Abstract
Retinal degenerations and optic neuropathies often lead to death of photoreceptors or retinal ganglion cells, respectively. Stem cell therapies are showing promise for these diseases in preclinical models and are beginning to transition into human trials, but cell delivery and integration remain major challenges. Focusing on photoreceptor- and progenitor-directed approaches, in this article, the authors review how advances in tissue engineering and cell scaffold design are enhancing cell therapies for retinal degeneration.
Keywords: photoreceptor, progenitor cell, scaffold, stem cell, tissue engineering, transplantation
Retinal degeneration often ends with death of retinal neurons, such as photoreceptors in retinitis pigmentosa and age-related macular degeneration, and retinal ganglion cells (RGCs) in glaucoma. Although neuroprotective therapies may save these cells before they die, for example, using neurotrophic factors, stem cells or gene therapy, for many patients who are later in the disease process and have lost these cells, cell replacement therapy remains a major goal. Transplanted photoreceptors and their precursor retinal progenitors cells (RPCs) may integrate into recipient retinas, form mature morphologies, create synapses with host retinal neurons and restore light-evoked electrical responses [1]. Furthermore, in rodent models lacking rod function, cell transplants are able to re-establish some functional vision, for example, in maze navigation tests [2]. While these early data are promising, cell transplant therapies remain plagued by low cell survival and integration at the transplant site, as well as difficulties in retaining injected cells in a targeted focal region, such as under the fovea.
One way to address these limitations is through the use of 2 or 3D tissue-engineered scaffolds. The field of tissue engineering is interdisciplinary, combining cells with physical supports and biochemical signals to create cell scaffolds capable of supporting cell delivery, survival and integration. For example, scaffolds have been developed to deliver corneal epithelial cells, keratocytes and mesechymal stem cells to the cornea [3–5], embryonic and mesechymal stem cells to the heart [6–8], and Schwann and neural stem cells into the peripheral and CNS [9–13].
Can similar approaches be developed to facilitate stem cell therapy for retinal diseases? Indeed, early steps are now being taken to facilitate retinal cell therapies with advanced tissue engineering approaches [14]. As these scaffolds are designed to be implanted into the subretinal space, and because it is necessary for outer segments of photoreceptors to interact with retinal pigment epithelial cells across this space, it is necessary for these scaffolds to be either biodegradable or resorbable. As a result, the scaffolds have primarily been constructed from polyesters such as polylactic acid (PLA), polylactic-co-glycolic acid (PLGA) or polycaprolactone (PCL) (Table 1). The degradation of these materials has been well characterized both in vitro [15] and in vivo [16], with degradation rates affected by the molecular weight of the polymer, the degree of crystallinity, and in the case of PLGA, the ratio of lactate to glycolate subunits in the material. Of these materials, PLGA has the fastest degradation rate and PCL has the slowest [15]. Polyglycerol sebacate (PGS) is a fourth, recently developed, polyester that has been synthesized in an effort to create a polyester with mechanical properties closer to that of native tissue [17]. Thus, specific scaffold materials may confer certain advantages in design characteristics for retinal repair.
Table 1.
Polymers used in the scaffold production for the transplantation of retinal progenitors cells and their properties.
| Polymer | Chemical structure | Scaffold type |
Mechanical stiffness (modulus) |
Biodegradation | Notes |
|---|---|---|---|---|---|
| PLA | 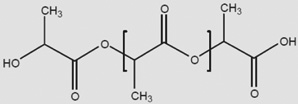 |
Cylinder | 2.8–4.1 GPa (l) 1.4–2.8 GPa (d,l) [73] |
12–24 months (depends on crystallinity) [15] | Modulus and degradation depends on crystallinity |
| PLGA |  |
Cylinder or fiber | 1.4–2.8 Gpa [73] | 1–6 months [15] | Modulus and degradation depends on lactide to glycolide ratio |
| PCL | 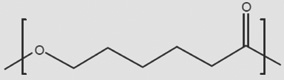 |
Cylinder or fiber | 206–344 Mpa [73] | >24 months [15] | |
| PGS | 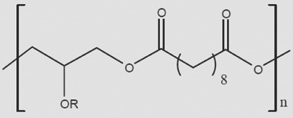 |
Cylinder | 0.282 ± 0.025 Mpa [74] | 1–2 months [74] | |
| PMMA | 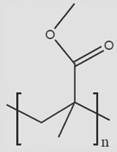 |
Cylinder | 1.8–3.1 Gpa [75] | Nondegradable | |
| Chitosan | 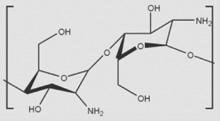 |
Fiber | 159.4 ± 40.0 Mpa (nanofibers) [76] | Enzymatically by chitosanase and lysozyme | Rate of degradation is dependent on primary nitrogen substitutions |
| HAMC | 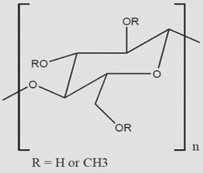 |
Hydrogel | 1–100 Pa [49] | 5–15 days [49] | Modulus and degradation rate are dependent on ratio of polymers |
| PNIPAAm | 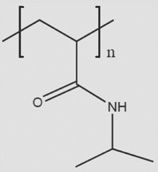 |
Hydrogel | N/A | N/A | Grafted to collagen to create self-aggregating hydrogel |
HAMC: Hyaluronan/methyl cellulose; N/A: Not applicable; PCL: Polycaprolactone; PGS: Polyglycerol sebacate; PLA: Polylactic acid; PLGA: Polylactic-co-glycolic acid; PMMA: Polymethyl methacrylate; PNIPAAm: Poly(N-isopropylacryl amide).
Scaffolds for the transplantation of RPCs can be loosely classified into three different types: cylindrical scaffolds designed to mimic the vertical 3D organization of cells found in the retina; fibrous scaffolds designed to mimic the microstructure of the extracellular matrix and hydrogel scaffolds designed to replicate the mechanical properties of the retina. In this article, the authors review the advances in scaffold design for retinal tissue engineering, and discuss the advantages and challenges remaining for the various scaffold polymer materials studied to date.
Microcylinder scaffolds
Vertical cylinder designs may allow for vertical integration of retinal cell communication, as is seen through much of the signaling of visual signals in the native retina. Several scaffolds have been designed with vertical pores (Figure 1), to orient the growth of the cells within the scaffold as well as shield the cells from sheer forces experienced during implantation. The first scaffolds of this design were created using a solid–liquid phase separation or solvent phase-inversion techniques to produce scaffolds from PLA and PLGA, which were 100–200 µm thick with 50-µm pores [18]. While the pores using this method were vertical, they were not continuous through the scaffold; and as a result, RPCs were limited primarily to the upper 30 µm of the scaffold. Upon implantation, cell survival was increased significantly, but cells were not able to migrate from the polymer scaffold into the retina [18,19].
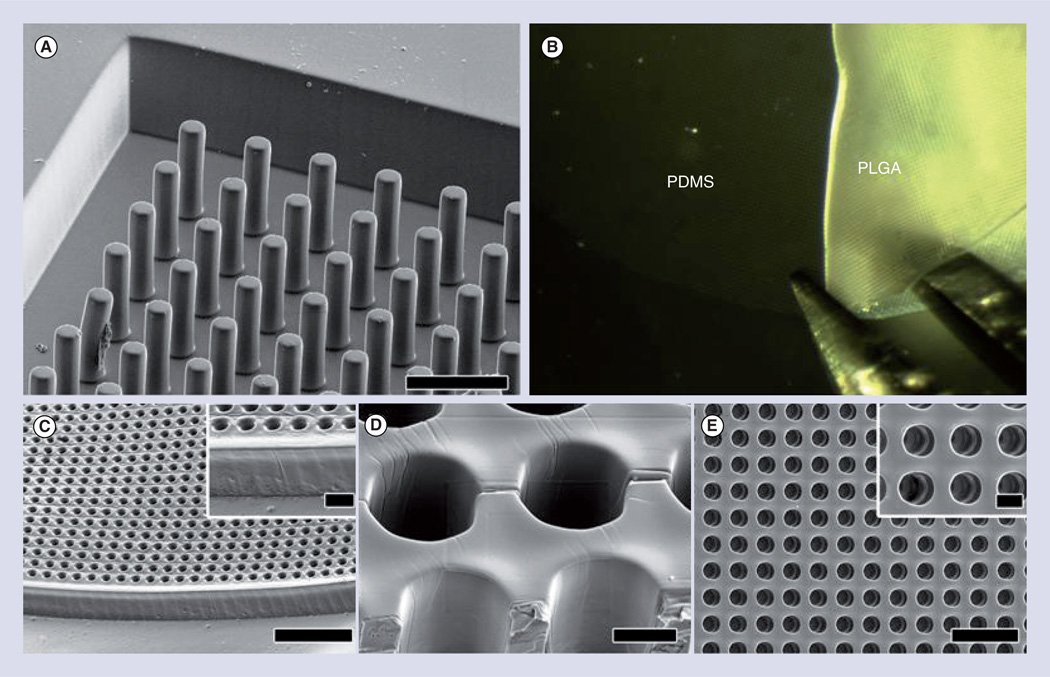
Figure 1. Microcylinder scaffolds are used to mimic the vertical structural organization of cells found in the retina.
