Abstract
The human brain shrinks with advancing age, but recent research suggests that it is also capable of remarkable plasticity, even in late life. In this review we summarize the research linking greater amounts of physical activity to less cortical atrophy, better brain function, and enhanced cognitive function, and argue that physical activity takes advantage of the brain's natural capacity for plasticity. Further, although the effects of physical activity on the brain are relatively widespread, there is also some specificity, such that prefrontal and hippocampal areas appear to be more influenced than other areas of the brain. The specificity of these effects, we argue, provides a biological basis for understanding the capacity for physical activity to influence neurocognitive and neuropsychiatric disorders such as depression. We conclude that physical activity is a promising intervention that can influence the endogenous pharmacology of the brain to enhance cognitive and emotional function in late adulthood.
Keywords: aging, physical activity, exercise, brain, plasticity, neuroplasticity
Abstract
Si bien el cerebro humano se reduce a medida que avanza la edad, la investigación reciente sugiere que también es capaz de una extraordinaria plasticidad, incluso hacia el final de la vida. En esta revisión se resume la investigación que relaciona una gran cantidad de actividad física con una menor atrofia cortical, una mejor función cerebral y un refuerzo de la función cognitiva, y se argumenta que la actividad física hace uso de la capacidad natural del cerebro para la plasticidad. Además, aunque los efectos de la actividad física en el cerebro son relativamente generalizados, también hay alguna especificidad, de tal modo que las áreas prefrontal e hipocámpica parecen estar más influenciadas que otras áreas cerebrales. Se argumenta que la especificidad de estos efectos proporciona las bases biológicas para comprender la capacidad de la actividad física para influenciar los trastornos neurocognitivos y neuropsiquiátricos como la depresión. Se concluye que la actividad física es una intervención prometedora que puede influir en la farmacología endógena del cerebro para reforzar las funciones cognitivas y emocionales en la adultez avanzada.
Abstract
Le cerveau humain se rétracte avec l'âge mais est aussi doté d'une plasticité remarquable, même à un âge avancé comme le montrent des études récentes. Nous résumons dans cet article les données de la recherche associant une activité physique plus importante à une moindre atrophie corticale, à un meilleur fonctionnement cérébral, et à des fonctions cognitives améliorées. Nous discutons du fait que l'activité physique bénéficie de l'aptitude naturelle du cerveau à la plasticité. De plus, bien que les effets d'une activité physique sur le cerveau soient assez généralisés, il existe certains effets specifiques comme ceux sur l'hippocampe et le cortex préfrontal qui semblent plus influencés que les autres aires cérébrales. Nous pensons que ces effets spécifiques fournissent une base biologique à la comprehension de l'influence exercée par l'activité physique sur les troubles neurocognitifs et neuropsychiatriques comme la dépression. Cette activité physique semble donc prometteuse en termes d'action sur la pharmacologie endogène du cerveau pour améliorer les fonctions émotionnelles et cognitives chez les personnes âgées.
Introduction
The proportion of adults over the age of 65 is expected to increase over the next 40 years. An anticipated rise in the number of older adults is expected to lead to an increase in the prevalence of age-related diseases, which in turn, might result in escalating health care costs and heightened distress among family and caregivers.1 Cognitive impairment, and more specifically Alzheimer's disease, is one of the most threatening age-related diseases, but even so-called “normal” age-related cognitive decline can cause agonizing distress and loss of personal identity. Unfortunately, pharmaceutical treatments or preventions for cognitive impairment are only modestly effective, resulting in the search for nonpharmaceutical approaches such as intellectually stimulating activities, dietary interventions, and physical activity, for preventing or treating cognitive decline.
A recent report estimated that modifiable risk factors including education, smoking, mid-life obesity, hypertension, diabetes, depression, and physical inactivity contribute significantly to the risk of Alzheimer's disease, and that a 10% to 25% reduction in these factors could prevent as many as 3 million cases worldwide.2 Yet, despite the recognition of the importance of modifiable risk factors in the incidence and prevalence of cognitive impairment, there is often a misunderstanding of the research that has been conducted examining whether intervening on these modifiable risk factors would have any noticeable effect on brain or cognitive health. In contrast, a good deal of research has been conducted to examine the effects of physical activity and cognitive stimulation on human brain morphology and function. The aim of this review is to summarize recent research findings that examine the potential for physical activity, cardiorespiratory fitness, and exercise interventions to enhance brain health in late life. The studies reviewed here support the position that physical activity influences the endogenous pharmacology of the brain and takes advantage of the brain's natural capacity for plasticity, well into late adulthood. Physical activity increases the lifespan,3 reduces the risk for many cardiovascular diseases4 and cancers,5 and also reduces the risk for cognitive decline6 and depression in late adulthood.7 In short, we argue that the influence of physical activity on brain plasticity might have consequences not only for memory and other cognitive functions, but also has implications for many different psychiatric and neurologic conditions through a set of common biological pathways.
Establishing the molecular basis of physical activity on brain health
Several recent reviews have comprehensively described the neuromolecular events resulting from physical activity.8,9 There are several reasons for briefly summarizing this literature here. First, studies using rodent models for exploring the ways in which physical activity influences the brain can control when and how much physical activity the animal receives. Hence, the nature of these systematic experiments allows for causal and directional conclusions about the effects of physical activity on learning and memory, neurotransmitter systems, metabolic and growth factors, and cell proliferation. Second, animal models allow for an examination of the cellular and molecular events resulting from physical activity that are simply impossible to study in humans. For these reasons, it is important to describe this literature since it provides a causal and low-level biological foundation to understand the effects observed in human neuroimaging and clinical studies.
