Abstract
Lung cancer is the leading cause of cancer deaths worldwide, and current therapies are disappointing. Elucidation of the cell(s) of origin of lung cancer may lead to new therapeutics. In addition, the discovery of putative cancer-initiating cells with stem cell properties in solid tumors has emerged as an important area of cancer research that may explain the resistance of these tumors to currently available therapeutics. Progress in our understanding of normal tissue stem cells, tumor cell of origin, and cancer stem cells has been hampered by the heterogeneity of the disease, the lack of good in vivo transplantation models to assess stem cell behavior, and an overall incomplete understanding of the epithelial stem cell hierarchy. As such, a systematic computerized literature search of the MEDLINE database was used to identify articles discussing current knowledge about normal lung and lung cancer stem cells or progenitor cells. In this review, we discuss what is currently known about the role of cancer-initiating cells and normal stem cells in the development of lung tumors.
Keywords: Cancer-initiating cells, lung cancer, progenitor cells, stem cells
INTRODUCTION
As the leading cause of cancer deaths worldwide,[1] lung cancer imposes a major therapeutic burden. Lung cancer can be divided into two main histopathological subtypes: non-small-cell lung cancer (NSCLC) and small-cell lung cancer (SCLC). Murine models of NSCLC and SCLC indicate that tumor phenotype is dictated by genetic alterations and the cell type in which these mutations occur.[2,3]
The cellular origin(s) of lung cancer remain largely unknown. It has been speculated that different tumor histopathological subtypes arise from distinct cells of origin localized in defined microenvironments. Primarily owing to their proximal-to-distal distribution pattern, squamous cell carcinoma (SCC) is generally thought to arise from the proximal airway and adenocarcinoma from more distal locations.[4] SCLC may arise from neuroendocrine cells. It is therefore speculated that different tumor subclasses arise from cells of origin located within a defined compartmental microenvironment.
Tissue stem cells are attractive candidates for the cell(s) of origin of tumors, as their long in vivo life span would allow them to accumulate genetic mutations driving tumorigenesis.[5] The possibility also exists that differentiated cells and committed progenitors serve as the primary targets, though this may depend on their turnover rates as well as the acquisition of specific and/or combinations of genetic modifications that confer self-renewal capacity.[6]
Most of our understanding regarding the existence of stem/progenitor cells originates from mouse model studies. The adult mouse lung has been described as three structurally distinct compartments: the first being composed of trachea and extrapulmonary bronchi, the second intrapulmonary bronchi, bronchioles, and terminal bronchioles, and the third alveoli.[7] The mouse tracheal epithelium contains ciliated epithelial lining cells, goblet cells, an abundance of basal cells, and Clara cells. Though solitary neuroendocrine cells are present, they are far rarer than in distal airways. In the trachea, Keratin (KRT) 5-positive basal cells have been shown to have the ability to self-renew and give rise to Clara cells.[8] In the more distal airways, the much more abundant Clara cells lining the bronchioles have demonstrated the ability to generate ciliated cells and self-renew in response to epithelial injury as well as homeostatic conditions.[9] The most distal compartment of the lung, comprised of alveolar ducts or gas-exchanging airspaces, is composed of alveolar type I and alveolar type II cells, the latter of which are considered to be the major stem/progenitor cell of the alveolar epithelium based on their ability to both give rise to alveolar type I cells and to self-renew.[10,11] The transition between the alveoli and terminal bronchioles is known as the bronchioalveolar duct junction (BADJ), where “variant” Clara cells with stem-like properties exist near neuroepithelial cells.[12]
Analysis of the underlying genetic alterations of lung cancer has revealed an association between the observed genetic lesions and the histopathology of the disease.[13] Although distinct lesions clearly are associated with specific tumor cell types, it remains unclear to what extent the cell of origin determines tumor phenotype.
