Abstract
Campylobacter jejuni subsp. jejuni and Campylobacter coli are leading causes of gastroenteritis, with virulence linked to cell surface carbohydrate diversity. Although the associated gene clusters are well studied for C. jejuni subsp. jejuni, C. coli has been largely neglected. Here we provide comparative analysis of the lipooligosacharide (LOS) and capsular polysaccharide (CPS) gene clusters, using genome and cluster sequence data for 36 C. coli strains, 67 C. jejuni subsp. jejuni strains and ten additional Campylobacter species. Similar to C. jejuni subsp. jejuni, C. coli showed high LOS/CPS gene diversity, with each cluster delineated into eight gene content classes. This diversity was predominantly due to extensive gene gain/loss, with the lateral transfer of genes likely occurring both within and between species and also between the LOS and CPS. Additional mechanisms responsible for LOS/CPS diversity included phase-variable homopolymeric repeats, gene duplication/inactivation, and possibly host environment selection pressure. Analyses also showed that (i) strains of C. coli and Campylobacter upsaliensis possessed genes homologous to the sialic acid genes implicated in the neurological disorder Guillain Barré syndrome (GBS), and (ii) C. coli LOS classes were differentiated between bovine and poultry hosts, potentially aiding post infection source tracking.
Keywords: Campylobacter, virulence gene clusters, lipooligosacharide, capsular polysaccharide, lateral gene transfer, genomics
1. Introduction
Campylobacter jejuni subsp. jejuni and Campylobacter coli are recognized as the leading causes of human bacterial gastroenteritis in the industrialized world (Alfredson and Korolik, 2007; Ketley, 1997; Moore et al., 2005), with reported incidences estimated to be between 27 and 880 cases per 100,000 individuals (Blumer et al., 2003; CDC, 2004; Friedman et al., 2000; Gallay et al., 2003; Takkinen et al., 2003; Unicomb et al., 2003; Unicomb et al., 2006). Campylobacter infections (Campylobacteriosis) can also lead to several complications including toxic mega-colon, hemolytic uremic syndrome, Reiter’s syndrome, Miller Fisher syndrome, and Guillain Barré syndrome (GBS).
Although C. coli accounts for far fewer infections than C. jejuni subsp. jejuni (Alfredson and Korolik, 2007; Ketley, 1997; Moore et al., 2005), its impact is still considerable. For example, in Israel, the proportion of C. coli contained within Campylobacter isolates from diarrheal specimens is consistently 24–30% (Bersudsky et al., 2000), and a 2000 survey of Campylobacter infection within the UK showed that C. coli accounted for over 25,000 cases of gastroenteritis, with 11 subsequent deaths (Tam et al., 2003).
No suitable animal models (non-primate) of human Campylobacteriosis are available. Consequently, little is known of how Campylobacter cause disease. However, there are several Campylobacter gene clusters involved in human epithelial cell invasion and attachment and are consequently implicated in pathogenesis (Wassenaar and Blaser, 1999). For example, the capsular polysaccharides (CPS) of many bacterial pathogens are known to play an important role in host invasion and subsequent evasion of the host immune response (Roberts, 1996). In C. jejuni subsp. jejuni, Bacon et al. (2001) demonstrated a role for the CPS in serum resistance, epithelial cell invasion, and diarrhoeal disease. More recently, Jones et al. (2004) demonstrated a role for the CPS in gastrointestinal tract invasion. Lipooligosacharides (LOS), are found on the surface of many mucosal pathogens, and in C. jejuni subsp. jejuni have been shown to be important in adhesion to human intestinal cells, invasion, and protection from complement-mediated killing (Guerry et al., 2002; McSweegan and Walker, 1986). The LOS are capable of mimicking human antigens (Guerry and Szymanski, 2008), and it is this mimicry that is implicated in GBS and Miller Fisher syndrome (Ang et al., 2004; Willison and O'Hanlon, 1999).
Studies focusing on C. jejuni subsp. jejuni have revealed a remarkable diversity in gene content for CPS and LOS (Dorrell et al., 2001; Godschalk et al., 2004; Karlyshev et al., 2005; Parker et al., 2008; Pearson et al., 2003). Comparative sequence analysis of the CPS and particularly the LOS for C. jejuni subsp. jejuni has provided a characterization of these gene clusters and subsequent delineation of strains into numerous gene content classes. Eleven classes are reported for CPS (Karlyshev et al., 2005; Poly et al., 2011) and 18 for LOS (Gilbert et al., 2002; Godschalk et al., 2004; Parker et al., 2008). Importantly, Godschalk et al. (2004) determined that specific LOS classes containing sialylation genes were associated with GBS. Structural variation of the CPS and LOS may represent important C. jejuni subsp. jejuni strategies for evading the immune response and genetic characterization of C. jejuni subsp. jejuni CPS and LOS genes have suggested multiple mechanisms responsible for such variation, including (i) lateral gene transfer, (ii) gene inactivation, duplication, deletion, and fusion, and (iii) phase variable homopolymeric tracts (Gilbert et al., 2002; Godschalk et al., 2004; Karlyshev et al., 2005; Parker et al., 2008; Parker et al., 2005; Parkhill et al., 2000).
In contrast to C. jejuni subsp. jejuni, the genetic characterization of the gene clusters described above have been largely neglected for C. coli. An exception is the study of Lang et al. (2010) who presented comparative genomic hybridization data showing a pattern of high gene content diversity for CPS and LOS, similar to that described for C. jejuni subsp. jejuni. Here we make use of an extensive C. coli genome sequence data set, generated as part of a previous study of ours that addressed bacteria species questions (Lefebure et al., 2010) (see below for details) to provide the first detailed characterization of the CPS and LOS gene clusters for this species. We make additional use of this earlier genome data set by providing new gene cluster data for strains of C. jejuni subsp. jejuni from multiple hosts, which we combine with data already available for ten additional Campylobacter species, to provide a comprehensive comparative perspective of the CPS and LOS gene clusters between C. coli and four thermophilic and seven non-thermophilic Campylobacter species.
2. Materials and methods
2.1. Strains, sequencing, and assembly
In the previous study mentioned above (Lefebure et al., 2010), Illumina GA II technology was used to sequence genomic DNA obtained from 42 C. coli strains isolated from human, turkey, chicken, swine, and bovine hosts, and 43 C. jejuni subsp. jejuni strains isolated from human, chicken, and bovine hosts (details of the sequencing procedure and original de novo assembly are provided therein). Annotation of these de novo assemblies was performed as part of this study, using the NCBI Prokaryotic Genomes Automatic Annotation Pipeline (PGAAP). LOS and CPS genes were further annotated using Blast2GO (Gotz et al., 2008). These Whole Genome Shotgun projects have been deposited at DDBJ/EMBL/GenBank under the accessions AIMI00000000 - AIPO00000000. The versions described in this paper are the first versions, AIMI 01000000 - AIPO01000000.
In order to generate contiguous sequence data completely spanning a gene cluster, strains with a cluster contained in multiple contigs in the original Velvet (Zerbino and Birney, 2008) assembly were further assembled using Geneious v5.1.2 (Drummond et al., 2010). Accuracy of these Geneious assemblies was confirmed using Sanger sequencing. The gene content of the CPS gene cluster for two C. coli strains (H8 and 2553) was of particular interest (see Results and Discussion). These strains required additional Sanger sequencing to generate contiguous assemblies spanning the complete CPS gene cluster. Table 1 presents details for the 34 C. coli strains and 21 C. jejuni subsp. jejuni strains for which we were able to assemble contiguous LOS and/or CPS gene clusters. The table also shows details for 10 additional C. coli and C. jejuni subsp. jejuni strains for which genome sequences were available and were included in our analyses. Several studies have shown that C. coli STs are delineated into three major groups, with one of these groups typically restricted to human and domestic livestock isolates (Colles et al., 2011; Sheppard et al., 2010; Sopwith et al., 2010). The 36 C. coli isolates analyzed here were all isolated from humans or domestic livestock and represented 29 distinct STs. Of these, 26 were included in the studies mentioned above and as expected all of them fell within the human/domestic livestock group.
Table 1.
| A. C. coli strain information | ||||||
|---|---|---|---|---|---|---|
| Strain | Strain ID | Source | ST | LOS | CPS | Accession# |
| LMG 23336 | cco76 | Human | 3868 | I | - | AINM00000000 |
| 2680 | cco111 | Turkey | 3872 | I | - | AIMN00000000 |
| 2692 | cco115 | Turkey | 860 | I | - | AIMQ00000000 |
| 86119 | cco16 | Chicken | 825 | I | - | AIMU00000000 |
| 202/04 | cco69 | Human | 1585 | II | - | AINH00000000 |
| Z163 | cco100 | Chicken | 3336 | II | V | AIMK00000000 |
| H8 | cco93 | Human | 901 | II | VII | AINU00000000 |
| 111-3 | cco1 | Swine | 1467 | II | AIMI00000000 | |
| 2548 | cco105 | Turkey | 1167 | II | VI | AIML00000000 |
| 2553 | cco106 | Turkey | 825 | II | VIII | AIMM00000000 |
| JV20 | JV20 | Human | 860 | II | - | AEER01000001 |
| LMG 23341 | cco77 | Human | 855 | III | - | AINN00000000 |
| 37/05 | cco74 | Human | 1191 | III | - | AINK00000000 |
| LMG 23342 | cco78 | Human | 855 | III | - | AINO00000000 |
| H6 | cco91 | Human | 3020 | III | - | AINT00000000 |
| 2688 | cco113 | Turkey | 1017 | III | III | AIMP00000000 |
| 2698 | cco117 | Turkey | 829 | III | - | AIMR00000000 |
| RM2228 | RM2W | Chicken | 1063 | III | I | AAFL01000001 |
| 1948 | cco61 | Bovine | 1104 | IV | - | AINE00000000 |
| 1961 | cco67 | Bovine | 1104 | IV | - | AING00000000 |
| LMG 9860 | cco88 | Human | 900 | IV | - | AINS00000000 |
| H56 | cco96 | Human | 1096 | IV | - | AINW00000000 |
| 1098 | cco23 | Bovine | 1104 | IV | - | AIMW00000000 |
| 67-8 | cco7 | Swine | 1061 | IV | IV | AINI00000000 |
| 151-9 | cco8 | Swine | 1102 | IV | - | AINQ00000000 |
| 1909 | cco55 | Bovine | 1104 | V | - | AINC00000000 |
| 1957 | cco65 | Bovine | 2698 | V | - | AINF00000000 |
| 1148 | cco25 | Bovine | 1068 | VI | - | AIMX00000000 |
| 1417 | cco37 | Bovine | 1436 | VI | III | AIMY00000000 |
| 7--1 | cco4 | Swine | 3860 | VI | IV | AIMZ00000000 |
| LMG 9853 | cco81 | Human | 3869 | VII | - | AINR00000000 |
| H9 | cco94 | Human | 825 | VIII | VI | AINV00000000 |
| 59-2 | cco6 | Swine | 890 | VIII | - | AIND00000000 |
| Z156 | cco99 | Chicken | 854 | - | V | AINX00000000 |
| 2685 | cco112 | Turkey | 1082 | - | II | AIMO00000000 |
| 132-6 | cco5 | Swine | 3861 | - | III | AINA00000000 |
| B. C. jejuni subsp. jejuni and C. jejuni subsp. doylei strain information | ||||||
|---|---|---|---|---|---|---|
| Strain | Strain ID | Source | ST | LOS | CPS | Accession# |
| 60004 | cje11 | Chicken | 4836 | A | - | AIOE00000000 |
| 1997-7 | cje21 | Human | 93 | A | - | AIOX00000000 |
| 87459 | cje34 | Chicken | 6224 | A | - | AIPE00000000 |
| 86605 | cje13 | Chicken | 6219 | A | - | AIOJ00000000 |
| LMG 9879 | cje120 | Human | 47 | A | - | AIOI00000000 |
| ICDCCJ07001 | jG7 | Human | 2993 | A | E | CP002029 |
| 81–176 | jG2 | Human | 604 | B | D | CP000538 |
| 1997-4 | cje19 | Human | 475 | B | - | AIOW00000000 |
| LMG 23218 | cje102 | Chicken | 48 | B | - | AIOB00000000 |
| 1893 | cje79 | Bovine | 38 | B | - | AIPK00000000 |
| 140-16 | cje4 | Bovine | 6217 | B | - | AIPF00000000 |
| IA3902 | jG4 | Sheep | 8 | C | K* | CP001876 |
| NCTC 11168 (RM1862) | jG6 | Human | 43 | C | A | AL111168 |
| LMG 23216 | cje100 | Chicken | 4835 | R | - | AIOA00000000 |
| LMG 23211 | cje96 | Chicken | 220 | R | H* | AIPO00000000 |
| 1997-14 | cje25 | Human | 5159 | E | F* | AIPA00000000 |
| 81116 | jG3 | Human | 267 | E | J* | CP000814 |
| RM1221 | jG1 | Chicken | 354 | F | I* | CP000025 |
| S3 | jG8 | Chicken | 354 | F | I* | CP001960 |
| 1997-10 | cje22 | Human | 6222 | H | - | AIOY00000000 |
| 1854 | cje77 | Bovine | 922 | H | - | AIPJ00000000 |
| 2008-894 | cje146 | Human | 1962 | H | - | AIOQ00000000 |
| 51494 | cje10 | Chicken | 4834 | P | - | AINZ00000000 |
| LMG 23223 | cje104 | Chicken | 791 | T* | - | AIOC00000000 |
| 2008-979 | cje160 | Human | 2274 | U* | - | AIOU00000000 |
| 2008-1025 | cje145 | Human | 50 | V* | - | AIOP00000000 |
| M1 | jG5 | Human | 137 | W* | L* | CP001900 |
| 51037 | cje28 | Chicken | 939 | - | F* | AIPB00000000 |
| 55037 | cje12 | Chicken | 2223 | - | G* | AIOH00000000 |
| 269-97 (C. j. doylei) | cjd | Human | N | M* | CP000768 | |
Dash (-) refers to strains lacking contiguous sequence for a particular gene cluster.