One of the earliest studies found that animals that were provided access to a running wheel in their cage tended to outperform their more sedentary counterparts on several different learning and memory tasks such as the t-maze and Morris water maze.10 In one version of the Morris water maze, rodents are made to swim in an opaque pool until they find the location of a submerged platform that sits just below the surface. By using cues located around the room, the rodent learns to navigate to the submerged platform more quickly after successive trials. In this task, both older and younger animals engaging in exercise demonstrate faster learning of the location of the submerged platform compared with rodents not engaging in exercise.11 Importantly, performance on the Morris water maze has been frequently linked to the hippocampus,12 a medial temporal lobe structure critical in memory formation. In fact, other studies utilizing hippocampus-sensitive tasks have also reported that exercise enhances both acquisition and rétention,13,14 suggesting that the hippocampus might be especially sensitive to the effects of exercise.
There is now substantial support for robust and consistent effects of physical activity on the morphology and function of the hippocampus.8 For example, one of the most consistent findings in this literature is that exercise has the capacity to increase cell prolifération in the dentate gyrus of the hippocampus, even in aged animals.8,11,13
Furthermore, increased proliferation and survival of neurons in the hippocampus mediates learning enhancements.13,15 These studies indicate that the hippocampus remains highly modifiable throughout the lifespan and that exercise has the capacity to take advantage of the plasticity of this structure.
Cell proliferation in the hippocampus leads to an increased demand for nutrients to support the new neural architecture. These regions acquire nutrients through increased vascularization of neural tissue. After exercise, increased vascularization has been routinely found in several different brain regions including the cerebellum, motor cortex, hippocampus, and frontal cortex.16-18 Increased proliferation of cells and capillaries in the hippocampus work in concert to enhance learning and memory in behavioral paradigms,19 but these effects can also be observed on the neurophysiological level. For example, exercise increases the number of synapses in the hippocampus,20 enhances indices of long-term memory formation,20 and elevates the rate of gene expression for molecules associated with learning and memory20 such as brain-derived neurotrophic factor (BDNF) and serotonin.8
It is clear from this literature that exercise influences the integrity of the hippocampus by influencing gene expression, cell proliferation and survival, vascularization, and synaptic plasticity. However, this literature has identified many different brain regions influenced by exercise, indicating that exercise has widespread effects. In conclusion, there are many different molecular and cellular pathways mediating the effects of exercise on cognitive and behavioral outcomes, including increased neurogenesis, angiogenesis, and the production of growth factors important in memory and cognitive function.
Effects of physical activity on cognitive function in humans
Greater amounts of physical activity and higher cardiorespiratory fitness levels are associated with better cognitive function in older adults. For example, older adult athletes outperform their more sedentary peers on many different cognitive tasks,21 and fitter individuals are faster and more accurate on executive functioning and memory tasks.22 Longitudinal studies of physical activity have also found that engaging in a greater amount of physical activity earlier in life is associated with better cognitive function later in life,23 with larger effects for individuals engaging in more intense exercises. However, cross-sectional and longitudinal observational studies are often plagued by confounding factors that make it challenging to make causal claims about the link between physical activity and cognitive function. In other words, in the studies described above it is equally likely that individuals with better cognitive function choose to participate in more physical activities than individuals with poorer cognitive function. Along these same lines, physical activity might instead be a proxy for better health habits more generally rather than being specific to physical activity per se.
Randomized controlled trials reduce or eliminate some of the limitations of cross-sectional and observational studies. These types of interventions in which older adults are randomized to either a moderate intensity physical activity group or to a non-active or less-active control group, routinely demonstrate that increasing physical activity for 3 to 6 months is effective at improving cognitive performance.24 For example, in one study, inactive older adults were randomized to either a moderate intensity physical activity group or to a stretching and toning control group for 6 months.25 Both groups came into the laboratory 3 days per week and the exercise group participated in moderate-intensity exercises for 30 to 45 minutes per day while the stretching group participated in stretching exercises for the same amount of time. Trained exercise physiologists monitored heart rates, intensity, and compliance in both groups for the duration of the exercise regimen. A comprehensive neuropsychological evaluation was conducted before and after the intervention. This study found that participation in moderate-intensity physical activity (eg, brisk walking) was effective at enhancing performance on tasks that measured executive functions, but was less effective at improving performance on tasks that measured other cognitive domains. In contrast, the stretching and toning group did not show significant improvements in performance over this same period. Meta-analyses of physical activity interventions have confirmed that the effects of exercise on cognitive function in late life are both general and specific.24 General in the sense that many different cognitive domains are improved after several months of exercise, but specific in the sense that executive functions are enhanced more than other cognitive functions.
Effects of physical activity and aerobic fitness on neuroimaging indices of brain health
The animal and human cognitive studies described above highlight a few key principles. First, in terms of cognitive function, the effects of exercise appear to be widespread, but most strongly associated with executive domains. This suggests that brain regions and networks that support executive functions might be more sensitive to the effects of exercise than other brain areas. The rodent literature largely supports this claim, with the largest and most consistent effects of exercise appearing in regions that support higher-level cognitive functions including the hippocampus, frontal cortex, and basal ganglia. The second key principle emerging from these studies is that the brain remains modifiable well into late adulthood, and physical activity has the capacity to take advantage of brain plasticity. Brain plasticity resulting from exercise can be detected at the molecular and cellular level in rodents and at the cognitive level in humans. These two points, specificity and plasticity, provide the foundation for neuroimaging methods to examine whether physical activity, fitness, or exercise has any appreciable effect on the morphology or function of the human brain. Given the principles described above, neuroimaging studies exploring these associations have hypothesized that physical activity would influence the morphology and function of the human brain and that the effects would be widespread but most consistently associated with regions that support higher-level cognitive functions such as the prefrontal cortex and hippocampus.
