Abstract
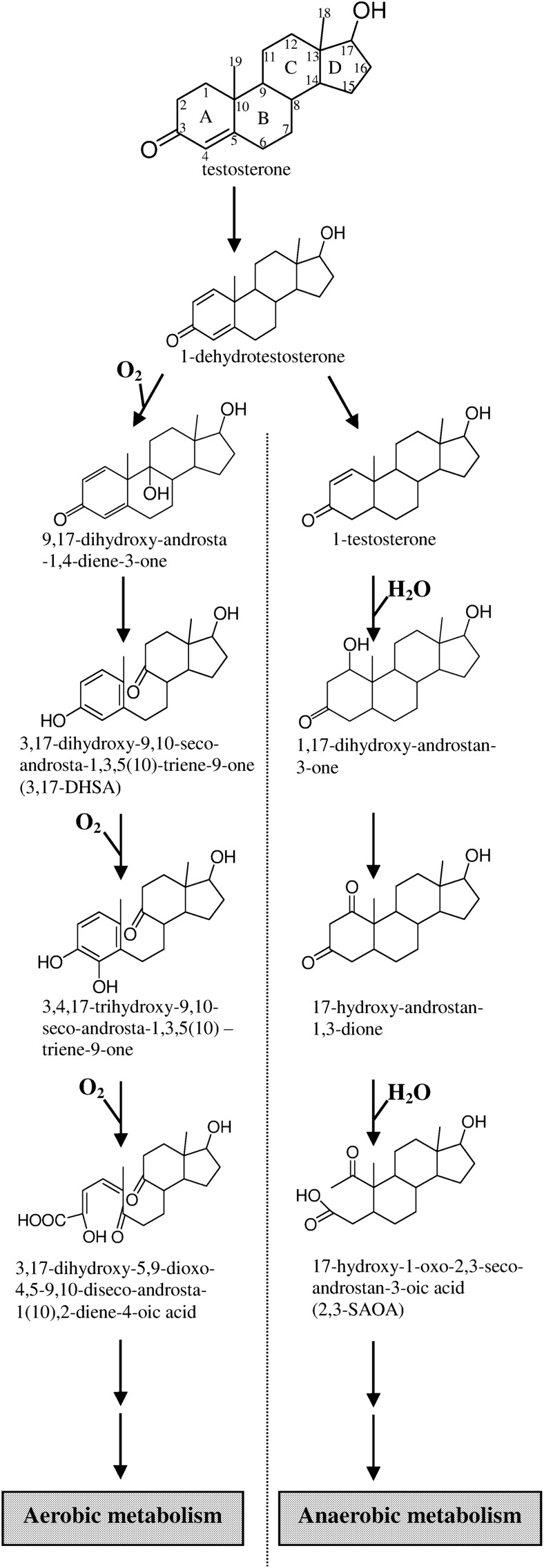
The aerobic degradation of steroids by bacteria has been studied in some detail. In contrast, only little is known about the anaerobic steroid catabolism. Steroidobacter denitrificans can utilize testosterone under both oxic and anoxic conditions. By conducting metabolomic investigations, we demonstrated that S. denitrificans adopts the 9,10-seco-pathway to degrade testosterone under oxic conditions. This pathway depends on the use of oxygenases for oxygenolytic ring fission. Conversely, the detected degradation intermediates under anoxic conditions suggest a novel, oxygenase-independent testosterone catabolic pathway, the 2,3-seco-pathway, which differs significantly from the aerobic route. In this anaerobic pathway, testosterone is first transformed to 1-dehydrotestosterone, which is then reduced to produce 1-testosterone followed by water addition to the C-1/C-2 double bond of 1-testosterone. Subsequently, the C-1 hydroxyl group is oxidized to produce 17-hydroxy-androstan-1,3-dione. The A-ring of this compound is cleaved by hydrolysis as evidenced by H218O-incorporation experiments. Regardless of the growth conditions, testosterone is initially transformed to 1-dehydrotestosterone. This intermediate is a divergence point at which the downstream degradation pathway is governed by oxygen availability. Our results shed light into the previously unknown cleavage of the sterane ring structure without oxygen. We show that, under anoxic conditions, the microbial cleavage of steroidal core ring system begins at the A-ring.
Keywords: steroid biodegradation, anaerobic metabolism, denitrifying bacteria, metabolomics, testosterone
Steroids are ubiquitous and abundant in nature. Considering their divergent functions, the structural similarity of steroids is remarkable. Mammals are unable to degrade steroids. After a modification (e.g., glucuronide and sulfate conjugations) to enhance the solubility, steroid hormones are excreted into the environment through the urinary tract of mammals (1). In addition, large amounts of steroid drugs are released from the pharmaceutical industry as environmental pollutants (2). Members of androgens and estrogens have been detected in a number of effluents of wastewater treatment plants and rivers at concentrations in the ng l−1 range (3–6). Because of the negative environmental effects of steroid hormones, the removal of these compounds from the environment has attracted considerable interest (7–9).
The biotransformation of steroids by microorganisms is a crucial example of the successful application of microbial technology in industrial processes (10). Several species of bacteria, such as Comamonas testosteroni, can degrade testosterone under oxic conditions. In 1968, Coulter and Talalay (11) established the oxygenase-dependent pathway (9,10-seco-pathway; Fig. 1) for the degradation of testosterone by aerobes. The aerobic testosterone degradation by C. testosteroni starts with the oxidation of the C-17 hydroxyl group and the introduction of a double bond at C-1/C-2. Subsequently, hydroxylation at C-9 occurs. The resulting compound, 9α-hydroxy-androsta-1,4-diene-3,17-dione, is unstable and undergoes spontaneous aromatization of the A-ring and nonenzymatic cleavage of the B-ring. The aromatized A-ring is subsequently split through the meta-cleavage. Investigations of the details of the aerobic steroid catabolic pathway are in progress. Overall, during the aerobic degradation of testosterone, three reactions are catalyzed by oxygenases. In addition to testosterone, cholesterol and phytosterols are also degraded through the common 9,10-seco-pathway, with C19 androgens as the intermediates (12).
Fig. 1.
The proposed aerobic and anaerobic catabolic pathways of testosterone demonstrated in S. denitrificans DSMZ18526. All these testosterone-derived intermediates were also observed as their 17-keto structures. The ring identification (A–D) and carbon numbering systems (1–19) are shown in testosterone.
By contrast, information on the biochemical and molecular details of anaerobic steroid degradation is very limited. Steroids, especially sterols, may remain in anoxic sediments over hundreds of millions of years (13, 14), indicating that steroids are not degraded facilely by anaerobes. Obviously, anaerobes must use a novel, oxygenase-independent catabolic strategy to degrade steroids in the absence of oxygen. Denitrifying bacteria are facultative aerobes that can use various aromatic compounds and terpenoids as sole sources of carbon and energy; thus, they play a crucial role in carbon cycling in the environment. In the last decade, a few denitrifying bacteria that can anaerobically mineralize steroids were isolated and characterized (15–18). Among them, S. denitrificans DSMZ18526 has an unusual ability to degrade testosterone under both oxic and anoxic conditions. The Blast results showed that S. denitrificans strains are widely distributed in diverse oxic and anoxic ecosystems, e.g., agriculture soil, bioremediated soil, anoxic sediment, activated sludge, and anoxic sludge (supplementary Fig. I).
Recently, the initial reactions involved in the anaerobic metabolism of cholesterol and testosterone were reported (19–21), albeit the ring cleavage details of the anaerobic pathways are yet to be unraveled. In this study, we adopted a 13C metabolomic approach to investigate the anaerobic degradation of testosterone using S. denitrificans as a model organism. The aerobic testosterone degradation by the same model organism was also studied for comparison. The results obtained shed light into the previously unknown cleavage of the sterane ring structure without oxygen. To our knowledge, this is the first study showing that under anoxic conditions, the microbial cleavage of steroidal core ring system begins at the A-ring.
MATERIALS AND METHODS
Chemicals and bacterial strain
The [2,3,4C-13C]testosterone was purchased from Isosciences. The chemicals were analytical grade and were purchased from Mallinckrodt Baker, Merck, or Sigma-Aldrich. Steroidobacter denitrificans DSMZ18526 was obtained from the Deutsche Sammlung für Mikroorganismen und Zellkulturen (Braunschweig, Germany).
