Abstract
Catecholic drugs had been reported to be metabolized through conjugation reactions, particularly methylation and sulfation. Whether and how these two Phase II conjugation reactions may occur in a concerted manner, however, remained unclear. The current study was designed to investigate the methylation and/or sulfation of five catecholic drugs. Analysis of the spent media of HepG2 cells metabolically labeled with [35S]sulfate in the presence of individual catecholic drugs revealed the presence of two [35S]sulfated metabolites for dopamine, epinephrine, isoproterenol, and isoetharine, but only one [35S]sulfated metabolite for apomorphine. Further analyses using tropolone, a catechol O-methyltransferase (COMT) inhibitor, indicated that one of the two [35S]sulfated metabolites of dopamine, epinephrine, isoproterenol, and isoetharine was a doubly conjugated (methylated and sulfated) product, since its level decreased proportionately with increasing concentrations of tropolone added to the labeling media. Moreover, while the inhibition of methylation resulted in a decrease of the total amount of [35S]sulfated metabolites, sulfation appeared to be capable of compensating the suppressed methylation in the metabolism of these four catecholic drugs. A two-stage enzymatic assay showed the sequential methylation and sulfation of dopamine, epinephrine, isoproterenol, and isoetharine mediated by, respectively, the COMT and the cytosolic sulfotransferase SULT1A3. Collectively, the results from the present study implied the concerted actions of the COMT and SULT1A3 in the metabolism of catecholic drugs.
Keywords: Methylation, Sulfation, COMTs, SULTs, Catecholic drugs
1. Introduction
A number of catecholic drugs, such as dopamine, dobutamine (Dobutrex), isoproterenol (Isuprel), inamrinone (Amrinone), and isoetharine (Bronkosol), are currently in use for treating a variety of diseases/disorders [1–5]. Previous studies have demonstrated that conjugation reactions, particularly methylation and sulfation, are involved in the metabolism of these drugs and the regulation of their pharmacological activity [6–10].
Methylation of catecholic compounds is mediated by the catechol O-methyltransferase (COMT). COMT catalyzes the transfer of a methyl group from S-adenosyl-L-methionine (AdoMet) to one of the two vicinal hydroxyl groups, mainly the 3-hydroxyl group, on the aromatic ring of endogenous and xenobiotic catecholic compounds, including catecholic drugs [11–14]. In humans, there is a single COMT gene encoding two forms of COMT that differ in their N-terminal region, a soluble form (S-COMT) present in the cytosol and a membrane-bound form (MB-COMT) located in the endoplasmic reticulum [15,16]. Previous studies have shown that MB-COMT has ~10-fold higher affinity toward catecholamines than does S-COMT; whereas S-COMT exists as the predominant form in most tissues except brain [15,17,18]. Sulfation of catecholic compounds is mediated by the cytosolic sulfotransferases (SULTs) which are a group of enzymes that catalyze the transfer of a sulfonate group from the “active” sulfate, 3′-phosphoadenosine 5′-phosphosulfate (PAPS), to a hydroxyl or amino group of substrate compounds [19]. Sulfation is a key process that serves for the biotransformation of endogenous catecholamines, steroid/thyroid hormones, cholesterol, and bile acids, as well as a variety of xenobiotics including catecholic compounds [20–22]. Sulfate conjugation by these enzymes generally results in the inactivation of the substrate compounds and/or increase in their water-solubility, thereby facilitating their removal from the body. For the sulfation of catecholamines such as dopamine and epinephrine, SULT1A3 (previously called the catecholamine-preferring phenol sulfotransferase) has been shown to be the major responsible enzyme among the eleven known human cytosolic SULTs [23,24]. For the sulfation of catecholestrogens, five different SULTs, SULT1A1, SULT1A2, SULT1A3, SULT1C4, and SULT1E1, are involved [25,26].
We report in this communication the generation and release of both singularly sulfated and doubly methylated–sulfated metabolites by HepG2 human hepatoma cells incubated in the presence of all tested catecholic drugs, except apomorphine. Enzymatic assays showed the sequential conjugation reactions of dopamine, epinephrine, isoproterenol, and isoetharine under the concerted actions of COMT and SULT1A3. The implications of the occurrence of dual conjugation of catecholic drugs are discussed in the context of their metabolism and regulation.
2. Materials and methods
2.1. Materials
Dopamine, epinephrine, (±)-isoproterenol hydrochloride, isoetharine mesylate salt, apomorphine hydrochloride, adenosine 5′-triphosphate (ATP), 3′-phosphoadenosine-5′-phosphosulfate (PAPS), 3-(N-morpholino)propanesulfonic acid (MOPS), Trizma base, sodium dodecyl sulfate (SDS), dithiothreitol (DTT), isopropyl β-D-thiogalac-topyranoside (IPTG), dimethyl sulfoxide (DMSO), 2-hydroxy-2,4,6-cycloheptatrien-1-one (tropolone), S-(5′-adenosyl)-L-methionine (AdoMet), and minimum essential medium (MEM) were from Sigma Chemical Company (St. Louis, MO). Protease inhibitor cocktail, EDTA-free, was a product of Roche Diagnostics (Mannheim, Germany). Carrier-free sodium [35S]sulfate and Ecolume scintillation cocktail were obtained from MP Biomedicals (Irvine, CA). S-[methyl-14C]-AdoMet was a product of PerkinElmer (Boston, MA). Fetal bovine serum was from Biomeda (Foster City, CA). HepG2 human hepatoma cells (ATCC HB-8065) were from American Type Culture Collection (Manassa, VA). Cellulose thin-layer chromatography (TLC) plates were products of EMD Chemicals (Gibbstown, NJ). Oligonucleotide primers were synthesized by MWG Biotech (Huntsville, AL). All other chemicals were of the highest grade commercially available.
2.2. Metabolic labeling of HepG2 human hepatoma cells
HepG2 cells were routinely maintained, under a 5% CO2 atmosphere, at 37 °C in MEM supplemented with 10% fetal bovine serum, penicillin G (30 μg/ml), and streptomycin sulfate (50 μg/ ml). Confluent HepG2 cells, grown in individual wells of a 24-well culture plate, preincubated in sulfate-free (prepared by omitting streptomycin sulfate and replacing magnesium sulfate with magnesium chloride) MEM for four hours, were labeled with 0.25 ml aliquots of the same medium containing [35S]sulfate (0.3 mCi/ml), and 50 μM of tested catecholic drugs, without or with tropolone (at concentrations ranging from 0 to 500 μM), an inhibitor of COMT. At the end of an 18-h labeling, the media were collected and spin-filtered. The filtrates were subjected to the analysis of [35S]sulfated products using a TLC procedure with n-butanol/isopropanol/formic acid/water (2:1:3:1; v/v/v/v) as the solvent system. Upon completion of TLC, an autoradiograph was taken from the TLC plate to reveal radioactive spots corresponding to [35S]sulfated derivatives of tested catecholic drugs added to the labeling media. Thereafter, the radioactive spots were cut out from the plate and the radioactive materials therein were eluted and counted for [35S]radioactivity using a liquid scintillation counter.
2.3. Preparation of purified human SULTs
Recombinant human SULTs, SULT1A1, SULT1A2, SULT1A3, SULT1B1, SULT1C2, SULT1C4, SULT1E1, SULT2A1, SULT2B1a, SULT2B1b, and SULT4A1, were expressed using pGEX-2TK or pET23c prokaryotic expression system, and purified as previously described [27–31].