One of the unfortunate characteristics of the brain is that it generally shrinks and atrophies with advancing age. In fact, both the prefrontal cortex and hippocampus shrink at roughly 1% to 2% annually in individuals over the age of 55,26 with more precipitous rates of atrophy when individuals begin experiencing cognitive impairment.27 Although the rate and trajectory of decline varies from region to region, the general finding is that regions that support memory and executive functions show the earliest and most rapid decline.26 Interestingly, the loss of brain volume is mirrored by age-related changes in cognitive function with the most significant losses occurring on memory and executive tasks.28 Yet, it is these cognitive domains and brain areas that appear the most sensitive to physical activity training. Would greater amounts of physical activity or higher cardiorespiratory fitness levels have any beneficial or positive associations with the morphology of the older adult brain?
There have now been several studies finding that older adults who are more fit,29-33 more physically active,34-36 and who participate in exercise interventions37,38 have greater brain volumes than their less fit and less active counterparts. In one cross-sectional study, cardiorespiratory fitness levels were assessed in a sample of cognitively healthy older adults and voxel-based morphometry was used to assess gray matter volume.29 Although increased age was associated with reductions in gray matter volume throughout the prefrontal, temporal, and parietal cortices, these same brain regions showed less atrophy in adults that were more fit. These results demonstrated that remaining more aerobically fit could help to preserve brain tissue that would normally atrophy with age. Higher fitness levels have now been associated with greater gray matter volume in other populations, including postmenopausal women receiving hormone therapy,39 a higher educated older adult sample,40 a sample with multiple sclerosis,41 and older adults with mild cognitive impairment.42 These studies have all derived a similar conclusion from these results: individuals with a higher level of fitness have greater gray matter volume than less fit individuals, and the associations are relatively specific to brain areas that support higher-level cognition, including the prefrontal cortex.
As described above, much of the animal literature has focused on the effects of exercise on hippocampal plasticity and memory functions supported by the hippocampus. Are higher cardiorespiratory fitness levels associated with larger hippocampal volumes in humans? This question is important since the hippocampus shrinks with advancing age and contributes to agerelated memory loss.26,27 In 165 cognitively normal older adults, cardiorespiratory fitness levels were recorded in addition to high-resolution anatomical images of the brain.30 The size of the hippocampus was assessed using an automated segmentation algorithm that uses a point distribution model to determine the location, size, and shape of the structure. A clear association was found between higher fitness levels and greater hippocampal volume, but importantly, greater hippocampal volume also mediated the fitness-memory association. This result suggests that greater hippocampal volume is not just a meaningless by-product of more vascularization, but rather has a meaningful impact on memory function in late life. This general association between higher fitness levels and larger hippocampal volume has now been replicated in individuals with mild cognitive impairment.31
Cross-sectional research defines important associations between variables of interest, such as cardiorespiratory fitness levels and cortical volume. Demonstrating these associations is necessary before embarking on a lengthy and expensive longitudinal randomized trial. However, there are inherent limitations to cross-sectional designs that prohibit the ability to draw conclusions about the causal nature of physical activity on brain plasticity. Several studies have now been conducted that examine these associations from a longitudinal and randomized perspective. For example, in the Cardiovascular Health Study at the Pittsburgh, Pennsylvania site, 1479 ambulatory adults over the age of 65 were enrolled into a longitudinal study on the incidence of cardiovascular diseases.34 Information about lifestyles and physical function were collected as part of this study including information on the frequency and duration of walking. Approximately 9 years after the original enrollment period these same participants were recruited to participate in a brain MRI study in which high-resolution brain images were collected. The brain images from 299 cognitively normal adults were selected from this sample and used in an analysis to examine whether greater amounts of self-reported walking 9 years earlier was predictive of gray matter volume later in life.34 The analysis of this data confirmed that greater amounts of physical activity was associated with greater gray matter volume in several different brain regions including the frontal cortex, parietal cortex, and temporal cortex including the hippocampus. Interestingly, after a period of 4 more years, 116 of these 299 adults were diagnosed with either mild cognitive impairment or dementia, but greater graymatter volume associated with physical activity was associated with a two-fold reduced risk of developing cognitive impairment.34 This study demonstrated for the first time the link between participation in physical activityearlier in life, greater gray matter volume, and the reduced risk for cognitive impairment later in life.
This study and others35 demonstrate that the effects of physical activity on brain plasticity might endure and influence the risk for cognitive impairment over a span of several years. Randomized interventions have also reported that assigning sedentary older adults to engage in more physical activity results in an increase in graymatter volume in several different brain areas. For example, Colcombe et al38 randomized a group of cognitively normal adults to either a moderate-intensity walking exercise program or to a stretching and toning control group. Similar to the study described above,29 this study required participants to report to the laboratory three times per week for a period of 6 months. High-resolution brain MRI scans were collected both before and after the intervention period. Interestingly, the walking exercise group showed a significant increase in the volume of prefrontal and temporal brain areas along with an increase in the volume of the frontal white matter tracts especially the genu of the corpus callosum.
Another randomized intervention of physical activity examined whether participation in 1 year of a structured exercise regimen would increase the volume of the hippocampus in older adults.37 In this study, 120 cognitively normal older adults participated in a similar exercise design as that described previously.29,38 High-resolution brain scans were collected before the intervention, after 6 months, and then at completion of the 1-year trial. Although the thalamus and caudate nucleus did not show significant changes in volume resulting from exercise, there was an effect of exercise on the size of the hippocampus. Whereas the stretching and toning control group displayed about a 1.4% decline in the size of the hippocampus the exercising group showed an increase of about 2% over this same 1-year period. This study demonstrated that the volume of the hippocampus remains modifiable into late adulthood, and participation in 1 year of consistent and moderate intensity exercise was sufficient for increasing the size of the structure. Furthermore, the changes in hippocampal volume for the exercising group were correlated with improvements in memory performance suggesting an important link between changes in volume induced by exercise and memory enhancement. In fact, more recent studies have found that the volumetric differences observed as a function of cardiorespiratory fitness mediate improvements in memory and executive function,30,32-33 again supporting the claim that these volumetric effects are not meaningless by-products, but important factors in promoting better cognitive function.