Anaerobic in vivo transformation of [2,3,4C-13C]testosterone
S. denitrificans was grown with 2 mM of unlabeled testosterone in a 250 ml glass bottle. After the unlabeled testosterone was completely consumed, 10 ml of the anoxic culture was transferred into a 12 ml glass bottle sealed with a rubber stopper. The S. denitrificans cells were subsequently fed with 2 mM testosterone (unlabeled testosterone and [2,3,4C-13C]testosterone were mixed in a 1:1 molar ratio) under denitrifying conditions. The samples (1 ml) were withdrawn after 10 min, 6 h, and 12 h of incubation at 28°C. After the second sampling (6 h), 0.5 mM of mercaptopropionic acid [an inhibitor of acyl-CoA dehydrogenase (22)] was immediately added to the anoxic culture. The culture samples were immediately extracted three times with the same volume of ethyl acetate to recover testosterone-derived intermediates. The ethyl acetate fractions were combined, the solvent was evaporated, and the residue was redissolved in 100 μl of methanol. The testosterone-derived intermediates were identified using UPLC-HRMS.
Anaerobic growth of S. denitrificans with unlabeled testosterone
The S. denitrificans was grown with 4 mM of unlabeled testosterone at 28°C in anoxic fed-batch cultures (2 l) according to published procedures (20). The amounts of residual testosterone in the anoxic culture were monitored using HPLC. After the consumption of 2 mM testosterone, 0.5 mM mercaptopropionic acid was added to the cultures, and incubation was continued for an additional 12 h. The cultures were subsequently extracted three times with the same volume of ethyl acetate to recover residual testosterone and its derivatives from the aqueous phase. Separation of ethyl acetate extracts was performed using silica gel chromatography, TLC, and HPLC. The structures of HPLC-purified intermediates were determined using NMR spectroscopy.
Aerobic growth of S. denitrificans with testosterone
The S. denitrificans was grown in phosphate-buffered shake-flask cultures (500 ml in 2 l Erlenmeyer flasks) containing 4 mM testosterone. The cultures were incubated at 28°C in an orbital shaker (180 rpm). After the consumption of 2 mM testosterone, 1 mM of 3-chlorocatechol [a meta-cleavage inhibitor (23)] was added to the cultures, and incubation continued for an additional 12 h. The cultures were extracted using ethyl acetate, and testosterone-derived intermediates present in the extract were analyzed using UPLC-HRMS. Separation of ethyl acetate extracts was performed using silica gel chromatography, TLC, and HPLC.
S. denitrificans grown under various concentrations of oxygen
A S. denitrificans culture (500 ml) was first anaerobically grown on 2 mM testosterone. After testosterone and its derivatives were completely consumed, 50 ml of the preculture was mixed with 450 ml of fresh phosphate-buffered medium (pH 7.0) containing 2.2 mM testosterone, 10 mM NH4Cl (the nitrogen source), and 10 mM NaNO3 (the potential electron acceptor). The resulting cultures (100 ml) were transferred to five 1 l glass bottles sealed with rubber stoppers and were incubated under various concentrations of oxygen [headspace (900 ml); 0, 2.5, 5, 10, and 20% (v/v)]. The culture containing 20% oxygen in headspace was prepared in air. The remaining four cultures were prepared in an anaerobic chamber containing 95% nitrogen and 5% hydrogen gas. Oxygen gas was injected into the headspace after passing through a 0.22 μm membrane filter (Millipore). The fed-batch cultures were incubated at 28°C with shaking (180 rpm). Samples (3 ml) were retrieved every 4 h to measure the growth of bacterial cells (measured as total proteins), the residual amount of nitrate and testosterone, and the production of ring cleavage intermediates (3,17-DHSA and 2,3-SAOA). NaNO3 was added continuously to 10 mM when the nitrate added initially was consumed. After the consumption of 1 mM testosterone, 0.5 mM mercaptopropionic acid and 1 mM 3-chlorocatechol were added to the cultures, and incubation was continued for an additional 12 h.
Measurement of protein content and nitrate
The protein content in the culture samples and in cell extracts was determined using a BCA protein assay according to manufacturer's instructions, with BSA as the standard. Nitrate was determined by using the 2,6-dimethylphenol photometric method as described elsewhere (21).
Silica gel chromatography
A 385 ml silica gel column (55 × 3 cm; SiliaFlash P60; Silicycle) was equilibrated with 2 bed volumes of dichloromethane/ethyl acetate/ethanol (14:4:1, v/v). The ethyl acetate extract (approximately 350 mg dissolved in 3 ml ethyl acetate) was loaded to the column and eluted with the same solvent system at a flow rate of 2 ml min−1. The eluate was collected in 5 ml fractions, and a 0.5 ml sample was withdrawn from each fraction. The solvent was evaporated until dry, and the residue was redissolved in 10 μl of methanol. The samples after silica gel chromatography (SGC) were analyzed using TLC. The fractions that contained the same compounds were pooled and evaporated to dryness, and 200 μl of methanol was used to redissolve the residue. Further purification of testosterone-derived intermediates was performed using TLC.
Thin layer chromatography
The steroid standards and products were separated on silica gel aluminum TLC plates (Silica gel 60 F254, thickness, 0.2 mm, 20 × 20 cm; Merck). The following developing solvent system was used: dichloromethane/ethyl acetate/methanol (14:4:1, v/v). The steroid compounds were visualized under UV light at 254 nm or by spraying the TLC plates with 30% (v/v) H2SO4.
High-performance liquid chromatography
A reversed-phase Hitachi high-performance liquid chromatography (HPLC) system was used for the final separation. The separation was achieved on an analytical RP-C18 column [Luna 18 (2), 5 μm, 150 × 4.6 mm; Phenomenex] with a flow rate of 0.5 ml min−1. The separation was performed isocratically at room temperature with 50% (v/v) methanol as an eluent. The steroid products were detected in the range of 200–300 nm using a photodiode array detector. In addition, HPLC was used for the quantification of steroids present in the S. denitrificans cultures. The quantity of steroids (testosterone, 3,17-DHSA, and 2,3-SAOA) was calculated from their respective peak areas using a standard curve of individual standards. The R2 values for the standard curves were greater than 0.98. Data are averages of three measurements.
18O-Incorporation experiments
The denitrifying growth of S. denitrificans with testosterone and the preparation of cell extracts were performed as previously described (21). To determine the origins of the oxygen atoms at C-1 and/or C-3 of 17-hydroxy-androstan-1,3-dione and 17-hydroxy-1-oxo-2,3-seco-androstan-3-oic acid (2,3-SAOA), two in vitro assays were performed. The two reaction mixtures (3 ml for each assay) were prepared anaerobically and were incubated at 30°C for 16 h with shaking. The steroid products were extracted from the assays using ethyl acetate, and the extracts were analyzed using UPLC-APCI-mass spectrometry.
Control assay.
The 3 ml reaction mixture contained 50 mM Tris-HCl buffer (pH 7), soluble proteins (15 mg) of S. denitrificans, 0.5 mM mercaptopropionic acid, and 200 μl of 67.5 mM 1-testosterone solution (in 2-propanol). The final concentration of the steroid substrate in the reaction mixture was 4.5 mM. The final 2-propanol content was 6.67%.
18O-Labeled water-treated assay.
A total of 1.5 ml of 18O-labeled water (97 atom %, Aldrich) was added to 1.5 ml of 100 mM Tris-HCl buffer (pH 7) containing soluble proteins of S. denitrificans (15 mg) and 1 mM mercaptopropionic acid. The final 18O-water content was approximately 48.5%. The reaction was started by adding 4.5 mM of 1-testosterone to the anoxic assay. The 2-propanol content was also 6.67%.
UPLC-APCI-HRMS
The ethyl acetate extractable samples or purified steroid intermediates were analyzed using UPLC-MS with UPLC coupled to an atmospheric pressure chemical ionization (APCI) high-resolution mass spectrometry (HRMS). Mass spectral data were obtained using a Waters HDMS-QTOF synapt mass spectrometer (Waters) equipped with a standard APCI source operating in the positive ion mode. Separation was achieved on a reversed-phase C18 column (Acquity UPLC BEH C18, 1.7 μm, 100 × 2.1 mm; Waters) with a flow rate of 0.4 ml min−1 at 35°C (column oven temperature). The mobile phase comprised a mixture of two solvents: Solvent A [2% (v/v) acetonitrile containing 0.1% formic acid to enable excellent ionization in the APCI] and Solvent B (methanol containing 0.1% formic acid). Separation was achieved with a linear gradient of Solvent B from 10% to 99% in 8 min. In APCI-MS analysis, the temperature of the ion source was maintained at 100°C. Nitrogen desolvation gas was set at a flow rate of 500 l h–1 and the probe was heated to 400°C. Nitrogen was used as the APCI nebulizer gas. The corona current was maintained at 20 μA, and the electron multiplier voltage was set to1700 eV. The parent scan was in the range of m/z 50–500. The predicted elemental composition of individual intermediates was calculated using MassLynx Mass Spectrometry Software (Waters).