Asterisk in Table 1B denotes new LOS or CPS classes. For one C. coli strain and five C. jejuni subsp. jejuni strains (omitted from table), none of the gene clusters could be assembled into contiguous sequence.
ST=multi locus sequence typing (MLST) sequence type.
2.2. Orthology assignment
Prior to the genomic era, the identification of orthologs (homologous genes related through speciation from a common ancestral gene present in their last common ancestor) and paralogs (homologous genes related through duplication) was typically accomplished using phylogenetic techniques. However, the high computational demand of these approaches coupled with the need to analyze large genomic data sets has led to the development of complementary approaches not requiring phylogenetic analyses. Rather, these approaches rely on the simplifying assumption that homologs can be identified via reciprocal best BLAST search hits. Here, we combine an implementation of this approach that incorporates a Markov Cluster algorithm with DNA sequence alignments to delineate multiple Campylobacter strains and species into gene content classes for CPS and LOS gene clusters. This approach allowed precise comparison of the gene content of these virulence gene clusters across multiple species and strains. DNA sequence alignments were performed using MAFFT v6.814b (Katoh et al., 2002) as implemented in Geneious v5.1.2, and OrthoMCL v2.0 (Li et al., 2003) was used to delineate orthologous and paralogous protein sequences. In addition to identifying orthologs, the program also attempts to differentiate between recent paralogs (in-paralogs) and those that pre-date the species split (out-paralogs) (the program groups orthologs with putative in-paralogs). We analyzed 114 Campylobacter strains as follows: (i) genome sequences from 36 C. coli strains (34 strains sequenced as part of this study plus two additional strain sequences obtained from NCBI), (ii) 29 genome sequences from C. jejuni subsp. jejuni strains (21 strains sequenced in this study plus eight additional strain sequences obtained from NCBI), (iii) genome sequences obtained from NCBI for three additional thermophilic Campylobacter species: Campylobacter jejuni subsp. doylei (n=1), Campylobacter lari (n=1) Campylobacter upsaliensis (n=2), (iv) single genome sequences obtained from NCBI for seven non-thermophilic Campylobacter species: Campylobacter rectus, Campylobacter showae, Campylobacter concisus, Campylobacter curvus, Campylobacter fetus subsp. fetus, Campylobacter gracilis, Campylobacter hominis, (v) NCBI sequences of the LOS gene cluster from 34 C. jejuni subsp. jejuni strains, and (vi) NCBI sequences of the CPS gene cluster from six C. jejuni subsp. jejuni strains. For C. jejuni subsp. jejuni strain 81–176, the analysis included two separate sequences. The first was a CPS sequence, and the second was a complete genome sequence. For C. jejuni subsp. jejuni strain RM1862, the analysis also included two separate sequences. The first was a LOS sequence, and the second was a complete genome sequence. See Tables 1, 2, 3, and 4 for Accession and strain ID numbers.
Table 2.
Strain ID, accession numbers, and gene cluster information for additional thermophilic and non-thermophilic Campylobacter species
| Species | Strain | Species ID | Accession# |
|---|---|---|---|
| C. lari | RM2100 | cla | CP000932 |
| C. upsaliensis | JV21 | cup1 | AEPU01000001 |
| C. upsaliensis | RM3195 | cup2 | AAFJ01000001 |
| C. rectus * | RM3267 | rec | ACFU01000001 |
| C. showae * | RM3277 | sho | ACVQ01000001 |
| C. concisus * | 13826 | con | CP000792 |
| C. curvus * | 525.92 | cur | CP000767 |
| C. fetus subsp. fetus * | 82-40 | fet | CP000487 |
| C. gracilis * | RM3268 | gra | ACYG01000001 |
| C. hominis * | ATCC BAA-381 | hom | CP000776 |
All species were human sourced isolates.
Asterisk shows non-thermophilic species.
Table 3.
C. jejuni subsp. jejuni strain information for previously sequenced CPS classes
Table 4.
C. jejuni subsp. jejuni strain information for previously sequenced LOS classes
| Strain | Strain ID | Source | LOS | Accession# |
|---|---|---|---|---|
| RM1048 | J1A | human | A | AF215659 |
| RM1556 (ATCC 43438) | J2A | human | A | AF400048 |
| ATCC 43446 | J3A | human | A | AF167344 |
| OH4384 | J4A | human | A | AF130984 |
| RM1052 | J1B | human | B | AF401528 |
| RM1050 | J2B | human | B | AF401529 |
| RM1046 | J1C | bovine | C | AF400047 |
| RM1862 (NCTC 11168) | J2C | human | C | AL139077 |
| RM1045 | J3C | human | C | AY044156 |
| LIO87 | J1D | undetermined | D | AF400669 |
| RM3418 | J2D | undetermined | D | EU404109 |
| RM1863 (81116) | J1E | human | E | AJ131360 |
| RM1552 | J2E | human | E | EU404105 |
| GB15 | J1F | human | F | AY423554 |
| RM1170 | J2F | chicken | F | AY434498 |
| RM3415 | J3F | undetermined | F | EU404108 |
| RM1555 (ATCC 43437) | J1G | goat | G | AY436358 |
| RM1047 (ATCC 43431) | J1H | human | H | AY800272 |
| RM1553 | J2H | human | H | EU404106 |
| RM1850 | J1I | chicken | I | EU404107 |
| RM1508 | J1J | human | J | EU404104 |
| GB24 | J1K | human | K | AY573819 |
| RM2227 | J2K | chicken | K | EF143353 |
| RM2229 | J3K | undetermined | K | EF143354 |
| RM1861 | J4K | undetermined | K | EU410350 |
| RM3435 | J1L | undetermined | L | EU404111 |
| RM1503 | J1M | undetermined | M | EF140720 |
| RM2095 | J1N | undetermined | N | AY816330 |
| RM3423 | J1O | undetermined | O | EF143352 |
| GB4 | J1P | human | P | AY943308 |
| RM3437 | J1Q | undetermined | Q | EU404112 |
| GC149 | J1R | undetermined | R | AY962325 |
| RM3419 | J1S | undetermined | S | EU404110 |
| RM2095 | J1N | undetermined | N | AY816330 |
The first step in the OrthoMCL procedure was to perform a reciprocal BLASTp among protein sequences. The resulting e-values were then used to build a normalized similarity matrix, which was analyzed using a Markov Cluster algorithm to delineate proteins into “OrthoMCL groups” containing sets of orthologs and/or recent paralogs. Proteins were considered recent paralogs if they were more similar to each other than to any protein from another strain. Following Li et al. (2003), an e-value cut-off of 1e-5 was used in the BLASTp. Throughout the manuscript, the term ortholog refers to an OrthoMCL group/cluster.
2.3. Phylogenetics, gene content, recombination, and homopolymeric tracts
Gene content similarity across strains for each of the gene clusters, as well as for the remainder of the genome, was explored using the presence or absence of the orthologs delineated using the orthology assignment procedure. Presence/absence of orthologs was used to generate binary sequences that were used to construct a split network using the Neighbor-Net procedure (Bryant and Moulton, 2004), as implemented in the program SplitsTree4 v4.9.1 (Huson and Bryant, 2006). We tested for evidence of recombination for each of the LOS and CPS orthologs as follows. First, nucleotide sequences were aligned using Probalign v1.1 (Roshan and Livesay, 2006). We then tested for recombination using a combination of three methods: Pairwise Homoplasy Index (PHI), Neighbour Similarity Score (NSS), and Maximum χ2, as implemented in the program PhiPack (Bruen et al., 2006). The first two methods (PHI and NSS) are compatibility methods that examine pairs of sites for homoplasy (Bruen et al., 2006; Jakobsen and Easteal, 1996). Maximum χ2 is a substitution distribution method that searches for significant clustering of substitutions at putative recombination break points (Maynard Smith, 1992).
Evolutionary relationships among strains were compared to the distribution of LOS and CPS classes among strains. For C. coli and C. jejuni subsp. jejuni the number of core orthologs (orthologs seen in all strains of each species) were 832 and 774 respectively. The nucleotide sequences for these orthologs were aligned using Probalign and then tested for evidence of recombination using the procedure outlined above. Orthologs showing evidence for recombination for one or more of the methods (C. coli = 433, C. jejuni subsp. jejuni = 568) were removed. Maximum Likelihood (ML) phylogenies (gene-trees) for each of the remaining orthologs (C. coli = 399, C. jejuni subsp. jejuni = 206) were constructed using PhyML v3.0. The GTR substitution model was employed. Using the two sets of gene trees, consensus phylogenies (species-trees) for each species were constructed using the Triple Construction Method as implemented in the program Triplec (Ewing et al., 2008). Species trees with four or more taxa can produce anomalous gene-trees (AGTs), which for a given data set, are more probable to observe than a tree that is congruent with the species-tree (Degnan and Rosenberg, 2006). Based on the observation that rooted three taxa trees (rooted triples) do dot exhibit AGTs, the Triple Construction Method searches all gene-trees for the most frequent of the three possible rooted triples for each set of three taxa. Once found, the set of rooted triples are joined to form the species-tree using the quartet puzzling heuristic (Strimmer and vonHaeseler, 1996). The procedure has been shown to be a statistically consistent estimator of the species-tree topology and to out perform majority-rule and greedy consensus methods (Degnan et al., 2009). The distribution of LOS and CPS classes among strains was overlain on each of the species-trees.
