Abstract
The guinea pig (Cavea porcellus) is a mammalian non-rodent species in the Caviidae family. The sensitivity of the respiratory system and the susceptibility to infectious diseases allows the guinea pig to be a useful model for both infectious and non-infectious lung diseases such as asthma and tuberculosis. In this report, we demonstrated for the first time, the major cell types and composition in the guinea pig airway epithelium, using cell type-specific markers by immunohistochemical staining using the commercial available immunological reagents that cross-react with guinea pig. Our results revealed the availability of antibodies cross-reacting with airway epithelial cell types of basal, non-ciliated columnar, ciliated, Clara, goblet and alveolar type II cells, as well as those cells expressing Mucin 5AC, Mucin 2, Aquaporin 4 and Calcitonin Gene Related Peptide. The distribution of these various cell types were quantified in the guinea pig airway by immunohistochemical staining and were comparable with morphometric studies using an electron microscopy assay. Moreover, this study also demonstrated that goblet cells are the main secretory cell type in the guinea pig's airway, distinguishing this species from rats and mice. These results provide useful information for the understanding of airway epithelial cell biology and mechanisms of epithelial–immune integration in guinea pig models.
Keywords: Guinea pig, Epithelial cells, Airway, Lung, Immunohistochemical staining, Morphometry
1. Introduction
Guinea pigs (Cavea porcellus) are mammals in the Caviidae family, which are currently designated as a nonrodent species (D'Erchia et al., 1996; Graur et al., 1991). They share many similarities with humans, including hormonal and immunologic responses, pulmonary physiology, exogenous vitamin C requirement and delayed-type hypersensitivity (DTH) reaction to infections such as tuberculosis (Padilla-Carlin et al., 2008). These biological characteristics make guinea pigs valuable animal models for studying developmental biology and the pathogenesis of numbers of diseases (Mess, 2007; Padilla-Carlin et al., 2008; Soliman, 1990). Of the similarities, the sensitivity of the respiratory system and susceptibility to infectious diseases lead guinea pigs to be broadly used as models of respiratory diseases such as asthma and tuberculosis (Kashino et al., 2008; Williams et al., 2009; Wright et al., 2007). With respect to the pathogenesis and immune response to these diseases, guinea pigs were more representative of a human than models using a rodent species such as mice.
The lung is an organ directly open to the environment, which is lined by many distinct types of epithelial cells in different anatomical regions. The respiratory epithelium constructs a large surface area in contact with particles of pollutants, microorganisms, and antigens in the environment. The respiratory epithelium and its antimicrobial products (such as lysozyme and lactoferrin), together inflammatory cells including macrophages, dendritic cells, neutrophils, natural killer cells and cytotoxic T cells—compose the main cellular components of innate immunity in the airway to deactivate or clear inhaled pathogens (Bartlett et al., 2008; Opitz et al., 2010). The respiratory epithelial cell biology in humans, rodents (rats and mice) and other laboratory animals such as ferrets, has been extensively investigated (Boers et al., 1996, 1998, 1999; Liu et al., 2006a; Mercer et al., 1994; Plopper et al., 1980a; Rogers, 2003; Wang et al., 2001). As an important animal model in the studies of both pulmonary allergic and infectious diseases (such as asthma and tuberculosis, respectively), little information on the airway epithelial cell biology is available for guinea pigs, mainly due to the lack of appropriate immunological reagents in comparison with other species. Using electron microscopy and morphological analysis, the morphology and ultrastructure of distal airway epithelium (Davis et al., 1984; Tyler, 1983) and non-ciliated epithelial (Clara) cells (Plopper et al., 1980a,b) of guinea pigs have been well documented. The morphometry of the developing lungs of fetal guinea pigs have also been investigated (Collins et al., 1986).
Markers for a diversity of airway epithelial cell types have been identified for humans and mice. This has made possible numerous studies on airway epithelial cell biology, stem cell biology, and immunology of specific epithelial cell populations in these species (Boers et al., 1998, 1999; Crosby and Waters, 2010; Liu et al., 2006a, 2009; Senju et al., 2000). However, unlike that demonstrated in other species of laboratory animals and humans, there is no report concerning the availability of epithelial cell type-specific markers for the epithelial cell types in the airway of guinea pigs. To this end, we have investigated the epithelial cell types of guinea pig airways using commercially available antibodies against epithelial cell type-specific markers of other species. Our results clarify that few of the available immunological reagents cross-reacting with guinea pigs and can be employed in the studies of guinea pig airway epithelial cell biology. However, several useful cross-reactive antibodies were identified that will facilitate future investigations in this species.
2. Materials and methods
2.1. Animals and tissue processing
The animal care and all experimental procedures were carried out according to ethical guidelines established by the Ningxia University. Three month-old healthy outbred Kunming White mice (23 ± 5 g) and outbred Hartley–Duncan guinea pigs of both sexes (300 ± 50 g) obtained from the Animal facility of Ningxia Medical University (Yinchuan, China). They were housed in the animal facility under clean condition (not specific-pathogen free, non-SPF) according to the Housing and Husbandry Guidelines for Laboratory Animals of Ningxia Medical University. The animal was euthanized with an overdose of intraperitoneal injection of sodium pentobarbital (50 mg/kg) in the facility, and the lungs were perfused with PBS prior to harvesting. The trachea and lungs were removed and fixed in 10% neutral buffered formalin or 2.5% glutaraldehyde (for electronic microscopy) immediately following the perfusion, immediately followed by being fixed and processed for embedding in a RMC Paraffin Tissue Processor (Model 1530, Research & Manufacturing Inc.). Nine animals from each species were evaluated in this study.
