Abstract
Background:
Tremor is an important cause of disability in patients with multiple sclerosis (MS). It is an ongoing debate as to which brain region should be targeted in MS patients with complex tremors.
Case Description:
Here, we describe our experience with targeting thalamic tremor cells in the ventro-intermediate/ventro-oralis posterior (Vim/Vop) region in a patient with MS related complex tremor. Intraoperative multiple-microelectrode recordings showed the existence of tremor cells. Test stimulation produced the best effect when performed at the regions where tremor cells were recorded. Postoperative examination revealed a substantial improvement of the tremor.
Conclusion:
Our case observation reveals the existence of a neurophysiological target for deep brain stimulation (DBS) in MS related tremor.
Keywords: Deep brain stimulation, multiple sclerosis, nucleus ventro-oralis posterior, thalamus, tremor cell, ventro-intermediate nucleus
INTRODUCTION
Tremor is an important cause of disability, affecting 25-58% of patients with multiple sclerosis (MS).[1,13] MS induced tremors can be present in rest, in action, or increase during an intended movement. Often it is a complex tremor, meaning that a combination of tremor types coexist in one patient. The first treatment option is pharmacotherapy. However, these tremors do not respond adequately to drug therapy. In these patients, deep brain stimulation (DBS) of the thalamus has been proposed as a potential therapy.[1,15] It is an ongoing debate as to which brain region should be targeted in MS patients with complex tremors. The most relevant options seem to be the ventro-intermediate (Vim) nucleus of the thalamus,[6,14] the ventro-oralis posterior (Vop) nucleus of the thalamus,[3] the ventro-oralis anterior of the thalamus, or the zona incerta.[9,15] It should also be noted that there is some discrepancy among the terminology used for thalamic nuclei.[7] Here, we describe our experience with targeting thalamic tremor cells in the Vim/Vop region in one patient with MS related complex tremor. Tremor cells are cells firing at the same frequency as the tremor and can be found in the thalamic Vim/Vop nuclei and in the subthalamic nucleus (STN).[4,11]
CASE REPORT
The patient was a 37-year-old right handed woman who developed lower extremity weakness at the age of 26. Brain magnetic resonance imaging (MRI) showed periventricular demyelinating lesions. Cerebrospinal fluid (CSF) examination revealed oligoclonal band positivity leading to the diagnosis of MS according to Mc Donald's criteria.[10] Despite immunomodulatory therapy, the patient suffered from relapsing and remitting episodes of pyramidal, sensory, and autonomous symptoms. In the past 5 years, she developed severe tremors in both upper extremities, more pronounced at the right side. This was substantially limiting her daily living activities. The patient's Expanded Disability Status Scale (EDSS) associated to tremor and ataxia increased up to 7.[8] After an unsuccessful course of different drug treatments including high doses of propranolol, primidon, isoniazide, and pramipexole, patient was referred to our clinic for DBS.
Upon examination, we found 7-10 Hz resting, action, and intention tremors, more pronounced at the right side. Furthermore, the patient suffered from mild postural-and truncal ataxia, but was still able to walk with bilateral support. In addition, the patient showed slight restriction of the horizontal movements in the left eye, and there were few beats of nystagmus in both eyes at horizontal sight. In addition, her speech showed mild dysarthria. Muscular strength was intact in four extremities.
More specific examination revealed that she was also unable to perform the 9-hole peg test with her right hand and scored 45.61 seconds with the left hand.[2] The total score of the Fahn Tolosa Marin tremor scale (FTMTS) was 72.[13] The patient failed the trail making test with the upper right extremity, and had very hard time performing the test with her left hand.
MRI of the brain revealed lesions that were iso or hypointense on T1-weighted images and hyperintense on T2-weighted and fluid attenuated inversion recovery (FLAIR) images in the right cerebellum, both middle cerebellar peduncles, periventricular region, in both centrum semiovale and juxtacortical localizations representing demyelinating lesions. Cervical MRI also revealed hyperintense demyelinating lesions on T2-weighted images at the level of C3-4.
