Abstract
Purpose
There is strong evidence supporting the link between nonadherence to antipsychotic medication and relapse of schizophrenia. However, less obvious are the economic consequences of nonadherence. The systematic review reported here evaluated the economic aspects of nonadherence to antipsychotic medication.
Methods
A systematic review of scientific papers in the PubMed MEDLINE, Embase, PsychINFO, BIOSIS, and Evidence-Based Medicine Reviews databases was undertaken. Studies that measured adherence to antipsychotic medication and that provided comparative information on health care costs were included.
Results
Eight studies met the inclusion criteria. All were observational. Despite the differences between the studies in terms of design, adherence measures, and cost components analyzed, the results of this systematic review indicate that nonadherence to antipsychotic medication is associated with increased hospitalization rates and resource utilization, resulting in increased direct health care costs.
Conclusion
Nonadherence to antipsychotic medication results in poor health and economic outcomes; therefore, the authors suggest endorsing interventions aimed at improving adherence because they can improve patient health without substantially increasing costs.
Keywords: adherence, costs, observational study, hospitalization rates, resource utilization
Introduction
According to the widely accepted World Health Organization general definition, “therapeutic adherence” is “the extent to which a person’s behavior corresponds with agreed recommendations from a health care provider.”1 Adherence to antipsychotic medication is essential to improve outcomes in most patients with schizophrenia.2 However, nonadherence to long-term therapy is common, and it is particularly problematic in this condition.3,4 A variety of factors can affect adherence in schizophrenia. Authors have categorized such factors in several ways, but commonly arrange them in terms of their relation to the patient, his/her relationship with health care providers and caregivers, the medication, the environment, and some features of the service delivery system.3,4 Difficulties arise when measuring adherence. Practicing psychiatrists frequently have trouble acquiring an accurate measure of their patients’ adherence level.5 Several methods have been used to this end, including both subjective (patient self-, caregiver, or physician report) and objective (pill counts, pharmacy records, electronic monitoring, or plasma concentrations). However, all are proxy measures of adherence, and none is devoid of significant limitations.6,7
Antipsychotic nonadherence is more than a conceptual problem. It has a very negative impact on patients and society as a whole.8 There is strong epidemiologic evidence that indicates nonadherence significantly increases the risk of relapse9–12 and is associated with impaired functional outcomes.13 Also, adherence issues make it particularly difficult for the psychiatrist to assess treatment response and make appropriate adjustments to therapy,5 not to mention the long-term consequences it may have by precluding patients from benefiting from prolonged periods of symptomatic remission.14,15
Schizophrenia is a devastating mental illness that carries a significant economic burden.16,17 Although many studies have addressed the clinical consequences of nonadherence in schizophrenia, utilization of health care resources and costs have received little attention. Nonadherence to antipsychotic medication also has a relevant socioeconomic facet, because it is likely to result in an increase in the frequency of relapse, more severe symptoms, and longer hospital stays, which may lead to increased utilization of health care resources18 and costs.19,20 In particular, relapse has been consistently noted to be an important predictor of subsequent relapse and treatment costs.21 Although relapse has been commonly associated with elevated costs of inpatient treatment, other cost components are also higher in patients who relapse than in those who do not.21
The current environment, dominated by attempts to curtail health care cost escalation, demands information about the economic consequences of nonadherence to antipsychotic medication.22 The few reviews available on this subject have focused almost entirely on hospitalization costs.19,20,23 As such, the purpose of the systematic review reported here was to gather evidence on the economic impact of patients with schizophrenia’s nonadherence to antipsychotic medication, focusing on studies that included the analysis of nonadherence and costs among their primary objectives. In addition to hospitalization costs, other cost components were also considered in this review.
Methods
Objectives and procedures
The objective of this review was to evaluate the economic consequences of nonadherence to antipsychotic medication in patients with schizophrenia in terms of resource utilization and health care costs. Studies that provided measures of adherence to antipsychotic medication of patients with schizophrenia, health care resource utilization, and costs were gathered to address this objective. This report was developed in accordance with the framework proposed in the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement.24
Eligibility criteria
Original research published in peer-reviewed journals, in English or Spanish, was checked. The review included studies that met all of the following four criteria: (1) the involvement of patients with declared diagnoses of schizophrenia or schizoaffective disorders, (2) the use of original data as opposed to data from previous publications (reviews were excluded), (3) the provision of direct or indirect measures of adherence, and (4) the provision of comparative information on costs between adherent and nonadherent patients with schizophrenia or schizoaffective disorders. The review excluded studies that provided comparative economic outcomes based on simulation modeling and not on real world data. We used these eligibility criteria to obtain empirical and explicit information on adherence measurements and health care costs simultaneously from the same studies.