(A) Scanning electron micrograph of PDMS negative mold. (B) Removal of PLGA scaffold from PDMS mold with forceps. (C–E) Scanning electron micrographs of top side of removed PLGA microcylinder scaffolds. (C) Overhead view. (C insert) Higher magnification of (C). (D) Alternate view at 45° tilt angle with cutaway showing inside walls of channels. (E) Alternate view at 45° tilt angle showing the edge of a scaffold. (E insert) Higher magnification of (E). Scale bars in (A) and (C) = 50 µm, (C insert) and (D) = 10 µm, (E) = 100 µm and (E insert) = 20 µm.
PDMS: Polydimethylsiloxane; PLGA: Polylactic-co-glycolic acid.
Reproduced with permission from [25]. © Elsevier (2012).
Other experiments using similarly prepared scaffolds showed a limited ability of RPCs cells to migrate from the polymer scaffold into normal control retinas, but no cells migrated into a rhodopsin knockout (Rho−/−) mouse [14], a model for photoreceptor degeneration that demonstrates associated glial scarring [20,21]. Furthermore, thick scaffolds created large retinal detachments as well as damage to the retina during implantation. As a result of the retinal damage from thick scaffolds, thinner scaffolds containing cylindrical pores were explored. Initial scaffolds were created from poly(methyl methacrylate) (PMMA) using a two-step process of photolithography and reactive ion etching to produce a 6-µm scaffold with 11-µm diameter pores gridded 63 µm apart [22]. As before, these scaffolds were coated with PDL and laminin to increase cell binding to the scaffold surface. The addition of cylinder pores to the scaffold allowed a 20-fold increase in the number of cells transplanted into the subretinal space when compared with thin films without architecture, and sections of the implanted 6-µm thick scaffold did not create large retinal deformations. This scaffold design by increasing the total number of cells transplanted and the residence time these cells have to interact with the retina also increased the number of cells migrating into the retina, with approximately half of all cells that survived transplantation migrating into the retina 4 weeks after implantation [22].
Because PMMA is not biodegradable, similar scaffolds were produced using the biodegradable polymer PCL. These scaffolds were created through solvent casting of PCL over a polydimethylsiloxane (PDMS) negative to produce a 5–6-µm thick scaffold with 25-µm diameter pores [23]. As with PMMA scaffolds, these scaffolds increased 20-fold the number of attached cells when compared with PCL thin films without architecture; however, these scaffolds were not implanted so potential for damage to the retina was not assessed. This technique for preparing scaffolds was further expanded in two different pore architectures in separate PCL films, which were then thermally bonded to each other to produce a scaffold <30 µm thick with pores in the upper film being either 60 or 200 µm in diameter and a lower film containing 10-µm diameter pores [24]. The theory behind this scaffold was that stem cell-derived photoreceptor cell bodies would be seeded into the upper film contained within the larger pores, while the pores in the lower scaffold would facilitate interaction of photoreceptor outer segments but not cell bodies to the retinal pigment epithelium (RPE). Such interactions between RPCs and RPCs, which have differentiated into photoreceptors, was previously explored using 50-µm PLGA scaffolds with 15-µm pores spaced 5 µm apart through solvent evaporation using a PDMS negative mold [25]. In these experiments, the scaffolds were incubated in media containing serum prior to being seeded with RPCs derived from human embryonic stem cells. Following culture in the scaffold, photoreceptors and glial cells oriented their growth along the length of the pore. When these scaffolds were transplanted in vitro onto a RPE explant, photoreceptor outer segments formed and were phagocytosed by RPE, as is seen in vivo. These scaffolds were not implanted in vivo so it is unknown what effect the increased scaffold thickness would have on the retina. Taken together, these data suggest that microcylinder scaffolds less than 50 µm in thickness can allow for the interaction of seeded cells with RPE; however, it is unknown whether this interaction will persist following migration into the retina as well as to what degree transplanted cells migrate from the biodegradable scaffolds into the retina.
As PGS contains mechanical properties, which more closely resembles those of the native retina, it was examined in thin microcylinder scaffolds, which were cured while using a PDMS negative to create 45-µm thick scaffolds containing 50-µm diameter pores separated 175 µm apart [26]. Using this material, it was first necessary to coat the scaffold with laminin prior to cell seeding. Once coated, these scaffolds supported RPC growth and proliferation. In an effort to improve transplantation efficiency and to limit the damage from the transplantation surgery, these scaffolds were scrolled, which was possible due to the higher elasticity of PGS when compared with PLA PLGA, and inserted into a 1-mm internal diameter needle, which could then inject the scaffold into the subretinal space [27]. Implanting this scaffold onto both control and Rho−/− mouse retinal explants revealed significant retinal integration of transplanted cells, with twice as many cells being able to migrate into the control retina. In addition, cells migrated to the outer nuclear layer (ONL), inner nuclear layer, ganglion cell layer (GCL) and nerve fiber layer. In vivo, transplanted cells migrated into both the ONL and inner nuclear layer twofold better in control than Rho−/− retinas (Figure 2). Thus, the use of the more elastic PGS material to recreate the thin microcylinder scaffolds was able to increase the migration of transplanted cells into the retina and also conferred the advantage of implantation through a less-invasive procedure.
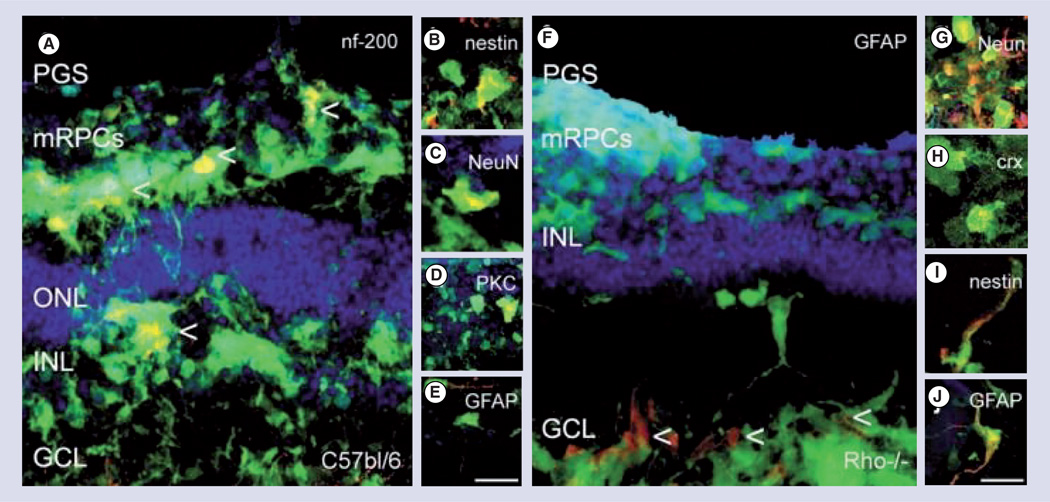
Figure 2. Polyglycerol sebacate microcylinder scaffold delivery of green fluorescent protein and retinal progenitors cells to control and rhodopsin knockout (Rho−/−) degenerated mouse retinal explants.
Retinal progenitors cells were capable of migrating to all layers of the retina and differentiating into several mature cell types (A–E) control wild-type retina, (F–J) Rho−/− retinas. (A) green fluorescent protein and mRPCs co-labeled (yellow) for nf200 (ganglion cell) labeled with arrows, (B) Nestin (immature), (C, G) NeuN, (D) PKC (bipolar), and (E,J) GFAP (glial) and (H) Crx (photoreceptor). Green: green fluorescent protein; red: rhodamine labeled marker; blue: topro-3 nuclei label; scale: 25 mm.
GCL: Ganglion cell layer; INL: Inner nuclear layer; NeuN: Neuronal nuclei; mRPCs: Murine retinal progenitors cells; ONL: Outer nuclear layer; PGS: Polyglycerol sebacate.
Reprinted with permission from [27]. © 2009 Elsevier.