UPLC-ESI-HRMS
The ethyl acetate extractable samples or TLC-purified testosterone-derived intermediates were also analyzed using UPLC-ESI-HRMS. The separation conditions for UPLC were the same as those for UPLC-APCI-HRMS. Mass spectral data were collected in +ESI mode in separate runs on a Waters HDMS-QTOF synapt mass spectrometer operated in a scan mode from m/z 50 to 500. The capillary voltage was set at 3000 V; the source and desolvation temperatures were 100°C and 250°C, respectively. The cone gas flow rate was 50 l h−1.
NMR spectroscopy
The 1H- and 13C-NMR spectra were recorded at 27°C using a Bruker AV600_GRC 600 MHz NMR (in the case of 2,3-SAOA) or a Bruker Avance-400 FT-NMR [for 3,17-dihydroxy-9,10-seco-androsta-1,3,5(10)-triene-9-one (3,17-DHSA)] spectrometer. Chemical shifts (δ) were recorded and shown as ppm values with deuterated methanol (99.8%, 1H: δ = 3.31 ppm; 13C: δ = 49.0 ppm; in the case of 2,3-SAOA) or chloroform (99.5%, 1H: δ = 7.26 ppm; 13C: δ = 77.0 ppm; for 3,17-DHSA) as the solvents and internal references.
Phylogenetic analysis of S. denitrificans strains
Detection of the phylogenetic relationship of S. denitrificans strains was conducted using Clustal W and MEGA 5.0 (24). Sixty-three 16S rRNA gene sequences of S. denitrificans were retrieved from the GenBank database of the National Center for Biotechnology Information (NCBI).
RESULTS
Aerobic testosterone catabolism by S. denitrificans
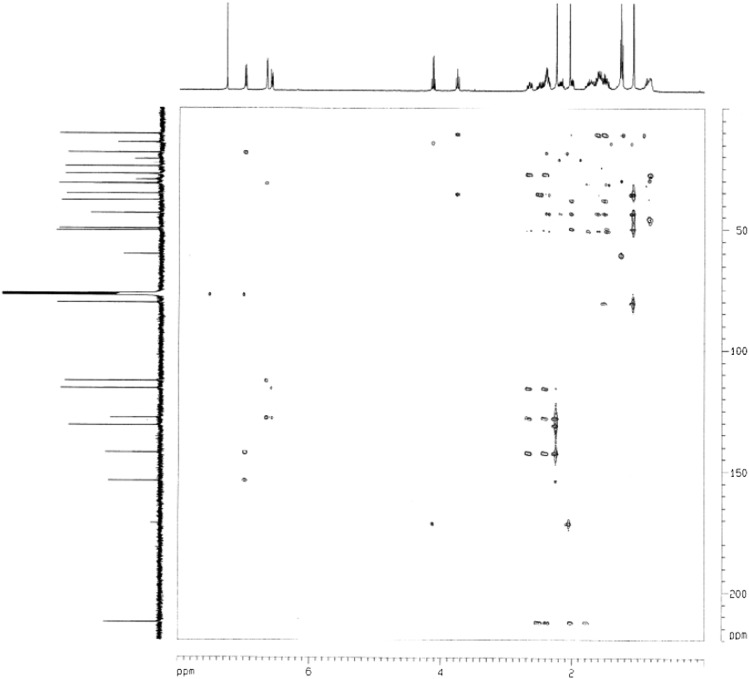
S. denitrificans is able to grow with testosterone under aerobic conditions. It was unclear whether the bacterium utilizes the well-studied 9,10-seco-pathway to degrade testosterone in the presence of oxygen. Metabolites of the aerobic testosterone degradation by S. denitrificans were extracted using ethyl acetate from shake-flask cultures. The addition of the meta-cleavage inhibitor 3-chlorocatechol resulted in the accumulation of at least seven intermediates, which were identified using UPLC-HRMS (Table 1). The initial intermediates (1-dehydrotestoterone, androst-4-en-3,17-dione, and androsta-1,4-diene-3,17-dione) were identified by comparison with authentic standards by UPLC-HRMS. The 3,17-dihydroxy-9,10-seco-androsta-1,3,5(10)-triene-9-one (3,17-DHSA), 3,4,17-trihydroxy-9,10-seco-androsta-1,3,5(10)-triene-9-one, and their 17-keto derivatives exhibited maximal UV absorption of approximately 280 nm, indicating the presence of the phenolic A-ring structure in these compounds. The chemical structure of 3,17-DHSA was further confirmed using NMR (Tables 2 and 3 for the 1H- and 13C-NMR spectral data, respectively). In the 1H-NMR spectrum of 3,17-DHSA, a set of mutually coupled aromatic protons was observed at δH 6.99 (1H, d, J = 8.0 Hz; H-1); 6.66 (1H, d, J = 2.8 Hz; H-4); and 6.59 (1H, dd, J = 8.0, 2.8 Hz; H-2). Furthermore, two methyl groups were present at 2.25 (3H, s; H-19) and 1.10 (3H, s; H-18). In the 13C-NMR spectrum of 3,17-DHSA, a carbonyl signal was observed at δC 212.7 (C-9). An additional oxygenated methine proton and a corresponding oxygenated carbon were present at δH 3.76 (1H, t, J = 8.4 Hz, H-17) and δC 80.8 (C-17), respectively. Final structural elucidation of this compound was performed using two-dimensional (2D) NMR. In the heteronuclear multiple bond coherence (HMBC) spectrum of 3,17-DHSA, the 2J long-range coupling signals were observed between δH 2.37, 2.52 (H-11)/δC 212.7 (C-9). In addition, the 3J-correlation signals between δH 1.79 (H-7) and δC 212.7 (C-9) and H-12 (δH 2.02)/δC 212.7 (C-9) were identified (Fig. 2). These results suggest that S. denitrificans adopts the 9,10-seco-pathway to degrade testosterone in the presence of oxygen, with 3,17-DHSA as the key intermediate (Fig. 1).
TABLE 1.
UPLC-HRMS analysis of the intermediates involved in aerobic testosterone catabolism by S. denitrificans
| Compound ID | UPLC Behavior (RT, min) | Molecular Formula (Predicted Molecular Mass)a | Dominant Ion Peaks | Identification of Product Ions | Mode Observed |
| Testosterone | 6.15 | C19H28O2 | 271.2059 | [M-H2O+H]+ | ESI and APCI |
| 288.2082 | 289.2161 | [M+H]+ | ESI and APCI | ||
| 311.1983 | [M+Na]+ | ESI | |||
| Androst-4-en-3,17-dione | 5.91 | C19H26O2 | 269.1909 | [M-H2O+H]+ | ESI and APCI |
| 286.1926 | 287.2023 | [M+H]+ | ESI and APCI | ||
| 309.1831 | [M+Na]+ | ESI | |||
| 1-Dehydrotestosterone | 5.77 | C19H26O2 | 269.1903 | [M-H2O+H]+ | ESI and APCI |
| 286.1926 | 287.2009 | [M+H]+ | ESI and APCI | ||
| 309.1823 | [M+Na]+ | ESI | |||
| Androsta-1,4-diene-3,17-dione | 5.45 | C19H24O2 | 267.1742 | [M-H2O+H]+ | ESI and APCI |
| 284.1770 | 285.1855 | [M+H]+ | ESI and APCI | ||
| 307.1666 | [M+Na]+ | ESI | |||
| 3,17-Dihydroxy-9,10-seco-androsta-1,3,5(10)-triene-9-one(3,17-DHSA) | 5.53 | C19H26O3 | 267.1740 | [M-2H2O+H]+ | ESI and APCI |
| 302.1875 | 285.1844 | [M-H2O+H]+ | ESI and APCI | ||
| 303.1946 | [M+H]+ | ESI and APCI | |||
| 325.1766 | [M+Na]+ | ESI | |||
| 3-Hydroxy-9,10-seco-androsta-1,3,5(10)-triene-9,17-dione | 5.35 | C19H24O3 | 283.1667 | [M-H2O+H]+ | ESI and APCI |
| 300.1719 | 301.1801 | [M+H]+ | ESI and APCI | ||
| 323.1625 | [M+Na]+ | ESI | |||
| 3,4,17-Trihydroxy-9,10-seco-androsta-1,3,5(10)-triene-9-one | 4.99 | C19H26O4 | 301.1762 | [M-H2O+H]+ | ESI and APCI |
| 318.1824 | 319.1958 | [M+H]+ | ESI and APCI | ||
| 341.1791 | [M+Na]+ | ESI | |||
| 3,4-Dihydroxy-9,10-seco-androsta-1,3,5(10)-triene-9,17-dione | 4.50 | C19H24O4 | 299. 1608 | [M-H2O+H]+ | ESI and APCI |
| 316.1668 | 317.1831 | [M+H]+ | ESI and APCI | ||
| 339. 1592 | [M+Na]+ | ESI |
RT, retention time.