Literature search and study selection
Relevant reports were identified through thorough literature searches of computerized databases including PubMed MEDLINE, Embase, PsychINFO, and BIOSIS (1990 to January 2012) as well as EBMR (2005 to 2011). Key search terms included “schizophrenia,” “nonadherence”/“non-adherence,” “noncompliance”/“non-compliance,” “cost,” “pharmacoeconomic,” “resource,” “economic,” “depot,” and “long-acting.” Several combinations of these keywords joined by the Boolean operators “and” or “or” were used in searches. In addition, the bibliographies of selected articles were reviewed to identify further reports. Previous reviews were not included, but their bibliographies were checked to ensure relevant reports were not overlooked.
Data collection
A structured form was prepared to gather information on the objectives, methodology, parameters and outcomes evaluated, and results of each study. Studies were coded by author and year of publication, objectives, design, total number of patients evaluated, and outcomes. The design of observational studies was classified as either cross-sectional, cohort (retrospective or prospective), or case-control. Mirror-image designs were frequent and were categorized as such. The quality of the studies was graded according to the STROBE Statement. The STROBE checklist was fulfilled for each of the reports included in the review (all studies were observational), and the number of items met was provided as an objective measure of reporting study quality.
Data synthesis and reporting
A descriptive summary of the results obtained in the studies is provided. Formal meta-analyses were not performed for several reasons; the paucity and heterogeneity of quantitative measures of nonadherence and cost estimates between studies particularly stand out.
Results
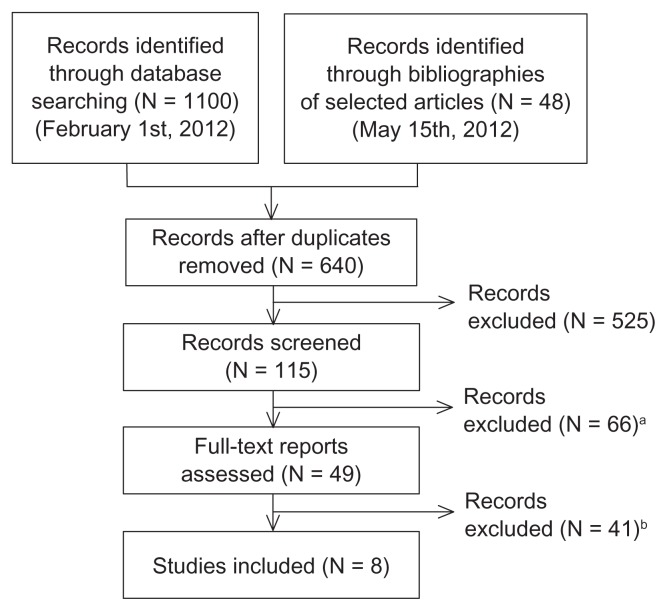
A total of 1148 records were initially retrieved (1100 from the database search and 48 through the bibliographies of selected studies). After discarding duplicates, 640 reports remained, 525 of which were excluded because of noncompliance with the selection criteria after reviewing the title or the abstract. Screening of the remaining 115 reports, either by reviewing the abstract or the full text, led to elimination of 66 more because of the reasons provided in Figure 1. Finally, 41 full-text reports were excluded (Figure 1), leaving eight studies, published between 2000 and 2011, that were included in the review.
Figure 1.
Flow diagram of the different phases of information retrieval.
Notes:aReasons for exclusion: missing/incomplete information on costs (N = 45), adherence (N = 11), or both (N = 9) (inclusion criteria 3 and 4); duplicated publication (N = 1) (inclusion criterion 2); breasons for exclusion: comparative cost information between adherent and nonadherent patients not provided (N = 19) (inclusion criterion 4); cost-effectiveness or based on simulated data (N = 16) (exclusion criterion); review of other studies (N = 3), duplicated publication (N = 2) (inclusion criterion 2); cost information not segregated for patients with schizophrenia (N = 1).
Among the selected reports, five were case-series studies, two were mirror-image studies, and one was a cross-sectional study. All of them used data collated retrospectively from various databases; none provided estimations of indirect costs. The studies included in the review met from 12 to 19 of the 22 items of the STROBE checklist. The retrospective medical record reviews featured large sample sizes, ranging from 619 to 35,815 patients. The sample sizes of the two mirror-image studies were of 147 and 443 patients. Six studies were performed in USA, one in the UK, and one in New Zealand. Table 1 provides an overview of the objectives, design, methods, and outcomes on a per-study basis.
Table 1.