2.4. SULT assay
The catecholic drug-sulfating activity of the recombinant human SULTs was assayed using [35S]PAPS as the sulfonate donor. The standard assay mixture, in a final volume of 20 μl, contained 50 mM of Mops buffer at pH 7.0, 1 mM DTT, and 14 μM [35S]PAPS. Stock solutions of the substrates (dopamine, epinephrine, isoproterenol, isoetharine, and apomorphine), prepared in H2O or DMSO, at 20 times the final concentration (50 μM), were used in the assay mixtures. The reaction was started by the addition of the SULT enzyme, allowed to proceed for 10 min at 37 °C, and terminated by placing the thin-walled tube containing the assay mixture on a heating block at 100 °C for 2 min. The precipitates were cleared by centrifugation at 13,000 × g for 3 min, and the supernatant was subjected to the analysis of [35S]sulfated product using a TLC procedure previously established with n-butanol/isopropanol/88% formic acid/water (3:1:1:1; v/v/v/v) as the solvent system [32]. Each experiment was performed in triplicate, together with a control without substrate. The results obtained were calculated and expressed in nanomoles of sulfated product formed/min/mg purified enzyme.
2.5. Cloning and bacterial expression of the human soluble COMT
To generate the human soluble COMT cDNA, sense (5′-ATGGGTGACACCAAGGAGCAGCGCATCCTGAACCACGTGC-3′) and antisense (5′-CGCGGATCCTCAGCTGCCTGGGCCCT-3′) oligonucleotide primers were designed based on 5′- and 3′-regeions of the coding sequence. Using this primer set, a PCR was carried out under the action of Ex Taq DNA polymerase, with the first-strand cDNA reverse-transcribed from the total RNA isolated from HepG2 cells as the template. Amplification conditions were 2 min 94 °C for initial denaturation followed by 20 cycles of 94 °C for 30 s, 60 °C for 40 s, and 72 °C for 1 min. The amplified cDNA was subcloned into the pETBlue vector. To express the recombinant human soluble COMT, purified pETBlue plasmid harboring the amplified COMT cDNA was transformed into E. coli BL21 (DE3) cells and the transformed cells were grown in 1 L LB medium supplemented with 100 μg/ml ampicillin. After the cell density reached ~0.2 OD600 nm, IPTG (at a final concentration of 1 mM) was added to induce the expression of the recombinant human COMT overnight at room temperature. Afterwards, the cells were collected and homogenized in 25 ml ice-cold lysis buffer (10 mM Tris–HCl, pH 8.0, 150 mM NaCl, and 1 mM EDTA) using a French Press. The crude homogenate supplemented with a protease inhibitor cocktail was subjected to centrifugation at 10,000 × g for 20 min at 4 °C. The supernatant collected was stored at −80 °C prior to being used for the enzymatic assay.
2.6. Enzymatic methylation–sulfation assay
In a two-stage methylation–sulfation assay, the methylation reaction was first performed using unlabeled or [14C]-labeled AdoMet as the methyl group donor. The standard assay mixture, in a final volume of 20 μl, contained 50 mM Tris–HCl buffer at pH 7.5, 5 mM DTT, 1.5 mM MgCl2, varying concentrations of unlabeled AdoMet (at final concentrations of 0, 2.5, 5, 10, 25, and 50 μM) or 50 μM [14C]AdoMet, and 5 or 50 μM substrate (dopamine, epinephrine, isoproterenol, isoetharine). The reaction was started by the addition of 50 μg COMT-expressing cell lysate and allowed to proceed for 30 min at 37 °C. Afterwards, 1.0 μl of SULT1A3 (at 1 mg/ml) and 1.25 μl of [35S]PAPS (at a final concentration of 14 μM) or varying concentrations of unlabeled PAPS (at final concentrations of 0, 10, 25, 50, and 100 μM) were added to each reaction mixture, and the sulfation reaction was allowed to proceed for another 10 or 30 min at 37 °C. The reaction was terminated by adding the 10 μl of 1 M HCl and the precipitates formed were cleared by centrifugation at 16,000 × g for 20 min. For the analysis of [35S]sulfated product, the supernatant was neutralized with 1 M NaOH and was subjected to the TLC analysis with n-butanol/isopropanol/formic acid/water (2:1:3:1; v/v/v/v) as the solvent system. For the analysis of [14C]methylated product, the supernatant was directly subjected to the TLC analysis with n-butanol/isopropanol/formic acid/water (3:1:1:1; v/v/v/v) as the solvent system. Upon completion of TLC, an autoradiograph was taken from the TLC plate to reveal radioactive spots corresponding to [35S]sulfated or [14C]methylated products of tested catecholic compounds. Thereafter, the radioactive spots were cut out from the plate, eluted, mixed with Ecolume scintillation cocktail, and counted using a liquid scintillation counter.
2.7. Miscellaneous methods
[35S]PAPS was synthesized from ATP and carrier-free [35S]sulfate using the recombinant human bifunctional PAPS synthase and its purity was determined as previously described [33]. The [35S]PAPS synthesized was adjusted to the required concentration and a specific activity of 15 Ci/mmol at 1.4 mM by the addition of unlabeled PAPS. SDS-polyacrylamide gel electrophoresis (SDS-PAGE) was performed on 12% polyacrylamide gels using the method of Laemmli [34]. Protein determination was based on the method of Bradford with bovine serum albumin as the standard [35].
3. Results
3.1. Generation and release of [35S]sulfated metabolites of catecholic drugs by HepG2 cells
Confluent HepG2 cells grown in individual wells of a 24-well plate were labeled with [35S]sulfate in sulfate-free medium containing 50 μM of dopamine, epinephrine, isoproterenol, isoetharine, or apomorphine. At the end of an 18-h incubation, the labeling media were collected and analyzed for the generation and release of [35S]sulfated metabolites by thin-layer chromatography. It is noted that the 18-h incubation period was selected in order to allow sufficient time for the cells to metabolize tested drugs while remaining fully viable in the sulfate-free medium used in the metabolic labeling experiment. Compared with the control without added drug, two distinct [35S]sulfated species were observed in the labeling media containing dopamine, epinephrine, or isoproterenol, whereas a major [35S]sulfated species overlapping with a slower-migrating minor [35S]sulfated species was observed in the labeling medium containing isoetharine (Fig. 1). In contrast, only one [35S]sulfated species was observed in the labeling medium containing apomorphine (Fig. 1).
Fig. 1.