METHODS
We performed a systematic computerized search of the MEDLINE (PubMed) database (last search: August 1, 2012) to identify all published articles from January 1, 2001 to July 31, 2012 dealing with the identification of the cell of origin of lung cancer using the algorithm: (lung OR lung cancer OR lung carcinoma OR nonsmall cell OR non small cell OR NSCLC OR small cell OR SCLC OR lung neoplasms) AND (stem cell OR progenitor cell OR cell of origin OR cancer-initiating cell). We hand searched journals known to publish data relevant to our search. The reference lists of all articles we recovered and those of relevant review articles were also cross-referenced. We considered only peer-reviewed published articles with data pertaining to the cell of origin of lung cancer in both NSCLC and SCLC. Abstracts and meeting proceedings were excluded and no language restriction was imposed. There was no exclusion based on completeness of field term identifiers per study. The final list of articles eligible for review was analyzed to identify articles in which there might be overlap in the data presented.
CELL OF ORIGIN OF NON-SMALL CELL LUNG CANCER
Adenocarcinoma
As discussed by others, putative cancer-initiating cells are likely to exhibit properties inherent to normal tissue stem cells.[14,15] Increasing data over recent years have demonstrated tumorigenic cells with stem cell characteristics in lung tumors. Further examination of mutant K-Ras-induced mouse lung adenocarcinomas revealed the presence of a rare population of double-positive cells (DPCs) shown to express the Clara cell marker Clara Cell Antigen 10 (CC10); also known as Clara cell secretory protein, uteroglobin, and Secretoglobin 1a1 (Scgb1a1) and the alveolar type II marker, Surfactant Protein C (SFTPC).[2] Kim et al.[16] demonstrated that these DPCs located at the BADJ were quiescent in normal lung homeostasis, but self-renew and give rise to bronchiolar and alveolar cells after naphthalene injury. These DPCs were further shown to express established stem cell surface markers of hematopoietic and skin stem cells, stem cell antigen-1 (Sca-1) and cluster of differentiation 34 (CD34) antigen, respectively.[17,18] Moreover, when plated in Matrigel, these DPCs gave rise to Clara, alveolar type II, and alveolar type I cells, suggesting that they exhibited progenitor cell-like features and lending support to their designation as bronchioalveolar stem cells (BASCs).[16]
Although other groups have demonstrated using a variety of in vitro and in vivo approaches that these putative BASCs may not act as normal tissue stem cells,[9,19] Kim et al. demonstrated that these cells proliferate when a K-Ras mutant is induced.[16] Early tumorigenic lesions in inducible K-Ras mutant mice showed elevated numbers of DPCs, and further, a continual expansion of these cell populations correlated with tumor progression in these mice. Moreover, combined naphthalene treatment with K-RasG12D activation resulted in an increase in the size and number of tumors. In addition, in vivo studies to date investigating the molecular mechanisms that regulate the maintenance and proliferation of BASCs by manipulating genes with established roles in lung development or carcinogenesis have shown increases in the abundance of DPCs with targeted loss of these genes.[20–23] Nevertheless, the tumorigenic capacity of these BASCs with respect to the remaining tumor population will need to be confirmed by tumor xenograft studies.
In order to assess whether putative BASCs are the only cell type capable of initiating K-Ras mutant adenocarcinoma in mice, our group utilized “knock-in” mouse models in which a codon 12 K-Ras mutant gene is induced specifically in either CC10-positive cells (Clara cells throughout the airway, putative BASCs at the BADJ, and about 10% of alveolar type II cells) or in SFTPC-positive cells (putative BASCs and all type II cells). When lineage tracing experiments were performed to track which cell types initiated the tumors in these models, we found that type II cells were clearly able to initiate adenocarcinoma in response to K-RasG12D. In the bronchioles, both SFTPC-positive and SFTPC-negative Clara cells initiated hyperplasia that does not progress to adenocarcinoma. Thus, we concluded that type II cells are the cell of origin of K-Ras-induced adenocarcinomas [Figure 1].[24]
Figure 1.