Overview of the studies selected for this review (those that provide empirical and explicit information on adherence measurements and health care costs), ordered by date of publication
| Study | Objective/s | Design | Methods/outcome measures | Results and conclusions |
|---|---|---|---|---|
| MarketScan database USA (Peng et al)25 | Determine change in hospitalization risk from 6 months pre- to 6 months post-initiation on any LAI antipsychotic. Determine changes in the utilization of outpatient services, patients’ adherence level, and total direct costs and cost components |
Mirror-image study, with evaluation of treatment periods lasting 12 months (6 months before and 6 months after starting treatment with LAI antipsychotics); information retrieved from a claims database | “Adherence” to antipsychotic medication, defined through the MPR; number of psychiatric hospital admissions; utilization of outpatient services (emergency room, day treatment and other outpatient visits); and total direct health care costs and selected cost components | N = 147; significant increase from 36.8% to 60.3% in MPR and decrease from 49.7% to 22.4% in the proportion of patients hospitalized for any psychiatric reason and the mean number of hospitalizations for schizophrenia from 0.53 to 0.29 after LAI initiation; decrease in mean (median) total direct costs from US$11,111.30 (US$7089.40) to US$7883.80 (US$4051.93) per patient, driven by reductions in psychiatric hospitalizations. In conclusion, there were declines in hospitalization rates and related costs after initiating LAI therapy |
| LAI risperidone in New Zealand (Carswell et al)26 | Explore costs of patients treated with LAI risperidone in New Zealand | Mirror-image (12 months pre- and 12 months post-initiation of LAI risperidone); data collected retrospectively from medical files, with cases ascertained through consulting an anonymous list of prescription approvals for LAI risperidone | “Nonadherence,” defined as any break in treatment recorded in the medical files during the follow-up period; number of admissions; length of bed stay; treatment data (risperidone LAI and all other antipsychotic medication, total daily dose, and route of administration); hospitalization costs associated with the use of LAI risperidone (cost per admission and per hospital day) | N = 443; decrease (from pre- to post-LAI risperidone period) in hospitalization costs by approximately NZ$1.7 million (when computed as cost per admission), increase in hospitalization costs by approximately NZ$3.5 million (when computed as cost per day spent in hospital); lower mean number of admissions (1.38 vs 0.61) but greater mean length of bed stay (37.2 vs 53.3 days); patients who remained on LAI risperidone 12 months after initiation had fewer admissions and smaller increases in days in hospital (increase from 38.4 to 41.8 days) than patients who discontinued LAI risperidone use in the first year (increase from 38.4 to 69.5 days). In conclusion, longer admissions were driven by those who discontinued treatment within 12 months and improved resource and cost outcomes are associated with continuation |
| California Medicaid (Medi-Cal) adapted to the US Medicaid (Marcus and Olfson)27 | Estimate the fraction of hospital admission and hospital days attributable to gaps in antipsychotic medication in California, in patients covered by Medi-Cal, derive the US national number and cost of acute care impatient admissions that are attributable to gaps in antipsychotic medication | Retrospectively collated information from the databases of two surveys and Medicaid prescription claims of patients treated with oral antipsychotics | “Nonadherence,” defined as gaps in antipsychotic coverage calculated from prescription claims; rate of inpatient admission per 100,000 eligible person-days; number of hospital admissions and inpatient days attributable to medication nonadherence; number of Medicaid-reimbursed acute care hospital admissions for schizophrenia; national number of inpatient days attributable to medication nonadherence; daily costs of inpatient hospitalization, taken from a survey of Medicare unit costs | N = 35,815, accounting for 1208 inpatient admissions, with 4.44 inpatient schizophrenia admissions per 100,000 person-days; 36.6% of inpatient admissions occurred within medication gaps, which were associated independently with a significant increase in the odds of admission (OR = 1.49); 12.3% (with 15-day gaps) and 9.5% (30-day gaps) of acute care inpatient admissions attributable to not receiving antipsychotic medication; the calculated national cost savings of these fractions was US$106 million per year. In conclusion, gaps in antipsychotic medication treatment appear to significantly contribute to the national costs of acute care inpatient of schizophrenia patients, thus enhancement of the continuity of antipsychotic treatment has the potential to lead to savings |
| Florida Medicaid recipients (Becker et al)28 | Compare medication adherence, service costs, and other outcomes among persons taking different classes of antipsychotic medication | Retrospectively collated information from a Medicaid prescription claims database of patients treated with antipsychotics and databases of other publicly funded behavioral health services | Four adherence levels, based on the proportion of prescriptions filled: (1) maximal adherence (75%–100% use over the 2-year study), (2) moderate adherence (50%–74.9%), (3) minimal adherence (25%–49.9%), and (4) negligible adherence (<25%); direct cost of health care services, including pharmacy, covered by Medicaid and other publicly funded behavioral health services | N = 10,330; inverse and significant relationship between health care costs and the level of adherence: lower resource utilization, to a mean calculated value of US$729 (if treated with FGA) and US$1189 (if treated with SGA) per patient per month by patients in the maximal adherence level (64% of the sample), compared with US$1102 (patients of FGA) and US$1238 (patients on SGA) by patients in the minimal adherence level (18.8% of the sample), and US$1023 (FGA) and US$1322 (SGA) by patients in the negligible adherence level (4.9% of the sample). In conclusion, treatment adherence is a key factor in the relationship between a physician’s drug treatment plan and a patient outcome; it may be as important to treatment costs and benefits as the class of medication used |