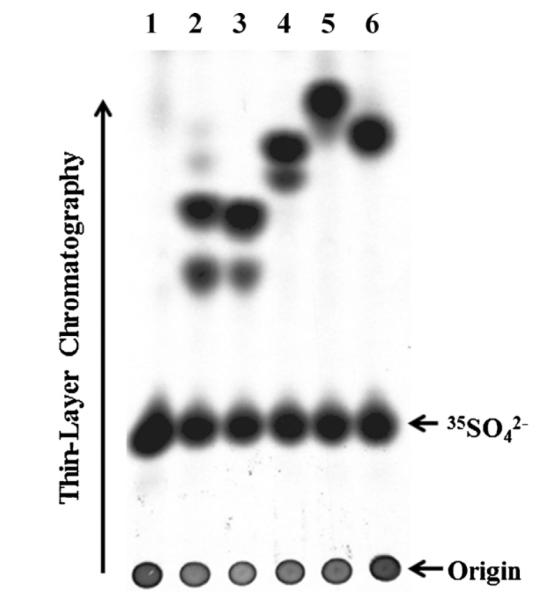
Analysis of [35S]sulfated metabolites generated and released by HepG2 cells labeled with [35S]sulfated in the presence of catecholic drugs. Confluent HepG2 cells were labeled with [35S]sulfate in the presence of 50 μM of different catecholic drugs. At the end of an 18-h labeling, the labeling media were collected and subjected to the TLC analysis for [35S]sulfated metabolites. The catecholic drugs tested were dopamine (lane 2), epinephrine (lane 3), isoproterenol (lane 4), isoetharine (lane 5), and apomorphine (lane6). Lane 1 shows the control without addition of catecholic drugs to the labeling medium. The figure is representative of three independent experiments.
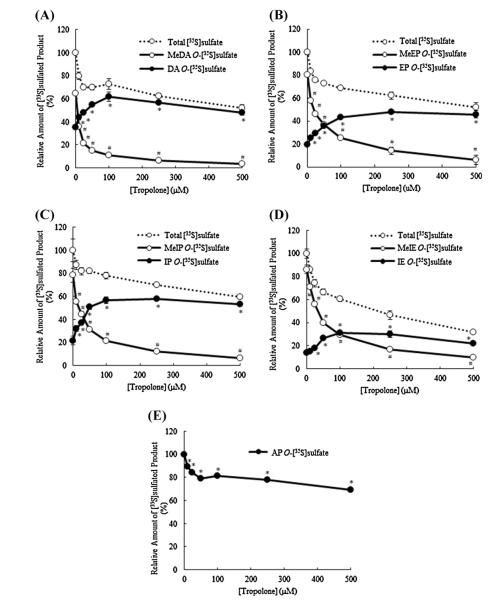
3.2. Effects of tropolone, a COMT inhibitor, on the generation and release of [35S]sulfated metabolites of catecholic drugs by HepG2 cells
To clarify the identity of the two [35S]sulfated species detected in the labeling media of HepG2 cells labeled in the presence of dopamine, a preliminary experiment using [35S]sulfated 3-O-methyldopamine and [35S]sulfated dopamine enzymatically synthesized using purified SULT1A3 was performed to compare their positions of migration with those of the two [35S]sulfated metabolites of dopamine generated by HepG2 cells upon TLC separation. Autoradiograph taken from the plate upon completion of TLC showed clearly co-migrations of enzymatically synthesized [35S]sulfated 3-O-methyldopamine and [35S]sulfated dopamine with, respectively, upper and lower [35S]sulfated species present in the labeling medium containing dopamine (figure not shown). To clarify further the occurrence of the metabolism of dopamine, epinephrine, isoproterenol, or isoetharine through single (sulfation only) and dual (methylation plus sulfation) conjugation reaction(s), a similar metabolic labeling study was performed in the presence of tropolone, a COMT inhibitor [36,37]. As shown in Fig. 2, in the labeling media containing dopamine, epinephrine, or isoproterenol, with increasing concentrations of tropolone, a proportionate decrease in the intensity of the upper [35S]sulfated species (as indicated by empty arrows) was observed with a concomitant increase in the intensity of the lower [35S]sulfated species (as indicated by solid arrows). It was noted that, in the labeling media containing isoetharine, while the lower [35S]sulfated species was barely visible in the absence of tropolone (also cf.Fig. 1), it became increasingly prominant and distinct with increasing concentrations of tropolone. At the same time, the upper [35S]sulfated species showed a proportionate decrease in intensity. Moreover, the migration positions, upon TLC analysis, of [35S]sulfated epinephrine, isoproterenol, and isoetharine enzymatically synthesized using purified SULT1A3 coincided to the lower [35S]sulfated species detected in the labeling media containing each of the four catecholic drugs (figure not shown). Collectively, these results indicated that the upper [35S]sulfated species in the labeling media in the presence of dopamine, epinephrine, isoproterenol, or isoetharine corresponded to the doubly conjugated (methylated–[35S]sulfated) metabolite of each of these four catecholic drugs. The lower [35S]sulfated species corresponded to the singly conjugated ([35S]sulfated) metabolite of these four catecholic drugs. It was noted that a single [35S]sulfated species was detected in the labeling media containing apomorphine, irrespective of the different concentrations of tropolone used (Fig. 2). The levels of the upper and lower [35S]sulfated species present in the spent media of HepG2 cells collected in the tropolone treatment experiment were determined. As shown in Fig. 3, in the absence of tropolone, the methylated–[35S]sulfated metabolites of dopamine, epinephrine, isoproterenol, and isoetharine produced by the HepG2 cells accounted for 64%, 80%, 78%, and 86% of the total [35S]sulfated metabolites of these four catecholic drugs. With increasing concentrations of tropolone, the amounts of these (methylated–[35S]sulfated) metabolites showed a proportionate decrease, while the lower [35S]sulfated metabolites showed a corresponding increase, reaching 85%, 63%, 73%, and 52% of the total [35S]sulfated metabolites, respectively, at 100 μM of tropolone. It was noted that with increasing tropolone concentrations, the combined amount of upper and lower [35S]sulfated species started decreasing, to 48% (dopamine), 46% (epinephrine), 59% (isoproterenol), and 32% (isoetharine) at 500 μM tropolone, compared with those detected in media without trpolone. For apomorphine which was conjugated exclusively by sulfation, there was also a decrease in the generation and release of [35S]sulfated apomorphine by HepG2 cells labeled in the presence of tropolone (decreased to 69% at 500 μM tropolone).
Fig. 2.
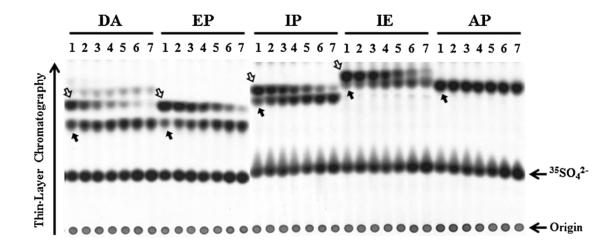
Analysis of [35S]sulfated metabolites generated and released by HepG2 cells labeled with [35S]sulfated in the presence of catecholic drugs plus different concentrations of tropolone. Confluent HepG2 cells were labeled with [35S]sulfate in the presence of 50 μM of different catecholic drugs plus varying concentrations (0, 10, 25, 50, 100, 250, and 500 μM) of tropolone. At the end of an 18-h labeling, the labeling media were collected and subjected to the TLC analysis for [35S]sulfated metabolites. Lanes 1–7 correspond to labeling media containing different catecholic drugs (dopamine (DA), epinephrine (EP), isoproterenol (IP), isoetharine (IE), and apomorphine (AP)) in the presence of different concentrations 0, 10, 25 μM (lane 3), 50, 100, 250, and 500 μM of tropolone. The empty and solid arrows indicate the [35S]sulfated derivatives of the catecholic drugs tested. The figure is representative of three independent experiments.
Fig. 3.