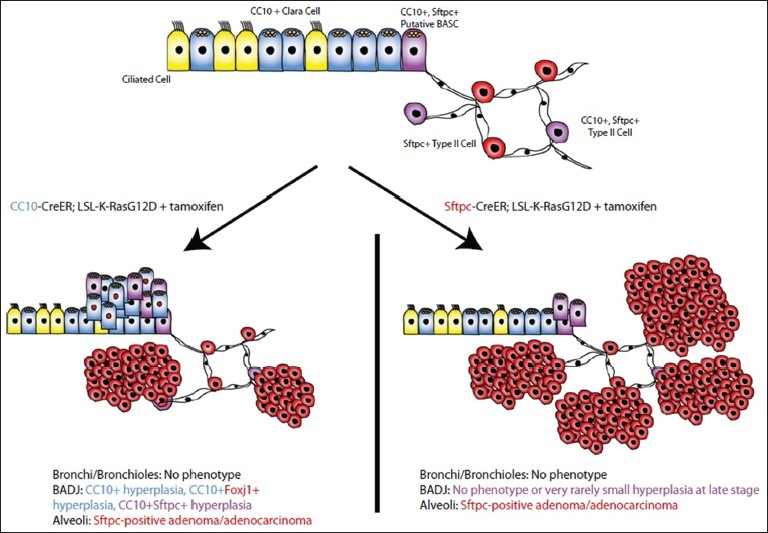
[24]: Model for response of various respiratory epithelial cells to K-RasG12D induction. Type II cells either express only Surfactant Protein C (SFTPC) (0red) or both Clara Cell Antigen (CC10) and SFTPC (purple) in the steady-state normal lung. When K-RasG12D is expressed in CC10-Cre-recombinase (CreER) mice (left), hyperplasia in the bronchioalveolar duct junction (BADJ) involves putative bronchioalveolar stem cells and Clara cells. When K-RasG12D is expressed in SFTPC-CreER mice (right), the BADJ is normal or contains rare small hyperplastic areas, and tumors arise only in the alveoli
SQUAMOUS CELL LUNG CANCER
Tracheal basal cell progenitors have been speculated to be the target cells of origin in mouse lung SCC, as the gene expression and histopathology patterns of these lesions frequently resemble these cells. Tracheal basal cells express the transcription factor p63, located at intercartilaginous boundaries or mucosal gland duct junctions, and are KRT 5/14 positive.[25–27] Rock et al.[8] generated a tamoxifen-inducible Cre-recombinase (CreERT2) transgenic mouse under the control of the human KRT5 gene promoter (KRT5-CreERT2) that demonstrated lacZ+ basal, Clara, and ciliated cells in the trachea of KRT5-CreERT2; Rosa26R-lacZ adult mice at defined time-points following tamoxifen administration in steady-state conditions and in response to epithelial injury. These findings lend evidence to the fact that KRT5+ basal cells have the ability to generate luminal cells in the trachea and self-renew. Further studies with mouse models whereby genetic modifications associated with SCC are restricted to basal progenitor cells will need to be conducted in order to establish a clear relationship between basal progenitors and lung SCC.
In addition, amplification of chromosome segment 3q26.33 is found in human lung and esophageal SCCs.[28] Interestingly, the high mobility group transcription factor Sox2, which is essential for the maintenance of both stem cells in multiple adult tissues and pluripotent embryonic stem cells and specifically plays a role in the differentiation and proliferation of basal progenitor cells, resides within 3q26.33 and has been shown to be the relevant gene for amplification.[28–30] We developed a conditional allele of the Rosa26 locus whereby Sox2 is overexpressed either during lung development using a SFTPC-Cre transgene, or in Clara epithelial cells and a proportion of type II cells in the adult lung using a CC10-CreER “knock-in” allele.[30] In both cases, overexpression led to extensive bronchial hyperplasia. In the terminal bronchioles, a trachea-like pseudostratified epithelium developed with p63-positive cells underlying columnar cells. Over 12-34 weeks, about half of the mice expressing the highest levels of Sox2 developed carcinoma resembling adenocarcinoma but expressing the squamous marker, Trp63 (p63).[30] Whether Clara and type II cells can initiate full-blown squamous cell lung cancer in response to other oncogenic stimuli remains to be elucidated.