2.2. Electron microscopy
Glutaraldehyde-fixed guinea pig and mouse tracheas were stained with 1.25% osmium tetroxide in PBS, dehydrated, and sputter coated prior to visualization on a Hitachi S-450 microscope (Tokyo, Japan) for scanning electron microscopy (SEM). For transmission electron microscopy (TEM), tissues were fixed as for SEM, followed by infiltration with Spurr resin following dehydration. 80 nm serial sections were then viewed on a Hitachi H-7000 Electron Microscope (Tokyo, Japan).
2.3. Immunohistochemical staining
The immunohistochemistry (IHC) staining was performed on 6 μm thick paraffin sections. Paraffin sections were dewaxed in xylene, followed by incubation in methanol containing 0.3% H2O2 for 30 min to inactivate endogenous peroxidase. Sections were then rehydrated in a series of graded ethanol according to histological standards. The antigen was retrieved by boiling in citrate buffer (pH 6.0) for 20 min, followed by to slow cooling down for 2 h at room temperature (RT). The sections were then blocked with blocking buffer (5% horse serum in PBS) at room temperature for 2 h before incubating with a primary antibody (diluted in blocking buffer) in a humid chamber at 4 °C overnight. Following washing three times for 5 min in PBS, a mixture of biotinylated horse anti-rabbit or mouse (Vector Laboratories, Burlingame, CA, USA) secondary antibodies was applied at room temperature for 2 h. The antigen was detected with an elite ABC kits and developed with a DAB Peroxidase substrate kit (Vector Laboratories, Burlingame, CA, USA). The antibodies and working dilutions used in this study were listed in Table 1. Finally, the stained sections were briefly (10 s) counter-stained with haematoxylin (Gill's formula) (Vector Laboratories, Burlingame, CA, USA). Followed by rinsing in running tap water for 5 min, dehydrated, cleared with xylene and mounted in Permount (Fisher Scientific, Pittsburgh, PA, USA). Periodic Acid Schiff's (PAS) staining was conducted using Schiff's reagents from Sigma (St. Louis, MO, USA). Synthesized Calcitonin Gene Related Peptide (CGRP) was product of Phoenix Pharmaceuticals, Inc. (Burlingame, CA, USA). The staining was visualized under a light microscope and the picture was captured using a Leica DFC300 F camera. For all antibodies tested, mouse positive tissues and negative tissues were utilized as positive and negative controls, respectively.
Table 1.
Primary antibodies used for immunohistochemical staining in guinea pig tissues.
| Antibody | Host | Dilution | Company | Catalog number | Works for IHC |
|---|---|---|---|---|---|
| Keratin 14 | Rabbit | 1:500 | Thermo Sci | RB-9020 | Yes |
| Keratin 14 | Mouse | 1:1000 | Thermo Sci | MS-115 | Yes |
| Keratin 18 | Rabbit | 1:200 | Abcam | Ab32118 | Yes |
| Keratin 18 | Mouse | 1:200 | Millipore | MAB1600 | No |
| Keratin 18 | Mouse | 1:200 | Lab vision | MS-1242 | No |
| Tubulin IV | Mouse | 1:2000 | BioGenex | MU178-UC | Yes |
| Mucin 5AC | Rabbit | 1:500 | Santa Cruz | sc-20118 | Yes |
| Mucin 5AC | Mouse | 1:200 | Thermo Sci | MS-145 | No |
| Mucin 2 | Rabbit | 1:500 | Santa Cruz | sc-15334 | Yes |
| Aquaporin 4 | Rabbit | 1:500 | Millipore | MAB3068 | Yes |
| CGRP | Rabbit | 1:5000 | Sigma | C8198 | Yes |
| CGRP | Rabbit | 1:200 | Abcam | Ab8056 | No |
| CGRP | Goat | 1:500 | Abcam | Ab36001 | No |
| pro-SPC | Rabbit | 1:5000 | Millipore | AB3786 | Yes |
| CCSP | Rabbit | 1:5000 | USBiological | C5828-03 | Yes |
| CCSP | Rabbit | 1:1000 | Millipore | 07-623 | No |
| CCSP | Goat | 1:2000 | Santa Cruz | Sc-9773 | No |
2.4. Quantification and cell counting
The composition of major epithelial cell types in the lung cell was ascertained by following a standard for histomorphometric analysis of the lung, recently set by American Thoracic Society (ATS) (Hsia et al., 2010). Following the Periodic Acid Schiff's (PAS) staining or immunohistochemical staining on the serial transverse sections, epithelial cell counts were blindly made on every tenth section and then the cells with cell type-specific staining were counted under a light microscope. The total number of epithelial cells on each section was ascertained by counting the Hematoxylin stained nuclei in the epithelial layer. The PAS staining was utilized as the standard for the identification of goblet cells in lungs (Lan et al., 2009; Rogers, 2003). For each staining, at least three sections from each animal were evaluated from each of six animals. Representative images are shown in the corresponding figures. For each cell type, the percentage of specific epithelial cell type was represented as the percentage of positive stained cells in total counted epithelial cells. Total of six animals were analyzed in this study.