Quantitative data on the [35S]sulfated metabolites generated and released by HepG2 cells labeled with [35S]sulfate in the presence of different catecholic drugs plus different concentrations of tropolone. Results shown correspond to those of the [35S]sulfated metabolites of dopamine (A), epinephrine (B), isoproterenol (C), isoetharine (D), and apomorphine (E) (cf. Fig. 2). The radioactivity of each [35S]sulfated metabolite separated on the TLC plates shown in Fig. 2 were counted and expressed in relative values (%) against the total amount of the upper (methylated–[35S]sulfated) and lower ([35S]sulfated) metabolites of the control sample (without tropolone). Data shown represent calculated mean ± SD derived from three independent analyses. Statistical significance versus the control sample (without tropolone) are indicated by #p < 0.05 for the level of the upper (methylated–[35S]sulfated) species or *p < 0.05 for the level of lower ([35S]sulfated) species, as analyzed by one-way ANOVA with Dunnett’s test.
3.3. Differential sulfating activity of the human SULTs toward catecholic drugs
A systematic analysis was performed to examine the sulfating activity of eleven human SULTs toward the tested catecholic drugs. Of the eleven human SULTs analyzed, six showed no detectable activity. The other five, SULT1A1, SULT1A2, SULT1A3, SULT1C4, and SULT1E1, exhibited differential sulfating activities toward the five drugs tested (Table 1). Of the five, SULT1A3 showed considerably higher sulfating activities toward all tested drugs except apomorphine. On the other hand, SULT1A1 and SULT1C4 showed stronger sulfating activities toward apomorphine than did SULT1A3. These two latter SULTs also exhibited moderate sulfating activities toward dopamine, epinephrine, isoproterenol, or isoetharine. The two remaining SULTs, SULT1A2 and SULT1E1, displayed sulfating activity toward apomorphine, with the former showing also weak, but significant, activity toward isoetharine.
Table 1.
Specific activities of the human SULT1A1, SULT1A2, SULT1A3, SULT1C4, and SULT1E1 with different catecholic drugs as substrates.a
| Substrate | Specific activity (nmol/min/mg) |
||||
|---|---|---|---|---|---|
| SULT1A1 | SULT1A2 | SULT1A3 | SULT1C4 | SULT1E1 | |
| Dopamine | 5.43 ± 0.31 | N.D.b | 99.00 ± 2.67 | N.D. | N.D. |
| Epinephrine | 3.75 ± 0.30 | N.D. | 55.49 ± 0.87 | 1.60 ± 0.19 | N.D. |
| Isoproterenol | 2.12 ± 0.11 | N.D. | 47.35 ± 0.49 | 0.19 ± 0.3 | N.D. |
| Isoetharine | 2.01 ± 0.06 | 0.12 ± 0.01 | 36.43 ± 0.59 | 1.68 ± 0.04 | N.D. |
| Apomorphine | 67.09 ± 0.60 | 14.85 ± 0.24 | 52.59 ± 1.82 | 72.65 ± 0.20 | 8.41 ± 0.26 |
Data represent mean ± SD derived from three determinations. The concentration of the substrate used in the assay mixture was 50 μM.
Specific activity determined was lower than the detection limit (estimated to be ~0.01 nmol/min/mg protein).
3.4. Concerted actions of COMT and SULT
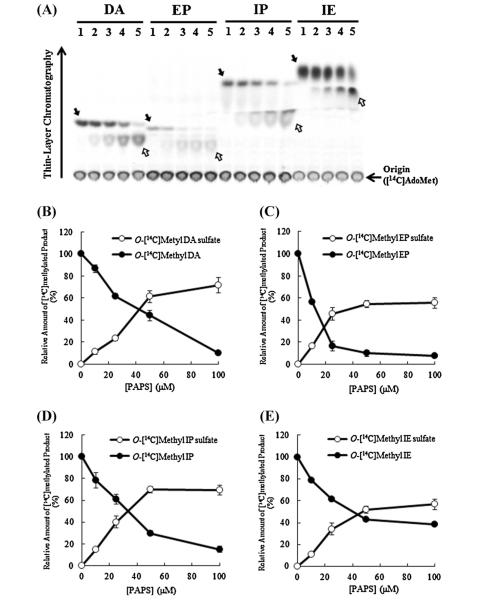
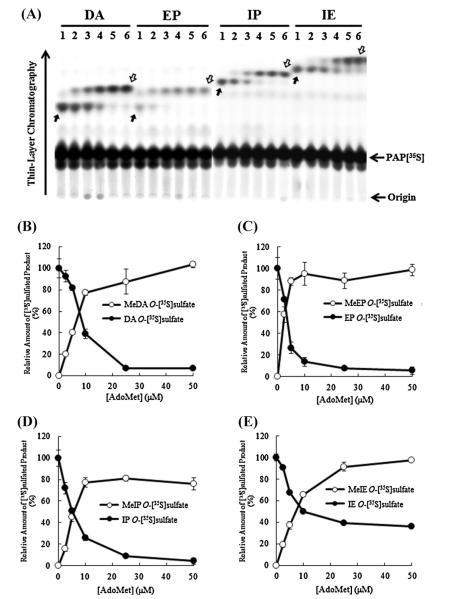
Since the results from the metabolic labeling experiments indicated the occurrence of doubly conjugated (methylated and sulfated) metabolites of dopamine, epinephrine, isoproterenol, and isoetharine, we were interested in clarifying the identity of the enzymes responsible for the sequential conjugations of these catecholic drugs. A two-stage methylation–sulfation assay involving first the methylation by recombinant human soluble COMT followed by a human SULT was established. Since unmethylated and methylated catecholic drugs are in fact different chemical entities, each of the eleven human SULTs was tested in the methylation–sulfation assay. A preliminary experiment revealed that SULT1A3 displayed strong sulfating activity toward methylated derivatives of all four catecholic drugs tested. Of the other 10 human SULTs, only SULT1A1 and SULT1C4 showed relatively weak sulfating activity toward methylated dopamine and methylated isoetharine, respectively (data not shown). To characterize further the dual conjugation of catecholic drugs by methylation and sulfation, the methylation–sulfation assays using recombinant soluble COMT and SULT1A3 were performed. In the first series of these assays, [14C]AdoMet at a fixed concentration of 50 μM in the initial methylation reaction and varying concentrations (ranging 0–100 μM) of nonradioactive PAPS were used in the subsequent sulfation reaction. As shown in Fig. 4A, with increasing concentrations of nonradioactive PAPS used in the sulfation reactions, increased amounts of [14C]-labeled methylated catecholic drugs were sulfated. Fig. 4B–E shows the quantitative data of the [14C]radioactivity associated with [14C]methylated (as indicated by solid arrows) or [14C]methylated–sulfated (as indicated by empty arrows) product of each of the four catecholic drugs generated during the two-stage methylation–sulfation assay. In a second series of the assays, varying concentrations (ranging 0–50 μM) of nonradioactive AdoMet were used in the initial methylation reaction, and a fixed concentration (14 μM) of PAP[35S] was used in the subsequent sulfation reaction. As shown in Fig. 5A, at low AdoMet concentrations, less doubly methylated–[35S]sulfated products of the catecholic drugs were generated and more singly [35S]sulfated products of the catecholic drugs were produced. With increasing concentrations of AdoMet used in the initial methylation reaction, more doubly methylated–[35S]sulfated products of the catecholic drugs were produced. Fig. 5B–E shows the quantitative data of the [35S]radioactivity associated with [35S] sulfated (as indicated by solid arrows) or methylated–[35S]sulfated (as indicated by empty arrows) product of each of the four catecholic drugs generated during the two-stage methylation– sulfation assay.