CELL OF ORIGIN OF SMALL-CELL LUNG CANCER
Though much progress has been made in identifying specific cell populations that give rise to NSCLC upon genetic alteration, it is unclear whether the same cell of origin is responsible for tumorigenesis in SCLC. This is largely due to the advanced stage of disease in most patients at the time of diagnosis.[30] However, it has been observed that at least a proportion of SCLCs display features of NSCLC, possibly arguing for a “common” cell of origin of lung cancer for those displaying mixed phenotypes, but it remains unclear whether the same cell of origin is responsible for initiating both of these types of cancers.[31] Otherwise, SCLCs have typically been shown to express a range of neuroendocrine markers and transcription factors that play important roles in neuroendocrine differentiation, prompting the hypothesis that a rare population of neuroendocrine cells may be the progenitors of SCLC.[3,32] Conversely, though microenvironments in the mouse lung found in close proximity to neuroepithelial bodies (NEBs) have been shown to maintain stem cell populations, pulmonary neuroendocrine cells associated with these NEBs exhibit properties of unipotent progenitor cells rather than stem cells. Nevertheless, the observation that SCLC can exhibit adenocarcinoma, epidermoid carcinoma, and/or large cell carcinoma-like features would argue for the existence of a “common” cell of origin for these lung cancers.[33]
CANCER STEM CELLS
Recently, Eramo et al. (2008) demonstrated that both SCLC and NSCLC tumors contain a rare population of undifferentiated cells that express a well-characterized marker of cancer stem cells, CD133.[34] In this study, injury to mouse lung epithelium was achieved through naphthalene administration. Following injury, a six-fold increase in CD133 cells was observed, leading the authors to speculate that progenitor cells, in this case, variant Clara cells, involved in regenerating the epithelial lining following injury are marked by CD133 cells. Moreover, this study demonstrated that when injected subcutaneously into immunocompromised mice, only CD133 positive lung cancer cells were able to form xenografts phenotypically identical to the original tumor. However, in normal lung tissue, it remains unclear which cell type expresses CD133, and immunohistochemical analysis has yet to demonstrate CD133.
The hallmark of cancer stem cells has long been claimed to be chemoresistance.[35] Interestingly, CD133 positive lung cancer cells exhibit chemoresistance in vitro to traditional therapeutic agents.[36] An independent study demonstrated an increase in the fraction of CD133 positive lung cancer cells present after treatment of mice carrying human lung cancer xenografts with cisplatin.[37] Despite these studies, it remains unclear whether a CD133 positive cell population is involved in chemoresistance in lung cancer. However, given that current therapies available in the treatment of lung cancer are not effectively able to completely eradicate the disease, clarifying the importance of CD133 and other cancer stem cell markers will be important.
FUTURE STRATEGIES/CONCLUSIONS
Although we have made progress regarding the cell of origin of lung cancer in the mouse, many questions remain to be answered. First, mouse correlates of human lung cancer subtypes must be refined and studied. Because unique combinations of oncogenic stimuli and cell of origin are likely, these must be identified. Second, the human lung is much more complex than the mouse lung. Methods for isolation and culture of human lung cells followed by lentiviral transduction of oncogenes are necessary to validate mouse experiments. Finally, human lung tumors of all subtypes must be studied for unique tumorigenic subpopulations in order to identify further surface markers. Cancer-initiating cells may then be studied for therapeutic vulnerabilities.
AUTHOR'S PROFILE
Dr. Jennifer M. Hanna, Department of Surgery, Division of Cardiovascular and Thoracic Surgery, Duke University Medical Center, Durham, North Carolina, USA.