Color figure can be found online at www.expert-reviews.com/doi/suppl/10.1586/eop.12.56
Differentiation of RPCs on microcylinder scaffolds
The differentiation of stem and progenitor cells has been shown to be a function of many biological and mechanical factors, including the age of the cells when they are isolated [28,29], molecular cues in which the cells are cultured [30,31] and the environment in which the cells are transplanted [32]. In addition, scaffold materials [33] and mechanical properties [34–36] can also affect the differentiation of stem cells.
For example, RPCs cultured on microcylinder scaffolds begin to differentiate within the scaffold during culture as determined through immunohistochemistry and reverse transcription-PCR [18]. RPCs down-regulated immature markers Nestin, Hes1 and Hes5 and upregulated mature differentiated markers GFAP (astrocytic), MAP2 (neuronal), PKC-α (bipolar), recoverin (photoreceptors) and rhodopsin (photoreceptors) [14,18]. Thin cylinder scaffolds also promoted cell differentiation with downregulation of the immature marker sox-2 and increased expression of recoverin, rhodopsin and GFAP [22,23]. In some cases, the differentiated cells would actually form laminae within the scaffold. In this scaffold, Pax6 was used to label cells of the inner retina and Otx2 was used to label photoreceptors. Upon seeding, Pax6-positive cells remained in the uppermost 20 µm of the scaffold, and Otx2-positive cells were observed in the bottom 10 µm, with a transition zone in the middle 20 µm, and GFAP-positive cells spanning the entire channel, similar to localization of Müller glial cell in vivo [25]. Thus scaffold-promoted RPC differentiation may also promote differential migration and lamination of the cellular progeny, as occurs in the developing retina.
Can scaffolds also inhibit differentiation or promote stem cell persistence? Unlike cells seeded on scaffolds prepared from PLA and PLGA, cells seeded on scaffolds prepared from PGS upregulated Ki67 and nestin, both immature markers [27]. For cells cultured within the scaffold, cells were only positive for the mature markers of GFAP and NF200 (RGCs) [26]. However, once these cells were transplanted onto explants or in vivo, they began to differentiate into several different types of cells including NF200-positive RGCs, PKC-positive bipolar cells, CRX-positive and rhodopsin-positive photoreceptors and GFAP-positive glial cells. Thus, there may be an interaction between scaffold-promoted stem cell differentiation and the environment in which that scaffold is placed. To date, however, microcylinder scaffolds have not been shown to direct the differentiation of RPCs into a single (e.g., photoreceptor) phenotype.
Fibrous scaffolds
Fibrous scaffolds (Figure 3) have become very common in the field of tissue engineering due to their mimicking of the natural architecture of the extracellular matrix. The fibers in these scaffolds provide a large surface area for cell attachment while also creating interconnected pores for transport of nutrients [37,38]. While there are several methods for creating micrometer or nanometer scale fibers, one of the most common is through electrospinning. In this process, a high voltage is applied to polymers dissolved in a volatile solvent and pumped through a needle until a continuous stream of solution is ejected from the needle to a collecting plate normally 10–20 cm from the needle tip. As the solution is ejected from the needle, the solvent evaporates forming individual continuous fibers deposited on the collecting plate. Fibrous electrospun scaffolds produced from PCL and the biomaterial chitosan, which has been covalently bonded to PCL, as well as blends of the two materials, were compared for their engineered properties and their cellular effects [39]. The scaffolds produced from the different materials formed similar scaffolds with fiber mats containing a random orientation with a mean diameter between 656 and 925 nm and containing a porosity between 77 and 89%, with smaller fiber diameter scaffolds being less porous. The water contact angle varied significantly between the different scaffolds based upon the materials used to produce them: scaffolds produced from the hydrophobic PCL contained a contact angle of 134° while scaffolds blended with the hydrophilic polymer chitosan were found to have a contact angle of 0°. Decreasing the hydrophobicity not only affected the ability of the RPCs to spread on the scaffold, but also increased the proliferation rate of seeded RPCs. RPCs grown on electrospun, random alignment fibrous PCL scaffolds with a mean fiber diameter of 3 µm, a porosity of 52% and mean pore size of 24.4 µm coated with laminin, were observed to grow both on the scaffold surface as well as in the pores [40]. Once transplanted onto retinal explants, these cells migrated into control and Rho−/− retinas at similar rates.
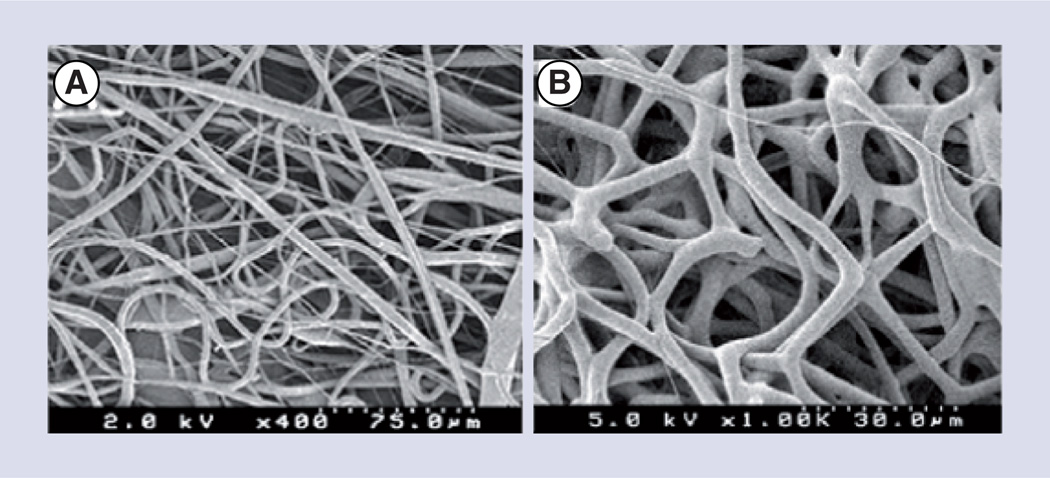
Figure 3. Electrospun fibrous scaffolds mimic the fibrous nature of the extracellular matrix found in the retina and other tissues.
Polycaprolactone electrospun fiber morphology analyzed by SEM at (A) 400× and (B) 1000× containing a mean fiber diameter of 2.962 ± 0.894 µm.
Reproduced with permission from [40]. © Taylor and Francis (2012).
Chemical and biological modification of fibrous scaffolds may be used to confer specific advantages. For example, to further enhance integration RPCs migration in the retina, electrospun fibers were incorporated with an aqueous domain at the core of the fibers that contained the enzyme MMP2. This zinc-dependent matrix metalloproteinase was previously demonstrated to enhance integration into degenerating retinas that show associated glial scarring by degrading inhibitory extracellular matrix proteins CD44 and neurocan that accumulate from the degeneration [41,42]. The addition of MMP2 to the scaffold fibers increased the total number of cells able to migrate into control retinas three- to fourfold when compared with control electrospun scaffolds, and significantly increased the number of RPC differentiated into photoreceptors transplanted 10–15-fold (Figure 4) [42].