Fig. 4.
Methylation–sulfation assays using [14C]AdoMet and unlabeled PAPS. COMT and SULT1A3 were used, respectively, in the methylation and sulfation reactions of dopamine (DA), epinephrine (EP), isoproterenol (IP), and isoetharine (IE). (A) TLC analysis of [14C]-labeled products of catecholic drugs generated during the two-stage methylation–sulfation assays. Methylation reaction was carried out using 50 μM [14C]AdoMet, followed by sulfation reaction using different concentrations (0 μM (lane 1), 10 μM (lane 2), 25 μM (lane 3), 50 μM (lane 4), 100 μM (lane 5)) of unlabeled PAPS. The solid and empty arrows indicate the two [14C]methylated derivatives of the catecholic drugs tested. The figure is representative of three independent experiments. (B–E) Quantitative analysis of [14C]methylated and [14C]methylated–sulfated products generated during the methylation–sulfation assays. Results obtained were expressed as relative value (%) against (unsulfated) [14C]methylated product produced with 0 μM of PAPS. Data shown correspond to calculated mean ± SD derived from three independent analyses.
Fig. 5.
Methylation–sulfation assays using unlabeled AdoMet and [35S]PAPS. COMT and SULT1A3 were used, respectively, in the methylation and sulfation reactions of dopamine (DA), epinephrine (EP), isoproterenol (IP), and isoetharine (IE). (A) TLC analysis of [35S]-labeled products of catecholic drugs generated during the two-stage methylation–sulfation assays. Methylation reaction was carried out using different concentrations of unlabeled AdoMet, 0 μM (lane 1), 2.5 μM (lane 2), 5 μM (lane 3), 10 μM (lane 4), 25 μM (lane 5), 50 μM (lane 6), followed by sulfation reaction by SULT1A3 with 14 μM [35S]PAPS as the sulfate donor. The figure is representative of three independent experiments. The solid and empty arrows indicate the two [35S]sulfated derivatives of the catecholic drugs tested. (B–E) Quantitative analysis of methylated–[35S]sulfated and [35S]sulfated products generated during the methylation–sulfation assays. Results obtained were expressed as relative value (%) against (unmethylated) [35S]sulfated product produced with 0 μM AdoMet. Data shown correspond to calculated mean ± SD derived from three independent analyses.
4. Discussion
Conjugation reactions, particularly methylation and sulfation, are known to be involved in the metabolism and regulation of catecholic compounds [6–10]. A previous study using SK-N-MC human neuroblastoma cells demonstrated that dopamine may be subjected to methylation and sulfation independently or in combination, forming two major sulfated metabolites, 3-O-methyldopamine 4-O-sulfate and dopamine O-sulfate [38]. It is therefore an interesting issue to clarify whether and how methylation and sulfation may act in concert in the metabolism of catecholic drugs.
A metabolic labeling study was initially performed to investigate the metabolism of catecholic drugs by methylation and/or sulfation using HepG2 human hepatoma cells, which are known to express the COMT and the SULTs including SULT1A1, SULT1A2, SULT1A3, SULT1E1, and SULT2A1 [39–41]. Results showed that HepG2 cells labeled with [35S]sulfate in the presence of dopamine, epinephrine, isoproterenol, or isoetharine produced and released two major [35S]sulfated metabolites. A subsequent experiment using tropolone, a COMT inhibitor, confirmed the identity of the fast migrating (upon TLC) [35S]sulfated metabolite being a doubly conjugated (methylated–sulfated) product (cf. Figs. 1 and 2). The slower-migrating [35S]sulfated metabolite, on the other hand, co-migrated with singularly [35S]sulfated products of the tested catecholic drugs synthesized enzymatically. Based on the [35S]radioactivity determination, [35S]sulfated–methylated dopamine, epinephrine, isoproterenol, and isoetharine accounted for, respectively, 64%, 80%, 78%, and 86% of the total [35S]sulfated products produced by HepG2 cells labeled in the presence of each of these four catecholic drugs. It therefore appears that dual conjugation by methylation and sulfation represented a major pathway for the metabolism of the four tested catecholic drugs. In contrast, a single [35S]sulfated metabolite of apomorphine was observed in the metabolic labeling experiment (cf. Figs. 1 and 2). While the reason for the inability of HepG2 cells to methylate apomorphine remains unclear, it is possible that the chemical structure of apomorphine may render it unable to be used as a substrate for the COMT. It is noted that although O-methylation of apomorphine had been demonstrated using rat liver COMT [42], neither methylation activity of the human COMT toward apomorphine nor the generation and release of O-methylated apomorphine by humans had been reported [13,43]. Moreover, in a COMT assay using human recombinant soluble COMT expressed in E. coli, no methylated product of apomorphine was detected (data not shown). For dopamine, epinephrine, isoproterenol, and isoetharine, the inhibition of methylation, upon treatment with tropolone, led to a concomitant increase in the production of singularly [35S]sulfated products. It appeared therefore that sulfation could compensate for the lack of methylation in the metabolism of catecholic drugs. It was noted also that treatment with tropolone led to a decrease in the amount of total (methylated–sulfated plus sulfated) [35S]sulfated products. While the exact mechanism underlying such a decrease remains to be clarified, one possibility is that the decrease could have been due to the cytotoxic effect of tropolone [44], resulting in decreased methylating and/or sulfating capacity of the cells. Additionally, tropolone, due to its structural similarity to catecholic drugs, may act as an inhibitor for SULT1A3, thereby decreasing its capacity to sulfate both unmethylated and methylated catecholic drugs. Moreover, the possibilities that other pathways, e.g., glucuronidation, may act to metabolize catecholic drugs when COMT is inhibited or that sulfated metabolites of catecholic drugs may be desulfated or otherwise degraded during the 18-hr incubation period should not be overlooked.
An important issue is with regard to the functional relevance of the dual conjugation of catecholic drugs. It has been proposed that methylation of catecholic compounds may lead to the inactivation of their physiological/pharmacological activity [6–10]. Some studies, however, showed that O-methyl norepinephrine and O-methyl epinephrine retained some affinity toward adrenergic receptors thereby displaying antagonist activity; whereas O-sulfate forms showed no affinity toward the receptors [45,46]. It is therefore possible that in the dual conjugation of catecholic drugs, methylation may rapidly inactivate the pharmacological activity and the subsequent sulfation may lead to the complete loss of their activity and, at the same time, facilitate their excretion from body. Such a dual conjugation of catecholic drugs may provide another advantage in terms of delaying or preventing the re-activation of conjugated catecholic compounds via deconjugation reactions, since both demethylation and desulfation would be required in order to recover their pharmacological activity. Although there is no information currently available concerning the demethylation or desulfation of methylated–sulfated catecholic compounds, O-methyl dopamine and O-methyl epinephrine have been shown to be de-methylated by enzymatic action for which the responsible enzyme has not been clearly defined [47,48]. Dopamine O-sulfate and epinephrine O-sulfate have also been shown to be de-sulfated by aryl sulfatases [49,50]. It is therefore an interesting question whether the dual conjugation of catecholic compounds may serve to prevent their deconjugation to revert back to the unconjugated, active form.
