Abstract
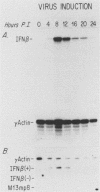
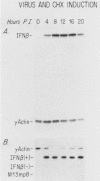
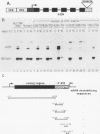
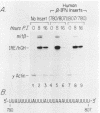
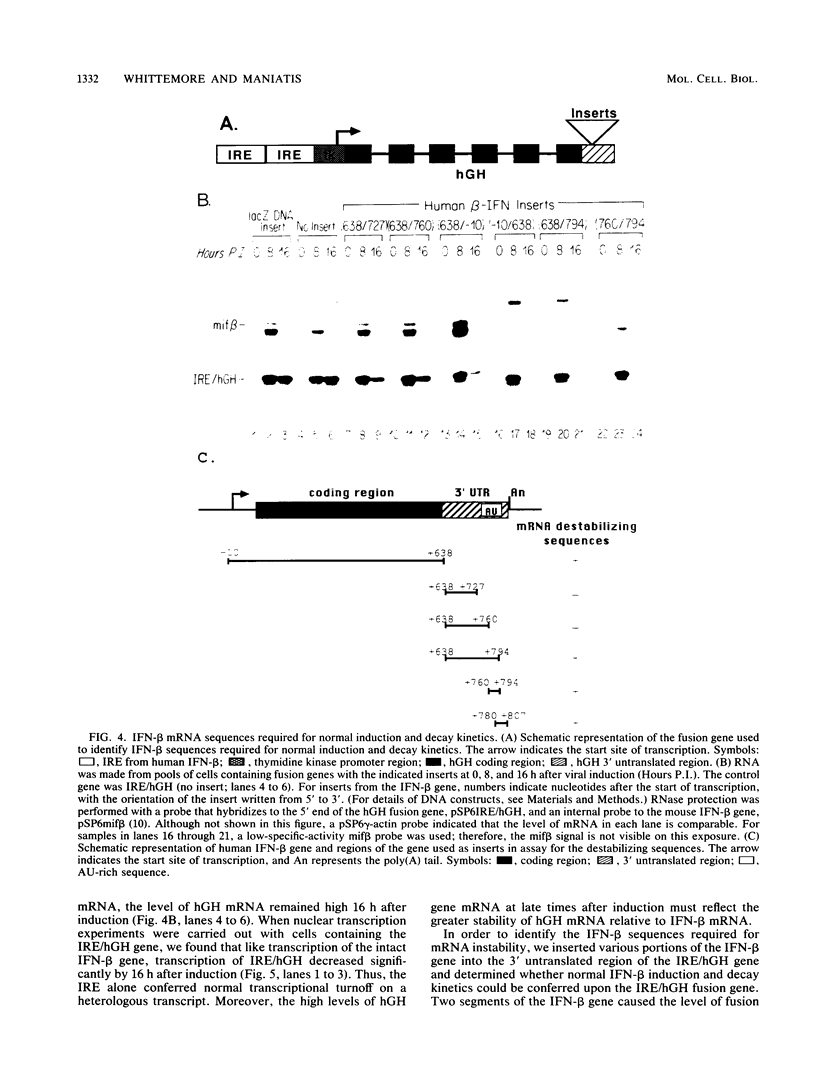
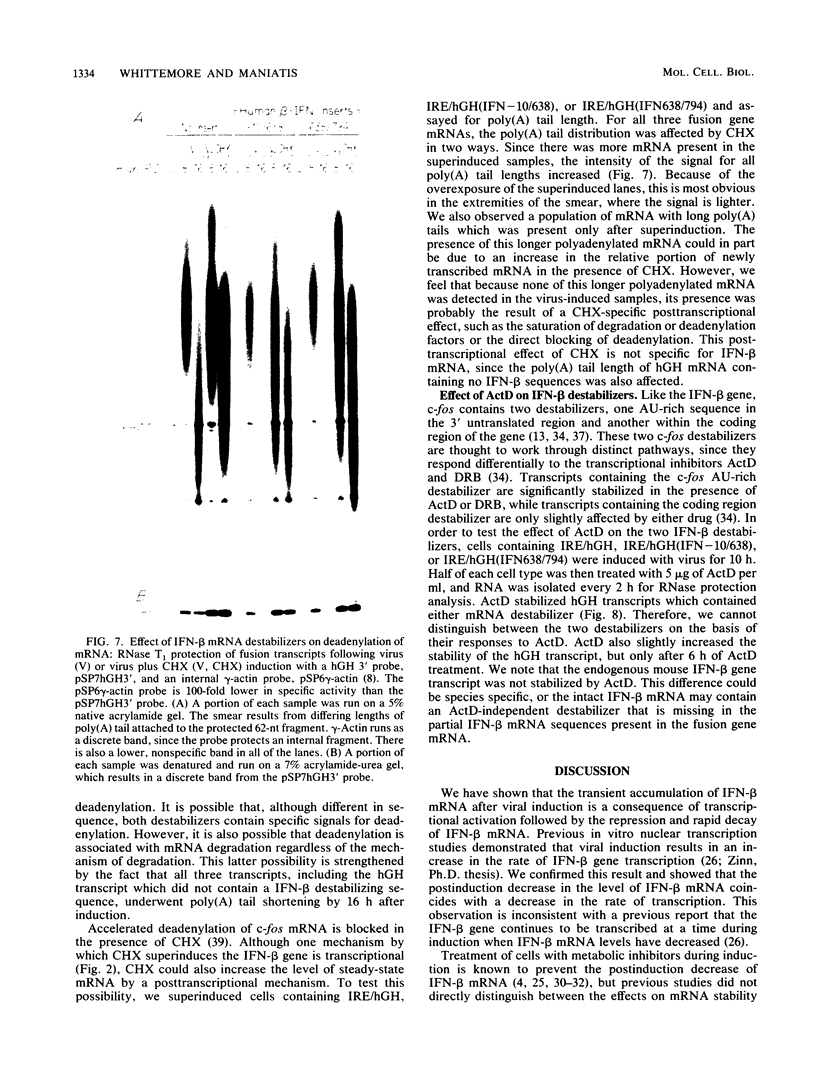
Viral induction of the human beta-interferon (IFN-beta) gene leads to a transient accumulation of high levels of IFN-beta mRNA. Previous studies have shown that the increase in IFN-beta mRNA levels after induction is due to an increase in the rate of IFN-beta gene transcription. In this paper, we show that the rapid postinduction decrease in the level of IFN-beta mRNA is due to a combination of transcriptional repression and rapid turnover of the mRNA. This transcriptional repression can be blocked with cycloheximide, suggesting that the synthesis of a virus-inducible repressor is necessary for the postinduction turnoff of the IFN-beta gene. Analysis of the sequence requirements for IFN-beta mRNA instability revealed two regions capable of destabilizing a heterologous mRNA. One destabilizer is an AU-rich sequence in the 3' untranslated region, and the other is located 5' to the translation stop codon.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brawerman G. mRNA decay: finding the right targets. Cell. 1989 Apr 7;57(1):9–10. doi: 10.1016/0092-8674(89)90166-9. [DOI] [PubMed] [Google Scholar]
- Brewer G., Ross J. Poly(A) shortening and degradation of the 3' A+U-rich sequences of human c-myc mRNA in a cell-free system. Mol Cell Biol. 1988 Apr;8(4):1697–1708. doi: 10.1128/mcb.8.4.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caput D., Beutler B., Hartog K., Thayer R., Brown-Shimer S., Cerami A. Identification of a common nucleotide sequence in the 3'-untranslated region of mRNA molecules specifying inflammatory mediators. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1670–1674. doi: 10.1073/pnas.83.6.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalieri R. L., Havell E. A., Vilcek J., Pestka S. Induction and decay of human fibroblast interferon mRNA. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4415–4419. doi: 10.1073/pnas.74.10.4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Dinter H., Hauser H. Superinduction of the human interferon-beta promoter. EMBO J. 1987 Mar;6(3):599–604. doi: 10.1002/j.1460-2075.1987.tb04796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch T., Zinn K., Maniatis T. Activation of the human beta-interferon gene requires an interferon-inducible factor. Mol Cell Biol. 1986 Mar;6(3):801–810. doi: 10.1128/mcb.6.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodbourn S., Burstein H., Maniatis T. The human beta-interferon gene enhancer is under negative control. Cell. 1986 May 23;45(4):601–610. doi: 10.1016/0092-8674(86)90292-8. [DOI] [PubMed] [Google Scholar]