Open reading frames from single representatives of each class for each gene cluster and species were searched for homopolymeric sequence repeats (i.e. An/Tn/Gn/Cn) using the script Poly (Gotz et al., 2008). Fragment ORFs clearly resulting from frame shifts caused by homopolymeric sequence repeats were deemed transient due to phase variation and not regarded as valid orthologs.
3. Results and discussion
3.1. Gene content diversity
Similar to that described for C. jejuni subsp. jejuni, our orthology assignment revealed a high level of gene content diversity in C. coli for both the LOS and CPS gene clusters. For example, we detected 51 distinct orthologs occurring within C. coli’s LOS, with these 51 orthologs occurring in eight distinct combinations, which we designated classes I through VIII (Table 1, Fig. 1). Following Gilbert et al. (2002), the conserved LOS biosynthesis genes waaC and waaF were considered the first and last genes of the cluster respectively. Orthologs occurring between (and including) these genes were considered as part of the LOS cluster. For C. jejuni subsp. jejuni, we detected 40 LOS orthologs, 11 fewer than observed for C. coli. However, these orthologs occurred in more combinations producing 22 distinct classes, which included four new classes not previously described. The previously described 18 LOS classes (Gilbert et al., 2002; Godschalk et al., 2004; Parker et al., 2008) are designated A through S (excluding N for C. jejuni subsp. doylei). Here we continue this nomenclature and designate the four new classes as T through W (Table 1, Fig. 1).
Fig. 1.
(A and B). C. coli and C. jejuni subsp. jejuni ortholog content for the LOS and CPS gene clusters. Arrows represent orthologs, and numbers are ortholog ID numbers (see Table S1 in the supplementary material for associated annotations). Grey arrows: orthologs belonging to the core genome (i.e. orthologs seen in all strains), with all remaining arrows representing orthologs belonging to the dispensable genome. Yellow arrows: orthologs present in the LOS of both species. White arrows: orthologs present in the CPS of both species. Green arrows: orthologs present in at least three of the following four possible locations (i) C. coli LOS, (ii) C. coli CPS, (iii) C. jejuni subsp. jejuni LOS, (iv) C. jejuni subsp. jejuni CPS. Only ortholog 6 was present in all four locations. Blue arrows: C. coli orthologs not present in the LOS or CPS of C. jejuni subsp. jejuni. Red arrows: C. jejuni subsp. jejuni orthologs not present in the LOS or CPS of C. coli. Orange arrows: orthologs present in the LOS of C. coli and the CPS of C. jejuni subsp. jejuni. Brown arrows: orthologs present in the CPS of C. coli and the LOS of C. jejuni subsp. jejuni. For reference, names of the first and last genes within each cluster and associated ortholog IDs were as follows: 25=waaC, 23=waaF, 35=kpsF, 31=kpsC. CDS for orthologs marked with (i) an asterisk were fragmented (see Table S1 in the supplementary material for details), and (ii) a plus sign showed evidence of phase variation. Grey shading highlights sialic acid gene cassette discussed in text. Note: C. jejuni subsp. doylei was omitted from the figure due to space considerations. However, gene content information for this species can be found in Table S1 in the supplementary material.
For the CPS, we followed Karlyshev et al. (2005) and considered the conserved genes kpsF and kpsC as the first and last genes of the cluster respectively. The CPS for both C. coli and C. jejuni subsp. jejuni contained almost twice the number orthologs than observed within the LOS (95 and 93 orthologs respectively). However, these orthologs were delineated into the same number of classes for C. coli (eight) and fewer classes for C. jejuni subsp. jejuni (12). The eight CPS classes for C. coli were designated I through VIII (Table 1, Fig. 1). The 12 CPS classes detected for C. jejuni subsp. jejuni included seven new classes not previously described, which we designated as classes F through L following Karlyshev et al. (2005) (Table 1, Fig. 1). C. jejuni subsp. doylei possessed a distinctive class which we designated M.
Subsequent to completion of our analyses, additional CPS sequence data for eight strains of C. jejuni subsp. jejuni were deposited at NCBI (Poly et al., 2011) (human isolates). An additional strain (ATCC43457) with the Accession EU200439 is also in the database, however, there appears to be no associated publication regarding generation of these data. Although the absence of these additional sequences from our analyses did not significantly affect the major findings of our study, we nevertheless proceeded to determine whether these sequences represented additional CPS classes. We constructed a database comprising 12 contiguous sequences representing each of the 12 classes described above and then used discontinuous Megablast to search it for the nine new sequences. Two pairs of the new sequences had identical gene content and none of the nine sequences had contiguous matches along their entire length with any of the sequences in the database, suggesting that these sequences represented an additional seven CPS classes for C. jejuni subsp. jejuni. Typically, there were matches for contiguous stretches of four to seven genes. However, strain ATCC43442 was very similar to class F, with this latter class having four additional genes. Accession numbers representing the seven additional classes are as follows: HQ343267, HQ343269, HQ343270 HQ343271, HQ343272, HQ343274, and EU200439.
3.2. Sialic acid biosynthesis orthologs
Previous studies have demonstrated that ganglioside mimicry is an important factor contributing to C. jejuni subsp. jejuni’s ability to cause Guillain Barré syndrome (GBS) (Gilbert et al., 2000; Godschalk et al., 2004). Three classes of C. jejuni subsp. jejuni LOS (A, B, and C) have been shown to be the most frequently associated with GBS (Godschalk et al., 2004; Parker et al., 2005), and these three classes possess genes capable of synthesizing and transferring sialic acid, which is an essential component of gangliosides (Godschalk et al., 2004). Specifically, these genes (which cluster together) are three sialic acid biosynthesis genes (neuBCA) and a gene encoding a sialic acid transferase (cst). More recently, Parker et al. (2008) discovered two new classes (R and M) that also contain these genes and thus have potential to cause GBS. Here we present an additional class (V) that also possess these genes and therefore likely has a similar potential (Table 1, Fig. 1).
The ortholog identification numbers arising from the OrthoMCL analysis corresponding to each of the sialic acid genes were as follows: cst = 1501, neuB = 9, neuC = 1645, neuA = 58 (however, also see discussion below for an expanded delineation of ortholog 58). The discussion that follows makes use of ortholog numerical identifiers, partly to make it easier to follow along with Fig. 1, and partly because the LOS cluster in particular has a history of various gene names being applied to the different loci; Table S1 in the supplementary material should be consulted for the associated locus tags/protein IDs, annotations, and GO-terms. Table S1 also contains the previous gene identifiers of Godschalk et al. (2004) and Parker et al. (2008) so that they can be cross-referenced to our ortholog identification numbers. Three of the sialic acid orthologs (9, 1645, and 58) were also found in a contiguous cluster in two strains of C. coli (H8 and 2553). However, rather than occurring in the LOS, these orthologs were found imbedded in the CPS. Strains H8 and 2553 represent two distinct CPS classes for C. coli (VII and VIII respectively). The fourth ortholog seen in the sialic acid gene cluster of C. jejuni subsp. jejuni (1501) was absent in C. coli. However, in its place was another ortholog (2742), which for strains H8 and 2553 had very similar functional annotation to the CDS representing 1501. The PGAAP annotation for 1501 was alpha-2,3 sialyltransferase, whereas 2742 in strains H8 and 2553 was annotated as a hypothetical protein. However, the Blast2GO annotation for both orthologs was alpha sialyltransferase. Furthermore, both orthologs shared the same molecular function GO-term of “transferase activity, transferring glycosyl groups,” suggesting a role as a sialic acid transferase.
Although preliminary without further functional analyses, these findings nevertheless suggest that (i) certain strains of C. coli may have the potential to cause GBS, and (ii) rather than the LOS, the CPS might be involved in ganglioside mimicry. Furthermore, our findings appear concordant with previous studies examining the potential of C. coli to induce GBS. For example, Bersudsky et al. (2000) examined a C. coli strain isolated from a patient with GBS and found evidence for a ganglioside-like epitope within its lipopolysaccharide (LPS) [subsequently, Karlyshev et al. (2000) demonstrated that the C. jejuni subsp. jejuni LPS was in fact the CPS]. Whereas Funakoshi et al. (2006) and van Belkum et al. (2009) found no evidence for ganglioside mimicry in the LOS of C. coli strains isolated from GBS patients.
It is of note that for C. coli only strains H8 and 2553 contained neuBCA and a sialyltransferase contiguously. Most of the remaining C. coli strains contained only one or two of these genes and they were located external to the LOS or CPS (Table S2 supplementary material). For C. jejuni subsp. jejuni, only classes A, B, C, M, R, and V contained neuBCA and cst contiguously. Typically, these classes also contained an additional copy of neuB external to the LOS. For the remaining classes, the majority had only one or two of these genes typically within the LOS.
We also found the four sialic acid orthologs (1501, 9, 1645, and 58) within the genome sequence of Campylobacter upsaliensis, strain JV21. Although the loci were contiguous, they were clustered external of the LOS (254 CDS separated them). Interestingly, a second C. upsaliensis strain (RM3195), which had been associated with GBS (Goddard et al., 1997), lacked these four orthologs. The sensitivity of C. upsaliensis isolation methods has been questioned in the literature (Byrne et al., 2001; Lastovica and Le Roux, 2001). Consequently, it’s possible that the GBS patient infected with strain RM3195 may have been carrying additional strain/s of C. upsaliensis that did possess the sialic acid genes. C. upsaliensis is an emerging human gastrointestinal pathogen with two previous reports of association with GBS (Bourke et al., 1998; Goddard et al., 1997; Ho et al., 1997). Furthermore, the pathogen is frequently isolated from domestic cats and dogs, with evidence of transmission to humans (Acke et al., 2009; Bourke et al., 1998; Goossens et al., 1991; Gurgan and Diker, 1994). Although C. jejuni subsp. jejuni is also associated with domestic pets, recent studies have shown C. upsaliensis to be the most frequently recovered species from dogs (results for cats were less conclusive) (Acke et al., 2009; Koene et al., 2009; Parsons et al., 2010; Westgarth et al., 2009). Our findings suggest that there is the genetic potential for some strains of C. upsaliensis to induce GBS, and this in turn suggests the potential importance of domestic dogs as a potential reservoir for pathogens linked to this sequela.