This discussion summarizes the relatively well-established scientific literature using cross-sectional, longitudinal, observational, and randomized controlled trials examining the effect of physical activity or cardiorespiratory fitness on regional gray matter volume. These studies have consistently reported that higher fitness levels are associated with larger brain volumes, and that participation in only modest amounts of physical activity is sufficient for increasing gray matter volume in select brain regions. In addition, these results are in line with the animal literature and human cognitive literature described in preceding sections demonstrating the brain plasticity and specificity of the effects of greater amounts of physical activity.
Volumetric data has proven useful in identifying how physical activity could alter the morphology of the adult brain. However, other neuroimaging methods including functional magnetic resonance imaging (fMRI) and resting state connectivity (rs) MRI approaches allow for an investigation of the effects of physical activity on brain network dynamics. In one of the earliest studies to examine this, Colcombe et al43 employed a task measuring selective attention and executive control in a two-part experiment. In the first experiment, higher cardiorespiratory fitness levels were associated with better performance on the task and this was paralleled by increases in fMRI activity in the dorsolateral prefrontal and parietal brain regions. The second experiment was a randomized exercise intervention in which adults were assigned to either receive a structured exercise regimen for 6 months or to a stretching and toning control group for the same amount of time. The participants performed the same selective attention task as the participants in the first experiment. The results from the randomized trial were strikingly similar to the results from the crosssectional study. That is, after 6 months of the intervention, the exercise group showed increased activity in the dorsolateral prefrontal cortex and parietal cortex and decreased activity in areas that support conflict monitoring such as the anterior cingulate cortex. These results are important because they demonstrate that in addition to volumetric changes resulting from exercise there are also significant changes in task-evoked brain function. Hence, the brain processes task demands more efficiently after only 6 months of exercise.
Although there are only several published studies using fMRI paradigms, each of these studies has found increased fMRI activity in prefrontal regions including during a semantic memory task,44 the digit symbol substitution task,45 and the Stroop task46 as a function of either higher cardiorespiratory fitness levels or greater physical activity levels. Yet, each of these studies also recognizes the complex nature of cognitive function and the necessity of understanding the networks of regions that support cognition and how physical activity exerts its effects on these networks. Hence, it is important to identify not only which brain areas are associated with physical activity, but also to understand how the communication between regions is influenced by physical activity. Could the functional connectedness of the network improve after several months of exercise and would these effects mediate improvements in memory and executive function?
The connectivity between regions can be examined using several different methods. Regions of interest can be used as seeds to examine whether regions that are functionally connected with the seed region vary as a function of some variable of interest (eg, cardiorespiratory fitness levels). Using a seed-based approach to examine functional connectivity, Voss et al47 found that older adults that had higher cardiorespiratory fitness levels had greater connectivity in the so-called default mode network. Further, they found that increased connectivity mediated the fitness related enhancements of executive control. Since the default mode network is reduced in older adults with mild cognitive impairment and dementia,48 increased functional connectivity indicates that physical activity might reduce the risk of impairment by elevating the cohesiveness of the default mode network. In fact, results from two randomized interventions indicate that the functional connectivity of these networks can be modified after several months of physical activity.49,50
The studies described above focus on three forms of brain health and integrity in late life: morphology, task-evoked functional dynamics, and connectivity. For each of these measures, cross-sectional, observational, and randomized interventions indicate that physical activity is capable of modifying age-related losses and that physical activity-induced changes in brain integrity and function mediate improvements in cognition. In summary, the human neuroimaging literature on physical activity indicates that the brain remains modifiable into late adulthood, the effects are distributed throughout the brain, but are most robust in the prefrontal and medial temporal lobe regions.
Common biological pathways in depression
The Cochrane Collaboration performed a systematic review of the effects of exercise on depression in adults of mixed ages.51 They identified 32 trials (1858 participants) that fulfilled their inclusion criteria, of which 30 (1101 participants) provided data for meta-analyses. Based on these 30 trials, the authors concluded that exercise seems to improve depressive symptoms in individuals with depression when compared with no treatment or a control intervention. In comparison to “no treatment,” exercise had a moderate effect size (standardized mean difference [SMD] -0.67, 95% confidence (CI) -0.90 to -0.43). There were no significant differences when comparing the effects of exercise with cognitive therapy or antidepressant treatment. When the authors limited the analyses to the four high-quality trials (326 participants), the pooled SMD was -0.31 (95% CI -0.63 to 0.01) indicating a small effect in favor of exercise.
Among the 32 trials identified that fulfilled the inclusion criteria, 8 studies were focused on or included adults older than 60 years.51-59 Six of the studies involved aerobic exercise and two studies progressive resistance training. Of the 6 studies that involved aerobic exercise, various exercise and comparator interventions were examined. Blumenthal and colleagues (1999) studied community volunteers with major depressive disorder (MDD) (n=156) mean (SD) age of 57 (6.5) randomized to aerobic exercise (group walking or jogging 3 times per week), antidepressant pharmacologic treatment (sertraline), or the combination.58 They found that all treatment groups had statistically significant improvement in depression scores, although participants receiving medication alone had the fastest initial response. After 16 weeks of treatment, exercise was equally effective in reducing depression among older adults with MDD. A limitation to this study was the absence of a placebo or control intervention. In a follow-up study, Blumenthal and colleagues59 examined community-dwelling older adults with MDD (n=202), mean (SD) age 52 (8), randomly assigned to home-based exercise, supervised exercise in a group setting, sertraline, or placebo for 16 weeks. While there was a high placebo response rate, the efficacy of exercise was comparable to antidepressant pharmacotherapy, and both were better than placebo. Brenes and colleagues studied 37 older adults with a mean (SD) age of 73.5 (7.8) with minor depression, randomized to exercise, antidepressant pharmacotherapy (sertraline), or usual care over 16 weeks.52 In the 32 participants who completed the study, they found trends for exercise and sertraline to be superior to usual care in improving emotional and physical functioning. Mather and colleagues examined whether exercise is effective as an adjunct to antidepressant pharmacotherapy in older adults. Eighty-six older adults with depression (mean age 65) were randomly assigned to attend exercise classes or health education talks for 10 weeks.53 At 10 weeks, a significantly higher proportion of the exercise group (55% versus 33%) experienced a greater than 30% decline in depressive symptoms as measured with the Hamilton Rating Scale for Depression. McNeil and colleagues (1991) randomly assigned 30 community dwelling, moderately depressed older adults with a mean (SD) age of 72.5 (6.9) to 1 of 3 interventions: experimenter-accompanied exercise (walking), social contact control condition, and a wait-list control.54 They found that exercise and social contact both resulted in reductions in the Beck Depression Inventory. Lastly, Williams and Tappen examined the effects of exercise training for depressed older adults with Alzheimer's disease.57 Subjects were randomly assigned to 16 weeks of comprehensive exercise, supervised walking, or social conversation. They found that all three groups had a reduction in depressive symptoms, with exercise showing a slightly greater benefit.