3. Results
3.1. Abundant ciliated cells on the surface of guinea pig trachea
The trachea is a major part of conductive airway of the respiratory system, which is lined by a pseudostratified epithelium in which ciliated and mucous cells predominate. Morphologic analysis by scanning electron microscopy of guinea pig tracheal epithelia demonstrated abundant ciliated cells and infrequent non-ciliated columnar cells on the surface of the guinea pig tracheas (Fig. 1A and B), which was similar to the observation in the tracheas of human, ferret and pig (Liu et al., 2007). By contrast, ciliated cells were less abundant and non-ciliated column cells predominated in the mouse trachea (40% ciliated and 60% non-ciliated epithelial cells), in comparison with those of guinea pig and other species as previous described (Fig. 1C and 1D) (Evans et al., 2004; Liu et al., 2006b; You et al., 2002). This finding may imply that guinea pig is a better model for study of airway epithelium in comparison with that of mouse.
Fig. 1.
Scanning electron microscopy (SEM). Tracheal epithelia from (A and B) guinea pig and (C and D) mouse were evaluated by SEM. More abundant non-ciliated column epithelial cells were observed in the mouse epithelia (C and D) as compared with guinea pigs (A and B). Ci, ciliated cell; Ncc, non-ciliated epithelial cell. Scale bars in A and C = 50 μm, B and D = 5 μm.
3.2. Immunohistochemical profile of the epithelial cell type-specific markers in the guinea pig lung
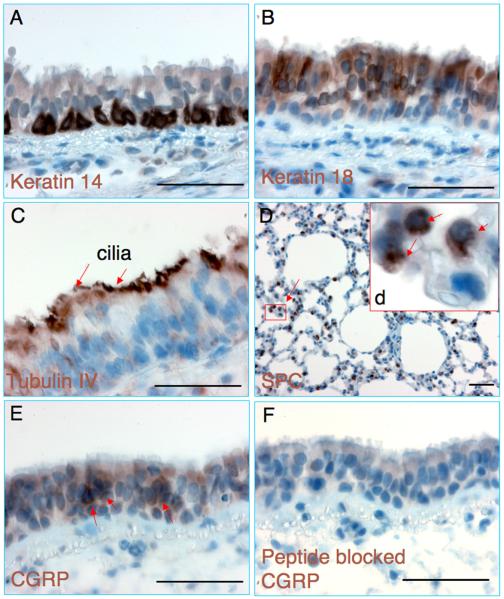
Commercially available antibodies against epithelial cell markers for other species have not been evaluated in guinea pig airways. To this end, we screened a number of antibodies by immunohistochemical (IHC) staining and the tested antibodies were listed in Table 1. The antibodies with cross-reaction to guinea pig were employed in this study, and it is worth noting that all the antibodies that worked for IHC staining also worked for immunofluorescent (IF) staining (data not shown). Keratin proteins are recognized as one type of intermediate filament forming the cytoskeleton of epithelial cells. IHC studies indicated the expression of basal cell marker of Keratin 14 in the basal layer of the tracheal epithelia (Fig. 2A)(Aitken et al., 1995), and the expression of the columnar epithelial cell marker Keratin 18 was seen throughout the epithelial layers of the trachea (Fig. 2B) (Shimizu et al., 1992). The expression of β-tubulin-IV in ciliary axonemes (a feature characteristic of ciliated cells), which utilized as a ciliated cell specific marker of the tracheal epithelia, was localized in the cilia of guinea pig tracheal epithelium (Fig. 2C) (Aitken et al., 1993). The expression of alveolar type II (AT2) cell type-specific marker, pro-SPC (Prosurfactant Protein C), was observed in the alveoli region of the lung (Fig. 2D) (Demaio et al., 2009). Calcitonin Gene Related Peptide (CGRP) has been suggested as a cellular marker for pulmonary Neuroendocrine cells (PNEC). The PNECs are normally found as either as clusters in neuroepithelial body (NEB) or as single cells scattered in the airway epithelium (Haworth et al., 2007). Scattered CGRP positive cells were found in the guinea pig tracheal epithelium (Fig. 2E) and submucosal glands (SMGs) (data not shown). The specificity of the anti-CGRP antibody was confirmed by blocking its cross-reaction with synthesized human CGRP peptide (1.0 ml of working antibody solution was pre-bound with 100 ng of CGRP peptide at RT for 1 h prior to be applied for IHC) (Fig. 2F). The expressions of proteins observed in the airway epithelia of other species, such as Aquaporin 4 (AQP4), a water channel protein was also observed in the guinea pig trachea (data not shown). These data suggested similarities in the expression of known epithelial markers among various species and the airway epithelial cell types of guinea pigs could be identified using available cross-reactive antibodies to other species.
Fig. 2.
Immunohistochemical staining of respiratory epithelial cells of guinea pigs. (A–F) Representative images of immunohistochemical staining of (A) Keratin 14, (B) Keratin 18, (C) Tubulin IV, (D) Surfactant Protein C (SPC), (E) Calcitonin Gene Related Peptide (CGRP) and (F) CGRP staining with anti-CGRP antibody pre-absorbed by synthesized CGRP peptide. Inset (d) depicts enlargement of boxed region in (D), shows SPC positive staining cells (arrows). Scale bar = 50 μm.