The predicated molecular mass was calculated using the atom mass of 12C (12.0000), 16O (15.9949), and 1H (1.0078).
TABLE 2.
1H-NMR chemical shifts (δH, ppm) of ring cleavage intermediates involved in aerobic or anaerobic testosterone catabolic pathways in S. denitrificans cells
| 1H | 3,17-DHSA | 2,3-SAOA |
| 1 | 6.99 (1H, d, J = 8.0 Hz) | − |
| 2 | 6.59 (1H, dd, J = 8.0, 2.8 Hz) | 2.18 (3H, s) |
| 3 | − | − |
| 4 | 6.66 (1H, d, J = 2.8 Hz) | 1.88 (1H, m) |
| 1.73 (1H, m) | ||
| 5 | − | 2.31 (1H, m) |
| 6 | 2.66 (1H, ddd, J = 13.2, 4.8, 4.8 Hz) | 1.78 (1H, m) |
| 2.43 (1H, m) | 1.23 (1H, m) | |
| 7 | 1.79 (1H, m) | 1.98 (1H, m) |
| 1.58 (1H, m) | 1.73 (1H, m) | |
| 8 | 2.41 (1H, m) | 1.36 (1H, m) |
| 9 | − | 1.47 (1H, m) |
| 10 | − | − |
| 11 | 2.52 (1H, m) | 1.62 (1H, m)a |
| 2.37 (1H, m) | 1.25 (1H, m) | |
| 12 | 2.02 (1H, ddd, J = 6.8, 6.0, 2.0 Hz) | 1.74 (1H, m) |
| 1.53 (1H, m) | 1.08 (1H, td, J = 13.2, 4.0 Hz) | |
| 13 | − | − |
| 14 | 1.61 (1H, m) | 1.03 (1H, m) |
| 15 | 1.71 (1H, m) | 1.38 (1H, m)a |
| 1.51 (1H, m) | 0.90 (1H, m) | |
| 16 | 2.19 (1H, m) | 1.75 (1H, m) |
| 1.65 (1H, m) | 1.03 (1H, m) | |
| 17 | 3.76 (1H, t, J = 8.4 Hz) | 3.57 (1H, t, J = 8.7 Hz) |
| 18 | 1.10 (3H, s) | 0.70 (3H, s) |
| 19 | 2.25 (3H, s) | 0.92 (3H, s) |
The signals of H-11 and H-15 of 2,3-SAOA are undistinguishable.
TABLE 3.
13C-NMR chemical shifts (δC, ppm) of ring cleavage intermediates involved in aerobic or anaerobic testosterone catabolic pathways in S. denitrificans cells
| 13C | 3,17-DHSA | 2,3-SAOA |
| 1 | 131.4 | 216.8 |
| 2 | 113.0 | 25.5 |
| 3 | 154.1 | 176.6 |
| 4 | 116.1 | 40.9 |
| 5 | 142.7 | 42.5 |
| 6 | 31.5 | 28.0 |
| 7 | 27.6 | 30.4 |
| 8 | 50.9 | 36.1 |
| 9 | 212.7 | 50.5 |
| 10 | 128.4 | 57.1 |
| 11 | 38.4 | 24.2a |
| 12 | 35.9 | 37.6 |
| 13 | 43.7 | 44.1 |
| 14 | 50.1 | 52.0 |
| 15 | 24.5 | 24.2a |
| 16 | 31.5 | 32.1 |
| 17 | 80.8 | 82.4 |
| 18 | 11.1 | 11.6 |
| 19 | 18.7 | 9.9 |
The signals of C-11 and C-15 of 2,3-SAOA are undistinguishable.
Fig. 2.
HMBC spectrum of the characteristic intermediate (3,17-DHSA) involved in aerobic testosterone catabolism by S. denitrificans.
Anaerobic testosterone catabolism by S. denitrificans
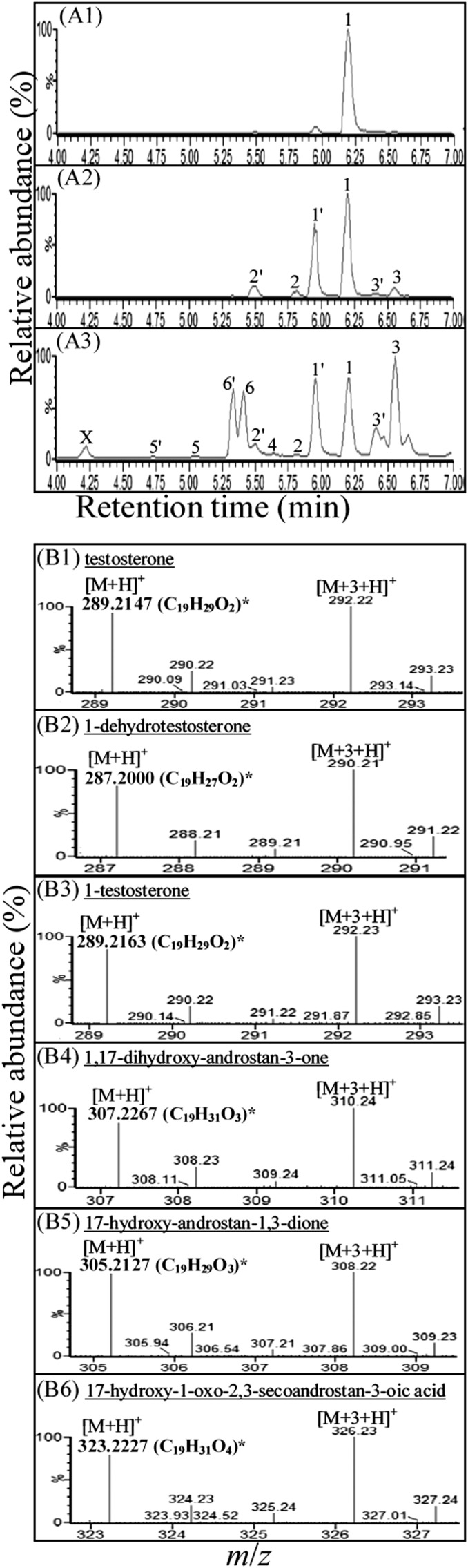
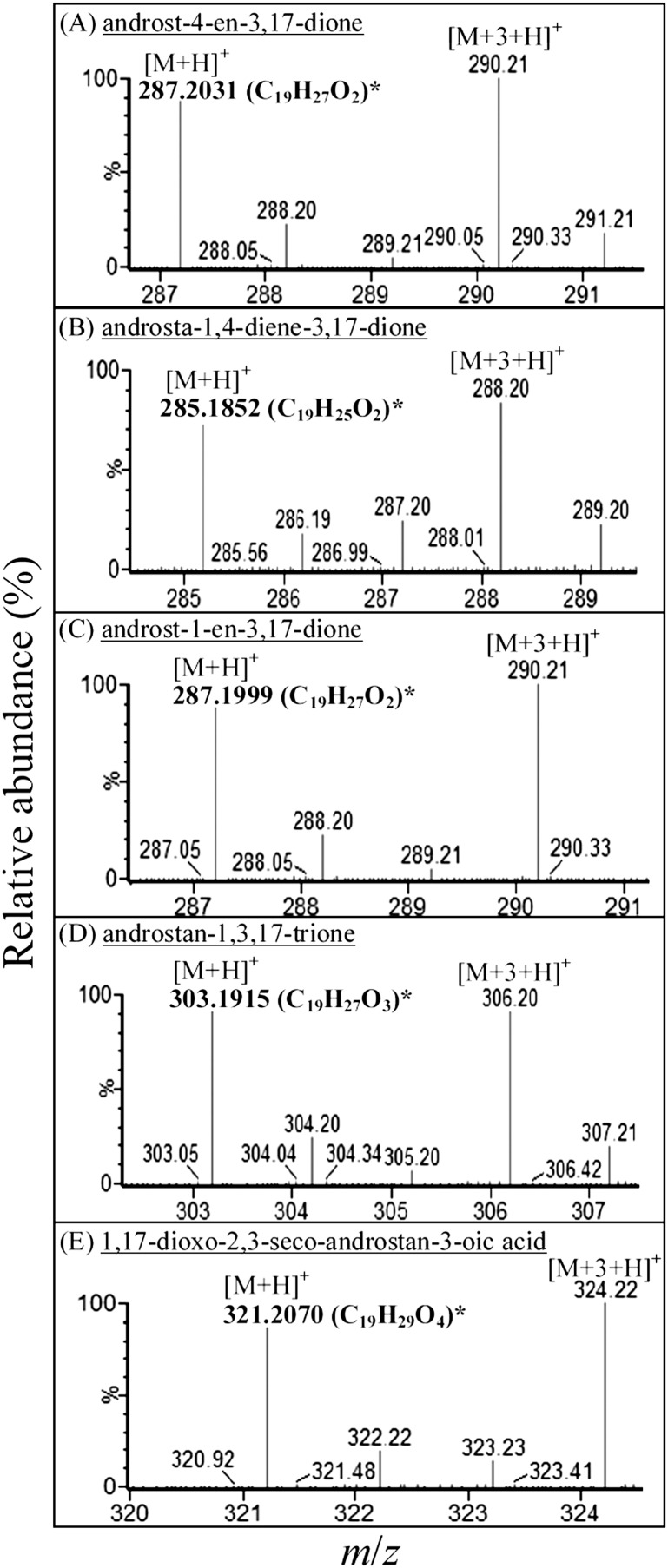
The metabolites of the anaerobic testosterone catabolism were extracted using ethyl acetate from in vivo transformation assays. These testosterone-derived intermediates were identified using UPLC-HRMS. The steroid substrate was composed of [2,3,4C-13C]testosterone and unlabeled testosterone (mixed in 1:1 molar ratio). Therefore, pairs of molecular adduct ions (with the m/z difference of 3) were observed in the mass spectra of testosterone-derived intermediates (Fig. 3). The mass spectra of their 17-keto structures are shown in Fig. 4. At the beginning of the assay, only testosterone was detected (Fig. 3A1). After 6 h of anaerobic incubation, 1-dehydrotestoterone, 1-testosterone, and their 17-keto derivatives appeared (Fig. 3A2). These steroid compounds were identified by comparison with authentic steroid standards by UPLC-HRMS. After a further incubation (6 h) with 0.5 mM mercaptopropionic acid (an acyl-CoA dehydrogenase inhibitor), a few new intermediates were present (Fig. 3A3). This phenomenon suggests that β-oxidation may play a role in the degradation of downstream intermediates. The testosterone-derived intermediates detected in the anaerobic S. denitrificans cultures are summarized in supplementary Table I.
