Ordonez-Llanos J., Sanchez-Quesada J. L. 2004. Impaired binding affinity of electronegative low-density lipoprotein (LDL) to the LDL receptor is related to nonesterified fatty acids and lysophosphatidylcholine content. Biochemistry. 43: 15863–15872 [DOI] [PubMed] [Google Scholar]
- 59.Benítez S., Camacho M., Arcelus R., Vila S., Bancells C., Ordonez-Llanos J., Sanchez-Quesada J. L. 2004. Increased lysophosphatidylcholine and non-esterified fatty acid content in LDL induces chemokine release in endothelial cells: relationship with electronegative LDL. Atherosclerosis. 177: 299–305 [DOI] [PubMed] [Google Scholar]
- 60.Mello A. P. Q., da Silva I. T., Abdalla D. S. P., Damasceno N. R. T. 2011. Electronegative low-density lipoprotein: origin and impact on health and disease. Atherosclerosis. 215: 257–265 [DOI] [PubMed] [Google Scholar]
- 61.Posner I., DeSanctis J. 1987. The effects of bovine serum albumin and oleic acid on rat pancreatic lipase and bovine milk lipoprotein lipase. Comp. Biochem. Physiol. B. 87: 137–141 [DOI] [PubMed] [Google Scholar]
- 62.Posner I., DeSanctis J. 1987. Kinetics of product inhibition and mechanisms of lipoprotein lipase activation by apolipoprotein C-II. Biochemistry. 26: 3711–3717 [DOI] [PubMed] [Google Scholar]
- 63.Amri E. Z., Teboul L., Vannier C., Grimaldi P. A., Ailhaud G. 1996. Fatty acids regulate the expression of lipoprotein lipase gene and activity in preadipose and adipose cells. Biochem. J. 314: 541–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Saxena U., Goldberg I. J. 1990. Interaction of lipoprotein lipase with glycosaminoglycans and apolipoproteins C-II: effects of free fatty acids. Biochim. Biophys. Acta. 1043: 161–168 [DOI] [PubMed] [Google Scholar]
- 65.Saxena U., Witte L. D., Goldberg I. J. 1989. Release of endothelial lipoprotein lipase by plasma lipoproteins and free fatty acids. J. Biol. Chem. 264: 4349–4355 [PubMed] [Google Scholar]
- 66.Olivecrona T., Bergo M., Hultin M., Olivecrona G. 1995. Nutritional regulation of lipoprotein lipase. Can. J. Cardiol. 11: 73G–78G [PubMed] [Google Scholar]
- 67.Sasaki A., Goldberg I. J. 1992. Lipoprotein lipase release from BFC-1 beta adipocytes. Effects of triglyceride-rich lipoproteins and lipolysis products. J. Biol. Chem. 267: 15198–15204 [PubMed] [Google Scholar]