3.3. Mucin secretory cells in guinea pig tracheal epithelia
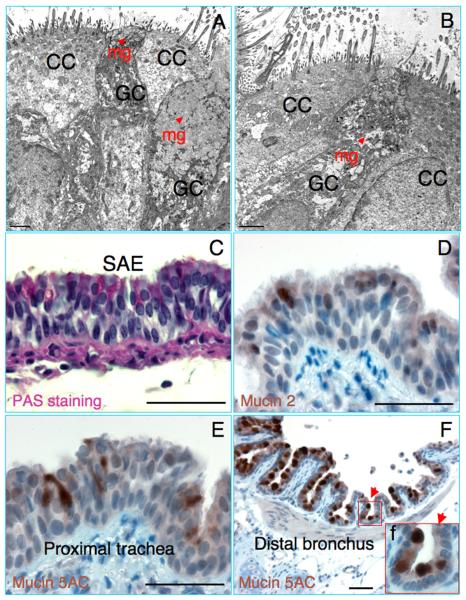
Mucus secretion and clearance of respiratory tract are extremely important for the integrity of respiratory epithelia and pulmonary defense. Mucus is the secretory product of the mucous cells, which is a non-homogeneous and viscoelastic gel composed of water, carbohydrates, proteins, and lipids. The mucous gel is primarily composed of macromolecular mucous glycoproteins or mucins that are encoded by mucin genes (MUC genes). The Mucin 5AC is currently considered as one of the major gel-forming mucins secreted in the large airway and Mucin 2 was found to be expressed in lower airway (Rose and Voynow, 2006). The goblet cells of the airway surface epithelium may contribute a great fraction of mucus volume in the rest state and distal airways where surface mucous cells are found in the absence of SMGs (Rogers, 2003). The sole function of goblet cells is to secrete mucin. Morphologic analysis by TEM revealed mucinogen granules in the cytoplasm of goblet cells, which are scattered among the lining epithelial cells of the large airway of guinea pigs (Fig. 3A and B) (Newman et al., 1996). The abundance of mucin-secreting goblet cells in the large airway and the presence of mucins in the SMGs of guinea pig were also demonstrated by PAS histochemical staining (Fig. 3C and data not shown, respectively) (Boers et al., 1999; Lan et al., 2009). Mucin 5AC was suggested to be a marker of goblet cell metaplasia in mice (Rose et al., 2000). Similar to that seen in PAS staining, the expression of Mucin 5AC was observed in a subset of epithelial cells of proximal tracheal epithelia (Fig. 3E), more abundant Mucin 5AC expressing cells were found in the distal bronchus (Fig. 3F) and SMGs (data not shown). The percentages of the Mucin 5AC positive cells and PAS staining positive cells in the tracheal epithelia were very comparable (data not shown). SMGs in the guinea pig trachea were rare, only found beneath the ventral aspect of epithelial layers between the cartilage rings (Widdicombe et al., 2001). Of note, more abundant PAS and Mucin 5AC positive cells were found in the ventral side of epithelium in comparison with the dorsal aspect (data not shown). Additionally, Mucin 2 was predominantly expressed in the intestinal epithelium (Rose and Voynow, 2006). Infrequent Mucin 2 expressing cells were also detected in the tracheal epithelium of guinea pig by IHC in this study (Fig. 3D). These results suggest that the abundant goblet cells in the epithelia contribute large part of mucus volume in the conductive airway of guinea pigs.
Fig. 3.
Mucin secretory cells in the tracheas of guinea pigs. (A and B) Transmission electron microscopy (TEM) images show goblet cells (GC) with mucin granules (mg, arrowhead) and ciliated cells (CC) in the tracheal epithelia of guinea pigs. (C) Periodic Acid Schiff's (PAS) staining reveals abundant PAS stained cells in the proximal tracheal epithelium of guinea pig trachea. (D) Immunohistochemical staining of Mucin 2 in the proximal tracheal epithelium. (E and F) Mucin 5AC indicates abundant Mucin 5AC secretory cells in the proximal tracheal epithelium (E) and bronchial epithelium (F) of guinea pig. Inset (f) depicts enlargement of boxed region in (F), shows Mucin 5AC positive staining cells. A and B scale bar = 2 μm, C–F scale bar = 50 μm.