Two studies found antidepressant effects of progressive resistance training in older adults with depression. Singh and colleagues studied the effects of progressive resistance training (PRT) on depressed adults 60 years and older with a mean (SD) age of 71.3 (1.2).55 Over 10 weeks compared with an attention-control group, PRT was associated with an improvement in the measures of depressive symptoms, quality of life, social functioning, and strength. In a follow-up study, Singh and colleagues found that higher-intensity PRT was more effective than low-intensity PRT on depression in older adults.56
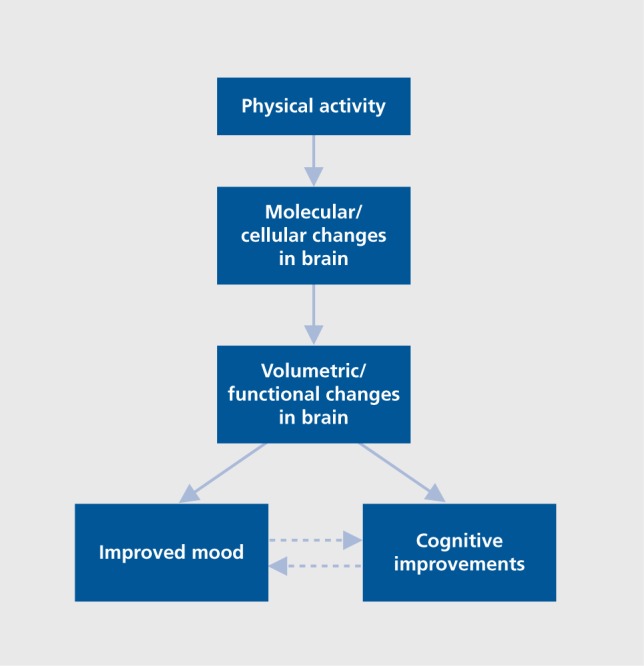
These studies support the argument that exercise has antidepressant effects in older adults, yet the mechanism of action remains unclear. The studies are careful to note the potential for the antidepressant effects of expectation and attention in research participation as well as for socialization when engaging in group exercises.51 Further, investigators have also suggested the effects of increased self-efficacy, a sense of mastery, positive thoughts, distraction from negative thoughts, and enhanced self-concept.59 However, biological mechanisms related to overall brain health are also likely related to the mood elevating properties of exercise. These mechanisms, described earlier, include enhanced gray matter volume in prefrontal cortex and hippocampal brain areas, elevated functioning of brain circuits involved in mood and emotional function such as subregions of the frontal cortex and medial temporal lobe, and improvements in functional connectivity of the default-mode network. In addition, it is also likely that exercise is having a pleiotropic effect on molecular systems related to the hypothalamic-pituitary-adrenocortical axis, dopaminergic, noradrenergic, serotonergic neurotransmission, immune function, and BDNF (Figure 1). 60,61 However, these biological mechanisms of exercise have not yet been carefully studied in older adults with depression. The role that changes in morphology and function could have on mitigating depressive symptoms remains speculative at this time.
Figure 1. A schematic representation of the general path by which cognitive function and mood are improved by physical activity, it could be hypothesized that improvements in cognitive function mediate the improvements in mood or that improvements in mood mediate some of the improvements in cognitive function. The dotted lines represent these hypothesized paths.
Conclusions and future directions
In this review we have briefly summarized the expansive and ever-growing literature on the effects of physical activity on brain health and plasticity. We can conclude from this overview that physical activity has consistent and robust effects on the brain, which mediate improvements in cognitive performance and reduce the risk for neuropsychiatric disorders. The beauty of this research is that the effects appear consistent across species and populations indicating an exceptional level of translation that is rare to find in other disciplines.
The research in this area has demonstrated that the effects of physical activity on brain plasticity in late life has a remarkable degree of specificity, such that some brain areas appear to be more commonly or easily influenced by physical activity than other areas. There might be several reasons for this specificity. One explanation might be that the hippocampus, frontal cortex, and neighboring areas are more inherently plastic than other brain areas and that the degree of specificity is simply a characteristic of the brain regions examined rather than anything specific to the capabilities of physical activity per se. However, an alternative explanation is that these brain areas contain some molecular or cellular process that is influenced by participation in physical activity. For example, BDNF has been described as one possible molecular pathway by which exercise improves cognitive function, but BDNF levels are found in different concentrations throughout the brain with higher levels in the hippocampus and cerebellum than in other areas.62 A third possible explanation could be that the brain areas that show the most amount of atrophy with age, including the frontal cortex and hippocampus, are the most sensitive to the effects of physical activity. Thus, the specificity of physical activity on the frontal cortex and hippocampus might be related to the atrophying nature of these areas. According to this reasoning, since these brain areas are shrinking with age, there is more room for them to grow with an intervention like physical activity. Therefore, the specificity of physical activity has to do with relatively little variation in the size and function of other brain regions with advancing age. Whatever the explanation for specificity, the effects appear to be both robust and consistent across samples and populations. This indicates that these effects are unlikely to be confounded by a particular sample characteristic (eg, gender) or comorbidity (eg, depression) and more likely reflects an adaptive biological importance of physical activity to enhance and maintain the body's organs, including the brain.