- Goodbourn S., Zinn K., Maniatis T. Human beta-interferon gene expression is regulated by an inducible enhancer element. Cell. 1985 Jun;41(2):509–520. doi: 10.1016/s0092-8674(85)80024-6. [DOI] [PubMed] [Google Scholar]
- Greenberg M. E., Ziff E. B. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature. 1984 Oct 4;311(5985):433–438. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- Jones T. R., Cole M. D. Rapid cytoplasmic turnover of c-myc mRNA: requirement of the 3' untranslated sequences. Mol Cell Biol. 1987 Dec;7(12):4513–4521. doi: 10.1128/mcb.7.12.4513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabnick K. S., Housman D. E. Determinants that contribute to cytoplasmic stability of human c-fos and beta-globin mRNAs are located at several sites in each mRNA. Mol Cell Biol. 1988 Aug;8(8):3244–3250. doi: 10.1128/mcb.8.8.3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruys V., Marinx O., Shaw G., Deschamps J., Huez G. Translational blockade imposed by cytokine-derived UA-rich sequences. Science. 1989 Aug 25;245(4920):852–855. doi: 10.1126/science.2672333. [DOI] [PubMed] [Google Scholar]
- Levy D. E., Kessler D. S., Pine R., Reich N., Darnell J. E., Jr Interferon-induced nuclear factors that bind a shared promoter element correlate with positive and negative transcriptional control. Genes Dev. 1988 Apr;2(4):383–393. doi: 10.1101/gad.2.4.383. [DOI] [PubMed] [Google Scholar]
- Lindstein T., June C. H., Ledbetter J. A., Stella G., Thompson C. B. Regulation of lymphokine messenger RNA stability by a surface-mediated T cell activation pathway. Science. 1989 Apr 21;244(4902):339–343. doi: 10.1126/science.2540528. [DOI] [PubMed] [Google Scholar]
- Malter J. S. Identification of an AUUUA-specific messenger RNA binding protein. Science. 1989 Nov 3;246(4930):664–666. doi: 10.1126/science.2814487. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Goodbourn S., Fischer J. A. Regulation of inducible and tissue-specific gene expression. Science. 1987 Jun 5;236(4806):1237–1245. doi: 10.1126/science.3296191. [DOI] [PubMed] [Google Scholar]
- Marcu K. B. Regulation of expression of the c-myc proto-oncogene. Bioessays. 1987 Jan;6(1):28–32. doi: 10.1002/bies.950060108. [DOI] [PubMed] [Google Scholar]
- McCormick F., Trahey M., Innis M., Dieckmann B., Ringold G. Inducible expression of amplified human beta interferon genes in CHO cells. Mol Cell Biol. 1984 Jan;4(1):166–172. doi: 10.1128/mcb.4.1.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell R. L., Henning-Chubb C., Huberman E., Verma I. M. c-fos expression is neither sufficient nor obligatory for differentiation of monomyelocytes to macrophages. Cell. 1986 May 23;45(4):497–504. doi: 10.1016/0092-8674(86)90281-3. [DOI] [PubMed] [Google Scholar]