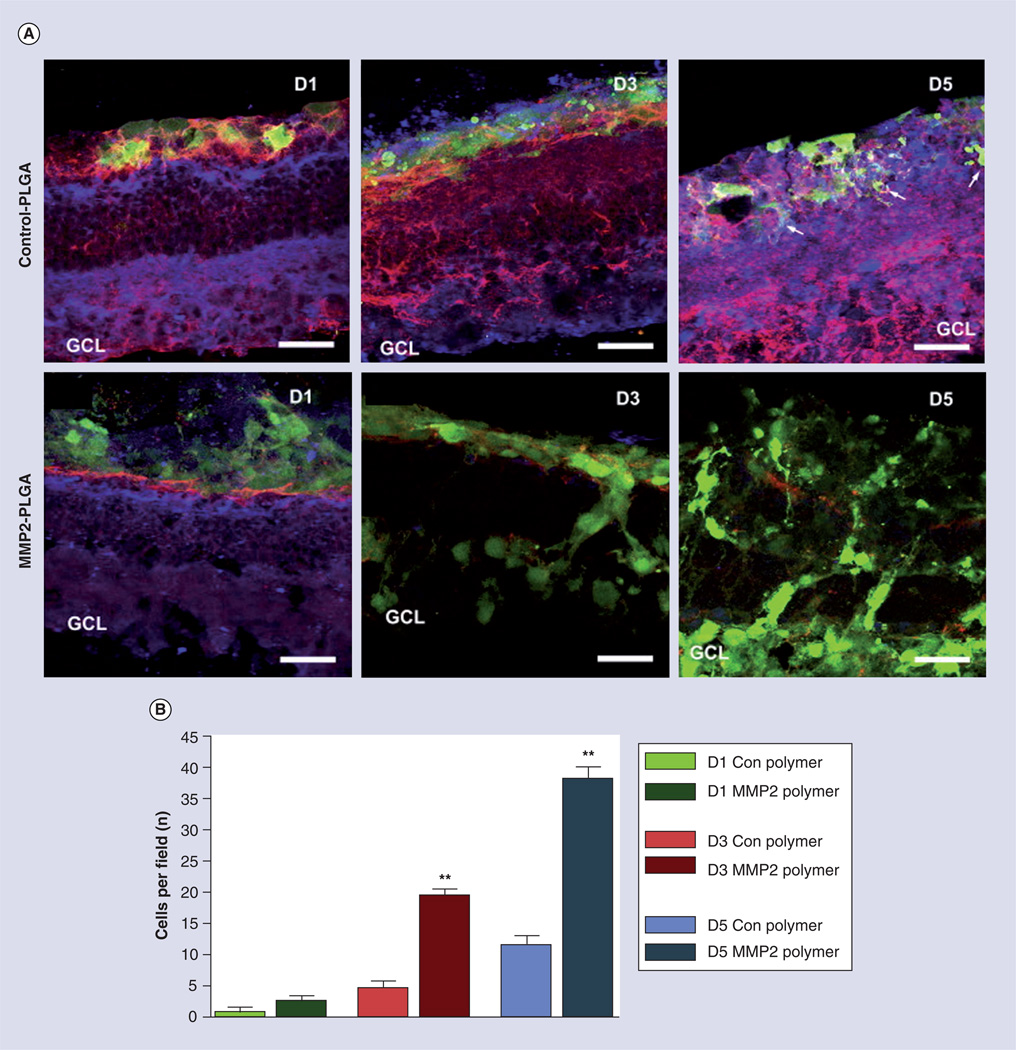
Figure 4. The controlled delivery of active MMP2 from electrospun fibrous scaffold enhances green fluorescent protein and retinal progenitors cells migration into degenerated Rho−/− retinal explants by degrading inhibitory matrix molecules CD44 and neurocan.
(A) Retinal explants were cultured in the absence (control PLGA) or presence (MMP2–PLGA) of active MMP2 for 1, 3 and 5 days at 37°C, and subsequently fixed, cryosectioned and immunolabeled for green fluorescent protein (green), CD44 (red) and neurocan (blue). (B) The average number of cells that cross from the polymer into the retinal explant per microscopic field.
(A) Magnification 40×, scale bar = 25 µm.
*p < 0.05; **p < 0.001.
GCL: Ganglion cell layer; PLGA: Polylactic-co-glycolic acid.
Reproduced with permission from [42]. © Elsevier (2009).
Color figure can be found online at www.expert-reviews.com/doi/suppl/10.1586/eop.12.56
Electrospinning is not the only mechanism to design fibrous scaffolds. By solvent casting PCL over an etched plate, fiber surface topographies were created on PCL thin films. Morphology of the nanowires could be controlled by the altering the processing temperature, creating scaffolds with long 27-µm or short 2.5-µm nanowires [43]. These were compared with control PCL thin films as RPC culture substrates. At 1 week, long nanowires increased the number of cells over those cultured on control thin films and short nanowire scaffolds. Long nanowire scaffolds also demonstrated an increased migration of RPCs into both control and Rho−/− retinas, with cells migrating to the correct lamina based upon their terminal differentiation. Thus, compared with microcylinder scaffolds, fibrous scaffolds better promote transplanted cell migration in both native and degenerated retinas. This migration was further enhanced by the controlled release of MMP2. Most importantly, differentiated cells were shown to migrate to the proper cell lamina within the retina when transplanted on fibrous scaffold. These data suggest that fibrous scaffolds may hold many advantages for retinal stem cell transplantation.
Differentiation of RPCs on fibrous scaffolds
RPC seeded on fibrous scaffolds have shown limited ability to differentiate following transplantation. In some cases, RPCs differentiated into only GFAP-positive glial cells [39,40]; in other cases, expression of photoreceptor markers recoverin and rhodopsin were upregulated in addition to GFAP, in the latter case when cells were cultured on chitosan/PCL composite scaffolds [39]. A more significant upregulation of recoverin and rhodopsin was seen using a PLGA nanofiber scaffold in the presence of MMP2, with a tenfold higher recoverin and rhodopsin expression compared with control PLGA scaffolds, although the mechanism associated with this increase was not investigated [42]. It is also important to note that the cell expression of the immature marker nestin and GFAP were not quantified, so it cannot be determined if there was an increase in the total number of cells differentiating or if there was a specific increase in photoreceptor differentiation.
In situ forming hydrogels
Hydrogels have been used extensively as scaffolds for the culture of neural cells because the mechanical properties; most importantly the stiffness, of these scaffolds can be tailored to match that of tissue in the CNS [35,44–47]. Due to the mechanical forces associated with implantation, however, transplanting a relatively soft cell-seeded hydrogel scaffold is extremely difficult. To overcome this limitation, research has begun into in situ-gelling hydrogels. Initial work studying scaffolds containing neural progenitors used photo-initiated free radical formation [34,45], but this technique proved cytotoxic to cells [48].
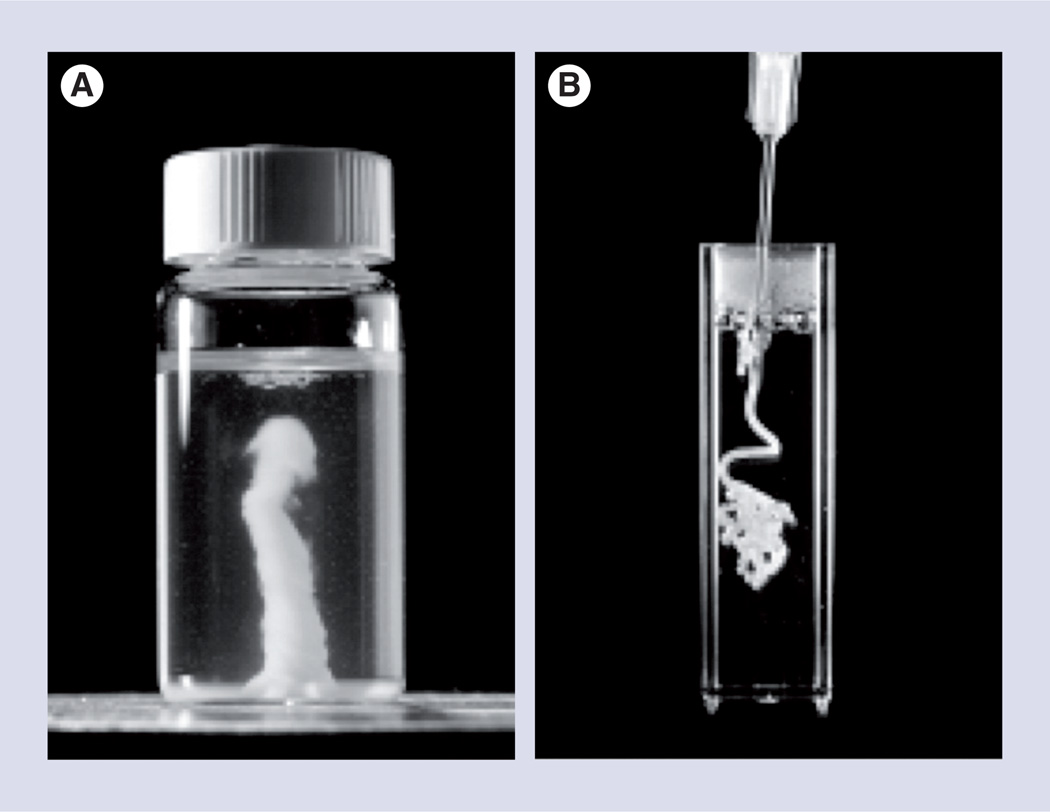
Two new innovations in in situ-gelling hydrogels (Figure 5) may prove useful for subretinal implantation. In one, mixing sodium haluronate and methylcellulose in equal proportions forms a temperature-responsive hydrogel whose elastic modulus and gelling time can be controlled by varying the total concentration of the two polymers in solution [49]. The polymer supported RPCs either as encapsulated whole neurospheres or as a single-cell suspension, and yielded the same survival as cells cultured in media without polymer [50]. When injected into the subretinal space, RPCs seeded in haluronate and methylcellulose formed a continuous layer, covering >90% of Bruch’s membrane, the basement membrane of the RPE, as opposed to RPCs injected in saline that covered approximately 50% (Figure 6). Using this material, RPCs primarily migrated into the RPE with a small number of cells migrating into the outer nuclear layer. This integration pattern could be due to interaction between the transplanted RPC monolayer formed on the RPE, creating interactions that result in the formation of tight junctions between the cells and their eventual differentiation and integration. This interaction is not seen with the other scaffold types where the scaffold forms a physical barrier to interaction with the RPE.