Despite the well-established literature linking physical activity to brain health and plasticity in late life, there remain many unanswered questions. First, although a number of studies described above have found effects with moderate intensity exercise for several months, the exact dose-response nature of the link between physical activity and mood, cognition, or brain health remains unknown. In other words, there is a very poor understanding of how much physical activity is necessary to observe effects. Second, individuals stop exercising for a variety of different reasons including injuries, illness, and personal issues (eg, mourning). Because of this it is important to examine whether the effects of a physically active lifestyle are retained or lost after some period of inactivity. Unfortunately we have a very poor understanding of the retention of the effects of physical activity. Third, we have a very poor understanding of the types of exercises that might be most useful to promote a healthier brain. It is conceivable that competitive sports like tennis offer additional benefits beyond noncompetitive sports because of their dependence on physical coordination, cognitive effort, and social interaction. In sum, although we have a solid understanding of the potential for physical activity to enhance cognitive and brain health in late life there remain many unanswered questions for future research to pursue.
Acknowledgments
KIE was supported by the University of Pittsburgh Alzheimer's Disease Research Center (P50 AG005133) and a research grant from the National Institutes of Health (R01 DK095172). AGG was supported by National Institutes of Health grants R01 MH084921 and ACISR P30 MH090333. MAB was supported by the National Institutes of Health's University of Pittsburgh Alzheimer's Disease Research Center (P50 AG005133), ACISR P30 MH090333 and R01 MH080240.
Contributor Information
Kirk I. Erickson, Department of Psychology, University of Pittsburgh, Pennsylvania, USA.
Ariel G. Gildengers, Department of Psychiatry, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, USA.
Meryl A. Butters, Department of Psychiatry, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, USA.
REFERENCES
- 1.Alzheimer's Association. 2012 Alzheimer's Disease Facts and Figures. Alzheimers Dement. 2012;8:131–168. doi: 10.1016/j.jalz.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Barnes DE., Yaffe K. The projected effect of risk factor reduction on Alzheimer's disease prevalence. Lancet Neurol. 2011;10:819–828. doi: 10.1016/S1474-4422(11)70072-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blair SN., Kohl HW., Paffenbarger RS., Clark DG., Cooper KH., Gibbons LW. Physical fitness and alll-cause mortality. JAMA. 1995;262:2395–2401. doi: 10.1001/jama.262.17.2395. [DOI] [PubMed] [Google Scholar]
- 4.Paffenbarger RS., Wing AL., Hyde RT. Physical activity as an index of heart attack risk in college alumni. Am J Epidemiol. 1978;108:12–18. doi: 10.1093/oxfordjournals.aje.a112608. [DOI] [PubMed] [Google Scholar]
- 5.McTiernan A., Kooperberg C., White E., et al Recreational physical activity and the risk of breast cancer in postmenopausal women: The Women's Health Initiative Cohort Study. JAMA. 2003;290:1331–1336. doi: 10.1001/jama.290.10.1331. [DOI] [PubMed] [Google Scholar]
- 6.Buchman AS., Boyle PA., Yu L., Shah RC., Wilson RS., Bennett DA. Total daily physical activity and the risk of AD and cognitive decline in older adults. Neurology. 2012;78:1323–1329. doi: 10.1212/WNL.0b013e3182535d35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dunn AL., Trivedi MH., Kampert JB., Clark CG., Chambliss HO. Exercise treatment for depression: efficacy and dose response. Am J Prev Med. 2005;28:1–8. doi: 10.1016/j.amepre.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 8.Vivar C., Potter MC., van Praag H. All about running: synaptic plasticity, growth factors and adult hippocampal neurogenesis. Curr Top Behav Neurosci. In press doi: 10.1007/7854_2012_220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Erickson Kl., Miller DL., Weinstein AM., Akl SL., Banducci SE. Physical activity and brain plasticity in late adulthood: a conceptual review. Ageing Res. 2012;4:34–47. [Google Scholar]
- 10.Fordyce DE., Wehner JM. Physical activity enhances spatial learning performance with an associated alteration in hippocampal protein kinase C activity in C57BL/6 and DBA/2 mice. Brain Res. 1993;619:111–119. doi: 10.1016/0006-8993(93)91602-o. [DOI] [PubMed] [Google Scholar]
- 11.Van Praag H., Shubert T., Zhao C., Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25:8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Izquierdo I., Bevilaqua LR., Rossato Jl., et al The connection between the hippocampal and the striatal memory systems of the brain: a review of recent findings. Neurotox Res. 2006;10:113–121. doi: 10.1007/BF03033240. [DOI] [PubMed] [Google Scholar]
- 13.Creer DJ., Romberg C., Saksida LM., van Praag H., Bussey TJ. Running enhances spatial pattern separation in mice. Proc Natl Acad Sci U S A. 2010;167:588–597. doi: 10.1073/pnas.0911725107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen HI., Lin LC., Liu YF., et al Treadmill exercise enhances passive avoidance learning in rats: the role of down-regulated serotonin system in the limbic system. Neurobiol Learn Mem. 2008;89:489–496. doi: 10.1016/j.nlm.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 15.Clark PJ., Brzezinska WJ., Thomas MW., Ryzhenko NA., Toshkov SA., Rhodes JS. Intact neurogenesis is required for benefits of exercise on spatial memory but not motor performance or contextual fear conditioning in C57BL/6J mice. Neuroscience. 2008;155:1048–1058. doi: 10.1016/j.neuroscience.2008.06.051. [DOI] [PubMed] [Google Scholar]