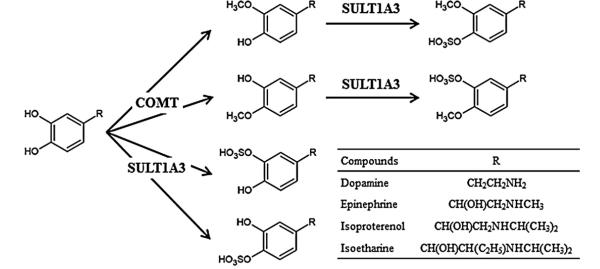
To clarify the SULT enzyme(s) responsible for the sulfation of unmethylated and methylated catecholic drugs, a systematic analysis of the sulfating activity of eleven known human SULTs was first performed. Five of the eleven, SULT1A1, SULT1A2, SULT1A3, SULT1C4, and SULT1E1, were found to display differential sulfating activities toward the five catecholic drugs tested (cf. Table 1). It should be pointed out that previous studies using human recombinant SULTs had demonstrated that SULT1A3 displayed considerably stronger sulfating activities toward dopamine, epinephrine, and isoproterenol than other human SULTs tested, and SULT1A1 and SULT1A3 displayed strong sulfating activities toward apomorphine [51,52]. Interestingly, our data revealed that SULT1A3 and SULT1C4 displayed the strongest sulfating activities toward isoetharine and apomorphine, respectively, among the eleven human SULTs tested. A two-stage sequential methylation–sulfation assay was subsequently established to examine the SULT enzyme(s) responsible for the sulfation of methylated catecholic drugs. Using the human soluble COMT in combination with individual SULTs, it was found that among the eleven human SULTs, SULT1A3 exhibited sulfating activity toward the methylated catecholic drugs generated under the action of COMT. It is to be noted that methylated catecholic drugs are different chemical entities from their unmethylated counterparts. It is therefore not surprising that while other SULTs such as SULT1A1, SULT1A2, SULT1C4, and SULT1E1 displayed sulfating activity toward unmethylated catecholic drugs, only SULT1A3 was able to sulfate methylated catecholic drugs. In methylation–sulfation assays, it was noted that the generation of doubly conjugated (methylated–sulfated) product was dependent on the levels of both the methyl donor (AdoMet) and sulfonate donor (PAPS). Another issue worth mentioning is with regard to the sequence of dual conjugation of catecholic drugs by methylation and sulfation. In a sulfation-methylation assay in which sulfation was carried out prior to methylation, no doubly conjugated (methylated–sulfated) products were detected (data not shown). This was not surprising since upon sulfation, sulfated catecholic drugs were no longer catecholic compounds and therefore could not serve as substrates for COMT. It is worthwhile mentioning that 3-O-methylated, 4-O-sulfated doubly conjugated dopamine, epinephrine, isoproterenol, or isoetharine had been identified in plasma and/or urine of human subjects as a major metabolite [53–56]. Fig. 6 summarizes the reactions and responsible enzymes in the single or dual conjugation of these catecholic drugs.
Fig. 6.
Proposed pathways for the methylation and/or sulfation of catecholic drugs as mediated by COMT or SULT1A3.
In conclusion, the current study showed clear evidence for the concerted actions of the COMT and SULT1A3 in mediating the dual conjugation (methylation and sulfation) for four of the five catecholic drugs tested. From the physiological standpoint, the functional relevance of the dual conjugation may lie in the irreversible metabolism of catecholic drugs during which methylation may serve to first inactivate their pharmacological activity followed by sulfation which then render the methylated derivatives more water-soluble so as to be more easily excreted. More work is warranted in order to validate these critical events.
Acknowledgments
This work was supported in part by a National Institutes of Health grant GM085756 and a startup fund from College of Pharmacy, The University of Toledo.
Footnotes
Contributors Participated in research design: Kurogi, Liu, M.-Y., Sakakibara, Suiko, Sugahara, Liu, M.-C.
Conducted experiments: Kurogi and Alazizi.
Performed data analysis: Kurogi and Liu, M.-C.
Wrote or contributed to the writing of the manuscript: Kurogi and Liu, M.-C.
References
- [1].Almquist A, Goldenberg IF, Milstein S, Chen MY, Chen XC, Hansen R, et al. Provocation of bradycardia and hypotension by isoproterenol and upright posture in patients with unexplained syncope. N Engl J Med. 1989;320:346–51. doi: 10.1056/NEJM198902093200603. [DOI] [PubMed] [Google Scholar]
- [2].Emerman CL, Shade B, Kubincanek J. A controlled trial of nebulized isoetharine in the prehospital treatment of acute asthma. Am J Emerg Med. 1990;8:512–4. doi: 10.1016/0735-6757(90)90153-q. [DOI] [PubMed] [Google Scholar]
- [3].Dupuis JY, Bondy R, Cattran C, Nathan HJ, Wynands JE. Amrinone and dobutamine as primary treatment of low cardiac output syndrome following coronary artery surgery: a comparison of their effects on hemodynamics and outcome. J Cardiothorac Vasc Anesth. 1992;6:542–53. doi: 10.1016/1053-0770(92)90096-p. [DOI] [PubMed] [Google Scholar]
- [4].Klarr JM, Faix RG, Pryce CJ, Bhatt-Mehta V. Randomized, blind trial of dopamine versus dobutamine for treatment of hypotension in preterm infants with respiratory distress syndrome. J Pediatr. 1994;125:117–22. doi: 10.1016/s0022-3476(94)70137-7. [DOI] [PubMed] [Google Scholar]
- [5].Furukawa CT, Lodewick MJ. β-Adrenergic agonists. In: Lieberman P, Anderson JA, editors. Current clinical practice: allergic diseases: diagnosis and treatment. Human Press; Totowa, NJ: 2007. pp. 335–42. [Google Scholar]
- [6].Axelrod J, Tomochick R. Enzymatic O-methylation of epinephrine and other catechols. J Biol Chem. 1958;233:702–5. [PubMed] [Google Scholar]
- [7].McFadden ER., Jr Beta 2 receptor agonist: metabolism and pharmacology. J Allergy Clin Immunol. 1981;68:91–7. doi: 10.1016/0091-6749(81)90164-0. [DOI] [PubMed] [Google Scholar]
- [8].Mulder GM, Jakoby WB. Sulfation in conjugation reactions. In: Mulder GJ, editor. Drug Metabolism. Taylor and Francis; London: 1990. pp. 107–61. [Google Scholar]
- [9].Coughtrie MW. Catecholamine sulfation in health and disease. Adv Pharmacol. 1998;42:339–42. doi: 10.1016/s1054-3589(08)60759-0. [DOI] [PubMed] [Google Scholar]