Surgery
A preoperative MRI was performed consisting of a double-dose contrast enhanced T1-weighted and T2-weighted 1 mm images. On the day of surgery, the stereotactic frame, Leksell G (Elekta Inc., Atlanta GA, USA) was mounted, while the patient received a local anesthetic. A stereotactic computed tomography (CT) was obtained with a slice thickness of 1 mm. We decided to perform 5 microelectrode-guided electrophysiology in the anatomically defined Vim/Vop region on fused CT/MRI-images (Framelink, Medtronic Inc. Minneapolis, MN, USA) with the following coordinates for the central electrode: 12 mm lateral to the AC–PC line, 1 mm posterior to the mid-commissural point, and 3 mm superior to the intercommissural line. Special attention was paid to the trajectory planning to avoid blood vessels during the insertion of the electrodes. After making a precoronally located burr hole, 5 microelectrodes (Leadpoint®, Medtronic) were introduced, 10 mm above the presumed target for electrophysiological recordings. Recordings were performed in steps of 0.5 mm till 7 mm below target. We found typical thalamic cells in all 5 electrode trajectories. Tremor cells were only found in the central trajectories at both sides. Tremor cells showed the same frequency as the tremor-induced potentials on the electromyogram (EMG) recorded from the upper extremities and did not show kineto-kinesthetic responses upon passive flexion/extension movements of the hands [Figure 1a]. These cells were found at 1.5 and 1 mm above the presumed target at the right side and at 0.5 and 1 mm below the presumed target at the left side. We performed macrostimulation in all trajectories and assessed the effect on different types of tremor. While we found some therapeutic effects in the medial, lateral, posterior, and anterior trajectories, examination showed the best effects at the central trajectories at the level where we recorded tremor cells. We decided to implant the final electrodes (model 3389, Medtronic) in these trajectories with contact 1 at this anatomical point. The position of the final electrodes was verified with fluoroscopy. The electrodes were finally fixed in the burr hole with methyl metacrylate and connected to an extension cable, which was externalized at a distance of about 7 cm from the burr hole, and connected to an external pulse generator (Model 3625, Medtronic). On the second postoperative day, the patient underwent MRI to evaluate the relative position of the electrodes and to detect (a) symptomatic bleedings or other complications [Figure 1b]. Two days later, the second operation was performed under general anesthesia, to implant the pulse generator infraclavicularly (Activa PC, Medtronic).
Figure 1.
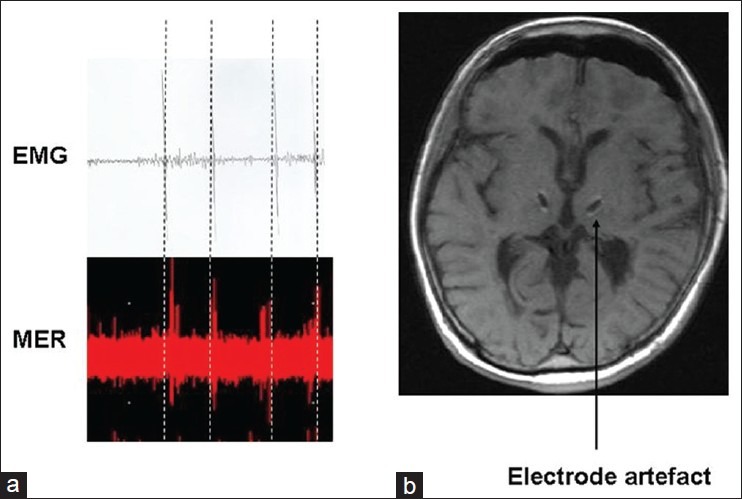
(a) Illustrates a representative microelectrode recording of a single thalamic cell in the Vim/Vop region, firing at the same frequency as the tremor recorded with the arm-EMG, (b)The bilateral electrode artefacts can be seen in the Vim/Vop region
Clinical outcome
The patient underwent programming of the stimulation parameters in the next days after surgery. The most optimal stimulation parameters were 3.2 V for the left and 2.6 V for the right side. The contact combinations were bipolar for the right side with contact 1 negative and contact 2 positive, and monopolar for the left side, with contacts 4 and 5 negative, and the case positive. The stimulation frequency was 130 Hz and the pulse width was 90 μs for both sides. No side effects of stimulation were noted.
After surgery the patient was able to eat and drink independently, which she could not perform preoperatively, and the tremor associated to posture in both upper extremities was substantially reduced. She was now able to stand without support and walk with unilateral support.
Sixth months later, the patient's EDSS was reduced from 7 to 6. The patient's 25-foot test score improved from 31.18 seconds with bilateral support to 20.2 seconds with unilateral support. Her performance with the 9-hole peg test improved substantially, scoring 127 seconds with her right and 23 seconds with her left hand in the. The FTMTS improved by 51%.
CONCLUSION
We performed thalamic DBS in a patient with MS-related tremor. The therapeutic effect was substantial and is in line with previous publications.[1,6,14,15] The most significant observation is probably the existence of tremor cells in the Vim/Vop area in a patient with MS. Tremor cells are usually found in patients with essential tremor or Parkinsonian tremor, again in the thalamic nuclei and also in the STN. Apparently, tremor, regardless the etiology (e.g., Parkinson's disease, MS), is linked to the presence of tremor cells, and this makes it more convenient for interventional purposes.[5,12] In this line, we believe that the existence of a neurophysiological target is helpful in extraordinary cases as described here.
ACKNOWLEDGMENTS
This study is part of the intensive collaboration between the departments of Neurosurgery of Maastricht University Medical Center (Netherlands) and Ondokuz Mayis University (Turkey). YT is a visiting professor at Ondokuz Mayis University and EK is a visiting scientist at Maastricht University.