3.3. Gene duplication
CDS representing ortholog 58 occurred frequently among and within C. jejuni subsp. jejuni LOS classes. For example, in addition to occurring in half of all the classes, this gene (or perhaps more accurately “gene-group”) also occurred twice in each of classes A and R and three times in class B. Furthermore, it also occurred occasionally within the CPS for both C. jejuni subsp. jejuni and C. coli. Using nucleotide sequences, we investigated the phylogenetic relationships of the members of this putative gene family. For C. jejuni subsp. jejuni LOS classes, CDS were sub-divided into six distinct groupings, which we designated 58-1a, 58-2, 58-3a, 58-3b, 58-4, and 58-5 (see Fig. S1 in the supplementary material). The family included CDS annotated as neuA (58-1a and 58-1b) and ctgA (58-2, 58-3a, 58-3b, and 58-5). Group 58-4 contained CDS that were an “in-frame fusion” of CDS from groups 58-1a and 58-2, as described previously by Gilbert et al. (2000). A single CDS designated 58-1b that was closely related to group 58-1a was found within the CPS of C. jejuni subsp. jejuni (class J). The LOS classes most frequently associated with GBS (A, B, and C) carried CDS from groups 58-1a and 58-4. However, classes R and M also contained CDS from group 58-1a, while class V contained CDS from group 58-4. CDS representing ortholog 58 from C. coli and C. upsaliensis that were contiguous with the other sialic acid genes formed two groups in the network that were designated 58-6a and 58-6b respectively. These CDS were closely related to CDS within group 58-1a (75.4% average pairwise nucleotide sequence identity), supporting their possible role sialic acid biosynthesis and GBS.
This analysis also suggests that neuA and ctgA are paralogs resulting from historical gene duplication. Subsequent to the neuA – ctgA duplication, ctgA appears to have undergone multiple additional duplications, with the 58-3a – 58-3b duplication being the most recent. Indeed, gene duplication appears to be a common feature of the evolutionary history of LOS and CPS for C. jejuni subsp. jejuni, with duplications in seven LOS classes and seven CPS classes (Fig. 1). Gene duplication events were also apparent in C. coli, occurring once in a LOS class and in two separate CPS classes. It seems likely that the fewer number of gene duplications observed for C. coli compared to C. jejuni subsp. jejuni is simply a reflection of the fewer number of C. coli LOS and CPS classes. Of the remaining Campylobacter species, we also detected duplication in C. lari, where ortholog 6 occurred three times (Fig. 2). The presence of multiple copies of an ortholog within a strain could also be due to lateral gene transfer.
Fig. 2.
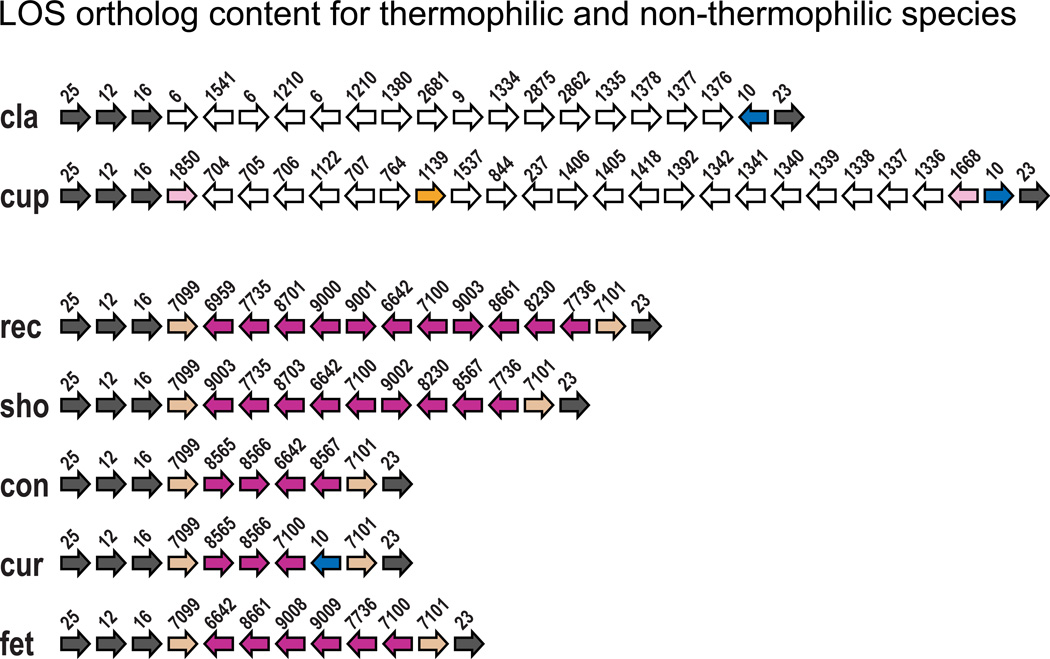
Ortholog content for the LOS gene cluster for thermophilic and non-thermophilic species other than C. jejuni subsp. jejuni and C. coli. Species abbreviations are as follows: cla=C. lari, cup=C. upsaliensis, rec=C. rectus, sho=C. showae, con=C. concisus, cur=C. curvus, fet=C. fetus subsp. fetus. Arrows represent orthologs, and numbers are ortholog ID numbers (see Table S1 for associated annotations). Grey arrows: with the exception of C. hominis and C. gracilis (see text for discussion), orthologs seen in all species. Blue arrows: ortholog is seen in all thermophilic species and two non-thermophilic species (C. curvus and C. hominis). Brown arrows: orthologs seen in all non-thermophilic species except C. hominis and C. gracilis. Purple arrows: dispensable non-thermophilic orthologs, none of which are seen in the thermophilic species. For C. upsaliensis, the pink and orange arrows show three dispensable orthologs shared with other thermophilic species. Pink orthologs occurred in the LOS of other species and the orange ortholog occurred in the CPS of other species. For reference, ortholog 25=waaC and ortholog 23=waaF.
3.4. Gene gain/loss and recombination
Numerous studies have provided evidence for lateral gene transfer (LGT) between the LOS of distinct strains of C. jejuni subsp. jejuni (Gilbert et al., 2004; Parker et al., 2008; Phongsisay et al., 2006). The bacterial core genome species concept first introduced by Dykhuizen and Green (1991) and later refined by Lan and Reeves (Lan and Reeves, 2000; 2001) proposed that the dispensable genome would turn over rapidly due to frequent LGT, and recent genomic work has supported this proposal (Donati et al., 2010). More specifically, Lefébure et al. (2010) showed that inter-species recombination for C. coli and C. jejuni subsp. jejuni predominated within the dispensable component of the genome. For the strains analyzed here, we regarded genes as dispensable for each species if they were not shared among all strains for the respective species. Core genes were shared among all strains of a species. With the exception of the five LOS and two CPS core flanking genes, all LOS and CPS genes for both species belonged to the dispensable genome.
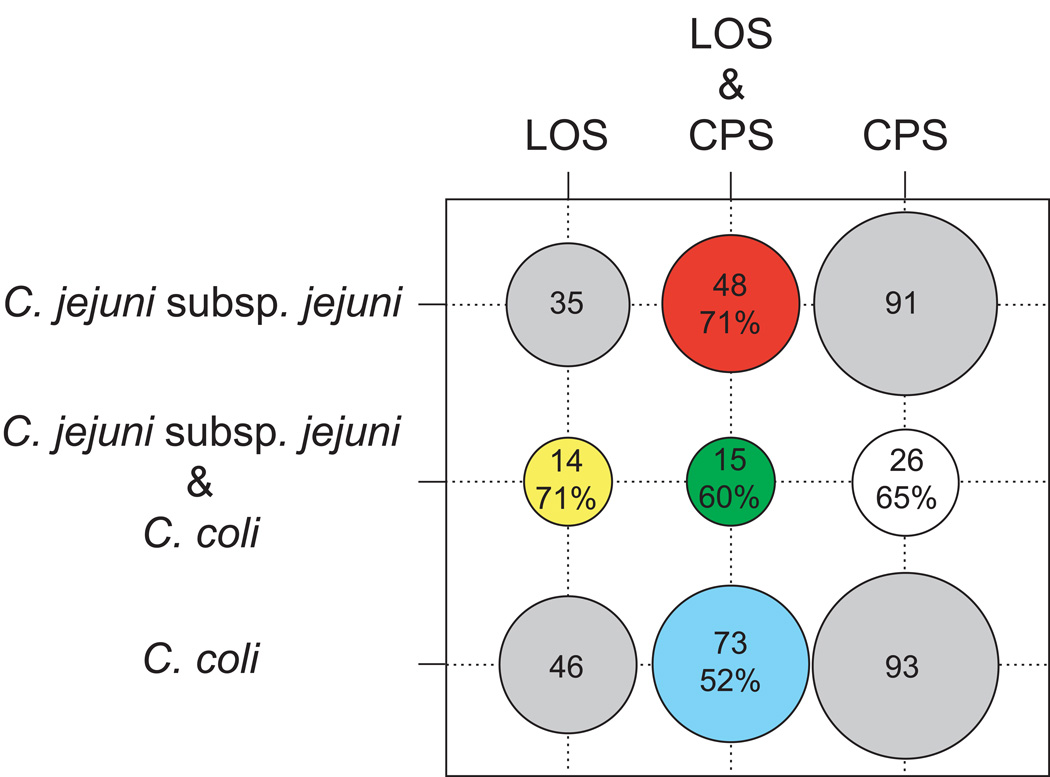
The principal forces that shape gene content diversity are gene gain/loss events within and among strains, and attempts at reconstructing evolutionary relationships using gene content are sensitive to these events (Kunin and Ouzounis, 2003). However, the number of genes shared by genomes has been shown to depend on evolutionary distance (Fitz-Gibbon and House, 1999; Snel et al., 1999; Tekaia et al., 1999). Therefore, if gene gain/loss events occur more frequently within the dispensable genome of strains of the same species, gene content analyses should group strains by species. We constructed a gene content network using genome wide orthologs not shared among all C. coli and C. jejuni subsp. jejuni strains (i. e. the collective dispensable genome) and C. coli and C. jejuni subsp. jejuni grouped strongly by species (Fig. S2 in the supplementary material). In contrast, gene content networks based exclusively on LOS and CPS orthologs showed the LOS and CPS classes to not group by species (Fig. 3). Two possible explanations for these observations are (i) frequent LGT between C. coli and C. jejuni subsp. jejuni for LOS and CPS genes, and (ii) gene loss events that are decreasing the gene content distinctiveness of each species (i.e. the loss of species-specific LOS/CPS dispensable genes). There were 188 distinct orthologs within the LOS and CPS for C. coli and C. jejuni subsp. jejuni, and 113 (60.1%) of these orthologs showed evidence for recombination for one or more of the recombination methods. This result supports frequent LGT for the LOS and CPS genes. It should be noted that our power to detect recombination for orthologs only occurring in a few strains was diminished due to the lower number of sequences in the respective ortholog alignment. The distribution of recombinant orthologs provides support for inter-species LGT (Fig. 4). For example, 71% of LOS ortholgs and 65% of CPS orthologs shared between both species were recombinant. On average, these frequencies were approximately equal to those for recombinant LOS/CPS orthologs specific to each species (C. coli = 52% and C. jejuni subsp. jejuni = 71%).
Fig. 3.