| San Diego Medicaid recipients (Gilmer et al)9 | Assess the level of antipsychotic medication adherence; examine the risk factors associated with nonadherence; examine the relationship of nonadherence to hospitalizations and health care costs | Retrospectively collated information from a claims database of Medicaid patients treated with oral antipsychotics, using patient-years of follow-up as the unit of analysis | “Nonadherence” defined as MPR between 0 and 0.49, partial adherence as MPR between 0.50 and 0.79, adherence between 0.80 and 1.10, and excess filling as MPR > 1.10; the annual cumulative MPR was calculated from prescription claims; number of medical and psychiatric hospitalizations; amount paid by Medicaid for inpatient, outpatient medical and mental health, and acute care | Data available from 2801 person-years; 24% of patients categorized as nonadherent, 16% partially adherent, 19% excess fillers, and 41% adherent; adherence generally increased with age; substance abusers and homeless patients were likely to be nonadherent. Nonadherent patients were 2.5 times more likely, and excess fillers or partially adherent patients 80% more likely, to be hospitalized for psychiatric reasons than adherent patients; mean yearly hospital expenditures of nonadherent patients (US$3413 per patient) were three times higher than the expenditures of adherent patients (US$1025 per patient), as were pharmacy expenditures of adherent patients (US$4463 vs US$1542 in nonadherent patients); cost savings for avoided hospitalizations for those who were adherent only partially offset the higher pharmacy costs associated with adherence. In conclusion, hospital costs were lower and pharmacy costs were higher in patients who were adherent than in those who were nonadherent |
| UK Psychiatric Morbidity Survey (Knapp et al)29 | Gain information on the nature of the relationship between nonadherence and resource use | Data from a cross-sectional survey of adults living in institutions that had been prescribed antipsychotic medication | Patient self-reported nonadherence to antipsychotic medication; utilization of health care resources during the preceding year; inpatient and external health care costs | N = 658; lower self-reported nonadherence among patients resident in hospital (11.2%) compared with among patients in other types of institutions (21.2%); only medication nonadherence appeared to exhibit a consistent association with greater resource use; patients reporting nonadherence were predicted to have an excess of inpatient costs of £2481 and total service use costs of £5231 per patient per year. In conclusion, medication nonadherence consistently exhibited an association with higher costs; interventions that improve adherence are likely to reduce service use costs |
| Wisconsin Medicaid recipients (Svarstad et al)30 | Assess the relationship between nonadherence, hospitalization, and costs among severely mentally ill patients | Retrospectively collated information from a database of individual data from patients treated with antipsychotics (oral or LAI), lithium, or antidepressants; medical record audits; and a survey of case managers, as well as evidence of prescription medication coverage and acute care hospital admissions from Medicaid | Irregular use of medication (having one or more year quarters during which no claim was made for an oral medication or two or more quarters during which no claim for a LAI medication was made) used as a proxy for nonadherence; hospitalization for psychiatric problems, total number of inpatient days, and actual hospital expenditures | N = 619; 424 with a diagnosis of schizophrenia or schizoaffective disorder, 96% treated with antipsychotics; 31% considered as nonadherent; greater proportion of nonadherent patients (33%) than adherent patients (18%) hospitalized during the study year; greater mean number of hospital days and hospital expenditures in nonadherent patients (13.9 days, US$3421 per year per patient) than in adherent patients (3.6 days, US$1799 per year per patient); nonadherent patients were two times (OR = 1.99) more likely to be hospitalized than adherent patients. In conclusion, nonadherence is a strong predictor of hospital outcomes |
| Impact of patterns of antipsychotic drug use in the Medi-Cal system (McCombs et al)31 | Evaluate whether suboptimal antipsychotic drug use patterns have an impact on direct health care costs | Retrospectively collated information from a claims database of Medicaid (averaged with Medicare data, for patients with dual eligibility) of outpatients with schizophrenia, with a minimum of 2 years of follow-up data available | “Nonadherence” defined as suboptimal use of antipsychotic medication in the form of delays in therapy, changes in therapy, or interrupted therapy; hospital, nursing, or care facility days, and other services provided by mental health providers; average per day cost reported by Medi-Cal or Medicare | N = 2476, 83.5% received antipsychotic medication (98.9% by oral route); association between pattern of active antipsychotic drug therapy (with respect to no therapy) and a significant reduction in total direct costs during a 2-year period of US$10,833 per patient, especially for psychiatric hospital care (US$8027 per patient); association between ; delays and changes in drug therapy were associated with increases of total direct health care costs of US$12,285 and US$17,644 per patient, respectively; continuous treatment did not have a significant effect over total direct costs of care. In conclusion, active drug therapy and continuous treatment were associated with a significant reduction in psychiatric hospital costs; continuous therapy was also associated with higher nursing home costs, possibly because of continuous monitoring of adherence, which offset the hospital cost savings |
Abbreviations: FGA, first-generation antipsychotics; LAI, long-acting injectable; Medi-Cal, California Medicaid; MPR, medication possession ratio; OR, odds ratio; SGA, second-generation antipsychotics.