Fig. 3.
UPLC-HRMS analysis of ethyl-acetate extracts of S. denitrificans cells grown anaerobically on testosterone (2 mM). In the in vivo assay, the steroid substrate was composed of [2,3,4C-13C]testosterone and unlabeled testosterone (mixed in 1:1 molar ratio). (A) UPLC chromatograms of ethyl-acetate extracts: (A1) 10 min after the anaerobic incubation; (A2) 6 h after the anaerobic incubation; and (A3) an additional 6 h anaerobic incubation with 0.5 mM mercaptopropionic acid. Abbreviations of testosterone-derived intermediates present in the chromatograms: 1: testosterone; 2: 1-dehydrotestosterone; 3: 1-testosterone; 4: 1,17-dihydroxy-androstan-3-one; 5: 17-hydroxy-androstan-1,3-dione; 6: 17-hydroxy-1-oxo-2,3-seco-androstan-3-oic acid. Numbers with an apostrophe represent their 17-keto derivatives (for their mass spectra, see Fig. 4). (B) The high-resolution mass spectra of 17-hydroxyl intermediates derived from testosterone. *The predicted elemental composition of individual intermediates was calculated using MassLynx Mass Spectrometry Software (Waters).
Fig. 4.
APCI-HRMS spectra data of 17-keto intermediates involved in anaerobic testosterone degradation by S. denitrificans. (A–E) In the in vivo assay, the steroid substrate was composed of [2,3,4C-13C]testosterone and unlabeled testosterone (mixed in 1:1 molar ratio). *The predicted elemental composition of individual molecular adduct ions was calculated using MassLynx Mass Spectrometry Software (Waters).
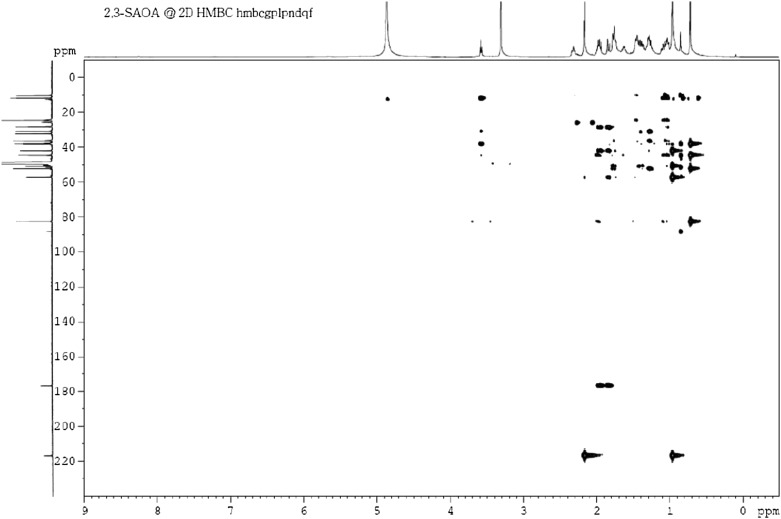
To produce a sufficient amount of intermediates for NMR analysis, an enlarged anoxic culture (2 l) containing S. denitrificans cells, testosterone (4 mM), and mercaptopropionic acid (0.5 mM) was produced. The intermediates were sequentially purified using liquid-liquid partition, silica gel chromatography, TLC, and HPLC. The structures of 17-hydroxy-1-oxo-2,3-seco-androstan-3-oic acid (2,3-SAOA) were elucidated using NMR spectroscopy. According to the UPLC-HRMS data, the elemental composition of 2,3-SAOA was calculated as C19H30O4 (Fig. 3B6). Its H-17, H-18, and H-19 signals were exhibited at δH 3.57 (1H, t, J = 8.7 Hz), 0.70 (3H, s), and 0.92 (3H, s), respectively, in the 1H-NMR spectrum (Table 2). An additional methyl signal (H-2) was observed at δH 2.18 (3H, s), indicating that the single bond between C-2 and C-3 was broken. In the 13C-NMR spectrum, a ketone group and a carboxyl group appeared at δC 216.8 and 176.6, respectively (Table 3). The investigation of the final structure of this compound was performed using 2D NMR. The 2J and 3J long-range coupling signals were observed between δH 2.18 (H-2)/δC 216.8 (C-1) and δH 0.92 (H-19)/δC 216.8 (C-1), respectively, in the HMBC spectrum (Fig. 5). The hydroxyl group at C-17 was confirmed by the appearance of a 3J-correlation signal between H-18 (δH 0.70) and the oxygenated carbon (δC 82.4).
Fig. 5.
HMBC spectrum of the characteristic intermediate (2,3-SAOA) involved in anaerobic testosterone catabolism by S. denitrificans.
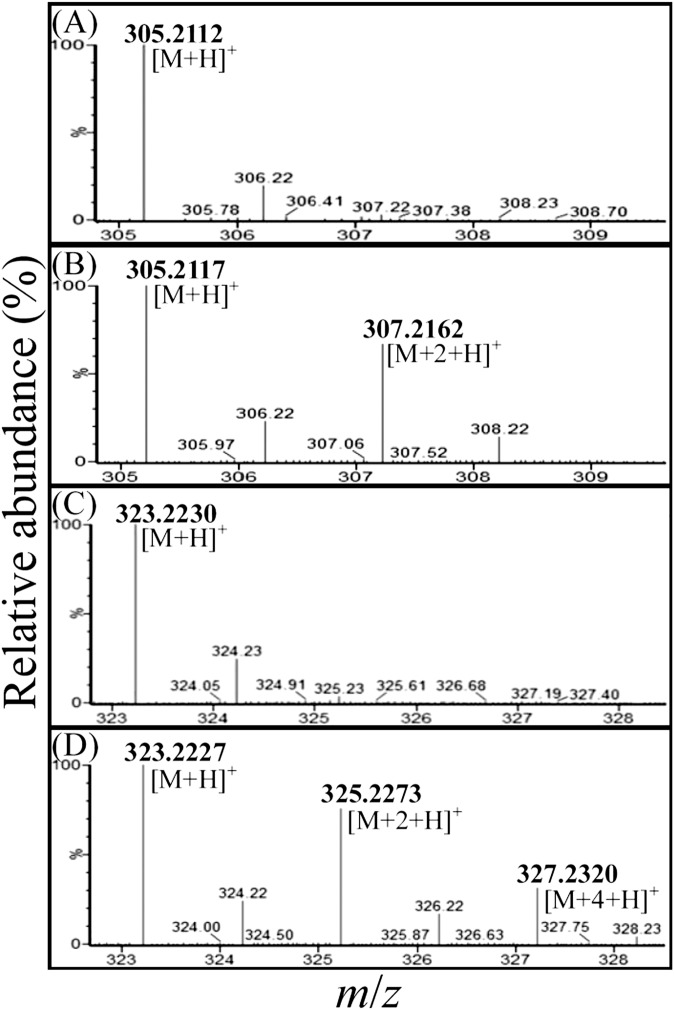
To determine the origins of the oxygen atoms at C-1 and/or C-3 of 17-hydroxy-androstan-1,3-dione and 2,3-SAOA, we conducted two in vitro transformation assays using 1-testosterone as the substrate, as follows: i) an 18O-labeled water-treated assay contained approximately 48.5% 18O-labeled water (mole/mole) in the anoxic reaction mixture; and ii) a control assay was incubated under anoxic conditions without the addition of 18O-labeled water. Compared with the 17-hydroxy-androstan-1,3-dione purified from the control assay (Fig. 6A), an additional 18O-isotopic molecular ion ([M+2+H]+, m/z 307.2162) was observed in the APCI-mass spectrum of the 1,3-dioxo product purified from the 18O-labeled water-treated assay (Fig. 6B). The APCI-mass spectrum of 2,3-SAOA purified from the 18O-labeled water-treated assay showed three dominant protonated molecular ions ([M+H]+, m/z 323.2227, 325.2273, and 327.2320; Fig. 6D). Their elemental composition was calculated as C19H3116O4, C19H3116O318O1, and C19H3116O218O2. By contrast, the APCI mass spectrum of 2,3-SAOA purified from the control assay showed only single protonated adduct ion (m/z 323.2230; Fig. 6C). These data indicated that under anoxic conditions, after the activation of the A-ring through a hydration reaction, the cleavage of the steroidal core ring system begins with the A-ring by a hydrolysis reaction. Moreover, the presence of 10 mM KCN [an inhibitor generally inactivates members of the xanthine oxidase family (25, 26)] considerably inhibited the in vitro transformation of 1-testosterone to 1,17-dihydroxy-androstan-3-one (data not shown), indicating that the hydration reaction occurring at C-1/C-2 of 1-testosterone may be catalyzed by a molybdopterin-containing enzyme.