3.4. Clara cells in the airway of guinea pigs
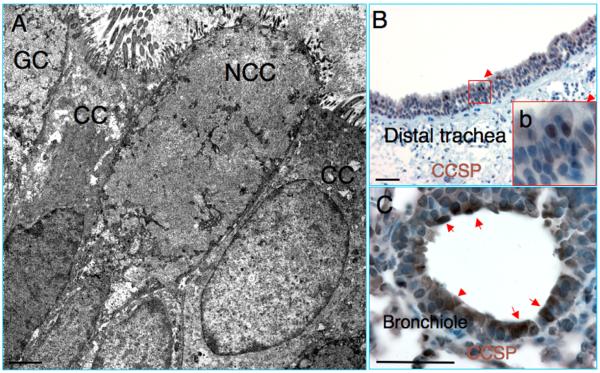
The major of the non-ciliated cells (95%) in the distal airway of mouse are Clara cells that express Clara cell secretory protein (CCSP) (Evans et al., 2004). The abundance and ultrastructure of Clara cells in the distal airway epithelium of guinea pig has been well characterized by morphological assays using electron microscopy (Davis et al., 1984; Evans et al., 2004; Plopper et al., 1980b). However, the identification of Clara cells using a cell type-specific marker has not been reported in the guinea pig lung. Morphological analysis demonstrated infrequent non-ciliated column epithelial cells (Clara cells) in the distal portion of the guinea pig tracheas (Fig. 4A). IHC staining using an antibody against Clara cell type-specific marker CCSP, revealed scattered CCSP-expressing Clara cells in the distal tracheas (Fig. 4B), although rare CCSP staining positive cell was observed in the proximal tracheas (data not shown). Consistent with that seen in the mouse airway epithelia, numbers of Clara cells increased along the proximal to distal axis of the trachea, and the predominant CCSP expressing Clara cells were observed in the intra-lobar bronchioles of guinea pig lungs (Fig. 4C) (Boers et al., 1999).
Fig. 4.
CCSP positive Clara cells in the respiratory epithelia of guinea pigs. (A) Transmission electron microscopy (TEM) images show non-ciliated column cells (NCC) in the tracheal epithelia of guinea pigs. (B) Infrequent Clara cell specific protein (CCSP) expressing in the distal tracheal epithelium. (C) Abundant CCSP positive cells in the intra-lobar small bronchiolar epithelium of guinea pig lung. CC, ciliated cell; GC, goblet cell. A scale bar = 2 μm, B and C scale bar = 50 μm.
3.5. Immunohistochemical quantification of epithelial cell types
To assess the cellular compositions in the tracheal epithelium and intra-lobar small bronchiolar epithelium of guinea pigs, quantification of epithelial cell type-specific marker positive cells was performed on IHC or PAS stained sections of proximal and distal tracheas and lungs, based on an ATS standard for quantitative histomorphometric analysis of the lung (Hsia et al., 2010). In these studies, the percentages of major cell types in conducting airway (basal, goblet, ciliated and non-ciliated columnar Clara cells) were evaluated (Table 2). No sexual dimorphism in the frequency and type of epithelial cells was observed (data not shown). However, a slight difference in the cellular composition of the quantified epithelial cell type between the proximal and distal trachea was observed. The predominant secretory cell type in the proximal tracheal epithelium of guinea pig was goblet cells, while both of Clara cells and goblet cells were major secretory cells in the distal tracheal epithelium of guinea pig. However, Clara cells were the most predominant cell type in the small intra-lobar bronchiolar epithelium (Table 2). These results were in agreement with previous observations of morphometric studies on airway epithelia of humans and rats (Mercer et al., 1994), and the distal airway epithelium of guinea pigs (Davis et al., 1984), using an electron microscopy assay.
Table 2.
Immunohistochemical quantification of epithelial cell types in guinea pig tracheas and intra-lobar small bronchioles.a
| Part of tracheab | Basal cells (%) | Ciliated cells (%) | Goblet cells (%) | Clara cellsc (%) |
|---|---|---|---|---|
| Proximalb | 25.2 ± 2.1 | 36.5 ± 4.3 | 19.4 ± 2.0 | 1.8 ± 0.8 |
| Distalb | 14.7 ± 1.1 | 42.6 ± 1.9 | 11.2 ± 1.5 | 17.7 ± 4.7 |
| Bronchiolard | 0e | 21.8 ± 4.3 | 1.7 ± 0.7 | 65.2 ± 7.4 |
Steady-state non-SPF guinea pig lungs were used in this study. Values represent the mean ± SD (standard deviation) from eighteen independent stained samples from six animals. At least 1000 cells were evaluated for each sample.
The epithelia of first three cartilage rings and the last three cartilage rings of tracheas were evaluated as proximal and distal parts of trachea in this study, respectively.
IHC staining of CCSP positive cells.
Intra-lobar small bronchiolar epithelium.
Small bronchiolar epithelium negative for K14 (basal cell marker).
4. Discussion
The airway epithelium is strategically positioned to interact with the outside environment in a dynamic fashion. The innate immunity of the airway plays a key role in both infectious and non-infectious lung diseases. The epithelial cells of the surface of airway epithelium and its products play a major role in innate immunity and protect the subepithelial compartments from the pathogens, toxic factors, allergens and other stresses (Swamy et al., 2010). The composition of epithelial cell types in the airway is distinct in different anatomical regions of the conducting airways and varies between species. For instance, basal, Clara, and ciliated cells are predominant cell types in the mouse trachea, and the non-ciliated, columnar and Clara cells are the major cell types in the distal airway of mice (Liu et al., 2006a). It has been demonstrated that Clara cells and serous cells are the predominant secretory cell types in mice and rat airways, respectively. However, Clara cells are limited to the bronchioles of human airways and goblet cells are the predominant secretory cell type in the human tracheobronchial airway (Liu et al., 2006a).