The most recently published study featured a comprehensive evaluation of overall direct health care costs under a mirror-image design.25 The authors compared the adherence, defined in terms of medication possession ratio (the proportion of days the patient is in possession of any antipsychotic during each 180-day observation period) and the overall costs from the 6 months preceding the initiation of a long-acting antipsychotic to the 6 months following. Together with a significant enhancement of the medication possession ratio from 36.8% to 60.0%, the mean overall cost declined significantly from US$11,111.30 to US$7883.80 per patient, mainly driven by the reduction of hospitalization costs.
Another mirror-image evaluation of patients starting long-acting risperidone was performed in New Zealand.26 Compared with the pre long-acting risperidone period, the mean number of admissions for the total study population decreased in the subsequent year (1.38 vs 0.61). However, the mean length of bed stay increased (37.2 vs 53.3 days), as did compulsory treatment use. Different methods were used to estimate hospital costs associated with the use of long-acting risperidone (cost estimates were influenced by the method used). Hospitalization costs decreased by approximately NZ$1.7 million in the post long-acting risperidone period when computed using a cost-per-admission approach, but an increase of NZ$3.5 million was observed when a daily hospitalization cost was applied. The authors analyzed the patients who remained on long-acting risperidone 12 months after initiation (58.3%) separately from those who “discontinued” – defined as any break in which three or more injections were not administered continuously – the drug over a 1-year period after treatment initiation (41.7%). Cost increase was significantly lower in patients who continued than in those who discontinued long-acting risperidone. In fact, the reduction in hospital admission rates between the two treatment periods was significantly greater in the continuation group, and the mean difference in length of hospital stays between the two treatment periods was also significantly lower for continuers (5.4 vs 31.1 days).
Marcus and Olfson27 estimated, by means of econometric analyses of data from California Medicaid files, that improving adherence to eliminate short antipsychotic medication gaps (≤15 days) could lower the number of acute care inpatient admissions (≤30 days) by approximately 12.3% and reduce the number of inpatient treatment days by approximately 13.1%, resulting in annual savings of approximately US$106 million (US$ adjusted to year 2005) in inpatient care costs for the national Medicaid system.27
Becker et al28 evaluated 24-month data from Medicaid and public behavioral service system databases in a single US state. They classified patients into four levels of adherence and compared the monthly average overall direct health care costs. Their analysis revealed a significant, inverse relationship between adherence and health care costs. The cost difference between adherent and nonadherent patients was greater among those treated with first-generation than among those treated with second-generation antipsychotics.
The research by Gilmer et al,9 also among Medicaid beneficiaries in a single US state, confirmed that nonadherent patients pose much higher hospital costs than adherent patients. This was the sole study we reviewed that evaluated several forms of nonadherence defined in terms of the medication possession ratio, including excess filling of medications. Excess fillers, possibly due to actual overuse and loss or theft of medications, posed higher economic burdens than any other patients. This study found drug acquisition costs were higher for those more adherent to their medication and even higher for excess fillers. Moreover, nonadherent patients were 2.5 times more likely to be hospitalized for psychiatric reasons than adherent patients. Overall cost savings for avoided hospitalizations only partially offset the higher drug acquisition costs associated with adherence. Mean yearly costs for adherent patients (US$9505) were US$102 and US$1337 higher than the costs for those who were partially adherent and nonadherent, respectively.
In the UK study, the authors used data from a public health survey to evaluate the adherence, based on self-report, and outcomes of patients living in institutions who received antipsychotic medications (72.0% of those resident in hospitals and 63.3% of those living in other institutions with a diagnosis of schizophrenia).29 In a model that evaluated the influence of various factors on total direct health care costs, nonadherence was associated with an excess of predicted inpatient hospital care and overall health care costs of £2481 and £5231 per patient per year, respectively.
Another study, restricted to Medicaid beneficiaries in a single US state that only evaluated hospitalization costs,30 reported higher hospitalization costs over 1 year for patients who were irregular users of medication (US$3421) than for patients who filled medication claims regularly (US$1799).
Finally, the study by McCombs et al31 featured an evaluation of overall direct health care costs over patients with dual eligibility (Medicaid and Medicare), of whom just 1.1% were being treated with second-generation antipsychotics. The authors evaluated the effects of suboptimal antipsychotic drug use over costs. Compared to the costs associated with other patterns of use, delays in drug therapy were associated with a significant increase in mean total costs of US$12,285 per patient, while continuous treatment with an antipsychotic drug over 2 years (only 3.2% of treated patients) did not have a significant effect on the direct health care costs.
Discussion
Despite the varied study designs, adherence measures, and costs considered in the analyses across studies, the results of the systematic review reported here support that poor adherence to antipsychotic treatment was linked to increased hospitalization rates and resource utilization which resulted in increased direct health care costs.