Figure 5. Hydrogels are used to approximate the mechanical stiffness of the native retina.
Self-aggregation of poly(N-isopropylacrylamide) into an amorphous hydrogel when (A) heated to or (B) injected into an aqueous solution at physiological temperature.
Reproduced with permission from [51]. © American Chemical Society (2010).
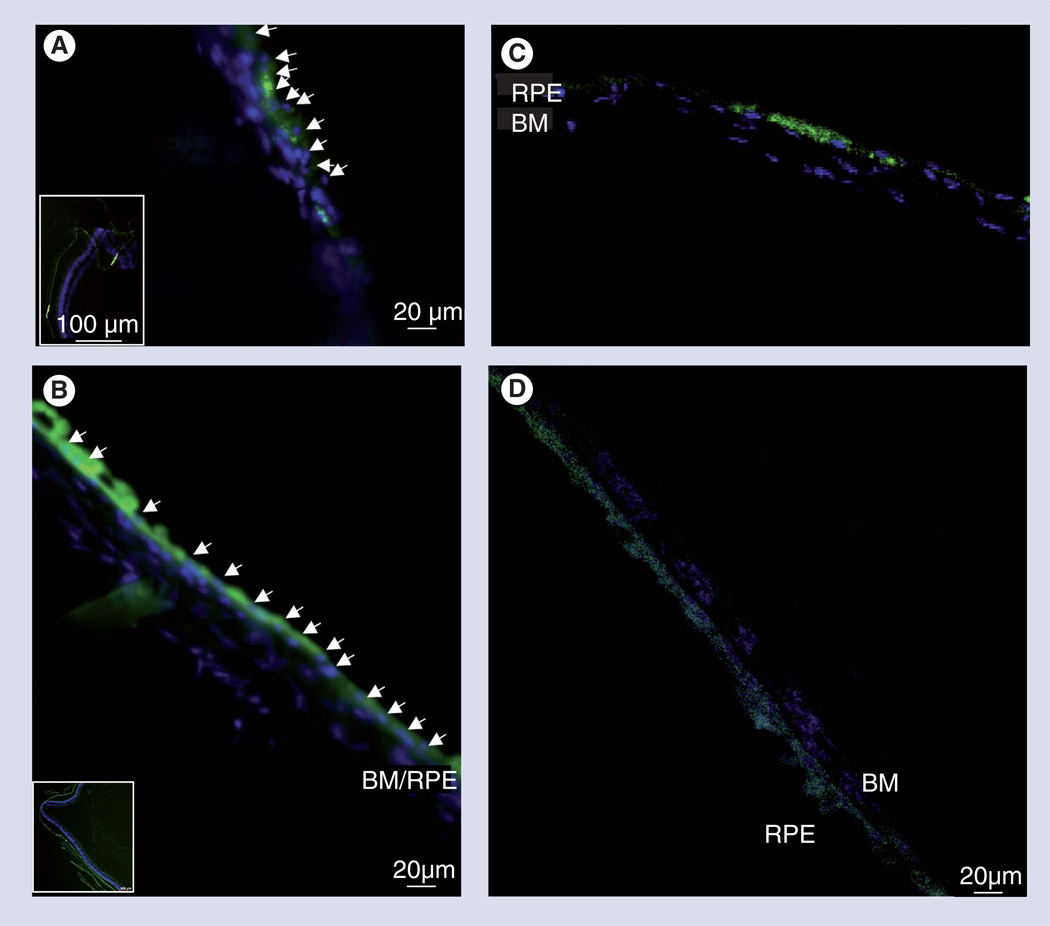
Figure 6. In vivo adult subretinal transplantation of green fluorescent protein and retinal progenitors cells in haluronate and methylcellulose self-aggregating hydrogel, assayed at 4 weeks post-transplantation.
(A) Control transplantation in saline vehicle show non-contiguous cellular integration and localized cellular groupings (inset) atop BM, suggestive of cellular aggregation pre- or post-transplantation. (B) Transplantation in haluronate and methylcellulose (HAMC) shows contiguous areas of RPE integration over large areas of retina (inset), suggesting HAMC maintains cellular distribution during injection and preventing aggregation pre- or post-transplantation. Arrowheads indicate location of nuclei of transplanted cells. Confocal images of cuboidal RPE cells sitting atop Bruch’s membrane after injection in (C) saline and (D) HAMC (Hoechst and green fluorescent protein).
BM: Bruch’s membrane; RPE: Retinal pigment epithelium.
Adapted with permission from [50]. © Elsevier 2010.
Color figure can be found online at www.expert-reviews.com/doi/suppl/10.1586/eop.12.56
In a second approach to in situ-gelling hydrogels, collagen modified with poly(N-isopropylacrylamide) undergoes a transition as the solution reaches physiological temperature, and gelling time is controlled through the method of grafting and the number of poly(N-isopropylacrylamide) chains polymerized to collagen [51]. This polymer supported >90% viability of RPE cells after 14 days in culture, but was not tested in vivo. Together, hydrogels can create a continuous covering of Bruch’s membrane, but exhibit a very limited ability to promote transplanted cells’ migration into the retina, with cells migrating primarily into the RPE. In addition, the actual terminal differentiation of RPCs seeded with the hydrogel scaffolds has yet to be determined; so, it is unknown whether the differentiation of RPCs to photoreceptors is supported.
Conclusion
RPCs are a potential tool in the treatment of retinal degenerative diseases. They are able to differentiate into different retinal cell types while still being able to proliferate, thereby providing an expandable source of cells for cellular replacement therapies. While these cells have previously been shown to create functional synapses to host retinas and restore vision, these methods show low cell survival and integration. Tissue-engineered scaffolds designed to mimic different aspects of the retina, used for cell expansion and delivery, have demonstrated significant survival of cells through transplantation into the subretinal space over cells injected alone or polymer films with no surface structures. These scaffolds also facilitate migration of transplanted cells into both uninjured controls and retinal degeneration models that include glial scarring, a major barrier to any cellular treatment of age-related macular degeneration. Challenges remain, as thickness, surface topography, mechanical properties and degradation characteristics must be considered in the design of the scaffold to prevent further damage to the retina or large retinal detachments, and to allow for interaction between the retina and the RPE. Together these data suggest that tissue engineering approaches will prove critical to enhancing our understanding of the nanoscale and microscale biology of retinal stem cells, and to providing meaningful mechanisms to promote regenerative cell transplantation for retinal and optic nerve degeneration.
Expert commentary
The ability of scaffolds to increase the delivery and survival of RPCs following transplantation into the subretinal space demonstrates the importance in the development of scaffolds for the treatment of retinal diseases. While significant research is being conducted on enhancing the migration of RPCs into the neural retina, less research is being conducted on controlling the differentiation of the RPCs on these scaffolds. In each of the cases described earlier where the differentiation of the RPCs was investigated, the predominant differentiation pathway was glial. While glial cells clearly provide several important functions in the retina, including promoting integration and formation of synapses [52–54], and acting as a favorable substrate for photoreceptor growth in vitro [55], glial cells may also create barriers to cell growth and migration of both native and transplanted cells [56,57] and can also lead to an increase in degeneration [58,59]. While the addition of the soluble factor MMP2 and the use of fibrous scaffolds were able to overcome some of the effects of glial scarring allowing RPCs to migrate into Rho−/− retinas, cellular transplantation has been shown to induce further gliosis, which could further affect cellular integration and repair [57].
In order to create a more suitable implant, it is possible that scaffolded RPCs should either be differentiated prior to implantation using the known soluble factors [31,60,61] or allow for the controlled release of these factors in a scaffold, similar to the retinoic acid releasing scaffold designed for mesenchymal stem cells [62]. A secondary advantage to the differentiation of the RPCs prior to implantation is that this could reduce the risk of tumor formation as has been seen with other stem cell implantations [63].