Fig. 6.
APCI-mass spectra (positive ion mode) of 17-hydroxy-androstan-1,3-dione and 2,3-SAOA. (A) 17-hydroxy-androstan-1,3-dione purified from the anaerobic control assay. (B) 17-hydroxy-androstan-1,3-dione purified from the 18O-labeled H2O-treated assay. (C) 2,3-SAOA purified from the anoxic control assay. (D) 2,3-SAOA purified from the 18O-labeled H2O-treated assay.
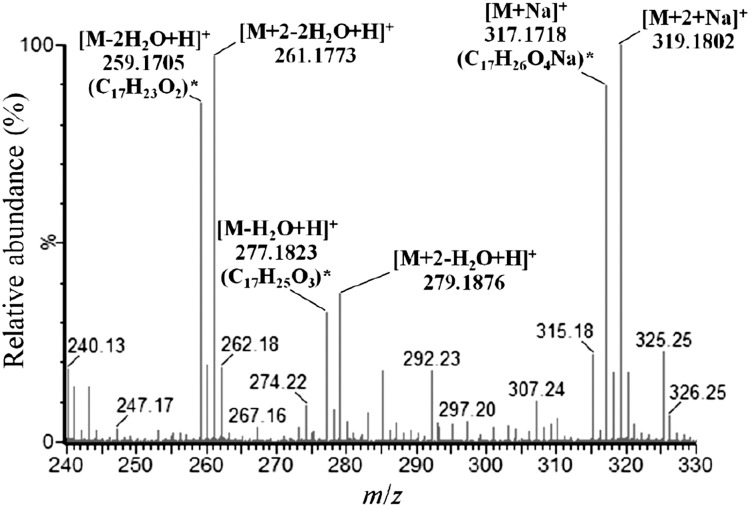
A testosterone-derived intermediate (compound X, Fig. 3A3) with 17 carbons was identified using UPLC-HRMS. Its ESI-mass spectrum (Fig. 7) indicated that this compound is labeled with two 13C. We assumed that the C-1 and C-2 of intermediate X was removed because i) testosterone, the steroid substrate, was labeled with three 13C at C-2/C-3/C-4, and ii) in the case of 2,3-SAOA, the single bond between C-2 and C-3 was broken. So far, we cannot produce a sufficient amount of compound X for NMR analysis. Therefore, the exact structure of the intermediate X remains unclear.
Fig. 7.
ESI-mass spectra of a C17 testosterone-derived intermediate involved in anaerobic testosterone degradation by S. denitrificans. *The predicted elemental composition of the product ions was calculated using MassLynx Mass Spectrometry Software (Waters).
Modes of respiration and testosterone catabolism by S. denitrificans grown under various oxygen concentrations
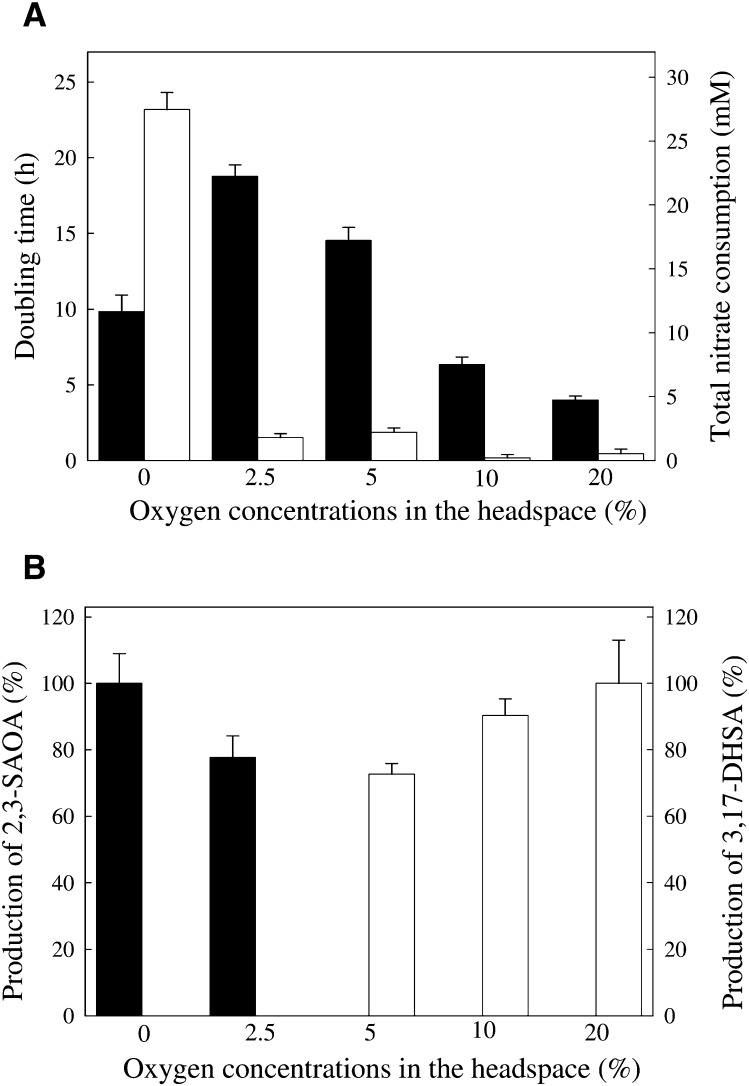
To test i) whether S. denitrificans adopts the oxygen-independent 2,3-seco-pathway under microaerobic conditions and ii) whether the coexistence of the 2,3-seco- and 9,10-seco-pathways is possible, we grew S. denitrificans under various oxygen concentrations (0, 2.5, 5, 10, and 20%, v/v). The volume ratio of the S. denitrificans cultures (100 ml) to its headspace (900 ml) was 1 to 9; thus, the oxygen concentration in the headspace was not heavily changed during bacterial growth. The bacterial growth was fastest in the presence 20% oxygen in the headspace, whereas the slowest growth was observed under 2.5% oxygen (Fig. 8A). S. denitrificans consumed nitrate as the terminal electron acceptor only under strictly anaerobic conditions (Fig. 8A). A tiny amount of nitrate was consumed in the other four cultures. The production of ring cleavage intermediates (3,17-DHSA and 2,3-SAOA) by S. denitrificans cells in five cultures was quantified using HPLC at 12 h after the addition of 3-chlorocatechol and mercaptopropionic acid. The HPLC detection limits of 2,3-SAOA and 3,17-DHSA concentrations in the bacterial cultures were 100 ng ml−1 and 25 ng ml−1, respectively.
Fig. 8.