- [10].Goldstein DS, Eisenhofer G, Kopin IJ. Sources and significance of plasma levels of catechols and their metabolites in human. J Pharmacol Exp Ther. 2003;305:800–1. doi: 10.1124/jpet.103.049270. [DOI] [PubMed] [Google Scholar]
- [11].Creveling CR, Morris N, Shimizu H, Ong HH, Daily J. Catechol O-methyltransferase. IV. Factors affecting m- and p-methylation of substituted catechols. Mol Pharmacol. 1972;8:398–409. [PubMed] [Google Scholar]
- [12].Männistö PT, Kaakkola S. Catechol-O-methyltransferase (COMT): biochemistry, molecular biology, pharmacology, and clinical efficacy of the new selective COMT inhibitors. Pharmacol Rev. 1999;51:593–628. [PubMed] [Google Scholar]
- [13].Lautala P, Ulmanen I, Taskinen J. Molecular mechanisms controlling the rate and specificity of catechol O-methylation by human soluble catechol O-methyltransferase. Mol Pharmacol. 2001;59:393–402. doi: 10.1124/mol.59.2.393. [DOI] [PubMed] [Google Scholar]
- [14].Zhu BT. Catechol-O-methyltransferase (COMT)-mediated methylation metabolism of endogenous bioactive catechols and modulation by endobiotics and xenobiotics: importance in pathophysiology and pathogenesis. Curr Drug Metab. 2002;3:321–49. doi: 10.2174/1389200023337586. [DOI] [PubMed] [Google Scholar]
- [15].Tenhunen J, Salminen M, Lundström K, Kiviluoto T, Savolainen R, Ulmanen I. Genomic organization of the human catechol O-methyltransferase gene and its expression from two distinct promoters. Eur J Biochem. 1994;223:1049–59. doi: 10.1111/j.1432-1033.1994.tb19083.x. [DOI] [PubMed] [Google Scholar]
- [16].Ulmanen I, Peränen J, Tenhunen J, Tilgmann C, Karhunen T, Panula P, et al. Expression and intracellular localization of catechol O-methyltransferase in transfected mammalian cells. Eur J Biochem. 1997;243:452–9. doi: 10.1111/j.1432-1033.1997.0452a.x. [DOI] [PubMed] [Google Scholar]
- [17].Lotta T, Vidgren J, Tilgmann C, Ulmanen I, Melén K, Julkunen I, et al. Kinetics of human soluble and membrane-bound catechol O-methyltransferase: a revised mechanism and description of the thermolabile variant of the enzyme. Biochemistry. 1995;34:4202–10. doi: 10.1021/bi00013a008. [DOI] [PubMed] [Google Scholar]
- [18].Bai HW, Shim JY, Yu J, Zhu BT. Biochemical and molecular modeling studies of the O-methylation of various endogenous and exogenous catechol substrates catalyzed by recombinant human soluble and membrane-bound catechol-O-methyltransferase. Chem Res Toxicol. 2007;20:1409–25. doi: 10.1021/tx700174w. [DOI] [PubMed] [Google Scholar]
- [19].Lipmann F. Biological sulfate activation and transfer. Science. 1958;128:575–80. doi: 10.1126/science.128.3324.575. [DOI] [PubMed] [Google Scholar]
- [20].Falany CN, Roth JA. Properties of human cytosolic sulfotransferase involved in the drug metabolism. In: Jeffery EH, editor. Human Drug Metabolism: From Molecular Biology to Man. CRC Press; Boca Raton, FL: 1993. pp. 101–15. [Google Scholar]
- [21].Weinshilboum R, Otterness DM. Sulfotransferase enzymes. In: Kaufman FC, editor. Conjugation–Deconjugation Reactions in Drug Metabolism and Toxicity. Springer-Verlag; Berlin: 1994. pp. 45–78. [Google Scholar]
- [22].Strott CA. Sulfonation and molecular action. Endocr Rev. 2002;23:703–32. doi: 10.1210/er.2001-0040. [DOI] [PubMed] [Google Scholar]
- [23].Falany CL. Enzymology of human cytosolic sulfotransferases. FASEB J. 1997;11:206–16. doi: 10.1096/fasebj.11.4.9068609. [DOI] [PubMed] [Google Scholar]
- [24].Dajani R, Cleasby A, Neu M, Wonacott AJ, Jhoti H, Hood AM, et al. X-ray crystal structure of human dopamine sulfotransferase, SULT1A3. Molecular modeling and quantitative structure-activity relationship analysis demonstrate a molecular basis for sulfotransferase substrate specificity. J Biol Chem. 1999;274:37862–68. doi: 10.1074/jbc.274.53.37862. [DOI] [PubMed] [Google Scholar]
- [25].Adjei AA, Weinshilboum RM. Catecholestrogen sulfation: possible role in carcinogenesis. Biochem Biophys Res Commun. 2002;292:402–8. doi: 10.1006/bbrc.2002.6658. [DOI] [PubMed] [Google Scholar]
- [26].Hui Y, Yasuda S, Liu M-Y, Wu Y-Y, Liu M-C. On the sulfation and methylation of catecholestrogens in human mammary epithelial cells and breast cancer cells. Biol Pharm Bull. 2008;31:769–73. doi: 10.1248/bpb.31.769. [DOI] [PubMed] [Google Scholar]
- [27].Sakakibara Y, Takami Y, Nakayama T, Suiko M, Liu M-C. Localization and functional analysis of the substrate specificity/catalytic domains of human M-form and P-form phenol sulfotransferases. J Biol Chem. 1998;273:6242–7. doi: 10.1074/jbc.273.11.6242. [DOI] [PubMed] [Google Scholar]
- [28].Sakakibara Y, Yanagisawa K, Katafuchi J, Ringer DP, Takami Y, Nakayama T, et al. Molecular cloning, expression, and characterization of novel human SULT1C sulfotransferases that catalyze the sulfonation of N-hydroxy-2-acetylaminofluorene. J Biol Chem. 1998;273:33929–35. doi: 10.1074/jbc.273.51.33929. [DOI] [PubMed] [Google Scholar]
- [29].Sakakibara Y, Suiko M, Pai TG, Nakayama T, Takami Y, Katafuchi J, et al. Highly conserved mouse and human brain sulfotransferases: molecular cloning, expression, and functional characterization. Gene. 2002;285:39–47. doi: 10.1016/s0378-1119(02)00431-6. [DOI] [PubMed] [Google Scholar]
- [30].Suiko M, Sakakibara Y, Liu M-C. Sulfation of environmental estrogen-like chemicals by human cytosolic sulfotransferases. Biochem Biophys Res Commun. 2000;2267:80–4. doi: 10.1006/bbrc.1999.1935. [DOI] [PubMed] [Google Scholar]
- [31].Pai TG, Sugahara T, Suiko M, Sakakibara Y, Xu F, Liu M-C. Differential xenoestrogen-sulfating activities of the human cytosolic sulfotransferase: molecular cloning, expression, and purification of human SULT2B1a and SULT2B1b sulfotransferases. Biochim Biophys Acta. 2002;1573:165–70. doi: 10.1016/s0304-4165(02)00416-6. [DOI] [PubMed] [Google Scholar]