A second area for study is on the long-term effects of implantation of the scaffolds in the subretinal space. In many of these studies, the polymer PCL was used, whose degradation rate is variable based upon factors listed above, can last as long as 1–2 years to fully degrade. PGS degrades at a faster rate, however, over an implantation time of 1 month, it is associated with a loss of existing photoreceptors [64]. The self-aggregating hydrogel scaffolds may provide the smallest barrier between RPE and neural retina, being resorbable in as little as 1 week [50]. The slow degradation rate of these materials leaves a solid barrier separating the neural retinal from the RPE for the lifespan of the implant. Interaction with the RPE is necessary for the formation of outer segments and increases photoreceptor survival and normal function [25,65,66]. As a result, it is unknown what long-term effect scaffold implantation will have on either the RPE or the photoreceptors being implanted.
Five-year view
While significant research has been conducted on the development of different scaffolds for the transplantation of progenitor cells towards the treatment of degenerative diseases of photoreceptors, scaffolds have yet to be developed for degenerative diseases of retinal ganglion cells such as glaucoma or optic nerve stroke. In these diseases, retinal ganglion cells are permanently damaged in the optic nerve, leading to interruption of retinal signaling to the brain and ultimately to cell death. Treatments for these diseases face similar obstacles to those of diseases of the photoreceptors, mainly transplantation and integration into the existing cellular circuitry of the outer retina and inner plexiform layer. However, retinal ganglion cell-replacement therapy is more complex, and also requires pathfinding from the retina to the optic nerve, extension through the optic nerve, pathfinding through the optic chiasm and to the visual centers of the brain.
Cellular treatments are further limited by degeneration within the optic nerve, which creates glial scarring and an environment inhibitory to axon growth. However, recent studies into the PTEN/mTOR and SOCS3 pathways demonstrate regeneration through optic nerve crush sites to the optic chiasm [67–69]. Several of the scaffolds discussed earlier when implanted into the subretinal space contained cells that migrated into the GCL, including one scaffold that was able to preferentially differentiate cells into NF200-positive cells [26]; however, these scaffolds were not capable of directing the cell growth towards the optic nerve head. Cells injected into the vitreous body are able to migrate into GCL, although again these studies have not shown an ability to direct cell growth towards the optic nerve head [70–72]; however, seeded cells that do reach the optic nerve head are able to send short processes into the optic nerve [72]. In order to create a scaffold capable of directing the radial growth of the ganglion cells towards the optic nerve head, it will be necessary to create scaffolds for implantation above the retina in the vitreous body. In the next 5 years, we can expect to see scaffolds prepared to direct cells to the optic nerve, with RPCs modified to allow migration through glial scarring and projecting dendrites into the inner plexiform layer.
Key issues.
Retinal progenitor cells offer a potential expandable cell source capable of differentiating into all retinal cell types.
Retinal progenitor cells have previously been shown to be capable of forming functional synapses when implanted into the subretinal space; however, the cellular injections have low survival.
Tissue-engineered scaffolds are 3D supports capable of acting as a cellular delivery device when implanted into the subretinal space, demonstrating a tenfold increase in cell survival and a fourfold increase in cellular migration into retina.
Several different scaffold architectures have been designed with fibrous scaffolds showing the greatest migration of cells into the retina, which was further enhanced by the incorporation of the enzyme MMP2 that helps to degrade inhibitory factors in glial scarred retinas.
A total scaffold thickness of greater than 50 µm has been shown to create large retinal detachments and further damage the retina in animal models during implantation.
The long-term effect of scaffold implantation, including degradation of the scaffold in the subretinal space, and long-term separation of the retina from direct contact with the retinal pigment epithelium have yet to be determined.
Acknowledgments
The authors were supported by grants from the NIH (EY020297 to JL Goldberg and P30-EY014801 to Univeristy of Miami [FL, USA]), and an unrestricted grant to the University of Miami from Research to Prevent Blindness. JL Goldberg is the WG Ross Distinguished Chair in Ophthalmic Research.
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
- 1. MacLaren RE, Pearson RA, MacNeil A, et al. Retinal repair by transplantation of photoreceptor precursors. Nature. 2006;444(7116):203–207. doi: 10.1038/nature05161. • Not only shows that photoreceptors can be transplanted into retinas, but that the cells can integrate into existing neural circuitry and respond to light responses.
- 2.Pearson RA, Barber AC, Rizzi M, et al. Restoration of vision after transplantation of photoreceptors. Nature. 2012;485(7396):99–103. doi: 10.1038/nature10997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deshpande P, McKean R, Blackwood KA, et al. Using poly(lactide-co-glycolide) electrospun scaffolds to deliver cultured epithelial cells to the cornea. Regen. Med. 2010;5(3):395–401. doi: 10.2217/rme.10.16. [DOI] [PubMed] [Google Scholar]
- 4.Pang K, Du L, Wu X. A rabbit anterior cornea replacement derived from acellular porcine cornea matrix, epithelial cells and keratocytes. Biomaterials. 2010;31(28):7257–7265. doi: 10.1016/j.biomaterials.2010.05.066. [DOI] [PubMed] [Google Scholar]
- 5.Zajicova A, Pokorna K, Lencova A, et al. Treatment of ocular surface injuries by limbal and mesenchymal stem cells growing on nanofiber scaffolds. Cell Transplant. 2010;19(10):1281–1290. doi: 10.3727/096368910X509040. [DOI] [PubMed] [Google Scholar]
- 6.Wei HJ, Chen CH, Lee WY, et al. Bioengineered cardiac patch constructed from multilayered mesenchymal stem cells for myocardial repair. Biomaterials. 2008;29(26):3547–3556. doi: 10.1016/j.biomaterials.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 7.Xiang Z, Liao R, Kelly MS, Spector M. Collagen-GAG scaffolds grafted onto myocardial infarcts in a rat model: a delivery vehicle for mesenchymal stem cells. Tissue Eng. 2006;12(9):2467–2478. doi: 10.1089/ten.2006.12.2467. [DOI] [PubMed] [Google Scholar]
- 8.Leor J, Gerecht S, Cohen S, et al. Human embryonic stem cell transplantation to repair the infarcted myocardium. Heart. 2007;93(10):1278–1284. doi: 10.1136/hrt.2006.093161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen G, Hu YR, Wan H, et al. Functional recovery following traumatic spinal cord injury mediated by a unique polymer scaffold seeded with neural stem cells and Schwann cells. Chin. Med. J. 2010;123(17):2424–2431. [PubMed] [Google Scholar]
- 10.Fansa H, Keilhoff G. Comparison of different biogenic matrices seeded with cultured Schwann cells for bridging peripheral nerve defects. Neurol. Res. 2004;26(2):167–173. doi: 10.1179/016164104225013842. [DOI] [PubMed] [Google Scholar]
- 11.Olson HE, Rooney GE, Gross L, et al. Neural stem cell- and Schwann cell-loaded biodegradable polymer scaffolds support axonal regeneration in the transected spinal cord. Tissue Eng. Part A. 2009;15(7):1797–1805. doi: 10.1089/ten.tea.2008.0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oudega M, Gautier SE, Chapon P, et al. Axonal regeneration into Schwann cell grafts within resorbable poly(α-hydroxyacid) guidance channels in the adult rat spinal cord. Biomaterials. 2001;22(10):1125–1136. doi: 10.1016/s0142-9612(00)00346-x. [DOI] [PubMed] [Google Scholar]
- 13.Xiong Y, Zhu JX, Fang ZY, et al. Coseeded Schwann cells myelinate neurites from differentiated neural stem cells in neurotrophin-3-loaded PLGA carriers. Int. J. Nanomedicine. 2012;7:1977–1989. doi: 10.2147/IJN.S30706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tomita M, Lavik E, Klassen H, Zahir T, Langer R, Young MJ. Biodegradable polymer composite grafts promote the survival and differentiation of retinal progenitor cells. Stem Cells. 2005;23(10):1579–1588. doi: 10.1634/stemcells.2005-0111. [DOI] [PubMed] [Google Scholar]
- 15.Pitt CG, Gu ZW. Modification of the rates of chain cleavage of poly(ε-caprolactone) and related polyesters in the solid state. J. Control. Rel. 1987;4:283–292. [Google Scholar]
- 16.Sung HJ, Meredith C, Johnson C, Galis ZS. The effect of scaffold degradation rate on three-dimensional cell growth and angiogenesis. Biomaterials. 2004;25(26):5735–5742. doi: 10.1016/j.biomaterials.2004.01.066. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, Kim YM, Langer R. In vivo degradation characteristics of poly(glycerol sebacate) J. Biomed. Mater. Res. A. 2003;66(1):192–197. doi: 10.1002/jbm.a.10534. [DOI] [PubMed] [Google Scholar]
- 18.Lavik EB, Klassen H, Warfvinge K, Langer R, Young MJ. Fabrication of degradable polymer scaffolds to direct the integration and differentiation of retinal progenitors. Biomaterials. 2005;26(16):3187–3196. doi: 10.1016/j.biomaterials.2004.08.022. [DOI] [PubMed] [Google Scholar]
- 19.Warfvinge K, Kiilgaard JF, Lavik EB, et al. Retinal progenitor cell xenografts to the pig retina: morphologic integration and cytochemical differentiation. Arch. Ophthalmol. 2005;123(10):1385–1393. doi: 10.1001/archopht.123.10.1385. [DOI] [PubMed] [Google Scholar]
- 20.Jones BW, Watt CB, Frederick JM, et al. Retinal remodeling triggered by photoreceptor degenerations. J. Comp. Neurol. 2003;464(1):1–16. doi: 10.1002/cne.10703. [DOI] [PubMed] [Google Scholar]
- 21.Humphries MM, Rancourt D, Farrar GJ, et al. Retinopathy induced in mice by targeted disruption of the rhodopsin gene. Nat. Genet. 1997;15(2):216–219. doi: 10.1038/ng0297-216. [DOI] [PubMed] [Google Scholar]
- 22. Tao S, Young C, Redenti S, et al. Survival, migration and differentiation of retinal progenitor cells transplanted on micro-machined poly(methyl methacrylate) scaffolds to the subretinal space. Lab Chip. 2007;7(6):695–701. doi: 10.1039/b618583e. • For the first time, it was shown that by using a scaffold as a cell delivery device that retinal progenitor cells (RPCs) could not only increase survival, but also increased migration of these cells into the retina.