- 16.Kerr AL., Steuer EL., Pochtarev V., Swain RA. Angiogenesis but not neurogenesis is critical for normal learning and memory acquisition. Neuroscience. 2010;171:214–226. doi: 10.1016/j.neuroscience.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 17.Black JE., Isaacs KR., Anderson BJ., Alcantara AA., Greenough WT. Learning causes synaptogenesis, whereas motor activity causes angiogenesis, in cerebellar cortex of adult rats. Proc Natl Acad Sci U S A. 1990;87:5568–5572. doi: 10.1073/pnas.87.14.5568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Swain RA., Harris AB., Wiener EC., et al Prolonged exercise induces angiogenesis and increases cerebral blood volume in primary motor cortex of the rat. Neuroscience. 2003;117:1037–1046. doi: 10.1016/s0306-4522(02)00664-4. [DOI] [PubMed] [Google Scholar]
- 19.Clark PJ., Brzezinska WJ., Puchalski EK., Krone DA., Rhodes JS. Functional analysis of neurovascular adaptations to exercise in the dentate gyrus of young adult mice associated with cognitive gain. Hippocampus. 2009;19:937–950. doi: 10.1002/hipo.20543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farmer J., Zhao X., van Praag H., Wodtke K., Gage FH., Christie BR. Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male Sprague-dawley rats in vivo. Neuroscience. 2004;124:71–79. doi: 10.1016/j.neuroscience.2003.09.029. [DOI] [PubMed] [Google Scholar]
- 21.Spirduso WW. Reaction and movement time as a function of age and physical activity level. J Gerontol. 1975;43:18–23. doi: 10.1093/geronj/30.4.435. [DOI] [PubMed] [Google Scholar]
- 22.Etnier JL., Nowell PM., Landers DM., Sibley BA. A meta-regression to examine the relationship between aerobic fitness and cognitive performance. Brain Res Rev. 2006;79:205–221. doi: 10.1016/j.brainresrev.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 23.Middleton LE., Barnes DE., Lui LY., Yaffe K. Physical activity over the life course and its association with cognitive performance and impairment in old age. J Am Geriatr Soc. 2010;58:1322–1326. doi: 10.1111/j.1532-5415.2010.02903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Colcombe S., Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci. 2003;13:125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- 25.Kramer AF., Hahn S., Cohen NJ., et al Ageing, fitness, and neurocognitive function. Nature. 1999;400:418–419. doi: 10.1038/22682. [DOI] [PubMed] [Google Scholar]
- 26.Raz N., Lindenberger U., Rodrigue K., et al Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex. 2005;15:1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- 27.Jack CR., Wiste HJ., Vemuri P., et al Alzheimer's Disease Neuroimaging Initiative. Brain beta-amyloid measures and magnetic resonance imaging atrophy both predict time-to-progression from mild cognitive impairment to Alzheimer's disease. Brain. 2010;133:3336–3348. doi: 10.1093/brain/awq277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reuter-Lorenz PA., Park DC. Human neuroscience and the aging mind: a new look at old problems. J Gerontol B Psychol Sci Soc Sci. 2010;65:405–415. doi: 10.1093/geronb/gbq035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Colcombe SJ., Erickson Kl., Raz N., et al Aerobic fitness reduces brain tissue loss in aging humans. J Gerontol A Biol Sci Med Sci. 2003;58:176–180. doi: 10.1093/gerona/58.2.m176. [DOI] [PubMed] [Google Scholar]
- 30.Erickson Kl., Prakash RS., Voss MW., et al Aerobic fitness is associated with hippocampal volume in elderly humans. Hippocampus. 2009;19:1030–1039. doi: 10.1002/hipo.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Honea RA., Thomas GP., Harsha A., et al Cardiorespiratory fitness and preserved medial temporal lobe volume in Alzheimer disease. Alzheimer Dis Assoc Disord. 2009;23:188–197. doi: 10.1097/WAD.0b013e31819cb8a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weinstein AM., Voss MW., Prakash RS., et al The association between aerobic fitness and executive function is mediated by prefrontal cortex volume. Brain Behav lmmun. 2012;26:811–819. doi: 10.1016/j.bbi.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Verstynen TD., Lynch B., Miller DL., et al Caudate nucleus volume mediates the link between cardiorespiratory fitness and cognitive flexibility in older adults. J Aging Res. 2012;2012:939285. doi: 10.1155/2012/939285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Erickson Kl., Raji CA., Lopez OL., et al Physical activity predicts gray matter volume in late adulthood: the Cardiovascular Health Study. Neurology. 2010;75:1415–1422. doi: 10.1212/WNL.0b013e3181f88359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rovio S., Spulber G., Nieminen LJ., et al The effect of midlife physical activity on structural brain changes in the elderly. Neurobiol Aging. 2010;31:1927–1936. doi: 10.1016/j.neurobiolaging.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 36.Bugg JM., Head D. Exercise moderates age-related atrophy of the medial temporal lobe. Neurobiol Aging. 2011;32:506–514. doi: 10.1016/j.neurobiolaging.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Erickson Kl., Voss MW., Prakash RS., et al Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. 2011;108:3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Colcombe SJ., Erickson Kl., Scalf PE., et al Aerobic exercise training increases brain volume in aging humans. J Gerontol A Biol Sci Med Sci. 2006;61:1166–1170. doi: 10.1093/gerona/61.11.1166. [DOI] [PubMed] [Google Scholar]