Footnotes
Available FREE in open access from: http://www.surgicalneurologyint.com/text.asp?2013/4/1/31/109464
Contributor Information
Ersoy Kocabicak, Email: ekocabicak@yahoo.com.
Murat Terzi, Email: mterzi@omu.edu.tr.
Onur Alptekin, Email: onur.alptekin@medtronic.com.
Yasin Temel, Email: y.temel@maastrichtuniversity.nl.
REFERENCES
- 1.Bittar RG, Hyam J, Nandi D, Wang S, Liu X, Joint C, et al. Thalamotomy versus thalamic stimulation for multiple sclerosis tremor. J Clin Neurosci. 2005;12:638–42. doi: 10.1016/j.jocn.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 2.Bosma LV, Kragt JJ, Knol DL, Polman CH, Uitdehaag BM. Clinical scales in progressive MS: Predicting long-term disability. Mult Scler. 2012;18:345–50. doi: 10.1177/1352458511419880. [DOI] [PubMed] [Google Scholar]
- 3.Foote KD, Seignourel P, Fernandez HH, Romrell J, Whidden E, Jacobson C, et al. Dual electrode thalamic deep brain stimulation for the treatment of posttraumatic and multiple sclerosis tremor. Neurosurgery. 2006;58:280–5. doi: 10.1227/01.NEU.0000192692.95455.FD. [DOI] [PubMed] [Google Scholar]
- 4.Gross RE, Krack P, Rodriguez-Oroz MC, Rezai AR, Benabid AL. Electrophysiological mapping for the implantation of deep brain stimulators for Parkinson's disease and tremor. Mov Disord. 2006;21(Suppl 14):S259–83. doi: 10.1002/mds.20960. [DOI] [PubMed] [Google Scholar]
- 5.Hamel W, Herzog J, Kopper F, Pinsker M, Weinert D, Muller D, et al. Deep brain stimulation in the subthalamic area is more effective than nucleus ventralis intermedius stimulation for bilateral intention tremor. Acta Neurochir (Wien) 2007;149:749–58. doi: 10.1007/s00701-007-1230-1. [DOI] [PubMed] [Google Scholar]
- 6.Hosseini H, Mandat T, Waubant E, Agid Y, Lubetzki C, Lyon-Caen O, et al. Unilateral thalamic deep brain stimulation for disabling kinetic tremor in multiple sclerosis. Neurosurgery. 2012;70:66–9. doi: 10.1227/NEU.0b013e31822da55c. [DOI] [PubMed] [Google Scholar]
- 7.Krack P, Dostrovsky J, Ilinsky I, Kultas-Ilinsky K, Lenz F, Lozano A, et al. Surgery of the motor thalamus: Problems with the present nomenclatures. Mov Disord. 2002;17(Suppl 3):S2–8. doi: 10.1002/mds.10136. [DOI] [PubMed] [Google Scholar]
- 8.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS) Neurology. 1983;33:1444–52. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 9.Nandi D, Chir M, Liu X, Bain P, Parkin S, Joint C, et al. Electrophysiological confirmation of the zona incerta as a target for surgical treatment of disabling involuntary arm movements in multiple sclerosis: Use of local field potentials. J Clin Neurosci. 2002;9:64–8. doi: 10.1054/jocn.2001.1012. [DOI] [PubMed] [Google Scholar]
- 10.Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69:292–302. doi: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reck C, Himmel M, Florin E, Maarouf M, Sturm V, Wojtecki L, et al. Coherence analysis of local field potentials in the subthalamic nucleus: Differences in parkinsonian rest and postural tremor. Eur J Neurosci. 2010;32:1202–14. doi: 10.1111/j.1460-9568.2010.07362.x. [DOI] [PubMed] [Google Scholar]
- 12.Sandvik U, Lars-Owe K, Anders L, Patric B. Thalamic and Subthalamic DBS for Essential Tremor: WhereIs the Optimal Target? Neurosurgery. 2011 [In Press] [Google Scholar]
- 13.Stacy MA, Elble RJ, Ondo WG, Wu SC, Hulihan J. Assessment of interrater and intrarater reliability of the Fahn-Tolosa-Marin Tremor Rating Scale in essential tremor. Mov Disord. 2007;22:833–8. doi: 10.1002/mds.21412. [DOI] [PubMed] [Google Scholar]
- 14.Torres CV, Moro E, Lopez-Rios AL, Hodaie M, Chen R, Laxton AW, et al. Deep brain stimulation of the ventral intermediate nucleus of the thalamus for tremor in patients with multiple sclerosis. Neurosurgery. 2010;67:646–51. doi: 10.1227/01.NEU.0000375506.18902.3E. [DOI] [PubMed] [Google Scholar]
- 15.Yap L, Kouyialis A, Varma TR. Stereotactic neurosurgery for disabling tremor in multiple sclerosis: Thalamotomy or deep brain stimulation? Br J Neurosurg. 2007;21:349–54. doi: 10.1080/02688690701544002. [DOI] [PubMed] [Google Scholar]