Dr. Mark W. Onaitis, Department of Surgery, Division of Cardiovascular and Thoracic Surgery, Duke University Medical Center, Durham, North Carolina, USA.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Jemal A, Thun MJ, Ries LA, Howe HL, Weir HK, Center MM, et al. Annual report to the nation on the status of cancer, 1975-2005, featuring trends in lung cancer, tobacco use, and tobacco control. J Natl Cancer Inst. 2008;100:1672–94. doi: 10.1093/jnci/djn389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jackson EL, Willis N, Mercer K, Bronson RT, Crowley D, Montoya R, et al. Analysis of lung tumor initiation and progression using conditional expression of oncogenic K-ras. Genes Dev. 2001;15:3243–8. doi: 10.1101/gad.943001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meuwissen R, Linn SC, Linnoila RI, Zevenhoven J, Mooi WJ, Berns A. Induction of small cell lung cancer by somatic inactivation of both Trp53 and Rb1 in a conditional mouse model. Cancer Cell. 2003;4:181–9. doi: 10.1016/s1535-6108(03)00220-4. [DOI] [PubMed] [Google Scholar]
- 4.Giangreco A, Groot KR, Janes SM. Lung cancer and lung stem cells: Strange bedfellows? Am J Respir Crit Care Med. 2007;175:547–53. doi: 10.1164/rccm.200607-984PP. [DOI] [PubMed] [Google Scholar]
- 5.Smalley M, Ashworth A. Stem cells and breast cancer: A field in transit. Nat Rev Cancer. 2003;3:832–44. doi: 10.1038/nrc1212. [DOI] [PubMed] [Google Scholar]
- 6.Sutherland KD, Proost N, Brouns I, Adriaensen D, Song JY, Berns A. Cell of origin of small cell lung cancer: Inactivation of Trp53 and Rb1 in distinct cell types of adult mouse lung. Cancer Cell. 2011;19:754–64. doi: 10.1016/j.ccr.2011.04.019. [DOI] [PubMed] [Google Scholar]
- 7.Rock JR, Randell SH, Hogan BL. Airway basal stem cells: A perspective on their roles in epithelial homeostasis and remodeling. Dis Model Mech. 2010;3:545–56. doi: 10.1242/dmm.006031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rock JR, Onaitis MW, Rawlins EL, Lu Y, Clark CP, Xue Y, et al. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc Natl Acad Sci USA. 2009;106:12771–5. doi: 10.1073/pnas.0906850106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rawlins EL, Okubo T, Xue Y, Brass DM, Auten RL, Hasegawa H, et al. The role of Scgb1a1+ Clara cells in the long-term maintenance and repair of lung airway, but not alveolar, epithelium. Cell Stem Cell. 2009;4:525–34. doi: 10.1016/j.stem.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adamson IY, Bowden DH. The type 2 cell as progenitor of alveolar epithelial regeneration. A cytodynamic study in mice after exposure to oxygen. Lab Invest. 1974;30:35–42. [PubMed] [Google Scholar]
- 11.Evans MJ, Cabral LJ, Stephens RJ, Freeman G. Transformation of alveolar type 2 cells to type 1 cells following exposure to NO2. Exp Mol Pathol. 1975;22:142–50. doi: 10.1016/0014-4800(75)90059-3. [DOI] [PubMed] [Google Scholar]
- 12.Giangreco A, Reynolds SD, Stripp BR. Terminal bronchioles harbor a unique airway stem cell population that localizes to the bronchoalveolar duct junction. Am J Pathol. 2002;161:173–82. doi: 10.1016/S0002-9440(10)64169-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sekido Y, Fong KM, Minna JD. Molecular genetics of lung cancer. Annu Rev Med. 2003;54:73–87. doi: 10.1146/annurev.med.54.101601.152202. [DOI] [PubMed] [Google Scholar]
- 14.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–11. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 15.Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: Accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8:755–68. doi: 10.1038/nrc2499. [DOI] [PubMed] [Google Scholar]
- 16.Kim CF, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel S, et al. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005;121:823–35. doi: 10.1016/j.cell.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 17.Blanpain C, Lowry WE, Geoghegan A, Polak L, Fuchs E. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell. 2004;118:635–48. doi: 10.1016/j.cell.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 18.Morrison SJ, Weissman IL. The long-term repopulating subset of hematopoietic stem cells is deterministic and isolatable by phenotype. Immunity. 1994;1:661–73. doi: 10.1016/1074-7613(94)90037-x. [DOI] [PubMed] [Google Scholar]
- 19.McQualter JL, Brouard N, Williams B, Baird BN, Sims-Lucas S, Yuen K, et al. Endogenous fibroblastic progenitor cells in the adult mouse lung are highly enriched in the sca-1 positive cell fraction. Stem Cells. 2009;27:623–33. doi: 10.1634/stemcells.2008-0866. [DOI] [PubMed] [Google Scholar]