Hospitalization was found to be less frequent in adherent rather than in nonadherent patients, and related costs were lower. Likewise, nonadherent patients consistently required more treatment interventions for relapses than their adherent counterparts. Based on the review results, adherence was associated with lower costs in six of the eight studies;25–30 and with higher costs in the remaining two.9,31 Reduced hospitalization rates in adherent patients accounted for a reduction in costs for psychiatric inpatient care in three of the eight studies included in this review, which were focused exclusively on hospitalization costs.26,27,30 In one of these studies, longer admissions were driven by treatment discontinuers and treatment continuation was associated with improved resource and cost outcomes compared with treatment discontinuation.26 Three studies found a reduction in overall direct health care costs,25,28,29 conflicting with another two studies that showed an increase.9,31 An increase in total costs was observed since expenditures for drug acquisition or adherence-enhancing treatments were slightly higher than savings accrued due to improved adherence.9,31
To the knowledge of the authors, this is the first review that has comprehensively evaluated the economic consequences of nonadherence, including – insofar as is possible – components of health care costs, without a preference for the costs of hospitalization. Prior evidence supports that poor treatment adherence leading to relapses of schizophrenic symptoms results in high costs of direct health care services.20 Most direct health care costs related to this mental illness are attributable to hospitalization for initial episodes and subsequent relapses.20 In addition to increasing inpatient care costs, repeated relapses may cause patients, family, and caregivers to become increasingly discouraged and pessimistic about the course of illness,9 which may produce secondary consequences of nonadherence: neurological deterioration,32 comorbid illness progression,33 substance use,34 criminal behavior,35 suicide attempts,36 re-hospitalization,23,37 or homelessness.38
A similar study carried out by Sun et al19 reported that all studies reviewed showed antipsychotic nonadherence was associated with an increase in hospitalization rate, hospital days, or hospital costs. As an example of how differences in methodology or cost components analyzed may affect cost outcomes, Sun et al calculated the US national re-hospitalization costs due to antipsychotic nonadherence using the data from two studies included in this review at US$1400 million per year.19 Such costs were much higher than those estimated by Marcus and Olfson (US$106 million),27 but these authors only considered acute inpatient hospital care costs attributable to gaps in antipsychotic medications of up to 30 days. The latter authors did not include other cost components, longer medication gaps, or other patterns of nonadherence in their analysis.
Although improved adherence to antipsychotic medication may not represent a substantial cost reduction in the short term, as some of the studies reviewed might suggest,9,31 investing resources in adherence and reinsertion programs might be more beneficial, from a societal perspective, than investing them in treating preventable relapses.
An important aspect that was not addressed in the studies reviewed relates to the impact of nonadherence on other outcomes, such as employment, quality of life, and, ultimately, the indirect costs associated with schizophrenia. The scarce data available suggest that indirect costs may pose an economic burden comparable to that of direct health care costs. For example, productivity loss may account for more than half of all excess annual costs of patients with schizophrenia.17 The resulting picture of the economic impact of nonadherence to antipsychotic medication in these patients may not ultimately be complete until indirect costs are accounted for as well. Presumably, improved adherence could reduce them considerably and thus lower the global economic burden. To advance in this direction, adherence to antipsychotic medication; use of non-pharmacological therapies; and patient-reported outcomes, such as quality of life, should be routinely included in the clinical research of schizophrenia in the future.
Limitations
When interpreting the results of this review, some limitations should be taken into account. Of note, none of the studies reviewed collected data prospectively. Other limitations concern the aforementioned difficulties inherent in measuring adherence. For example, some studies used patients’ self-reports or physicians’ reports, which tend to overestimate adherence, and others used prescription claims that are only a proxy for adherence.3 Further, the effects of nonadherence should also be evaluated in stable patients on prolonged symptomatic remission who may be engaged in recovery-oriented therapies, and these studies should account, as well, for indirect costs. In addition, most of the data come from the USA and, particularly, from Medicaid services, which may not represent other countries or health service systems. Some studies used a mirror-image design, which has been criticized because of selection bias and artifacts created by variables changing between the control and the test periods.39 Finally, at the review level, publication bias could not be quantified because the data was analyzed qualitatively.
Conclusion
Evidence in the literature supports that nonadherence to antipsychotic medication results in poor health and economic outcomes for patients with schizophrenia. In this systematic review, nonadherence to antipsychotic medication was found to be associated with increased hospitalization rates and resource utilization, which resulted in increased direct health care costs. Investing resources on interventions aimed at improving adherence should be endorsed due to the beneficial effect on patient health without a substantial increase in health care costs. Future studies should evaluate the economic aspects of adherence to antipsychotic medication in stable patients and strive to provide long-term estimations of the indirect costs, in addition to the direct costs, resulting from schizophrenia.
Acknowledgment
The authors acknowledge the contribution made by Jesus Villoria (medical writer, Medicxact, SL) in the preparation of this manuscript.
Footnotes
Disclosure
Tatiana Dilla, Antonio Ciudad, and María Álvarez are full-time employees of Lilly, SA, an affiliate of Eli Lilly and Company. The research reported here was funded by Lilly, SA.