Several studies involving genetics and immunity of guinea pigs have revealed striking immunologic similarities between guinea pigs and humans (Padilla-Carlin et al., 2008), which allow guinea pigs to be extremely useful models for the study of lung diseases (Balasubramanian et al., 1994; Mukaiyama et al., 2004). Given the similar cell types found in the guinea pigs trachea as compared to humans, the guinea pig may also be a useful model for studying airway stem cell biology. The ability to demonstrate an epithelial cell type in guinea pigs using a cell type-specific marker will facilitate the studies of the cellular functional properties of each epithelial cell type in their airway. Consequentially, it will aid in the understanding of the mechanisms of epithelial–immune integration and lung cell biology in this species. However, compared to massive immunological reagents are available to mice, much less information is available regarding the immunoreactivity of antibodies against guinea pigs, including the airway epithelial cells. In an attempt to screen available airway epithelial cell type-specific antibodies with cross-reactivity to the guinea pig, immunohistochemical staining was performed on the sections of tracheas and lungs of guinea pigs. Unexpectedly, most tested antibodies of Keratin 18, CGRP, CCSP and Mucin 5AC, those react with other species (humans, mice, or rats), exhibited no cross-reaction with guinea pigs (Table 1). Nonetheless, through this screen we were able to identified antibodies that cross-reacted guinea pigs by immunostaining (Table 1). Our morphological observations using an immunohistochemical technique to quantify airway epithelial cells in the guinea pigs generally confirmed with previous studies using light and electron microscopy.
Respiratory tract secretions consist of mucus, surfactant, and periciliary fluid. Mucus composes the thin layer of liquid overlying the epithelium of the airway. The mucins of mucus are vital to airway homeostasis by conferring the viscoelasticity of mucus for mucociliary clearance in the airway, where the mucus is transported from the lower respiratory tract into the pharynx by airflow and mucociliary clearance. The mucins are predominantly secreted by the goblet cells in the surface epithelium and by the mucous cells in the SMGs of the trachea (Newman et al., 1996; Rogers, 2003). IHC staining of Mucin 5AC and PAS histochemical staining demonstrated abundant goblet cells were found to reside in the epithelium along the trachea, suggesting the goblet cells are the main secretory cell type in the guinea pig airway. This observation was similar to that seen in that of humans (Rogers, 2003), but differed from that described in mice and rats, which the Clara cells and serous cells are the predominant secretory cell type in these two species, respectively (Liu et al., 2006a). It should be noted that the previous reported species differences of secretory cell types were mainly from studies using steady-state specific-pathogen free (SPF) status of the animals. The change of SPF status and/or a stress state (such as infection, smoking or lung injury) may alter the abundance of secretory cells (Jeffery and Li, 1997). For instance, the predominant secretory cell type in the rat conducting airway is the serous cell. However, goblet cells in mice and rats can be induced by specific cytokine stimuli and/or injury (Liu et al., 2006a). The guinea pig used in this study was non-SPF animal, we could not rule out whether the non-SPF status caused the abundance of goblet cells seen in the guinea pig conducting airway epithelium.
In summary, we have evaluated the epithelial cell types in guinea pig airways using cell type-specific markers by immunostaining methods and screened commercially available immunological reagents. Our results revealed antibodies that cross-reacted with various airway epithelial cell types in guinea pigs. The compositions of epithelial cell types evaluated by immunohistochemical staining were in agreement with previous observations using an electron microscopy. Moreover, this study also demonstrated that the goblet cells might be a major secretory cell type in guinea pig tracheobronchial epithelium. These results lay a foundation for the further understanding of airway epithelial cell biology and mechanisms of epithelial–immune integration in the guinea pig models.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (Nos: 30860207, 31060335), Grants of Science and Technology Program of Ningxia to YJW (No: KGZ-12-10-02), and the Natural Science Foundation of Ningxia Hui Autonomous Region (NZ0905, AZ09149).
Footnotes
Conflict of interest The authors declare that there are no conflicts of interest.
References
- Aitken ML, Villalon M, Pier M, Verdugo P, Nameroff M. Characterization of a marker of differentiation for tracheal ciliated cells independent of ciliation. Am. J. Respir. Cell Mol. Biol. 1993;9:26–32. doi: 10.1165/ajrcmb/9.1.26. [DOI] [PubMed] [Google Scholar]