- 20.Ventura JJ, Tenbaum S, Perdiguero E, Huth M, Guerra C, Barbacid M, et al. p38α MAP kinase is essential in lung stem and progenitor cell proliferation and differentiation. Nat Genet. 2007;39:750–8. doi: 10.1038/ng2037. [DOI] [PubMed] [Google Scholar]
- 21.Yanagi S, Kishimoto H, Kawahara K, Sasaki T, Sasaki M, Nishio M, et al. Pten controls lung morphogenesis, bronchioalveolar stem cells, and onset of lung adenocarcinomas in mice. J Clin Invest. 2007;117:2929–40. doi: 10.1172/JCI31854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang Y, Iwanaga K, Raso MG, Wislez M, Hanna AE, Wieder ED, et al. Phosphatidylinositol 3-kinase mediates bronchioalveolar stem cell expansion in mouse models of oncogenic K-ras-induced lung cancer. PLoS ONE. 2008;3:e2220. doi: 10.1371/journal.pone.0002220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Y, Goss AM, Cohen ED, Kadzik R, Lepore JJ, Muthukumaraswamy K, et al. A Gata6-Wnt pathway required for epithelial stem cell development and airway regeneration. Nat Genet. 2008;40:862–70. doi: 10.1038/ng.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu X, Rock JR, Lu Y, Futtner C, Schwab B, Guinney J, et al. Evidence for type II cells as cells of origin of K-Ras-induced distal lung adenocarcinoma. PNAS. 2012;109:4910–5. doi: 10.1073/pnas.1112499109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Borthwick DW, Shahbazian M, Krantz QT, Dorin JR, Randell SH. Evidence for stem-cell niches in the tracheal epithelium. Am J Respir Cell Mol Biol. 2001;24:662–70. doi: 10.1165/ajrcmb.24.6.4217. [DOI] [PubMed] [Google Scholar]
- 26.Hong KU, Reynolds SD, Watkins S, Fuchs E, Stripp BR. Basal cells are a multipotent progenitor capable of renewing the bronchial epithelium. Am J Pathol. 2004;164:577–88. doi: 10.1016/S0002-9440(10)63147-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hong KU, Reynolds SD, Watkins S, Fuchs E, Stripp BR. In vivo differentiation potential of tracheal basal cells: Evidence for multipotent and unipotent subpopulations. Am J Physiol Lung Cell Mol Physiol. 2004;286:L643–9. doi: 10.1152/ajplung.00155.2003. [DOI] [PubMed] [Google Scholar]
- 28.Bass AJ, Watanabe H, Mermel CH, Yu S, Perner S, Verhaak RG, et al. SOX2 is an amplified lineage-survival oncogene in lung and esophageal squamous cell carcinomas. Nat Genet. 2009;41:1238–42. doi: 10.1038/ng.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Que J, Luo X, Schwartz RJ, Hogan BL. Multiple roles for Sox2 in the developing and adult mouse trachea. Development. 2009;136:1899–907. doi: 10.1242/dev.034629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu Y, Futtner C, Rock JR, Xu X, Whitworth W, Hogan BL, et al. Evidence that SOX2 overexpression is oncogenic in the lung. PLoS ONE. 2010;5:e11022. doi: 10.1371/journal.pone.0011022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jackman DM, Johnson BE. Small-cell lung cancer. Lancet. 2005;366:1385–96. doi: 10.1016/S0140-6736(05)67569-1. [DOI] [PubMed] [Google Scholar]
- 32.Yesner R. Heterogeneity of so-called neuroendocrine lung tumors. Exp Mol Pathol. 2001;70:179–82. doi: 10.1006/exmp.2001.2373. [DOI] [PubMed] [Google Scholar]
- 33.Wistuba II, Gazdar AF, Minna JD. Molecular genetics of small cell lung carcinoma. Semin Oncol. 2001;28:3–13. [PubMed] [Google Scholar]
- 34.Travis WD. Pathology of lung cancer. Clin Chest Med. 2002;23:65–81. doi: 10.1016/s0272-5231(03)00061-3. [DOI] [PubMed] [Google Scholar]
- 35.Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5:275–84. doi: 10.1038/nrc1590. [DOI] [PubMed] [Google Scholar]
- 36.Eramo A, Lotti F, Sette G, Pilozzi E, Biffoni M, Di Virgilio A, et al. Identification and expansion of the tumorigenic lung cancer stem cell population. Cell Death Differ. 2008;15:504–14. doi: 10.1038/sj.cdd.4402283. [DOI] [PubMed] [Google Scholar]
- 37.Bertolini G, Roz L, Perego P, Tortoreto M, Fontanella E, Gatti L, et al. Highly tumorigenic lung cancer CD133+cells display stem-like features and are spared by cisplatin treatment. Proc Natl Acad Sci USA. 2009;106:16281–6. doi: 10.1073/pnas.0905653106. [DOI] [PMC free article] [PubMed] [Google Scholar]