- Mosca J. D., Pitha P. M. Transcriptional and posttranscriptional regulation of exogenous human beta interferon gene in simian cells defective in interferon synthesis. Mol Cell Biol. 1986 Jun;6(6):2279–2283. doi: 10.1128/mcb.6.6.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nepveu A., Levine R. A., Campisi J., Greenberg M. E., Ziff E. B., Marcu K. B. Alternative modes of c-myc regulation in growth factor-stimulated and differentiating cells. Oncogene. 1987;1(3):243–250. [PubMed] [Google Scholar]
- Nir U., Cohen B., Chen L., Revel M. A human IFN-beta 1 gene deleted of promoter sequences upstream from the TATA box is controlled post-transcriptionally by dsRNA. Nucleic Acids Res. 1984 Sep 25;12(18):6979–6993. doi: 10.1093/nar/12.18.6979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj N. B., Pitha P. M. Analysis of interferon mRNA in human fibroblast cells induced to produce interferon. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7426–7430. doi: 10.1073/pnas.78.12.7426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj N. B., Pitha P. M. Two levels of regulation of beta-interferon gene expression in human cells. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3923–3927. doi: 10.1073/pnas.80.13.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves R., Elton T. S., Nissen M. S., Lehn D., Johnson K. R. Posttranscriptional gene regulation and specific binding of the nonhistone protein HMG-I by the 3' untranslated region of bovine interleukin 2 cDNA. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6531–6535. doi: 10.1073/pnas.84.18.6531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringold G. M., Dieckmann B., Vannice J. L., Trahey M., McCormick F. Inhibition of protein synthesis stimulates the transcription of human beta-interferon genes in Chinese hamster ovary cells. Proc Natl Acad Sci U S A. 1984 Jul;81(13):3964–3968. doi: 10.1073/pnas.81.13.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuler G. D., Cole M. D. GM-CSF and oncogene mRNA stabilities are independently regulated in trans in a mouse monocytic tumor. Cell. 1988 Dec 23;55(6):1115–1122. doi: 10.1016/0092-8674(88)90256-5. [DOI] [PubMed] [Google Scholar]
- Sehgal P. B., Dobberstein B., Tamm I. Interferon messenger RNA content of human fibroblasts during induction, shutoff, and superinduction of interferon production. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3409–3413. doi: 10.1073/pnas.74.8.3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehgal P. B., Lyles D. S., Tamm I. Superinduction of human fibroblast interferon production: further evidence for increased stability of interferon mRNA. Virology. 1978 Aug;89(1):186–198. doi: 10.1016/0042-6822(78)90051-x. [DOI] [PubMed] [Google Scholar]