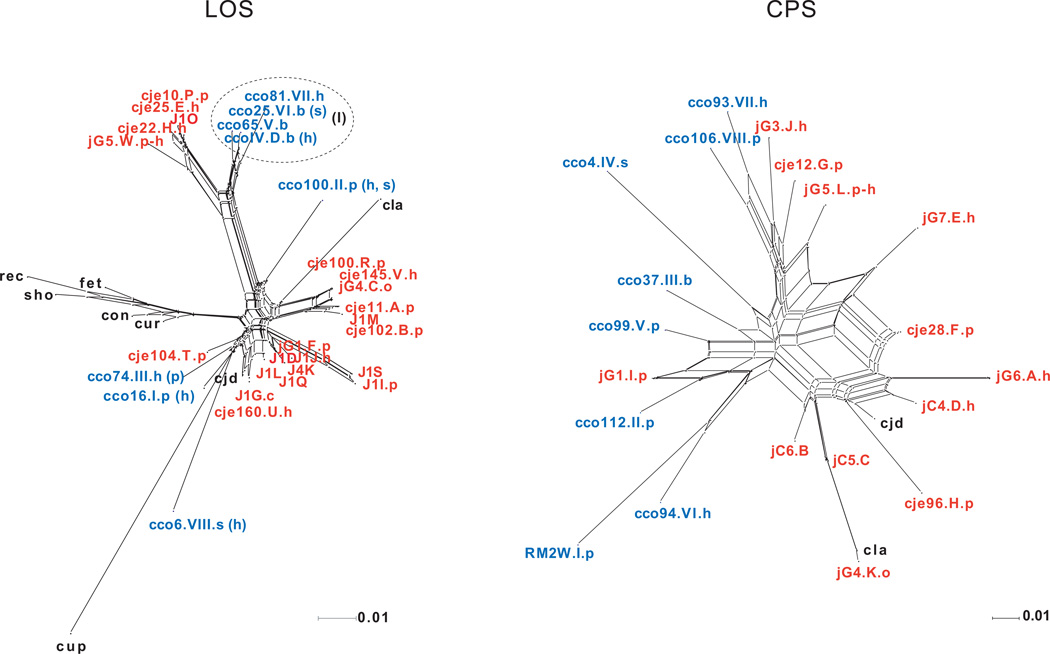
Split networks based on presence/absence of orthologs depicting gene content similarities among LOS and CPS gene cluster classes. Networks contain single representative C. coli and C. jejuni subsp. jejuni strains for each class (class IDs are shown in upper case following strain ID). C. coli strains are shown in blue and C. jejuni subsp. jejuni strains in red. Remaining taxa codes are as follows: cjd=C. jejuni subsp. doylei, cla=C. lari, cup=C. upsaliensis, rec=C. rectus, sho=C. showae, con=C. concisus, cur=C. curvus, fet=C. fetus subsp. fetus (also see Table 1 for additional strain information). Where present, lower case letters following class IDs for C. coli and C. jejuni subsp. jejuni show strain isolation source: h=human, p=poultry, b=bovine, s=swine, o=ovine, c=caprine. Many classes were isolated from multiple sources. For C. coli additional sources for each LOS class are shown in parentheses (see Table 1 for complete source distribution). The cluster of C. coli LOS classes (I) restricted to human, bovine, and swine sources is indicated with a dashed oval.
Fig. 4.
Distribution and overlap of the dispensable orthologs present in the LOS and CPS of C. coli and C. jejuni subsp. jejuni (the five core LOS and two core CPS orthologs are excluded). Circles shaded grey show the number of distinct orthologs observed in each gene cluster for each species. Circles shaded red, yellow, green, white, and blue correspond to the arrows (orthologs) in Fig.1 and show the number of distinct orthologs in each category (see Fig. 1 caption for a description of the categories). The proportion of orthologs in each of these categories showing evidence of recombination is also shown.
The frequency of recombinant orthologs present in at least three of the following four possible locations (i) C. coli LOS, (ii) C. coli CPS, (iii) C. jejuni subsp. jejuni LOS, and (iv) C. jejuni subsp. jejuni CPS was 60%, providing support for LGT between the LOS and CPS. The sialic acid cluster of orthologs (1501-9-1645-58) provide a specific example of this type of exchange: these orthologs are common in the LOS of many strains of C. jejuni subsp. jejuni, absent in the LOS of C. coli, and yet three of them are present in the CPS of C. coli, suggesting that they have been exchanged inter-specifically between the LOS and CPS. The much higher frequency of these orthologs in C. jejuni subsp. jejuni suggests that they may have been transferred from this species to C. coli. Indeed, Sheppard et al. (2008) recently showed genetic exchange between these species to be strongly biased in the C. jejuni subsp. jejuni to C. coli direction. Furthermore, for both C. coli CPS classes VII and VIII, the sialic acid genes were part of a five-gene cassette (58, 1645, 9, 2742, and 2688) that was oriented in the opposite direction to all remaining CPS genes in all classes (Fig. 1), again suggesting that these genes were imported into the CPS. A previous comparison of C. coli (RM2228) and C. jejuni subsp. jejuni (RM1221) suggested that LGT between these two strains was biased towards certain biological functional categories. Here we highlight that LGT for LOS and CPS genes is an important component of genetic exchange both within and between C. coli and C. jejuni subsp. jejuni.
While LOS and CPS genes are fundamental virulence components for C. coli and C. jejuni subsp. jejuni, with the exception of the conserved flanking genes, they are components of a large collective pool of dispensable genes. It appears that many of these genes can be assembled into multiple combinations of LOS and CPS classes, providing both these species with the ability to adapt and evade patient defenses. The extent of this diversity is apparent when you compare the much higher LOS ortholog diversity within C. coli and C. jejuni subsp. jejuni to the diversity observed among all the non-thermophilic species combined (Fig. 3), which likely reflects the greater virulence potential of C. coli and C. jejuni subsp. jejuni. Furthermore, this shared dispensable gene pool may extend to other thermophilic species, but exclude the non-thermophilic species. For example, C. jejuni subsp. doylei, and C. lari shared at least 70% of their LOS and CPS orthologs with other thermophilic species, whereas the thermophilic and non-thermophilic species shared no LOS dispensable orthologs (Fig. 2). A phylogeny for the genus supports the non-thermophilic and thermophilic species as two distinct clades (Lefebure and Stanhope, 2009), suggesting the dispensable LOS loci first appeared on the branch leading to the thermophilic species. The LOS of C. upsaliensis was unusual among the thermophiles in that it only shared three dispensable orthologs with the other thermophilic species (Fig. 2). C. upsaliensis was particularly distinctive in that it also lacked a characteristic CPS (see below). However, despite the apparent lack of lateral exchange involving the LOS for C. upsaliensis, this species nevertheless may have obtained the sialic acid genes via LGT, as the absence of these genes from C. upsaliensis strain RM3195 suggests they were either gained via LGT or present in the ancestor and lost in RM3195.
3.5. Comparison to non-thermophilic Campylobacter
With the exception of two non-thermophilic species (C. hominis and C. gracilis), all Campylobacter species possessed LOS gene content types unique to each taxon. The diversity of LOS types is undoubtedly a reflection of the fact that there are many genes serving as potential LOS loci and thus many combinations are possible. For several of these taxa there is at present only a single genome sequence. However, it is of note that with the LOS cluster sequenced for 33 C. coli and 60 C. jejuni subsp. jejuni strains, we found no evidence of interspecific overlap in LOS class, but plenty of intraspecific/interstrain overlap in LOS class, tending to support a biological distinction between these two taxa. Typically, genes waaCM (5’ end) and waaVF (3’ end) flank the LOS gene cluster. However, orthologs corresponding to waaC, and waaF were absent from C. hominis, and orthologs corresponding to waaC, waaV, and waaF were absent from C. gracilis, with both species lacking a characteristic cluster of LOS biosynthesis genes. For C. hominis, this may reflect the fact that this species is likely a non-pathogenic commensal of the human gastrointestinal tract (strains have been repeatedly isolated from human fecal material obtained from healthy individuals) (Lawson et al., 2001). In addition, C. hominis and C. gracilis also share an “unusual aflagellate rod-like cell structure” and high 16S rDNA sequence similarity (Lawson et al., 2001) suggesting a close relationship between these two species.
With the exception of C. upsaliensis, the CPS gene cluster of the thermophilic Campylobacter species was flanked by genes kpsFDETM (5’ end) and kpsCS (3’ end). Although C. upsaliensis possessed both of these sets of genes, they were separated by approximately 83kbp, and there were no genes characteristic of the CPS immediately down-stream of kpsFDETM (e.g. capsular polysaccharide biosynthesis and transfer). None of the non-thermophilic species possessed the cluster of CPS biosynthesis genes typical of the thermophilic species. For example, with the exception of kpsF and kpsT, all orthologs corresponding to the kps genes were absent from the non-thermophilic species (the ortholog corresponding to kpsT was only present in C. rectus, C. showae, and C. fetus subsp. fetus).
3.6. Distribution of LOS and CPS classes among hosts
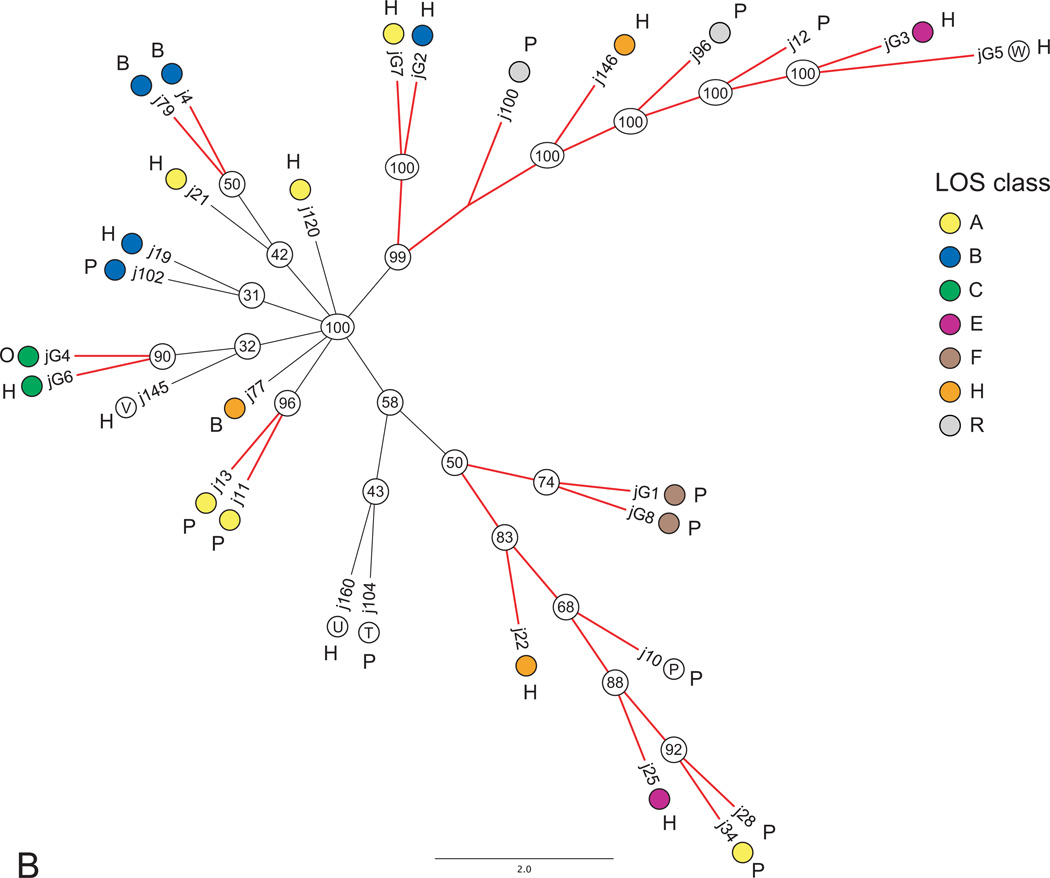
Previous studies for C. jejuni subsp. jejuni and C. coli have shown genetic partitioning among isolates with regard to source of isolation (Champion et al., 2005; Colles et al., 2011; Miller et al., 2006; Sheppard et al., 2010). Therefore, employing split networks depicting shared ortholog content, we investigated the possibility that LOS and CPS classes might have non-random distributions among hosts. The results revealed a non-random pattern for the LOS of C. coli. For example, all bovine strains were restricted to classes IV, V, and VI (Table 1), and strains possessing these LOS classes formed a tight cluster (I) in the split network (Fig. 3). This cluster also included the sole representative of class VII, a human sourced isolate. Our analysis included 33 C. coli strains isolated from four different host groups: human (n=12), poultry (turkey=6, chicken=3), bovine (n=7), and swine (n=5). Although there was overlap regarding human and swine sourced isolates between cluster I and all remaining strains, none of the poultry sourced isolates occurred in cluster I. Consequently, our results show strong partitioning between bovine and poultry sourced isolates, with bovine sourced isolates restricted to classes IV, V, and VI and poultry sourced isolates restricted to classes I, II, and III (Fisher exact test: P = 0.0006). In addition, the phylogenetic analysis (Fig. 5A) showed strong support for a grouping that contained all but one of the bovine sourced isolates.