The modes of respiration and testosterone catabolism adopted by S. denitrificans grown under various oxygen concentrations. Samples were withdrawn after 12 h incubation with 0.5 mM mercaptopropionic acid and 1 mM 3-chlorocatechol. The data shown are from five independent experiments. Data are means ± SE of three replicates in the representative experiment. (A) Bacterial growth (black column) and nitrate consumption (white column) of S. denitrificans cultures. (B) The production of 2,3-SAOA (black column) and 3,17-DHSA (white column) by S. denitrificans cultures. For white columns, the amount of 3,17-DHSA produced by S. denitrificans grown under 20% oxygen concentration (aerobic treatment) was set at 100%, and those of other four treatments are shown relative to that of the aerobic treatment. For black columns, the amount of 2,3-SAOA produced by S. denitrificans grown under strictly anaerobic conditions was set at 100%, and those of other four treatments are shown relative to that of the anaerobic treatment.
The characteristic ring cleavage intermediate of the 9,10-seco-pathway, 3,17-DHSA, was produced by S. denitrificans cells at 5∼20% oxygen in the headspace (Fig. 8B). Under 20% oxygen, the concentration of 3,17-DHSA in the culture was 24 ± 3 μg ml−1. On the other hand, the production of 2,3-SAOA, the key intermediate of the 2,3-seco-pathway, was only observed in the complete absence of oxygen and under 2.5% oxygen (Fig. 8B). 2,3-SAOA concentration in the strictly anaerobic culture was 7 ± 1 μg ml−1. It is worth mentioning that the two ring cleavage intermediates apparently never coexisted in any tested bacterial cultures. Our data showed that S. denitrificans adopts only one testosterone catabolic pathway at any time, depending on oxygen availability.
DISCUSSION
Under oxic conditions, the degradation of testosterone by S. denitrificans appeared to follow the 9,10-seco-pathway reported previously (27). The microbial oxygenolytic cleavage of cycloalkane and aromatic rings under oxic conditions is widely distributed in nature, for example, aerobic catabolism of cyclohexanol and phenol by bacteria (28). The insertion of hydroxyl groups into the organic substrates by oxygenases is a common catabolic strategy that enables the microbial cells to overcome the inherent inertness of these compounds (29, 30).
The metabolomic data presented in this study show that anaerobic degradation of testosterone by S. denitrificans occurs through a catabolic route that differs fundamentally from the aerobic degradation pathway. The crucial differences appear in the mechanisms adopted for the cleavage of the core ring structure of testosterone (Fig. 1). In the aerobic pathway, the cleavage starts with the B-ring, whereas in the anaerobic pathway, the A-ring is opened first. Another significant difference nicely reflects the influence of oxygen availability on the actual ring cleavage mechanism. In contrast to the oxygenase-catalyzed oxygenolytic ring fission under oxic conditions, the opening of the A-ring under anoxic conditions occurs through the oxygen-independent hydrolytic mechanism. The 18O-incorporation experiments corroborate the proposed hydrolytic ring cleavage mechanism.
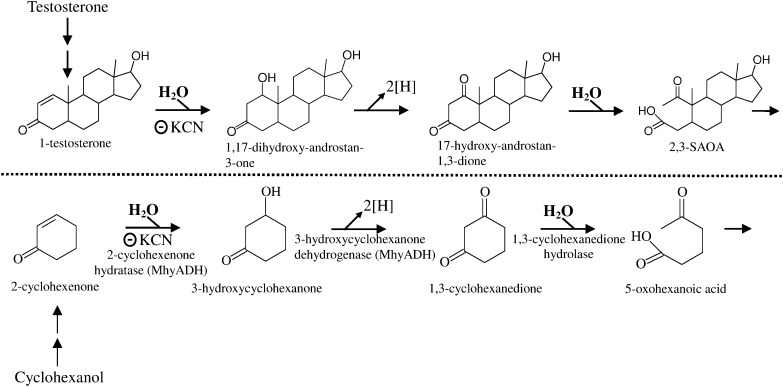
It is well established that the microbial cleavage of the rings of cycloalkane and aromatic compounds under anoxic conditions usually proceeds through hydrolytic mechanism (31–34). Dangel et al. (31, 32) showed that the hydrolytic ring cleavage substrate in anaerobic cyclohexanol degradation is 1,3-cyclohexanedione. Interestingly, in the anaerobic testosterone catabolism, the hydrolytic ring cleavage substrate, 17-hydroxy-androstan-1,3-dione, has a 1,3-dioxo structure in its A-ring (Fig. 9). Recently, 2-cyclohexenone hydratase catalyzing the addition of water to the C = C bond of α,β-unsaturated carbonyl compounds was purified and characterized from the cyclohexanol-degrading bacterium Alicycliphilus denitrificans (35). This heterotrimeric enzyme (MhyADH) contains molybdopterin, FAD, and [2Fe-2S] clusters and belongs to the xanthine oxidase family. Our data showed that KCN effectively inhibited the hydration reaction of 1-testosterone, suggesting that this reaction may be catalyzed by a similar molybdopterin-binding enzyme. So far, the ring cleavage enzyme involved in anaerobic cyclohexanol catabolism, 1,3-cyclohexanedione hydrolase, was only partly characterized. 1,2-cyclohexanedione serves as a competitive inhibitor for this enzyme (32). However, the addition of 1,2-cyclohexanedione (up to 1 mM) to the in vivo or in vitro assays did not inhibit the hydrolytic A-ring cleavage of steroid substrates (data not shown).
Fig. 9.
Comparison of the intermediates involved in anaerobic testosterone catabolic pathway (demonstrated in S. denitrificans DSMZ 18526) with those of anaerobic cyclohexanol catabolism (by Alicycliphilus denitrificans DSMZ 14773). The scheme of anaerobic cyclohexanol catabolic pathway is based on Jin et al. (36). 2-cyclohexenone hydratase belongs to the xanthine oxidase family, which is inactivated by KCN. This bifunctional molybdoenzyme (MhyADH) also catalyzes the subsequent alcohol oxidation to form 1,3-cyclohexanedione. According to our data, KCN effectively inhibited the hydration reaction of 1-testosterone.
A crucial finding of our studies is that testosterone is transformed to 1-dehydrotestosterone regardless of the growth conditions. Subsequently, the catabolism proceeds through divergent pathways depending on the availability of oxygen (Fig. 1). Therefore, 1-dehydrotestosterone can be considered a common intermediate or a divergence point for testosterone degradation. According to our current data, S. denitrificans adopts only one catabolic pathway (either the 2,3-seco- or 9,10-seco-pathway) to degrade testosterone, depending on oxygen tension. This mode of catabolism might have an ecological significance. It is more energetically efficient to start the degradation of a substrate through some common intermediate(s) with the same enzyme(s), regardless of the prevailing conditions. Subsequently, the last common intermediate can be channeled into the relevant pathways. This will help denitrifying bacteria to readily switch their catabolic enzyme inventory between the oxic and anoxic mode and consequently increase their metabolic competence. This hypothesis is supported by the wide distribution of 16S rRNA gene sequences of S. denitrificans strains in oxic and anoxic environments. Similar (but not as extreme) cases were reported in the literature for a number of facultative anaerobes, such as Thauera aromatica (36) and Azoarcus evansii (37). These bacteria can use benzoate and phenylacetate under oxic and anoxic conditions (34, 36–38). In both cases, the substrate is initially transformed to a common intermediate benzoyl-CoA or phenylacetyl-CoA by the same or isoenyzmes. Then, depending on the availability of oxygen, the common substrate is driven into the relevant pathway. Under oxic conditions, the recently disclosed epoxybenzoyl-CoA or epoxyphenylacetyl-CoA pathway is used; while in the absence of oxygen, the anaerobic benzoyl-CoA is preferred.
Supplementary Material
Acknowledgments
The authors thank the Small Molecule Metabolomics core facility sponsored by the Institute of Plant and Microbial Biology (IPMB), Academia Sinica, for UPLC-MS analysis.
Footnotes
Abbreviations:
- APCI
- atmosphere pressure chemical ionization
- 2D
- two-dimensional
- 3
- 17-DHSA, 3,17-dihydroxy-9,10-seco-androsta-1,3,5(10)-triene-9-one
- HMBC
- heteronuclear multiple bond coherence
- 2
- 3-SAOA, 17-hydroxy-1-oxo-2,3-seco-androstan-3-oic acid
- HRMS
- high-resolution mass spectrometry
- UPLC
- ultra-performance liquid chromatography
This work was supported by the National Science Council (NSC 100-2311-B-182-005-MY3) and Chang-Gung Memorial Hospital (CMRPD1A0071) of Taiwan.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of one figure and one table.