References
- 1.World Health Organization. Adherence to Long-Term Therapies: Evidence for Action. Geneva: World Health Organization; 2003. [Google Scholar]
- 2.Mueser KT, McGurk SR. Schizophrenia. Lancet. 2004;363(9426):2063–2072. doi: 10.1016/S0140-6736(04)16458-1. [DOI] [PubMed] [Google Scholar]
- 3.Lacro JP, Dunn LB, Dolder CR, Leckband SG, Jeste DV. Prevalence of and risk factors for medication nonadherence in patients with schizophrenia: a comprehensive review of recent literature. J Clin Psychiatry. 2002;63(10):892–909. doi: 10.4088/jcp.v63n1007. [DOI] [PubMed] [Google Scholar]
- 4.Weiden PJ. Understanding and addressing adherence issues in schizophrenia: from theory to practice. J Clin Psychiatry. 2007;68( Suppl 14):14–19. [PubMed] [Google Scholar]
- 5.Velligan DI, Wang M, Diamond P, et al. Relationships among subjective and objective measures of adherence to oral antipsychotic medications. Psychiatr Serv. 2007;58(9):1187–1192. doi: 10.1176/ps.2007.58.9.1187. [DOI] [PubMed] [Google Scholar]
- 6.Cramer JA, Rosenheck R. Compliance with medication regimens for mental and physical disorders. Psychiatr Serv. 1998;49(2):196–201. doi: 10.1176/ps.49.2.196. [DOI] [PubMed] [Google Scholar]
- 7.Velligan DI, Lam YW, Glahn DC, et al. Defining and assessing adherence to oral antipsychotics: a review of the literature. Schizophr Bull. 2006;32(4):724–742. doi: 10.1093/schbul/sbj075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leucht S, Heres S. Epidemiology, clinical consequences, and psychosocial treatment of nonadherence in schizophrenia. J Clin Psychiatry. 2006;67( Suppl 5):3–8. [PubMed] [Google Scholar]
- 9.Gilmer TP, Dolder CR, Lacro JP, et al. Adherence to treatment with antipsychotic medication and health care costs among Medicaid beneficiaries with schizophrenia. Am J Psychiatry. 2004;161(4):692–699. doi: 10.1176/appi.ajp.161.4.692. [DOI] [PubMed] [Google Scholar]
- 10.Robinson D, Woerner MG, Alvir JM, et al. Predictors of relapse following response from a first episode of schizophrenia or schizoaffective disorder. Arch Gen Psychiatry. 1999;56(3):241–247. doi: 10.1001/archpsyc.56.3.241. [DOI] [PubMed] [Google Scholar]
- 11.Valenstein M, Copeland LA, Blow FC, et al. Pharmacy data identify poorly adherent patients with schizophrenia at increased risk for admission. Med Care. 2002;40(8):630–639. doi: 10.1097/00005650-200208000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Weiden PJ, Kozma C, Grogg A, Locklear J. Partial compliance and risk of rehospitalization among California Medicaid patients with schizophrenia. Psychiatr Serv. 2004;55(8):886–891. doi: 10.1176/appi.ps.55.8.886. [DOI] [PubMed] [Google Scholar]
- 13.Ascher-Svanum H, Faries DE, Zhu B, Ernst FR, Swartz MS, Swanson JW. Medication adherence and long-term functional outcomes in the treatment of schizophrenia in usual care. J Clin Psychiatry. 2006;67(3):453–460. doi: 10.4088/jcp.v67n0317. [DOI] [PubMed] [Google Scholar]
- 14.Lieberman JA. Neurobiology and the natural history of schizophrenia. J Clin Psychiatry. 2006;67(10):e14. [PubMed] [Google Scholar]
- 15.Haro JM, Novick D, Suarez D, Ochoa S, Roca M. Predictors of the course of illness in outpatients with schizophrenia: a prospective three year study. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(5):1287–1292. doi: 10.1016/j.pnpbp.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 16.Salize HJ, McCabe R, Bullenkamp J, et al. Cost of treatment of schizophrenia in six European countries. Schizophr Res. 2009;111(1–3):70–77. doi: 10.1016/j.schres.2009.03.027. [DOI] [PubMed] [Google Scholar]
- 17.Wu EQ, Birnbaum HG, Shi L, et al. The economic burden of schizophrenia in the United States in 2002. J Clin Psychiatry. 2005;66(9):1122–1129. doi: 10.4088/jcp.v66n0906. [DOI] [PubMed] [Google Scholar]
- 18.Ascher-Svanum H, Zhu B, Faries DE, Furiak NM, Montgomery W. Medication adherence levels and differential use of mental-health services in the treatment of schizophrenia. BMC Res Notes. 2009;2:6. doi: 10.1186/1756-0500-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun SX, Liu GG, Christensen DB, Fu AZ. Review and analysis of hospitalization costs associated with antipsychotic nonadherence in the treatment of schizophrenia in the United States. Curr Med Res Opin. 2007;23(10):2305–2312. doi: 10.1185/030079907X226050. [DOI] [PubMed] [Google Scholar]