- [32].Liu M-C, Lipmann F. Decrease of tyrosine-O-sulfate-containing proteins found in rat fibroblasts infected with Rous sarcoma virus or Fujinami sarcoma virus. Proc Natl Acad Sci USA. 1984;81:3695–8. doi: 10.1073/pnas.81.12.3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Yanagisawa K, Sakakibara Y, Suiko M, Takami Y, Nakayama T, Nakajima H, et al. cDNA cloning, expression, and characterization of the human bifunctional ATP sulfurylase/adenosine 5′-phosphosulfate kinase enzyme. Biosci Biotechnol Biochem. 1998;62:1037–40. doi: 10.1271/bbb.62.1037. [DOI] [PubMed] [Google Scholar]
- [34].Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- [35].Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- [36].Belleau B, Burba J. Occupancy of adrenergic receptors and inhibition of catechol o-methyl transferase by tropolones. J Med Chem. 1963;6:755–9. doi: 10.1021/jm00342a028. [DOI] [PubMed] [Google Scholar]
- [37].Borchardt RT. Catechol O-methyltransferase. 1. Kinetics of tropolone inhibition. J Med Chem. 1973;16:377–82. doi: 10.1021/jm00262a015. [DOI] [PubMed] [Google Scholar]
- [38].Yasuda S, Yasuda T, Hui Y, Liu M-Y, Suiko M, Sakakibara Y, et al. Concerted action of the cytosolic sulfotransferase, SULT1A3, and catechol-O-methyltransferase in the metabolism of dopamine in SK-N-MC human neuroblastoma cells. Neurosci Res. 2009;64:273–9. doi: 10.1016/j.neures.2009.03.011. [DOI] [PubMed] [Google Scholar]
- [39].Malherbe P, Bertocci B, Caspers P, Zürcher G, Da Prada M. Expression of functional membrane-bound and soluble catechol-O-methyltransferase in Escherichia coli and a mammalian cell line. J Neurochem. 1992;58:1782–9. doi: 10.1111/j.1471-4159.1992.tb10054.x. [DOI] [PubMed] [Google Scholar]
- [40].Miyano J, Yamamoto S, Hanioka N, Narimatsu S, Ishikawa T, Ogura K, et al. Involvement of SULT1A3 in elevated sulfation of 4-hydroxypropranolol in Hep G2 cells pretreated with beta-naphthoflavone. Biochem Pharmacol. 2005;69:941–50. doi: 10.1016/j.bcp.2004.12.012. [DOI] [PubMed] [Google Scholar]
- [41].Westerink WM, Schoonen WG, Phase II. enzyme levels in HepG2 cells and cryopreserved primary human hepatocytes and their induction in HepG2 cells. Toxicol In Vitro. 2007;21:1592–602. doi: 10.1016/j.tiv.2007.06.017. [DOI] [PubMed] [Google Scholar]
- [42].Missala K, Lal S, Sourkes TL. O-Methylation of apomorphine and the metabolic prolongation of apomorphine-induced stereotyped behavior. Eur J Pharmacol. 1973;22:54–8. doi: 10.1016/0014-2999(73)90183-0. [DOI] [PubMed] [Google Scholar]
- [43].Van der Geest R, Kruger P, Gubbens-Stibbe JM, Van Laar T, Bodde HE, Danhof M. Assay of R-apomorphine, S-apomorphine, apocodein, isoapocodein and their glucuronide and sulfate conjugates in plasma and urine of patients with Parkinson’s disease. J Chromatogr B Biomed Sci Appl. 1997;702:131–41. doi: 10.1016/s0378-4347(97)00370-8. [DOI] [PubMed] [Google Scholar]
- [44].Inamori Y, Tsujibo H, Ohishi H, Ishii F, Mizugaki M, Aso H, et al. Cytotoxic effect of hinokitiol and tropolone on the growth of mammalian cells and on blastogenesis of mouse splenic T cells. Biol Pharm Bull. 1993;16:521–3. doi: 10.1248/bpb.16.521. [DOI] [PubMed] [Google Scholar]
- [45].Michel GL, Lenz T, Lernhardt U, Weicker H, Bieger WP, Werle E. Sulfoconjugated catecholamines: lack of beta-adrenoceptor binding and adenylate cyclase stimulation in human mononuclear leukocytes. Eur J Pharmacol. 1987;143:179–88. doi: 10.1016/0014-2999(87)90531-0. [DOI] [PubMed] [Google Scholar]
- [46].Lenz T, Werle E, Strobel G, Weicker H. O-Methylated and sulfoconjugated catecholamines: differential activities at human platelet alpha 2-adrenoceptors. Can J Physiol Pharmacol. 1991;69:929–37. doi: 10.1139/y91-141. [DOI] [PubMed] [Google Scholar]
- [47].Daly JW, Axelrod J, Witkop B. Dynamic aspects of enzymatic O-methylation and -demethylation of catechols in vitro and in vivo. J Biol Chem. 1960;235:1155–9. [PubMed] [Google Scholar]
- [48].Tyce GM, Sharpless NS, Owen CA., Jr Demethylation in erythrocytes: a reaction involving hemoglobin. Am J Physiol. 1978;235:E150–7. doi: 10.1152/ajpendo.1978.235.2.E150. [DOI] [PubMed] [Google Scholar]
- [49].Hashizume K, Yamatodani A, Yamamoto T, Wada H, Ogihara T. Contents of dopamine sulfoconjugate isomers and their desulfation in dog arteries. Biochem Pharmacol. 1989;38:1891–5. doi: 10.1016/0006-2952(89)90486-3. [DOI] [PubMed] [Google Scholar]
- [50].Strobel G, Werle E, Weicker H. Isomer specific kinetics of dopamine beta-hydroxylase and arylsulfatase towards catecholamine sulfates. Biochem Int. 1990;20:343–51. [PubMed] [Google Scholar]
- [51].Taskinen J, Ethell BT, Pihlavisto P, Hood AM, Burchell B, Coughtrie MW. Conjugation of catechols by recombinant human sulfotransferases, UDP-glucuronosyl-transferases, and soluble catechol O-methyltransferase: structure-conjugation relationships and predictive models. Drug Metab Dispos. 2003;31:1187–97. doi: 10.1124/dmd.31.9.1187. [DOI] [PubMed] [Google Scholar]
- [52].Allali-Hassani A, Pan PW, Dombrovski L, Najmanovich R, Tempel W, Dong A, et al. Structural and chemical profiling of the human cytosolic sulfotransferases. PLoS Biol. 2007;5:e97. doi: 10.1371/journal.pbio.0050097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Goodall M, Alton H. Metabolism of 3-hydroxytyramine (dopamine) in human subjects. Biochem Pharmacol. 1968;17:905–14. doi: 10.1016/0006-2952(68)90350-x. [DOI] [PubMed] [Google Scholar]
- [54].Labrosse EH, Axelrod J, Kopin IJ, Kety SS. Metabolism of 7-H3-epinephrine-d-bitartrate in normal young men. J Clin Invest. 1961;40:253–60. doi: 10.1172/JCI104251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Causon RC, Brown MJ, Davies DS. Reversed-phase high-performance liquid chromatography and amperometric detection of 3-O-methyl isoprenaline sulfate: application to studies on the presystemic metabolism of d-isoprenaline in man. J Chromatogr. 1985;337:311–20. doi: 10.1016/0378-4347(85)80044-x. [DOI] [PubMed] [Google Scholar]
- [56].Williams FM, Briant RH, Dollery CT, Davies DS. The influence of the route of administration on urinary metabolites of isoetharine. Xenobiotica. 1974;4:345–53. doi: 10.3109/00498257409052110. [DOI] [PubMed] [Google Scholar]