- 39.Erickson Kl., Colcombe SJ., Elavsky S., et al Interactive effects of fitness and hormone treatment on brain health in postmenopausal women. Neurobiol Aging. 2007;28:179–185. doi: 10.1016/j.neurobiolaging.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 40.Gordon BA., Rykhlevskaia El., Brumback CR., et al Neuroanatomical correlates of aging, cardiopulmonary fitness level, and education. Psychophysiology. 2008;45:825–838. doi: 10.1111/j.1469-8986.2008.00676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prakash RS., Snook EM., Motl RW., Kramer AF. Aerobic fitness is associated with gray matter volume and white matter integrity in multiple sclerosis. Brain Res. 2010;23:1341–1351. doi: 10.1016/j.brainres.2009.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burns JM., Cronk BB., Anderson HS., et al Cardiorespiratory fitness and brain atrophy in early Alzheimer disease. Neurology. 2008;71:210–216. doi: 10.1212/01.wnl.0000317094.86209.cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Colcombe SJ., Kramer AF., Erickson Kl., et al Cardiovascular fitness, cortical plasticity, and aging. Proc Natl Acad Sci U S A. 2004;101:3316–3321. doi: 10.1073/pnas.0400266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith JC., Nielson KA., Woodard JL., et al Interactive effects of physical activity and APOE-epsilon4 on BOLD semantic memory activation in healthy elders. Neuroimage. 2011;54:635–644. doi: 10.1016/j.neuroimage.2010.07.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rosano C., Venkatraman VK., Guralnik J., et al Psychomotor speed and functional brain MRI 2 years after completing a physical activity treatment. J Gerontol A Biol Sci Med Sci. 2010;65:639–647. doi: 10.1093/gerona/glq038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Prakash RS., Voss MW., Erickson Kl., et al Cardiorespiratory fitness and attentional control in the aging brain. Front Hum Neurosci. 2011;4:1–12. doi: 10.3389/fnhum.2010.00229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Voss MW., Erickson Kl., Prakash RS., et al Functional connectivity: a source of variance in the association between cardiorespiratory fitness and cognition? Neuropsychologia. 2010;48:1394–1406. doi: 10.1016/j.neuropsychologia.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Andrews-Hanna JR., Snyder AZ., Vincent JL., et al Disruption of largescale brain systems in advanced aging. Neuron. 2007;56:924–935. doi: 10.1016/j.neuron.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Voss MW., Prakash RS., Erickson Kl., et al Plasticity of brain networks in a randomized intervention trial of exercise training in older adults. Front Aging Neurosci. 2010;26:2. doi: 10.3389/fnagi.2010.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Burdette JH., Laurienti PJ., Espeland MA., et al Using network science to evaluate exercise-associated brain changes in older adults. Front Aging Neurosci. 2010;2:23. doi: 10.3389/fnagi.2010.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rimer J., Dwan K., Greig CA., et al Exercise for depression. Cochrane Database Syst Rev. 2012;7:CD004366. doi: 10.1002/14651858.CD004366.pub5. [DOI] [PubMed] [Google Scholar]
- 52.Brenes GA., Williamson JD., Messier SP., et al Treatment of minor depression in older adults: a pilot study comparing sertraline and exercise. Aging Merit Health. 2007;11:61–68. doi: 10.1080/13607860600736372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mather AS., Rodriguez C., Guthrie MF., McHarg AM., Reid IC., McMurdo ME. Effects of exercise on depressive symptoms in older adults with poorly responsive depressive disorderrandomised controlled trial. Br J Psychiatry. 2002;180:411–415. doi: 10.1192/bjp.180.5.411. [DOI] [PubMed] [Google Scholar]
- 54.McNeil JK., LeBlanc EM., Joyner M. The effect of exercise on depressive symptoms in the moderately depressed elderly. Psychol Aging. 1991;6:487–488. doi: 10.1037//0882-7974.6.3.487. [DOI] [PubMed] [Google Scholar]
- 55.Singh NA., Clements KM., Fiatarone MA. A randomized controlled trial of progressive resistance training in depressed elders. J Gerontol A Biol Sci Med Sci. 1997;52:M27–M35. doi: 10.1093/gerona/52a.1.m27. [DOI] [PubMed] [Google Scholar]
- 56.Singh NA., Stavrinos TM., Scarbek Y., Galambos G., Liber C., FiataroneSingh MA. A randomized controlled trial of high versus low intensity weight training versus general practitioner care for clinical depression in older adults. J Gerontol A Biol Sci Med Sci. 2005;60:768–776. doi: 10.1093/gerona/60.6.768. [DOI] [PubMed] [Google Scholar]
- 57.Williams CL., Tappen RM. Exercise training for depressed older adults with Alzheimer's disease. Aging Ment Health. 2008;12:72–80. doi: 10.1080/13607860701529932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Blumenthal JA., Babyak MA., Moore KA., et al Effects of exercise training on older patients with major depression. Arch Intern Med. 1999;159:2349–2356. doi: 10.1001/archinte.159.19.2349. [DOI] [PubMed] [Google Scholar]
- 59.Blumenthal JA., Babyak MA., Doraiswamy PM., et al Exercise and pharmacotherapy in the treatment of major depressive disorder. Psychosom Med. 2007;69:587–596. doi: 10.1097/PSY.0b013e318148c19a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Droste SK., Gesing A., Ulbricht S., Muller MB., Linthorst AC., Reul JM. Effects of long-term voluntary exercise on the mouse hypothalamic-pituitary-adrenocortical axis. Endocrinology. 2003;144:3012–3023. doi: 10.1210/en.2003-0097. [DOI] [PubMed] [Google Scholar]
- 61.Rubin RT. Pharmacoendocrinology of major depression. Eur Arch Psychiatry Neurol Sci. 1989;238:259–267. doi: 10.1007/BF00449807. [DOI] [PubMed] [Google Scholar]
- 62.Erickson Kl., Miller DL., Roecklein KA. The aging hippocampus: interactions between exercise, depression, and BDNF. Neuroscientist. 2012;18:82–97. doi: 10.1177/1073858410397054. [DOI] [PMC free article] [PubMed] [Google Scholar]