- Sehgal P. B., Tamm I. Two mechanisms contribute to the superinduction of poly(I).poly(C)-induced human fibroblast interferon production. Virology. 1979 Jan 15;92(1):240–244. doi: 10.1016/0042-6822(79)90230-7. [DOI] [PubMed] [Google Scholar]
- Shaw G., Kamen R. A conserved AU sequence from the 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986 Aug 29;46(5):659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- Shyu A. B., Greenberg M. E., Belasco J. G. The c-fos transcript is targeted for rapid decay by two distinct mRNA degradation pathways. Genes Dev. 1989 Jan;3(1):60–72. doi: 10.1101/gad.3.1.60. [DOI] [PubMed] [Google Scholar]
- Tan Y. H., Berthold W. A mechanism for the induction and regulation of human fibroblastoid interferon genetic expression. J Gen Virol. 1977 Mar;34(3):401–411. doi: 10.1099/0022-1317-34-3-401. [DOI] [PubMed] [Google Scholar]
- Taniguchi T. Regulation of cytokine gene expression. Annu Rev Immunol. 1988;6:439–464. doi: 10.1146/annurev.iy.06.040188.002255. [DOI] [PubMed] [Google Scholar]
- Treisman R. Transient accumulation of c-fos RNA following serum stimulation requires a conserved 5' element and c-fos 3' sequences. Cell. 1985 Oct;42(3):889–902. doi: 10.1016/0092-8674(85)90285-5. [DOI] [PubMed] [Google Scholar]
- Wilson T., Treisman R. Removal of poly(A) and consequent degradation of c-fos mRNA facilitated by 3' AU-rich sequences. Nature. 1988 Nov 24;336(6197):396–399. doi: 10.1038/336396a0. [DOI] [PubMed] [Google Scholar]
- Zinn K., DiMaio D., Maniatis T. Identification of two distinct regulatory regions adjacent to the human beta-interferon gene. Cell. 1983 Oct;34(3):865–879. doi: 10.1016/0092-8674(83)90544-5. [DOI] [PubMed] [Google Scholar]
- Zinn K., Keller A., Whittemore L. A., Maniatis T. 2-Aminopurine selectively inhibits the induction of beta-interferon, c-fos, and c-myc gene expression. Science. 1988 Apr 8;240(4849):210–213. doi: 10.1126/science.3281258. [DOI] [PubMed] [Google Scholar]
- Zinn K., Maniatis T. Detection of factors that interact with the human beta-interferon regulatory region in vivo by DNAase I footprinting. Cell. 1986 May 23;45(4):611–618. doi: 10.1016/0092-8674(86)90293-x. [DOI] [PubMed] [Google Scholar]
- van Heuvel M., Bosveld I. J., Luyten W., Trapman J., Zwarthoff E. C. Transient expression of murine interferon-alpha genes in mouse and monkey cells. Gene. 1986;45(2):159–165. doi: 10.1016/0378-1119(86)90250-7. [DOI] [PubMed] [Google Scholar]