REFERENCES
- 1.Shore L. S., Shemesh M. 2003. Naturally produced steroid hormones and their release into the environment. Pure Appl. Chem. 75: 1859–1871 [Google Scholar]
- 2.Daughton C. G. 2008. Pharmaceuticals as environmental pollutants: the ramifications for human exposure. In International Encyclopedia of Public Health. Vol. 5. K. Heggenhougen and S. Quah, editors. Academic Press, Oxford 66–102 [Google Scholar]
- 3.Belfroid A. C., Van der Horst A., Vethaak A. D., Schäfer A. J., Rijs G. B. J., Wegener J., Cofino W. P. 1999. Analysis and occurrence of estrogenic hormones and their glucuronides in surface water and waste water in the Netherlands. Sci. Total Environ. 225: 101–108 [DOI] [PubMed] [Google Scholar]
- 4.Huang C. H., Sedlak D. L. 2001. Analysis of estrogenic hormones in municipal wastewater effluent and surface water using enzyme-linked immunosorbent assay and gas chromatography/tandem mass spectrometry. Environ. Toxicol. Chem. 20: 133–139 [PubMed] [Google Scholar]
- 5.Kolodziej E. P., Gray J. L., Sedlak D. L. 2003. Quantification of steroid hormones with pheromonal properties in municipal wastewater effluent. Environ. Toxicol. Chem. 22: 2622–2629 [DOI] [PubMed] [Google Scholar]
- 6.Ternes T. A., Stumpf M., Mueller J., Haberer K., Wilken R. D., Servos M. 1999. Behavior and occurrence of estrogens in municipal sewage treatment plants--I. Investigations in Germany, Canada, and Brazil. Sci. Total Environ. 225: 81–90 [DOI] [PubMed] [Google Scholar]
- 7.Panter G. H., Thompson R. S., Sumpter J. P. 1998. Adverse reproductive effects in male fathead minnows (Pimephales promelas) exposed to environmentally relevant concentrations of the natural oestrogens, oestradiol and oestrone. Aquat. Toxicol. 42: 243–253 [Google Scholar]
- 8.Larsson D. G. J., Hällman H., Förlin L. 2000. More male fish embryos near a pulp mill. Environ. Toxicol. Chem. 19: 2911–2917 [Google Scholar]
- 9.Teles M., Gravato C., Pacheco M., Santos M. A. 2004. Juvenile sea bass biotransformation, genotoxic and endocrine responses to β-naphthoflavone, 4-nonylphenol and 17β-estradiol individual and combined exposures. Chemosphere. 57: 147–158 [DOI] [PubMed] [Google Scholar]
- 10.Fernandes P., Cruz A., Angelova B., Pinheiro H. M., Cabral J. M. S. 2003. Microbial conversion of steroid compounds: recent developments. Enzyme Microb. Technol. 32: 688–705 [Google Scholar]
- 11.Coulter A. W., Talalay P. 1968. Studies on the microbiological degradation of steroid ring A. J. Biol. Chem. 243: 3238–3247 [PubMed] [Google Scholar]
- 12.Kieslich K. 1985. Microbial side-chain degradation of sterols. J. Basic Microbiol. 25: 461–474 [DOI] [PubMed] [Google Scholar]
- 13.Mackenzie A. S., Brassell S. C., Eglinton G., Maxwell J. R. 1982. Chemical fossils: the geological fate of steroids. Science. 217: 491–504 [DOI] [PubMed] [Google Scholar]
- 14.Wakeham S. G. 1989. Reduction of stenols to stanols in particulate matter at oxic-anoxic boundaries in sea water. Nature. 342: 787–790 [Google Scholar]
- 15.Harder J., Probian C. 1997. Anaerobic mineralization of cholesterol by a novel type of denitrifying bacterium. Arch. Microbiol. 167: 269–274 [DOI] [PubMed] [Google Scholar]
- 16.Tarlera S., Denner E. B. M. 2003. Sterolibacterium denitrificans gen. nov., sp. nov., a novel cholesterol-oxidizing, denitrifying member of the β-Proteobacteria. Int. J. Syst. Evol. Microbiol. 53: 1085–1091 [DOI] [PubMed] [Google Scholar]
- 17.Fahrbach M., Kuever J., Meinke R., Kämpfer P., Hollender J. 2006. Denitratisoma oestradiolicum gen. nov., sp. nov., a 17β-oestradiol-degrading, denitrifying betaproteobacterium. Int. J. Syst. Evol. Microbiol. 56: 1547–1552 [DOI] [PubMed] [Google Scholar]
- 18.Fahrbach M., Kuever J., Remesch M., Huber B. E., Kämpfer P., Dott W., Hollender J. 2008. Steroidobacter denitrificans gen. nov., sp. nov., a steroidal hormone-degrading gammaproteobacterium. Int. J. Syst. Evol. Microbiol. 58: 2215–2223 [DOI] [PubMed] [Google Scholar]
- 19.Chiang Y. R., Ismail W., Müller M., Fuchs G. 2007. Study of anoxic and oxic cholesterol metabolism by Sterolibacterium denitrificans. J. Biol. Chem. 282: 13240–13249 [DOI] [PubMed] [Google Scholar]
- 20.Chiang Y. R., Fang J. Y., Ismail W., Wang P. H. 2010. Initial steps in anoxic testosterone degradation by Steroidobacter denitrificans. Microbiology. 156: 2253–2259 [DOI] [PubMed] [Google Scholar]
- 21.Leu Y. L., Wang P. H., Shiao M. S., Ismail W., Chiang Y. R. 2011. A novel testosterone catabolic pathway in bacteria. J. Bacteriol. 193: 4447–4455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sabbagh E., Cuebas D., Schulz H. 1985. 3-Mercaptopropionic acid, a potent inhibitor of fatty acid oxidation in rat heart mitochondria. J. Biol. Chem. 260: 7337–7342 [PubMed] [Google Scholar]
- 23.Klecˇka G. M., Gibson D. T. 1981. Inhibition of catechol 2,3-dioxygenase from Psudomonas putida by 3-chlorocatechol. Appl. Environ. Microbiol. 41: 1159–1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tamura K., Peterson D., Peterson N., Stecher G., Nei M., Kumar S. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28: 2731–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coughlan M. P., Johnson J. L., Rajagopalan K. V. 1980. Mechanisms of inactivation of molybdoenzymes by cyanide. J. Biol. Chem. 255: 2694–2699 [PubMed] [Google Scholar]
- 26.Hille R. 1996. The mononuclear molybdenum enzymes. Chem. Rev. 96: 2757–2816 [DOI] [PubMed] [Google Scholar]
- 27.Horinouchi M., Hayashi T., Kudo T. 2012. Steroid degradation in Comamonas testosteroni. J. Steroid Biochem. Mol. Biol. 129: 4–14 [DOI] [PubMed] [Google Scholar]
- 28.Norris D. B., Trudgill P. W. 1971. The metabolism of cyclohexanol by Nocardia globerula CLl. Biochem. J. 121: 363–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stanier R. Y., Ornston L. N. 1973. The β-ketoadipate pathway. Adv. Microb. Physiol. 9: 89–151 [PubMed] [Google Scholar]
- 30.Harwood C. S., Parales R. E. 1996. The β-ketoadipate pathway and the biology of self-identity. Annu. Rev. Microbiol. 50: 553–590 [DOI] [PubMed] [Google Scholar]
- 31.Dangel W., Tschech A., Fuchs G. 1988. Anaerobic metabolism of cyclohexanol by denitrifying bacteria. Arch. Microbiol. 150: 358–362 [DOI] [PubMed] [Google Scholar]
- 32.Dangel W., Tschech A., Fuchs G. 1989. Enzyme reactions involved in anaerobic cyclohexanol metabolism by a denitrifying Pseudomonas species. Arch. Microbiol. 152: 271–279 [DOI] [PubMed] [Google Scholar]
- 33.Boll M., Fuchs G., Heider J. 2002. Anaerobic oxidation of aromatic compounds and hydrocarbons. Curr. Opin. Chem. Biol. 6: 604–611 [DOI] [PubMed] [Google Scholar]
- 34.Fuchs G. 2008. Anaerobic metabolism of aromatic compounds. Ann. N. Y. Acad. Sci. 1125: 82–99 [DOI] [PubMed] [Google Scholar]
- 35.Jin J., Straathof A. J. J., Pinkse M. W. H., Hanefeld U. 2011. Purification, characterization, and cloning of a bifunctional molybdoenzyme with hydratase and alcohol dehydrogenase activity. Appl. Microbiol. Biotechnol. 89: 1831–1840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schühle K., Gescher J., Feil U., Paul M., Jahn M., Schagger H., Fuchs G. 2003. Benzoate-coenzyme A ligase from Thauera aromatica: an enzyme acting in anaerobic and aerobic pathways. J. Bacteriol. 185: 4920–4929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gescher J., Zaar A., Mohamed M., Schägger H., Fuchs G. 2002. Genes coding for a new pathway of aerobic benzoate metabolism in Azoarcus evansii. J. Bacteriol. 184: 6301–6315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fuchs G., Boll M., Heider J. 2011. Microbial degradation of aromatic compounds – from one strategy to four. Nat. Rev. Microbiol. 9: 803–816 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.