- 23.Steedman MR, Tao SL, Klassen H, Desai TA. Enhanced differentiation of retinal progenitor cells using microfabricated topographical cues. Biomed. Microdevices. 2010;12(3):363–369. doi: 10.1007/s10544-009-9392-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sodha S, Wall K, Redenti S, Klassen H, Young MJ, Tao SL. Microfabrication of a three-dimensional polycaprolactone thin-film scaffold for retinal progenitor cell encapsulation. J. Biomater. Sci. Polym. Ed. 2011;22(4–6):443–456. doi: 10.1163/092050610X487738. [DOI] [PubMed] [Google Scholar]
- 25. McUsic AC, Lamba DA, Reh TA. Guiding the morphogenesis of dissociated newborn mouse retinal cells and hES cell-derived retinal cells by soft lithography-patterned microchannel PLGA scaffolds. Biomaterials. 2012;33(5):1396–1405. doi: 10.1016/j.biomaterials.2011.10.083. • Describes the method for making 3D cylinder scaffolds from biomaterials including the creation of both the positive and negative molds as well as the effects of solvent and evaporation conditions on cylinder formation. This paper also describes differentiation which occurs within the biodegradable scaffold, with images showing differentiation markers as well as organization of these cells.
- 26.Neeley WL, Redenti S, Klassen H, et al. A microfabricated scaffold for retinal progenitor cell grafting. Biomaterials. 2008;29(4):418–426. doi: 10.1016/j.biomaterials.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Redenti S, Neeley WL, Rompani S, et al. Engineering retinal progenitor cell and scrollable poly(glycerol-sebacate) composites for expansion and subretinal transplantation. Biomaterials. 2009;30(20):3405–3414. doi: 10.1016/j.biomaterials.2009.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Livesey FJ, Cepko CL. Vertebrate neural cell-fate determination: lessons from the retina. Nat. Rev. Neurosci. 2001;2(2):109–118. doi: 10.1038/35053522. [DOI] [PubMed] [Google Scholar]
- 29.Okano H, Temple S. Cell types to order: temporal specification of CNS stem cells. Curr. Opin. Neurobiol. 2009;19(2):112–119. doi: 10.1016/j.conb.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 30.Eiraku M, Takata N, Ishibashi H, et al. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature. 2011;472(7341):51–56. doi: 10.1038/nature09941. [DOI] [PubMed] [Google Scholar]
- 31.Osakada F, Jin ZB, Hirami Y, et al. In vitro differentiation of retinal cells from human pluripotent stem cells by small-molecule induction. J. Cell. Sci. 2009;122(Pt 17):3169–3179. doi: 10.1242/jcs.050393. [DOI] [PubMed] [Google Scholar]
- 32.Sakaguchi DS, Van Hoffelen SJ, Theusch E, et al. Transplantation of neural progenitor cells into the developing retina of the Brazilian opossum: an in vivo system for studying stem/progenitor cell plasticity. Dev. Neurosci. 2004;26(5–6):336–345. doi: 10.1159/000082275. [DOI] [PubMed] [Google Scholar]
- 33.Bhang SH, Lim JS, Choi CY, Kwon YK, Kim BS. The behavior of neural stem cells on biodegradable synthetic polymers. J. Biomater. Sci. Polym. Ed. 2007;18(2):223–239. doi: 10.1163/156856207779116711. [DOI] [PubMed] [Google Scholar]
- 34.Leipzig ND, Shoichet MS. The effect of substrate stiffness on adult neural stem cell behavior. Biomaterials. 2009;30(36):6867–6878. doi: 10.1016/j.biomaterials.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 35.Saha K, Keung AJ, Irwin EF, et al. Substrate modulus directs neural stem cell behavior. Biophys. J. 2008;95(9):4426–4438. doi: 10.1529/biophysj.108.132217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Banerjee A, Arha M, Choudhary S, et al. The influence of hydrogel modulus on the proliferation and differentiation of encapsulated neural stem cells. Biomaterials. 2009;30(27):4695–4699. doi: 10.1016/j.biomaterials.2009.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vasita R, Katti DS. Nanofibers and their applications in tissue engineering. Int. J. Nanomedicine. 2006;1(1):15–30. doi: 10.2147/nano.2006.1.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ma PX, Zhang R. Synthetic nano-scale fibrous extracellular matrix. J. Biomed. Mater. Res. 1999;46(1):60–72. doi: 10.1002/(sici)1097-4636(199907)46:1<60::aid-jbm7>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 39.Chen H, Fan X, Xia J, et al. Electrospun chitosan-graft-poly (ε-caprolactone)/poly (ε-caprolactone) nanofibrous scaffolds for retinal tissue engineering. Int. J. Nanomedicine. 2011;6:453–461. doi: 10.2147/IJN.S17057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cai S, Smith ME, Redenti SM, Wnek GE, Young MJ. Mouse retinal progenitor cell dynamics on electrospun poly(ε-caprolactone) J. Biomater. Sci. Polymer. Ed. 2011 doi: 10.1163/092050611X584388. (Epub ahead of print). • Describes the process of electrospinning as well as showing a diagram of the setup to produce scaffolds through this method. Using these scaffolds, the authors demonstrated migration into both control and glial scarred retinas.
- 41.Zhang Y, Klassen HJ, Tucker BA, Perez MT, Young MJ. CNS progenitor cells promote a permissive environment for neurite outgrowth via a matrix metalloproteinase-2-dependent mechanism. J. Neurosci. 2007;27(17):4499–4506. doi: 10.1523/JNEUROSCI.0200-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tucker BA, Redenti SM, Jiang C, et al. The use of progenitor cell/biodegradable MMP2–PLGA polymer constructs to enhance cellular integration and retinal repopulation. Biomaterials. 2010;31(1):9–19. doi: 10.1016/j.biomaterials.2009.09.015. • Describes how the controlled release of the enzyme MMP2 degrades neurocan and CD44, two extracellular matrix proteins associated with glial scarring, a major barrier to cellular infiltration and regeneration. This method demonstrated a significant increase in migration of RPCs into a glial scarred model retina.