Fig. 5.
(A and B). Phylogenies showing the consensus of 399 and 206 gene-trees for C. coli (A) and C. jejuni subsp. jejuni (B) respectively. Numbers in circles show the proportion of gene-trees that supported a particular grouping. Branches for groupings with greater than 50% support are shown in red. Colored circles identify the LOS class possessed by a strain. Strain isolation source is shown with a single letter code (H=human, B=bovine, S=swine, and P=poultry). A well supported grouping of bovine sourced isolates for C. coli is indicated with a dashed line.
Our findings are concordant with the previous genomic hybridization work of Lang et al. (2010) that showed a tendency for strains to cluster by isolation source and the study of Miller et al. (2006), which showed MLST alleles to be source associated. However, our findings additionally suggest a possibly more precise basis for this association, in that strains possessing a specific LOS structure might be more successful at colonizing certain species. More specifically, classes in cluster I possessed seven orthologs (8, 1742, 1743, 1790, 1959, 2066, and 2089) not seen in any other C. coli LOS class (see Table S1 in the supplementary material for annotations). Two of these orthologs (1742 [glucose-1-phosphate thymidylyltransferase] and 1743 [dTDP-glucose dehydratase]) were involved in glucose metabolism. With the exception of class VIII, none of the other C. coli classes possessed genes involved in glucose metabolism. Although class VIII also possessed glucose metabolism genes, it was distinct from all other LOS classes (including C. jejuni subsp. jejuni) as it contained two genes for mannose metabolism. Concordant with Miller et al. (2006), we also detected less genetic diversity for bovine C. coli, suggesting a more recent colonization. The partitioning of C. coli strains by source, could lead to more efficient source tracking following human infection (Miller et al., 2006).
3.7. Convergence
Comparison of the evolutionary relationships among strains to the distribution of LOS classes among strains can be seen in Fig. 5. Despite low support for several sections of the C. coli phylogeny (species-tree), many groupings were supported by greater than 50% of the gene-trees. Focusing on these groupings, there were multiple examples of incongruence between evolutionary relationships among strains and the distribution of LOS classes. Specifically, there were examples of strains with different evolutionary histories converging on the same class for six of the eight classes (I, II, III, IV, V, and VI) It was impossible to assess convergence for class VII as it was only represented by a single strain. A possible explanation for this observation may be related, in part, to the source partitioning as described above. For example, classes I, II, and III have converged to poultry whereas classes IV, V, and VI have converged to bovine, with this convergence possibly driven by source environment selection. A similar pattern has been observed for mammalian gut bacterial communities converging on diet rather than the mammalian phylogeny (Ley et al., 2008; Muegge et al., 2011). Nucleotide sequence identities among C. coli LOS orthologs provide support for this hypothesis. For example, orthologs 6 (putative glycosyltransferase), 1541 (glycosyltransferase), and 1715 (hypothetical protein) were among those seen in both classes II and IV (Fig. 1). Average pairwise identities among strains within each class for each of these orthologs was very high (ranging between 99.8% and 100%). Whereas identities between the classes for these orthologs was considerably lower. For example, pairwise identity between two strains from each class was 81.7% (6), 80.4% (1541), and 76.5% (1715). For a particular ortholog seen in different classes, these observations suggest an older split between the classes, with a more recent proliferation within a class, possibly driven by selection. The distribution of CPS classes among C. coli strains was less conclusive with only two examples of convergence (classes III and VI) (Fig. S3 in the supplementary material). However, additional sampling will be required to more accurately assess the distribution of C. coli CPS classes among sources (see Table 1).
Overall, groupings within the C. jejuni subsp. jejuni phylogeny (species-tree) were well supported (Fig. 5). Seven classes were represented by multiple strains. Of these, five showed examples of convergence: A, B, E, H, and R. Sequence identities among C. jejuni subsp. jejuni LOS orthologs also showed a similar pattern to C. coli. For example, orthologs 6 (galactosyltransferase), 9 (neuB), and 1645 (neuC) were among those seen in classes A, B, C and R (Fig 1). Average pairwise identities among strains within each class for each of these orthologs was again high (ranging between 96.9% and 100%). Whereas identities between the classes for these orthologs was considerably lower. For example, pairwise identity between single strains from each class was 87.7% (6), 85.8% (9), and 84.3% (1645). Following the same logic as for C. coli, these observations again suggest recent selection for these classes. However, the factors responsible are less clear, as unlike C. coli, there is no apparent source partitioning for C. jejuni subsp. jejuni. For the C. jejuni subsp. jejuni CPS, there were only four strains that could be compared: two for class I, and two for class F. Interestingly, neither showed a pattern of convergence (phylogeny not shown). However, additional samples should be analyzed before convergence is rejected.
3.8. Additional mechanisms creating diversity
Phase variation due to homopolymeric tracts is often reported as an additional mechanism responsible for LOS and CPS structural variation in C. jejuni subsp. jejuni (Gilbert et al., 2002; Godschalk et al., 2004; Karlyshev et al., 2005; Parker et al., 2008; Parker et al., 2005; Parkhill et al., 2000). We assessed the potential for this mechanism to cause similar variation in C. coli and the other Campylobacter species included in this study by searching the nucleotide sequence of open reading frames (ORFs) from the LOS and CPS for homopolymeric sequence repeats. Results showed C. coli to have a comparable number of homopolymeric sequence repeats to C. jejuni subsp. jejuni (Table S3 in the supplementary material), suggesting that C. coli had a similar capacity for phase variation as that previously reported for C. jejuni subsp. jejuni. Indeed, for those C. coli classes containing multiple strains, sequence alignments revealed four clear instances where a homopolymeric tract appeared to have caused a reading frame shift (see Fig. 1). However, the possibility that sequencing error may have caused these shifts should also be considered.
The potential for phase variation was also detected for C. upsaliensis. Strain JV21 contained a homopolymeric C tract (C13) at the 3’ end of ortholog 1501 (one of the four orthologs associated with GBS). The homopolymeric tract appeared to have shifted the reading frame for this ortholog resulting in its premature termination and subsequent creation of a small 156bp ORF. Alignment to C. jejuni subsp. jejuni showed this small ORF to be a fragment of ortholog 1501, and the addition of one extra C to the C13 tract via manual editing extended the ORF to encompass the entire length of ortholog 1501. This suggests that C. upsaliensis might be able to phase modulate the genes implicated in GBS. Overall, the thermophilic species had more repeats than the non-thermophilic species, suggesting the potential for adaptive flexibility and increased virulence of the thermophilic Campylobacter species. Another mechanism likely responsible for LOS and CPS structural diversity is gene inactivation due to the disruption of reading frames by substitutions, insertions and deletions. An example of this process for C. coli involves class III where multiple indels have fragmented ortholog 1501 into several distinct ORFs.
4. Conclusion
Although mechanisms such as phase variation and gene duplication are important factors creating LOS and CPS structural diversity for both C. coli and C. jejuni subsp. jejuni, the most important factor appears to be that these genes comprise a highly dynamic component of the dispensable genome and that frequent LGT has likely contributed to their assembly into a highly diverse array of combinations. This genetic exchange might also extend to other thermophilic Campylobacter species such as C. jejuni subsp. doylei and C. lari. In addition to suggesting a close association among these thermophilic Campylobacter species, this also suggests repeated LGT within a shared gastrointestinal environment. Selection pressure of source environment might be an additional factor creating LOS gene content diversity for C. coli. The thermophilic species had in general much higher diversity than the non-thermophilic species likely reflecting increased virulence potential. In addition to evading patient defenses, the highly dynamic nature of the LOS/CPS gene content likely has important epidemiological implications. For example, recent bovine colonization by C. coli may have been facilitated by the capacity to rapidly produce a distinct combination of LOS genes, with the resulting source differentiation in turn providing a valuable aid for source tracking. Furthermore, repeated LGT of the dispensable LOS/CPS genes may have transferred genes implicated in GBS from C. jejuni subsp. jejuni to C. coli, with C. upsaliensis possibly involved in a similar type of exchange. Although preliminary without further functional analyses, these findings highlight the potential for GBS development following Campylobacter infection from swine, and domestic cat/dog sources.