- Aitken ML, Villalon M, Pier M, Verdugo P, Nameroff M. Characterization of a marker for tracheal basal cells. Exp. Lung Res. 1995;21:1–16. doi: 10.3109/01902149509031741. [DOI] [PubMed] [Google Scholar]
- Balasubramanian V, Wiegeshaus EH, Smith DW. Mycobacterial infection in guinea pigs. Immunobiology. 1994;191:395–401. doi: 10.1016/S0171-2985(11)80445-6. [DOI] [PubMed] [Google Scholar]
- Bartlett JA, Fischer AJ, McCray PB., Jr. Innate immune functions of the airway epithelium. Contrib. Microbiol. 2008;15:147–163. doi: 10.1159/000136349. [DOI] [PubMed] [Google Scholar]
- Boers JE, Ambergen AW, Thunnissen FB. Number and proliferation of basal and parabasal cells in normal human airway epithelium. Am. J. Respir. Crit. Care Med. 1998;157:2000–2006. doi: 10.1164/ajrccm.157.6.9707011. [DOI] [PubMed] [Google Scholar]
- Boers JE, Ambergen AW, Thunnissen FB. Number and proliferation of clara cells in normal human airway epithelium. Am. J. Respir. Crit. Care Med. 1999;159:1585–1591. doi: 10.1164/ajrccm.159.5.9806044. [DOI] [PubMed] [Google Scholar]
- Boers JE, den Brok JL, Koudstaal J, Arends JW, Thunnissen FB. Number and proliferation of neuroendocrine cells in normal human airway epithelium. Am. J. Respir. Crit. Care Med. 1996;154:758–763. doi: 10.1164/ajrccm.154.3.8810616. [DOI] [PubMed] [Google Scholar]
- Collins MH, Kleinerman J, Moessinger AC, Collins AH, James LS, Blanc WA. Morphometric analysis of the growth of the normal fetal guinea pig lung. Anat. Rec. 1986;216:381–391. doi: 10.1002/ar.1092160307. [DOI] [PubMed] [Google Scholar]
- Crosby LM, Waters CM. Epithelial repair mechanisms in the lung. Am. J. Physiol. Lung Cell. Mol. Physiol. 2010;298:L715–731. doi: 10.1152/ajplung.00361.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Erchia AM, Gissi C, Pesole G, Saccone C, Arnason U. The guinea-pig is not a rodent. Nature. 1996;381:597–600. doi: 10.1038/381597a0. [DOI] [PubMed] [Google Scholar]
- Davis ML, Lewandowski J, Dodson RF. Morphology and ultrastructure of the distal airway epithelium in the guinea pig. Anat. Rec. 1984;209:509–522. doi: 10.1002/ar.1092090411. [DOI] [PubMed] [Google Scholar]
- Demaio L, Tseng W, Balverde Z, Alvarez JR, Kim KJ, Kelley DG, Senior RM, Crandall ED, Borok Z. Characterization of mouse alveolar epithelial cell monolayers. Am. J. Physiol. Lung Cell. Mol. Physiol. 2009;296:L1051–1058. doi: 10.1152/ajplung.00021.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans CM, Williams OW, Tuvim MJ, Nigam R, Mixides GP, Blackburn MR, DeMayo FJ, Burns AR, Smith C, Reynolds SD, Stripp BR, Dickey BF. Mucin is produced by clara cells in the proximal airways of antigen-challenged mice. Am. J. Respir. Cell Mol. Biol. 2004;31:382–394. doi: 10.1165/rcmb.2004-0060OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graur D, Hide WA, Li WH. Is the guinea-pig a rodent? Nature. 1991;351:649–652. doi: 10.1038/351649a0. [DOI] [PubMed] [Google Scholar]
- Haworth R, Woodfine J, McCawley S, Pilling AM, Lewis DJ, Williams TC. Pulmonary neuroendocrine cell hyperplasia: identification, diagnostic criteria and incidence in untreated ageing rats of different strains. Toxicol. Pathol. 2007;35:735–740. doi: 10.1080/01926230701460000. [DOI] [PubMed] [Google Scholar]
- Hsia CC, Hyde DM, Ochs M, Weibel ER. An official research policy statement of the American Thoracic Society/European Respiratory Society: standards for quantitative assessment of lung structure. Am. J. Respir. Crit. Care Med. 2010;181:394–418. doi: 10.1164/rccm.200809-1522ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery PK, Li D. Airway mucosa: secretory cells, mucus and mucin genes. Eur. Respir. J. 1997;10:1655–1662. doi: 10.1183/09031936.97.10071655. [DOI] [PubMed] [Google Scholar]
- Kashino SS, Napolitano DR, Skobe Z, Campos-Neto A. Guinea pig model of Mycobacterium tuberculosis latent/dormant infection. Microbes Infect. 2008;10:1469–1476. doi: 10.1016/j.micinf.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan J, Ribeiro L, Mandeville I, Nadeau K, Bao T, Cornejo S, Sweezey NB, Kaplan F. Inflammatory cytokines, goblet cell hyperplasia and altered lung mechanics in Lgl1+/− mice. Respir. Res. 2009;10:83. doi: 10.1186/1465-9921-10-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Driskell RR, Engelhardt JF. Stem cells in the lung. Methods Enzymol. 2006a;419:285–321. doi: 10.1016/S0076-6879(06)19012-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Luo M, Guo C, Yan Z, Wang Y, Engelhardt JF. Comparative biology of rAAV transduction in ferret, pig and human airway epithelia. Gene Ther. 2007;14:1543–1548. doi: 10.1038/sj.gt.3303014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Luo M, Guo C, Yan Z, Wang Y, Lei-Butters DC, Engelhardt JF. Analysis of adeno-associated virus progenitor cell transduction in mouse lung. Mol. Ther. 2009;17:285–293. doi: 10.1038/mt.2008.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Yan Z, Luo M, Engelhardt JF. Species-specific differences in mouse and human airway epithelial biology of recombinant adeno-associated virus transduction. Am. J. Respir. Cell Mol. Biol. 2006b;34:56–64. doi: 10.1165/rcmb.2005-0189OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer RR, Russell ML, Roggli VL, Crapo JD. Cell number and distribution in human and rat airways. Am. J. Respir. Cell Mol. Biol. 1994;10:613–624. doi: 10.1165/ajrcmb.10.6.8003339. [DOI] [PubMed] [Google Scholar]