- 43.Redenti S, Tao S, Yang J, et al. Retinal tissue engineering using mouse retinal progenitor cells and a novel biodegradable, thin-film poly(e-caprolactone) nanowire scaffold. J. Ocul. Biol. Dis. Infor. 2008;1(1):19–29. doi: 10.1007/s12177-008-9005-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bakshi A, Fisher O, Dagci T, Himes BT, Fischer I, Lowman A. Mechanically engineered hydrogel scaffolds for axonal growth and angiogenesis after transplantation in spinal cord injury. J. Neurosurg. Spine. 2004;1(3):322–329. doi: 10.3171/spi.2004.1.3.0322. [DOI] [PubMed] [Google Scholar]
- 45.Royce Hynes S, McGregor LM, Ford Rauch M, Lavik EB. Photopolymerized poly(ethylene glycol)/poly(l-lysine) hydrogels for the delivery of neural progenitor cells. J. Biomater. Sci. Polym. Ed. 2007;18(8):1017–1030. doi: 10.1163/156856207781494368. [DOI] [PubMed] [Google Scholar]
- 46.Bellamkonda R, Ranieri JP, Bouche N, Aebischer P. Hydrogel-based three-dimensional matrix for neural cells. J. Biomed. Mater. Res. 1995;29(5):663–671. doi: 10.1002/jbm.820290514. [DOI] [PubMed] [Google Scholar]
- 47.Hynes SR, Rauch MF, Bertram JP, Lavik EB. A library of tunable poly(ethylene glycol)/poly(L-lysine) hydrogels to investigate the material cues that influence neural stem cell differentiation. J. Biomed. Mater. Res. A. 2009;89(2):499–509. doi: 10.1002/jbm.a.31987. [DOI] [PubMed] [Google Scholar]
- 48.Bryant SJ, Nuttelman CR, Anseth KS. Cytocompatibility of UV and visible light photoinitiating systems on cultured NIH/3T3 fibroblasts in vitro. J. Biomater. Sci. Polym. Ed. 2000;11(5):439–457. doi: 10.1163/156856200743805. [DOI] [PubMed] [Google Scholar]
- 49.Gupta D, Tator CH, Shoichet MS. Fast-gelling injectable blend of hyaluronan and methylcellulose for intrathecal, localized delivery to the injured spinal cord. Biomaterials. 2006;27(11):2370–2379. doi: 10.1016/j.biomaterials.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 50. Ballios BG, Cooke MJ, van der Kooy D, Shoichet MS. A hydrogel-based stem cell delivery system to treat retinal degenerative diseases. Biomaterials. 2010;31(9):2555–2564. doi: 10.1016/j.biomaterials.2009.12.004. • Implanted RPCs into the subretinal space using a self-aggregating hydrogel that showed the formation of a monolayer covering Bruch’s membrane and integrating into the retinal pigment epithelium.
- 51.Fitzpatrick SD, Jafar Mazumder MA, Lasowski F, Fitzpatrick LE, Sheardown H. PNIPAAm-grafted-collagen as an injectable, in situ gelling, bioactive cell delivery scaffold. Biomacromolecules. 2010;11(9):2261–2267. doi: 10.1021/bm100299j. [DOI] [PubMed] [Google Scholar]
- 52.Nägler K, Mauch DH, Pfrieger FW. Glia-derived signals induce synapse formation in neurones of the rat central nervous system. J. Physiol. (Lond.) 2001;533(Pt 3):665–679. doi: 10.1111/j.1469-7793.2001.00665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barker AJ, Koch SM, Reed J, Barres BA, Ullian EM. Developmental control of synaptic receptivity. J. Neurosci. 2008;28(33):8150–8160. doi: 10.1523/JNEUROSCI.1744-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kucukdereli H, Allen NJ, Lee AT, et al. Control of excitatory CNS synaptogenesis by astrocyte-secreted proteins Hevin and SPARC. Proc. Natl Acad. Sci. USA. 2011;108(32):E440–E449. doi: 10.1073/pnas.1104977108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kljavin IJ, Reh TA. Mueller cells are a preferred substrate for in vitro neurite extension by rod photoreceptor cells. J. Neurosci. 1991;11(10):2985–2994. doi: 10.1523/JNEUROSCI.11-10-02985.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Johnson TV, Bull ND, Martin KR. Identification of barriers to retinal engraftment of transplanted stem cells. Invest. Ophthalmol. Vis. Sci. 2010;51(2):960–970. doi: 10.1167/iovs.09-3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kinouchi R, Takeda M, Yang L, et al. Robust neural integration from retinal transplants in mice deficient in GFAP and vimentin. Nat. Neurosci. 2003;6(8):863–868. doi: 10.1038/nn1088. [DOI] [PubMed] [Google Scholar]
- 58.Profyris C, Cheema SS, Zang D, Azari MF, Boyle K, Petratos S. Degenerative and regenerative mechanisms governing spinal cord injury. Neurobiol. Dis. 2004;15(3):415–436. doi: 10.1016/j.nbd.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 59.Bringmann A, Iandiev I, Pannicke T, et al. Cellular signaling and factors involved in Müller cell gliosis: neuroprotective and detrimental effects. Prog. Retin. Eye Res. 2009;28(6):423–451. doi: 10.1016/j.preteyeres.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 60.Hirami Y, Osakada F, Takahashi K, et al. Generation of retinal cells from mouse and human induced pluripotent stem cells. Neurosci. Lett. 2009;458(3):126–131. doi: 10.1016/j.neulet.2009.04.035. [DOI] [PubMed] [Google Scholar]
- 61.Kelley MW, Turner JK, Reh TA. Retinoic acid promotes differentiation of photoreceptors in vitro. Development. 1994;120(8):2091–2102. doi: 10.1242/dev.120.8.2091. [DOI] [PubMed] [Google Scholar]
- 62.Jiang X, Cao HQ, Shi LY, Ng SY, Stanton LW, Chew SY. Nanofiber topography and sustained biochemical signaling enhance human mesenchymal stem cell neural commitment. Acta Biomater. 2012;8(3):1290–1302. doi: 10.1016/j.actbio.2011.11.019. [DOI] [PubMed] [Google Scholar]
- 63.Arnhold S, Klein H, Semkova I, Addicks K, Schraermeyer U. Neurally selected embryonic stem cells induce tumor formation after long-term survival following engraftment into the subretinal space. Invest. Ophthalmol. Vis. Sci. 2004;45(12):4251–4255. doi: 10.1167/iovs.03-1108. [DOI] [PubMed] [Google Scholar]
- 64.Ghosh F, Neeley WL, Arnér K, Langer R. Selective removal of photoreceptor cells in vivo using the biodegradable elastomer poly(glycerol sebacate) Tissue Eng. Part A. 2011;17(13–14):1675–1682. doi: 10.1089/ten.tea.2008.0450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bandyopadhyay M, Rohrer B. Photoreceptor structure and function is maintained in organotypic cultures of mouse retinas. Mol. Vis. 2010;16:1178–1185. [PMC free article] [PubMed] [Google Scholar]
- 66.Jacobson SG, Aleman TS, Cideciyan AV, et al. Human cone photoreceptor dependence on RPE65 isomerase. Proc. Natl Acad. Sci. USA. 2007;104(38):15123–15128. doi: 10.1073/pnas.0706367104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Park KK, Liu K, Hu Y, et al. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science. 2008;322(5903):963–966. doi: 10.1126/science.1161566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Park KK, Liu K, Hu Y, Kanter JL, He Z. PTEN/mTOR and axon regeneration. Exp. Neurol. 2010;223(1):45–50. doi: 10.1016/j.expneurol.2009.12.032. [DOI] [PubMed] [Google Scholar]
- 69.Sun F, Park KK, Belin S, et al. Sustained axon regeneration induced by co-deletion of PTEN and SOCS3. Nature. 2011;480(7377):372–375. doi: 10.1038/nature10594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hill AJ, Zwart I, Tam HH, et al. Human umbilical cord blood-derived mesenchymal stem cells do not differentiate into neural cell types or integrate into the retina after intravitreal grafting in neonatal rats. Stem Cells Dev. 2009;18(3):399–409. doi: 10.1089/scd.2008.0084. [DOI] [PubMed] [Google Scholar]
- 71.Hara A, Taguchi A, Aoki H, et al. Folate antagonist, methotrexate induces neuronal differentiation of human embryonic stem cells transplanted into nude mouse retina. Neurosci. Lett. 2010;477(3):138–143. doi: 10.1016/j.neulet.2010.04.050. [DOI] [PubMed] [Google Scholar]
- 72.Aoki H, Hara A, Niwa M, Motohashi T, Suzuki T, Kunisada T. Transplantation of cells from eye-like structures differentiated from embryonic stem cells in vitro and in vivo regeneration of retinal ganglion-like cells. Graefes Arch. Clin. Exp. Ophthalmol. 2008;246(2):255–265. doi: 10.1007/s00417-007-0710-6. [DOI] [PubMed] [Google Scholar]
- 73.Jones D. Pharmaceutical applications of polymers for drug delivery. Rapra Rev. Rep. 2004;15(6):3–40. [Google Scholar]
- 74.Wang Y, Ameer GA, Sheppard BJ, Langer R. A tough biodegradable elastomer. Nat. Biotechnol. 2002;20(6):602–606. doi: 10.1038/nbt0602-602. [DOI] [PubMed] [Google Scholar]
- 75.Mark JE, editor. Polymer Data Handbook. New York, NY, USA: Oxford University Press Inc.; 1999. p. 656. [Google Scholar]
- 76.Schiffman JD, Schauer CL. Cross-linking chitosan nanofibers. Biomacromolecules. 2007;8(2):594–601. doi: 10.1021/bm060804s. [DOI] [PubMed] [Google Scholar]