Supplementary Material
High C. coli LOS/CPS gene diversity: each cluster delineated into eight gene content classes
Diversity predominantly due to extensive gene gain/loss both within and between species and also between the LOS and CPS
Additional mechanisms: phase-variation, gene duplication/inactivation, selection pressure
Sialic acid biosynthesis homologs detected in C. coli and C. upsaliensis
C. coli LOS classes differentiated between bovine and poultry hosts
Acknowledgements
This project was supported by the Cornell University Zoonotic Research Unit of the Food and Waterborne Diseases Integrated Research Network, which was funded by the National Institute of Allergy and Infectious Diseases, US National Institutes of Health, under contract number N01-AI-30054 (ZC003-05 and ZC010-09) awarded to M.J.S.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acke E, McGill K, Golden O, Jones BR, Fanning S, Whyte P. Prevalence of thermophilic Campylobacter species in household cats and dogs in Ireland. Vet Rec. 2009;164:44–47. doi: 10.1136/vr.164.2.44. [DOI] [PubMed] [Google Scholar]
- Alfredson DA, Korolik V. Antibiotic resistance and resistance mechanisms in Campylobacter jejuni and Campylobacter coli. FEMS Microbiol Lett. 2007;277:123–132. doi: 10.1111/j.1574-6968.2007.00935.x. [DOI] [PubMed] [Google Scholar]
- Ang CW, Jacobs BC, Laman JD. The Guillain-Barre syndrome: a true case of molecular mimicry. Trends Immunol. 2004;25:61–66. doi: 10.1016/j.it.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Bacon DJ, Szymanski CM, Burr DH, Silver RP, Alm RA, Guerry P. A phase-variable capsule is involved in virulence of Campylobacter jejuni 81–176. Mol Microbiol. 2001;40:769–777. doi: 10.1046/j.1365-2958.2001.02431.x. [DOI] [PubMed] [Google Scholar]
- Bersudsky M, Rosenberg P, Rudensky B, Wirguin I. Lipopolysaccharides of a Campylobacter coli isolate from a patient with Guillain-Barre syndrome display ganglioside mimicry. Neuromuscul Disord. 2000;10:182–186. doi: 10.1016/s0960-8966(99)00106-6. [DOI] [PubMed] [Google Scholar]
- Blumer C, Roche P, Spencer J, Lin M, Milton A, Bunn C, Gidding H, Kaldor J, Kirk M, Hall R, Della-Porta T, Leader R, Wright P. Australia's notifiable diseases status, 2001: annual report of the National Notifiable Diseases Surveillance System. Commun Dis Intell. 2003;27:1–78. doi: 10.33321/cdi.2003.27.1. [DOI] [PubMed] [Google Scholar]
- Bourke B, Chan VL, Sherman P. Campylobacter upsaliensis : waiting in the wings. Clin Microbiol Rev. 1998;11:440–449. doi: 10.1128/cmr.11.3.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruen TC, Philippe H, Bryant D. A simple and robust statistical test for detecting the presence of recombination. Genetics. 2006;172:2665–2681. doi: 10.1534/genetics.105.048975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant D, Moulton V. Neighbor-net: an agglomerative method for the construction of phylogenetic networks. Mol Biol Evol. 2004;21:255–265. doi: 10.1093/molbev/msh018. [DOI] [PubMed] [Google Scholar]
- Byrne C, Doherty D, Mooney A, Byrne M, Woodward D, Johnson W, Rodgers F, Bourke B. Basis of the superiority of cefoperazone amphotericin teicoplanin for isolating Campylobacter upsaliensis from stools. J Clin Microbiol. 2001;39:2713–2716. doi: 10.1128/JCM.39.7.2713-2716.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. Preliminary FoodNet data on the incidence of infection with pathogens transmitted commonly through food – selected sites, United States. Morbidity and Mortality Weekly Report. 2004;53:338–343. [PubMed] [Google Scholar]
- Champion OL, Gaunt MW, Gundogdu O, Elmi A, Witney AA, Hinds J, Dorrell N, Wren BW. Comparative phylogenomics of the food-borne pathogen Campylobacter jejuni reveals genetic markers predictive of infection source. Proc. Natl. Acad. Sci. U. S. A. 2005;102:16043–16048. doi: 10.1073/pnas.0503252102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colles FM, Ali JS, Sheppard SK, McCarthy ND, Maiden MC. Campylobacter populations in wild and domesticated Mallard ducks (Anas platyrhynchos) Environ Microbiol Rep. 2011;3:574–580. doi: 10.1111/j.1758-2229.2011.00265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degnan JH, DeGiorgio M, Bryant D, Rosenberg NA. Properties of consensus methods for inferring species trees from gene trees. Syst Biol. 2009;58:35–54. doi: 10.1093/sysbio/syp008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degnan JH, Rosenberg NA. Discordance of species trees with their most likely gene trees. PLoS Genet. 2006;2:e68. doi: 10.1371/journal.pgen.0020068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donati C, Hiller NL, Tettelin H, Muzzi A, Croucher NJ, Angiuoli SV, Oggioni M, Dunning Hotopp JC, Hu FZ, Riley DR, Covacci A, Mitchell TJ, Bentley SD, Kilian M, Ehrlich GD, Rappuoli R, Moxon ER, Masignani V. Structure and dynamics of the pan-genome of Streptococcus pneumoniae and closely related species. Genome Biol. 2010;11:R107. doi: 10.1186/gb-2010-11-10-r107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorrell N, Mangan JA, Laing KG, Hinds J, Linton D, Al-Ghusein H, Barrell BG, Parkhill J, Stoker NG, Karlyshev AV, Butcher PD, Wren BW. Whole genome comparison of Campylobacter jejuni human isolates using a low-cost microarray reveals extensive genetic diversity. Genome Res. 2001;11:1706–1715. doi: 10.1101/gr.185801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond A, Ashton B, Cheung M, Heled J, Kearse M, Moir R, Stones-Havas S, Thierer T, Wilson A. Geneious. (5.1.2 ed.) 2010 [Google Scholar]
- Dykhuizen DE, Green L. Recombination in Escherichia coli and the definition of biological species. J. Bacteriol. 1991;173:7257–7268. doi: 10.1128/jb.173.22.7257-7268.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewing GB, Ebersberger I, Schmidt HA, von Haeseler A. Rooted triple consensus and anomalous gene trees. BMC Evol Biol. 2008;8:118. doi: 10.1186/1471-2148-8-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitz-Gibbon ST, House CH. Whole genome-based phylogenetic analysis of free-living microorganisms. Nucleic Acids Res. 1999;27:4218–4222. doi: 10.1093/nar/27.21.4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman C, Neimann J, Wegener H, Tauxe R. Campylobacter. 2nd ed. Washington, DC: ASM Press; 2000. Epidemiology of Campylobacter jejuni infections in the United States and other industrialized nations; pp. 121–138. [Google Scholar]
- Funakoshi K, Koga M, Takahashi M, Hirata K, Yuki N. Campylobacter coli enteritis and Guillain-Barre syndrome: no evidence of molecular mimicry and serological relationship. J Neurol Sci. 2006;246:163–168. doi: 10.1016/j.jns.2006.02.010. [DOI] [PubMed] [Google Scholar]
- Gallay A, Simon F, Megraud F. Surveillance of human Campylobacter infections in France--part 1--which data? A study of microbiological laboratories, 2000. Euro Surveill. 2003;8:213–217. doi: 10.2807/esm.08.11.00431-en. [DOI] [PubMed] [Google Scholar]
- Gilbert M, Brisson JR, Karwaski MF, Michniewicz J, Cunningham AM, Wu Y, Young NM, Wakarchuk WW. Biosynthesis of ganglioside mimics in Campylobacter jejuni OH4384. Identification of the glycosyltransferase genes, enzymatic synthesis of model compounds, and characterization of nanomole amounts by 600-mhz (1)h and (13)c NMR analysis. J Biol Chem. 2000;275:3896–3906. doi: 10.1074/jbc.275.6.3896. [DOI] [PubMed] [Google Scholar]
- Gilbert M, Godschalk PC, Parker C, Endtz H, Wakarchuk WW. Genetic basis for the variation in the lipooligosaccharide outer core of Campylobacter jejuni and possible association of glycosyltransferase genes with post-infectious neuropathies. In: Ketley JM, Konkel ME, editors. Campylobacter : molecular and cellular biology. Norfolk: Horizon Bioscience; 2004. [Google Scholar]
- Gilbert M, Karwaski MF, Bernatchez S, Young NM, Taboada E, Michniewicz J, Cunningham AM, Wakarchuk WW. The genetic bases for the variation in the lipo-oligosaccharide of the mucosal pathogen, Campylobacter jejuni Biosynthesis of sialylated ganglioside mimics in the core oligosaccharide. J Biol Chem. 2002;277:327–337. doi: 10.1074/jbc.M108452200. [DOI] [PubMed] [Google Scholar]
- Goddard EA, Lastovica AJ, Argent AC. Campylobacter 0:41 isolation in Guillain-Barre syndrome. Arch Dis Child. 1997;76:526–528. doi: 10.1136/adc.76.6.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godschalk PC, Heikema AP, Gilbert M, Komagamine T, Ang CW, Glerum J, Brochu D, Li J, Yuki N, Jacobs BC, van Belkum A, Endtz HP. The crucial role of Campylobacter jejuni genes in anti-ganglioside antibody induction in Guillain-Barre syndrome. J Clin Invest. 2004;114:1659–1665. doi: 10.1172/JCI15707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goossens H, Vlaes L, Butzler JP, Adnet A, Hanicq P, N'Jufom S, Massart D, de Schrijver G, Blomme W. Campylobacter upsaliensis enteritis associated with canine infections. Lancet. 1991;337:1486–1487. doi: 10.1016/0140-6736(91)93182-9. [DOI] [PubMed] [Google Scholar]
- Gotz S, Garcia-Gomez JM, Terol J, Williams TD, Nagaraj SH, Nueda MJ, Robles M, Talon M, Dopazo J, Conesa A. High-throughput functional annotation and data mining with the Blast2GO suite. Nucleic Acids Res. 2008;36:3420–3435. doi: 10.1093/nar/gkn176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerry P, Szymanski CM. Campylobacter sugars sticking out. Trends Microbiol. 2008;16:428–435. doi: 10.1016/j.tim.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Guerry P, Szymanski CM, Prendergast MM, Hickey TE, Ewing CP, Pattarini DL, Moran AP. Phase variation of Campylobacter jejuni 81–176 lipooligosaccharide affects ganglioside mimicry and invasiveness in vitro. Infect. Immun. 2002;70:787–793. doi: 10.1128/iai.70.2.787-793.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurgan T, Diker KS. Abortion associated with Campylobacter upsaliensis. J Clin Microbiol. 1994;32:3093–3094. doi: 10.1128/jcm.32.12.3093-3094.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho TW, Hsieh ST, Nachamkin I, Willison HJ, Sheikh K, Kiehlbauch J, Flanigan K, McArthur JC, Cornblath DR, McKhann GM, Griffin JW. Motor nerve terminal degeneration provides a potential mechanism for rapid recovery in acute motor axonal neuropathy after Campylobacter infection. Neurology. 1997;48:717–724. doi: 10.1212/wnl.48.3.717. [DOI] [PubMed] [Google Scholar]
- Huson DH, Bryant D. Application of phylogenetic networks in evolutionary studies. Mol Biol Evol. 2006;23:254–267. doi: 10.1093/molbev/msj030. [DOI] [PubMed] [Google Scholar]
- Jakobsen IB, Easteal S. A program for calculating and displaying compatibility matrices as an aid in determining reticulate evolution in molecular sequences. Comput. Appl. Biosci. 1996;12:291–295. doi: 10.1093/bioinformatics/12.4.291. [DOI] [PubMed] [Google Scholar]
- Jones MA, Marston KL, Woodall CA, Maskell DJ, Linton D, Karlyshev AV, Dorrell N, Wren BW, Barrow PA. Adaptation of Campylobacter jejuni NCTC11168 to high-level colonization of the avian gastrointestinal tract. Infect Immun. 2004;72:3769–3776. doi: 10.1128/IAI.72.7.3769-3776.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlyshev AV, Champion OL, Churcher C, Brisson JR, Jarrell HC, Gilbert M, Brochu D, St Michael F, Li J, Wakarchuk WW, Goodhead I, Sanders M, Stevens K, White B, Parkhill J, Wren BW, Szymanski CM. Analysis of Campylobacter jejuni capsular loci reveals multiple mechanisms for the generation of structural diversity and the ability to form complex heptoses. Mol Microbiol. 2005;55:90–103. doi: 10.1111/j.1365-2958.2004.04374.x. [DOI] [PubMed] [Google Scholar]