- 20.Thieda P, Beard S, Richter A, Kane J. An economic review of compliance with medication therapy in the treatment of schizophrenia. Psychiatr Serv. 2003;54(4):508–516. doi: 10.1176/appi.ps.54.4.508. [DOI] [PubMed] [Google Scholar]
- 21.Ascher-Svanum H, Zhu B, Faries DE, et al. The cost of relapse and the predictors of relapse in the treatment of schizophrenia. BMC Psychiatry. 2010;10:2. doi: 10.1186/1471-244X-10-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hughes DA, Bagust A, Haycox A, Walley T. Accounting for noncompliance in pharmacoeconomic evaluations. Pharmacoeconomics. 2001;19(12):1185–1197. doi: 10.2165/00019053-200119120-00001. [DOI] [PubMed] [Google Scholar]
- 23.Weiden PJ, Olfson M. Cost of relapse in schizophrenia. Schizophr Bull. 1995;21(3):419–429. doi: 10.1093/schbul/21.3.419. [DOI] [PubMed] [Google Scholar]
- 24.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peng X, Ascher-Svanum H, Faries D, Conley RR, Schuh KJ. Decline in hospitalization risk and health care cost after initiation of depot antipsychotics in the treatment of schizophrenia. Clinicoecon Outcomes Res. 2011;3:9–14. doi: 10.2147/CEOR.S16061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carswell C, Wheeler A, Vanderpyl J, Robinson E. Comparative effectiveness of long-acting risperidone in New Zealand: a report of resource utilization and costs in a 12-month mirror-image analysis. Clin Drug Investig. 2010;30(11):777–787. doi: 10.2165/11537680-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 27.Marcus SC, Olfson M. Outpatient antipsychotic treatment and inpatient costs of schizophrenia. Schizophr Bull. 2008;34(1):173–180. doi: 10.1093/schbul/sbm061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Becker MA, Young MS, Ochshorn E, Diamond RJ. The relationship of antipsychotic medication class and adherence with treatment outcomes and costs for Florida Medicaid beneficiaries with schizophrenia. Adm Policy Ment Health. 2007;34(3):307–314. doi: 10.1007/s10488-006-0108-5. [DOI] [PubMed] [Google Scholar]
- 29.Knapp M, King D, Pugner K, Lapuerta P. Non-adherence to antipsychotic medication regimens: associations with resource use and costs. Br J Psychiatry. 2004;184:509–516. doi: 10.1192/bjp.184.6.509. [DOI] [PubMed] [Google Scholar]
- 30.Svarstad BL, Shireman TI, Sweeney JK. Using drug claims data to assess the relationship of medication adherence with hospitalization and costs. Psychiatr Serv. 2001;52(6):805–811. doi: 10.1176/appi.ps.52.6.805. [DOI] [PubMed] [Google Scholar]
- 31.McCombs JS, Luo M, Johnstone BM, Shi L. The use of conventional antipsychotic medications for patients with schizophrenia in a medicaid population: therapeutic and cost outcomes over 2 years. Value Health. 2000;3(3):222–231. doi: 10.1046/j.1524-4733.2000.33004.x. [DOI] [PubMed] [Google Scholar]
- 32.Lieberman J, Chakos M, Wu H, et al. Longitudinal study of brain morphology in first episode schizophrenia. Biol Psychiatry. 2001;49(6):487–499. doi: 10.1016/s0006-3223(01)01067-8. [DOI] [PubMed] [Google Scholar]
- 33.Sokal J, Messias E, Dickerson FB, et al. Comorbidity of medical illnesses among adults with serious mental illness who are receiving community psychiatric services. J Nerv Ment Dis. 2004;192(6):421–427. doi: 10.1097/01.nmd.0000130135.78017.96. [DOI] [PubMed] [Google Scholar]
- 34.Regier DA, Farmer ME, Rae DS, et al. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) Study. JAMA. 1990;264(19):2511–2518. [PubMed] [Google Scholar]
- 35.Modestin J, Ammann R. Mental disorder and criminality: male schizophrenia. Schizophr Bull. 1996;22(1):69–82. doi: 10.1093/schbul/22.1.69. [DOI] [PubMed] [Google Scholar]
- 36.Herings RM, Erkens JA. Increased suicide attempt rate among patients interrupting use of atypical antipsychotics. Pharmacoepidemiol Drug Saf. 2003;12(5):423–424. doi: 10.1002/pds.837. [DOI] [PubMed] [Google Scholar]
- 37.Misdrahi D, Llorca PM, Lançon C, Bayle FJ. Compliance in schizophrenia: predictive factors, therapeutical considerations and research implications. Encephale. 2002;28(3 Pt 1):266–272. French. [PubMed] [Google Scholar]
- 38.Olfson M, Mechanic D, Hansell S, Boyer CA, Walkup J. Prediction of homelessness within three months of discharge among inpatients with schizophrenia. Psychiatr Serv. 1999;50(5):667–673. doi: 10.1176/ps.50.5.667. [DOI] [PubMed] [Google Scholar]
- 39.Hargreaves WA, Shumway M. Pharmacoeconomics of antipsychotic drug therapy. J Clin Psychiatry. 1996;57( Suppl 9):66–76. [PubMed] [Google Scholar]