- Mess A. The Guinea pig placenta: model of placental growth dynamics. Placenta. 2007;28:812–815. doi: 10.1016/j.placenta.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Mukaiyama O, Morimoto K, Nosaka E, Takahashi S, Yamashita M. Involvement of enhanced neurokinin NK3 receptor expression in the severe asthma guinea pig model. Eur. J. Pharmacol. 2004;498:287–294. doi: 10.1016/j.ejphar.2004.07.071. [DOI] [PubMed] [Google Scholar]
- Newman TM, Robichaud A, Rogers DF. Microanatomy of secretory granule release from guinea pig tracheal goblet cells. Am. J. Respir. Cell Mol. Biol. 1996;15:529–539. doi: 10.1165/ajrcmb.15.4.8879187. [DOI] [PubMed] [Google Scholar]
- Opitz B, van Laak V, Eitel J, Suttorp N. Innate immune recognition in infectious and noninfectious diseases of the lung. Am. J. Respir. Crit. Care Med. 2010;181:1294–1309. doi: 10.1164/rccm.200909-1427SO. [DOI] [PubMed] [Google Scholar]
- Padilla-Carlin DJ, McMurray DN, Hickey AJ. The guinea pig as a model of infectious diseases. Comp. Med. 2008;58:324–340. [PMC free article] [PubMed] [Google Scholar]
- Plopper CG, Hill LH, Mariassy AT. Ultrastructure of the nonciliated bronchiolar epithelial (Clara) cell of mammalian lung. III. A study of man with comparison of 15 mammalian species. Exp. Lung Res. 1980a;1:171–180. doi: 10.3109/01902148009069646. [DOI] [PubMed] [Google Scholar]
- Plopper CG, Mariassy AT, Hill LH. Ultrastructure of the nonciliated bronchiolar epithelial (Clara) cell of mammalian lung: I. A comparison of rabbit, guinea pig, rat, hamster, and mouse. Exp. Lung Res. 1980b;1:139–154. doi: 10.3109/01902148009069644. [DOI] [PubMed] [Google Scholar]
- Rogers DF. The airway goblet cell. Int. J. Biochem. Cell Biol. 2003;35:1–6. doi: 10.1016/s1357-2725(02)00083-3. [DOI] [PubMed] [Google Scholar]
- Rose MC, Piazza FM, Chen YA, Alimam MZ, Bautista MV, Letwin N, Rajput B. Model systems for investigating mucin gene expression in airway diseases. J. Aerosol Med. 2000;13:245–261. doi: 10.1089/jam.2000.13.245. [DOI] [PubMed] [Google Scholar]
- Rose MC, Voynow JA. Respiratory tract mucin genes and mucin glycoproteins in health and disease. Physiol. Rev. 2006;86:245–278. doi: 10.1152/physrev.00010.2005. [DOI] [PubMed] [Google Scholar]
- Senju S, Iyama K, Kudo H, Aizawa S, Nishimura Y. Immunocytochemical analyses and targeted gene disruption of GTPBP1. Mol. Cell. Biol. 2000;20:6195–6200. doi: 10.1128/mcb.20.17.6195-6200.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T, Nettesheim P, Ramaekers FC, Randell SH. Expression of “cell-type-specific” markers during rat tracheal epithelial regeneration. Am. J. Respir. Cell Mol. Biol. 1992;7:30–41. doi: 10.1165/ajrcmb/7.1.30. [DOI] [PubMed] [Google Scholar]
- Soliman AM. Type II collagen-induced inner ear disease: critical evaluation of the guinea pig model. Am. J. Otolaryngol. 1990;11:27–32. [PubMed] [Google Scholar]
- Swamy M, Jamora C, Havran W, Hayday A. Epithelial decision makers: in search of the `epimmunome'. Nat. Immunol. 2010;11:656–665. doi: 10.1038/ni.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler WS. Comparative subgross anatomy of lungs, pleuras, interlobular septa, and distal airways. Am. Rev. Respir. Dis. 1983;128:S32–36. doi: 10.1164/arrd.1983.128.2P2.S32. [DOI] [PubMed] [Google Scholar]
- Wang X, Zhang Y, Amberson A, Engelhardt JF. New models of the tracheal airway define the glandular contribution to airway surface fluid and electrolyte composition. Am. J. Respir. Cell Mol. Biol. 2001;24:195–202. doi: 10.1165/ajrcmb.24.2.3918. [DOI] [PubMed] [Google Scholar]
- Widdicombe JH, Chen LL, Sporer H, Choi HK, Pecson IS, Bastacky SJ. Distribution of tracheal and laryngeal mucous glands in some rodents and the rabbit. J. Anat. 2001;198:207–221. doi: 10.1046/j.1469-7580.2001.19820207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams A, Hall Y, Orme IM. Evaluation of new vaccines for tuberculosis in the guinea pig model. Tuberculosis (Edinb) 2009;89:389–397. doi: 10.1016/j.tube.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Wright JL, Postma DS, Kerstjens HA, Timens W, Whittaker P, Churg A. Airway remodeling in the smoke exposed guinea pig model. Inhal. Toxicol. 2007;19:915–923. doi: 10.1080/08958370701515563. [DOI] [PubMed] [Google Scholar]
- You Y, Richer EJ, Huang T, Brody SL. Growth and differentiation of mouse tracheal epithelial cells: selection of a proliferative population. Am. J. Physiol. Lung Cell. Mol. Physiol. 2002;283:L1315–1321. doi: 10.1152/ajplung.00169.2002. [DOI] [PubMed] [Google Scholar]