- Karlyshev AV, Linton D, Gregson NA, Lastovica AJ, Wren BW. Genetic and biochemical evidence of a Campylobacter jejuni capsular polysaccharide that accounts for Penner serotype specificity. Mol Microbiol. 2000;35:529–541. doi: 10.1046/j.1365-2958.2000.01717.x. [DOI] [PubMed] [Google Scholar]
- Katoh K, Misawa K, Kuma K, Miyata T. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002;30:3059–3066. doi: 10.1093/nar/gkf436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketley JM. Pathogenesis of enteric infection by Campylobacter. Microbiology. 1997;143(Pt 1):5–21. doi: 10.1099/00221287-143-1-5. [DOI] [PubMed] [Google Scholar]
- Koene MG, Houwers DJ, Dijkstra JR, Duim B, Wagenaar JA. Strain variation within Campylobacter species in fecal samples from dogs and cats. Vet Microbiol. 2009;133:199–205. doi: 10.1016/j.vetmic.2008.06.022. [DOI] [PubMed] [Google Scholar]
- Kunin V, Ouzounis CA. The balance of driving forces during genome evolution in prokaryotes. Genome Res. 2003;13:1589–1594. doi: 10.1101/gr.1092603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan R, Reeves PR. Intraspecies variation in bacterial genomes: the need for a species genome concept. Trends Microbiol. 2000;8:396–401. doi: 10.1016/s0966-842x(00)01791-1. [DOI] [PubMed] [Google Scholar]
- Lan R, Reeves PR. When does a clone deserve a name? A perspective on bacterial species based on population genetics. Trends Microbiol. 2001;9:419–424. doi: 10.1016/s0966-842x(01)02133-3. [DOI] [PubMed] [Google Scholar]
- Lang P, Lefebure T, Wang W, Pavinski Bitar P, Meinersmann RJ, Kaya K, Stanhope MJ. Expanded multilocus sequence typing and comparative genomic hybridization of Campylobacter coli isolates from multiple hosts. Appl Environ Microbiol. 2010;76:1913–1925. doi: 10.1128/AEM.01753-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lastovica AJ, Le Roux E. Efficient isolation of Campylobacter upsaliensis from stools. J Clin Microbiol. 2001;39:4222–4223. doi: 10.1128/JCM.39.11.4222-4223.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson AJ, On SL, Logan JM, Stanley J. Campylobacter hominis sp. nov., from the human gastrointestinal tract. Int J Syst Evol Microbiol. 2001;51:651–660. doi: 10.1099/00207713-51-2-651. [DOI] [PubMed] [Google Scholar]
- Lefebure T, Bitar PD, Suzuki H, Stanhope MJ. Evolutionary dynamics of complete Campylobacter pan-genomes and the bacterial species concept. Genome Biol Evol. 2010;2:646–655. doi: 10.1093/gbe/evq048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebure T, Stanhope MJ. Pervasive, genome-wide positive selection leading to functional divergence in the bacterial genus Campylobacter. Genome Res. 2009;19:1224–1232. doi: 10.1101/gr.089250.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley RE, Hamady M, Lozupone C, Turnbaugh PJ, Ramey RR, Bircher JS, Schlegel ML, Tucker TA, Schrenzel MD, Knight R, Gordon JI. Evolution of mammals and their gut microbes. Science. 2008;320:1647–1651. doi: 10.1126/science.1155725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Stoeckert CJ, Jr, Roos DS. OrthoMCL: identification of ortholog groups for eukaryotic genomes. Genome Res. 2003;13:2178–2189. doi: 10.1101/gr.1224503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard Smith J. Analyzing the mosaic structure of genes. J. Mol. Evol. 1992;34:126–129. doi: 10.1007/BF00182389. [DOI] [PubMed] [Google Scholar]
- McSweegan E, Walker RI. Identification and characterization of two Campylobacter jejuni adhesins for cellular and mucous substrates. Infect. Immun. 1986;53:141–148. doi: 10.1128/iai.53.1.141-148.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller WG, Englen MD, Kathariou S, Wesley IV, Wang G, Pittenger-Alley L, Siletz RM, Muraoka W, Fedorka-Cray PJ, Mandrell RE. Identification of host-associated alleles by multilocus sequence typing of Campylobacter coli strains from food animals. Microbiology. 2006;152:245–255. doi: 10.1099/mic.0.28348-0. [DOI] [PubMed] [Google Scholar]
- Moore JE, Corcoran D, Dooley JS, Fanning S, Lucey B, Matsuda M, McDowell DA, Megraud F, Millar BC, O'Mahony R, O'Riordan L, O'Rourke M, Rao JR, Rooney PJ, Sails A, Whyte P. Campylobacter. Vet Res. 2005;36:351–382. doi: 10.1051/vetres:2005012. [DOI] [PubMed] [Google Scholar]
- Muegge BD, Kuczynski J, Knights D, Clemente JC, Gonzalez A, Fontana L, Henrissat B, Knight R, Gordon JI. Diet drives convergence in gut microbiome functions across mammalian phylogeny and within humans. Science. 2011;332:970–974. doi: 10.1126/science.1198719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker CT, Gilbert M, Yuki N, Endtz HP, Mandrell RE. Characterization of lipooligosaccharide-biosynthetic loci of Campylobacter jejuni reveals new lipooligosaccharide classes: evidence of mosaic organizations. J Bacteriol. 2008;190:5681–5689. doi: 10.1128/JB.00254-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker CT, Horn ST, Gilbert M, Miller WG, Woodward DL, Mandrell RE. Comparison of Campylobacter jejuni lipooligosaccharide biosynthesis loci from a variety of sources. J Clin Microbiol. 2005;43:2771–2781. doi: 10.1128/JCM.43.6.2771-2781.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkhill J, Wren BW, Mungall K, Ketley JM, Churcher C, Basham D, Chillingworth T, Davies RM, Feltwell T, Holroyd S, Jagels K, Karlyshev AV, Moule S, Pallen MJ, Penn CW, Quail MA, Rajandream MA, Rutherford KM, van Vliet AH, Whitehead S, Barrell BG. The genome sequence of the food-borne pathogen Campylobacter jejuni reveals hypervariable sequences. Nature. 2000;403:665–668. doi: 10.1038/35001088. [DOI] [PubMed] [Google Scholar]
- Parsons BN, Porter CJ, Ryvar R, Stavisky J, Williams NJ, Pinchbeck GL, Birtles RJ, Christley RM, German AJ, Radford AD, Hart CA, Gaskell RM, Dawson S. Prevalence of Campylobacter spp. in a cross-sectional study of dogs attending veterinary practices in the UK and risk indicators associated with shedding. Vet J. 2010;184:66–70. doi: 10.1016/j.tvjl.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Pearson BM, Pin C, Wright J, I'Anson K, Humphrey T, Wells JM. Comparative genome analysis of Campylobacter jejuni using whole genome DNA microarrays. FEBS Lett. 2003;554:224–230. doi: 10.1016/s0014-5793(03)01164-5. [DOI] [PubMed] [Google Scholar]
- Phongsisay V, Perera VN, Fry BN. Exchange of lipooligosaccharide synthesis genes creates potential Guillain-Barre syndrome-inducible strains of Campylobacter jejuni. Infect Immun. 2006;74:1368–1372. doi: 10.1128/IAI.74.2.1368-1372.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poly F, Serichatalergs O, Schulman M, Ju J, Cates CN, Kanipes M, Mason C, Guerry P. Discrimination of major capsular types of Campylobacter jejuni by multiplex PCR. J. Clin. Microbiol. 2011;49:1750–1757. doi: 10.1128/JCM.02348-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts IS. The biochemistry and genetics of capsular polysaccharide production in bacteria. Annu Rev Microbiol. 1996;50:285–315. doi: 10.1146/annurev.micro.50.1.285. [DOI] [PubMed] [Google Scholar]
- Roshan U, Livesay DR. Probalign: multiple sequence alignment using partition function posterior probabilities. Bioinformatics. 2006;22:2715–2721. doi: 10.1093/bioinformatics/btl472. [DOI] [PubMed] [Google Scholar]
- Sheppard SK, Dallas JF, Wilson DJ, Strachan NJ, McCarthy ND, Jolley KA, Colles FM, Rotariu O, Ogden ID, Forbes KJ, Maiden MC. Evolution of an agriculture-associated disease causing Campylobacter coli clade: evidence from national surveillance data in Scotland. PLoS One. 2010;5:e15708. doi: 10.1371/journal.pone.0015708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard SK, McCarthy ND, Falush D, Maiden MC. Convergence of Campylobacter species: implications for bacterial evolution. Science. 2008;320:237–239. doi: 10.1126/science.1155532. [DOI] [PubMed] [Google Scholar]
- Snel B, Bork P, Huynen MA. Genome phylogeny based on gene content. Nat. Genet. 1999;21:108–110. doi: 10.1038/5052. [DOI] [PubMed] [Google Scholar]
- Sopwith W, Birtles A, Matthews M, Fox A, Gee S, James S, Kempster J, Painter M, Edwards-Jones V, Osborn K, Regan M, Syed Q, Bolton E. Investigation of food and environmental exposures relating to the epidemiology of Campylobacter coli in humans in Northwest England. Appl. Environ. Microbiol. 2010;76:129–135. doi: 10.1128/AEM.00942-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strimmer K, vonHaeseler A. Quartet puzzling: A quartet maximum-likelihood method for reconstructing tree topologies. Mol. Biol. Evol. 1996;13:964–969. [Google Scholar]
- Takkinen J, Ammon A, Robstad O, Breuer T. European survey on Campylobacter surveillance and diagnosis 2001. Euro Surveill. 2003;8:207–213. doi: 10.2807/esm.08.11.00430-en. [DOI] [PubMed] [Google Scholar]
- Tam CC, O'Brien SJ, Adak GK, Meakins SM, Frost JA. Campylobacter coli - an important foodborne pathogen. J Infect. 2003;47:28–32. doi: 10.1016/s0163-4453(03)00042-2. [DOI] [PubMed] [Google Scholar]
- Tekaia F, Lazcano A, Dujon B. The genomic tree as revealed from whole proteome comparisons. Genome Res. 1999;9:550–557. [PMC free article] [PubMed] [Google Scholar]
- Unicomb L, Ferguson J, Riley TV, Collignon P. Fluoroquinolone resistance in Campylobacter absent from isolates, Australia. Emerg Infect Dis. 2003;9:1482–1483. doi: 10.3201/eid0911.030336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unicomb LE, Ferguson J, Stafford RJ, Ashbolt R, Kirk MD, Becker NG, Patel MS, Gilbert GL, Valcanis M, Mickan L. Low-level fluoroquinolone resistance among Campylobacter jejuni isolates in Australia. Clin Infect Dis. 2006;42:1368–1374. doi: 10.1086/503426. [DOI] [PubMed] [Google Scholar]
- van Belkum A, Jacobs B, van Beek E, Louwen R, van Rijs W, Debruyne L, Gilbert M, Li J, Jansz A, Megraud F, Endtz H. Can Campylobacter coli induce Guillain-Barre syndrome? Eur J Clin Microbiol Infect Dis. 2009;28:557–560. doi: 10.1007/s10096-008-0661-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassenaar TM, Blaser MJ. Pathophysiology of Campylobacter jejuni infections of humans. Microbes Infect. 1999;1:1023–1033. doi: 10.1016/s1286-4579(99)80520-6. [DOI] [PubMed] [Google Scholar]
- Westgarth C, Porter CJ, Nicolson L, Birtles RJ, Williams NJ, Hart CA, Pinchbeck GL, Gaskell RM, Christley RM, Dawson S. Risk factors for the carriage of Campylobacter upsaliensis by dogs in a community in Cheshire. Vet Rec. 2009;165:526–530. doi: 10.1136/vr.165.18.526. [DOI] [PubMed] [Google Scholar]
- Willison HJ, O'Hanlon GM. The immunopathogenesis of Miller Fisher syndrome. J Neuroimmunol. 1999;100:3–12. doi: 10.1016/s0165-5728(99)00213-1. [DOI] [PubMed] [Google Scholar]
- Zerbino DR, Birney E. Velvet: algorithms for de novo short read assembly using de Bruijn graphs. Genome Res. 2008;18:821–829. doi: 10.1101/gr.074492.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.