Abstract
Background
Tumor necrosis factor-α (TNF-α) inhibitors represent efficacious therapeutic agents in many chronic inflammatory diseases such as psoriasis and rheumatoid arthritis. However they have been connected with increased risk of infection and reactivation of a variety of infectious agents, such as viruses. The reactivation of varicella zoster virus infection causes herpes zoster (HZ), a self-limiting, dermatomally localized, vesicular rash that can be accompanied by postherpetic neuralgia and severe neurological complications.
Main observations
Limited information has been published regarding HZ during therapy with TNF-α inhibitors especially for the occurrence of HZ during adalimumab treatment. We report the case of a 58-year-old immunocompetent man with a 18-year history of plaque psoriasis who develops ophthalmic HZ during treatment with adalimumab.
Conclusion
We report this case to enrich the literature and to highlight the increased risk of HZ infections in patient on anti-TNF-α therapy (incidence of HZ is about 3-fold increased respect to general population). Clinically, these infections often have atypical presentations that may hamper prompt diagnosis. Therefore, it is very important to identify early signs and symptoms of herpes zoster in patients on biologic therapy in order to start prompt efficient antiviral treatment to prevent the development of severe complications.
Keywords: adalimumab, biological therapy, etanercept, herpes zoster, infliximab, psoriasis, viral infection
Introduction
The course of chronic inflammatory diseases, such as psoriasis and rheumatoid arthritis, has been greatly modified by the relatively recent introduction of biologic drugs. Biologic agents include tumor necrosis factor alpha (TNF-α) inhibitors, namely adalimumab, infliximab, golimumab (monoclonal antibodies) and etanercept (dimeric fusion protein).[1] Varicella zoster virus (VZV) is an herpes-virus which establishes latency in neural tissue following primary infection. Reactivation of latent VZV from dorsal root ganglia causes herpes zoster (HZ), a neurocutaneous, painful and dermatomally localized vesicular rash disease. We report the case of a 58-year-old man suffering from psoriasis who develops ophthalmic HZ during treatment with adalimumab.
Case Report
A 58-year-old immunocompetent man with a 18-year history of plaque psoriasis presented to our outpatient clinic with some confluent hemorrhagic vesicles within an erythematous background localized on the scalp and the right side of the frontal region including the tip of the nose [Fig. 1]. These lesions, measuring from 2 to 8 mm in diameter, were characterized by intense itching. In addition they presented a distinctive localization and an unilateral distribution indicating the involvement of the ophthalmic area of the right side of the face. The patient reported an intermittent sensation of tingling, pricking and pain in the same area; these symptoms had preceded the vesicular eruption by 3 days. Patient medical history was not relevant apart from psoriasis and psoriatic arthritis, for which he was receiving an anti- TNF-α, adalimumab, 40 mg subcutaneously every 2 weeks for the last 2 years. The patient was not taking any other medications, had not been ill recently and denied any recent exposure to VZV. However he was reported to have suffered from chickenpox during childhood. Laboratory findings were within normal ranges. Antibodies to hepatitis A, B, and C viruses were negative. A Tzanck smear and a skin biopsy from vesicles showed typical signs of herpetic infection involving the epidermis with ballooning degeneration and multinucleated giant cells containing intranuclear inclusions. Polymerase chain reaction studies showed VZV DNA in the vesicles. Basing on these findings and on the characteristic distribution of the lesions a diagnosis of ophthalmic herpes zoster was made. We sent him for an ophthalmological consult because of the risk of ocular complications: the examination did not show any alterations or pathologic conditions. Anti-TNF-α therapy was temporarily discontinued and the patient was treated with acyclovir 400 mg x 2/die per os for 7 days; because of the involvement of the ophthalmic area, acyclovir ointment was applied into the conjuncitval fornix of the right eye three times a day for 14 days. The patient began to improve early and experienced complete resolution of lesions without sequelae within two weeks. Therefore adalimumab therapy was restarted: the patient had continued to do well on therapy with good control of the psoriatic disease.
Figure 1.
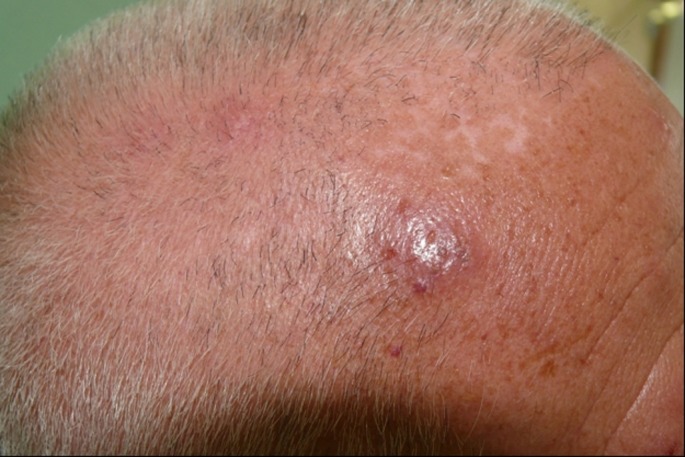
Confluent vesicles within an erythematous background localized on the scalp.
Discussion
TNF-α inhibitors represent efficacious and relatively safe therapeutic agents in many chronic inflammatory diseases. An association between their use and an increased risk of severe bacterial infections and the reactivation of tuberculosis has received much attention in the past but the influence on viral infections has not been extensively investigated.[2-4] The reactivation of VZV infection causes HZ which can be accompanied by postherpetic neuralgia and other rare but severe neurological complications including optic neuritis, aseptic meningitis and meningo-encephalitis. Limited information has been published to date regarding HZ during therapy with TNF-α inhibitors. One case report describing the occurrence of a severe HZ in a 20-year-old man with Crohn's disease at the site of infliximab's 7th and 9th infusion was published by Cruz et al.[5] while a case of disseminated HZ mimicking vasculitis in a rheumatoid arthritis patient on etanercept was reported by Tresch et al.[6] In a survey in 2007, the authors identified reactivation of VZV in 9 of 300 patients on treatment with TNF-α antagonists prescribed for chronic inflammatory disease.[7] McDonald et al. in 2009 reported 96 subjects with incident HZ among the 3.661 rheumatoid arthritis patients prescribed TNF-α antagonists analyzed; of these, 59 occurred on etanercept, 33 aroused on infliximab, and only 4 on adalimumab treatment.[8] Moreover Dreiher et al. in 2011, analyzed the risk of HZ in a group of 22.330 psoriatic patients treated with systemic therapies: among the anti-TNF-α, only the association with infliximab approached statistical significance with the risk of HZ while no cases of HZ were seen among patients treated with adalimumab.[9] A detailed cumulative analysis of studies which reported the occurrence of HZ during anti-TNF-α treatment it is shown in Table 1.[3,5-17] TNF-α inhibits replication of VZV and VZV antigen expression and it has been shown that blocking of TNF-α by monoclonal antibodies completely inhibits this antiviral activity.[18] As a result, blocking of TNF-α may have a severe impact on host defence including viral infections, and a possible role of biologic drugs in the development of the presented VZV infections cannot be ruled out. In fact, the incidence of HZ in the general population ranges from 3.2-4.2 cases per 1000 persons per year[19] while it is reported that rate is increased up to 10.60 per 1000 patient-years in patients on anti-TNF-α therapy.[8] Nevertheless, anti-TNF therapy can generally be safely restarted in the majority of these patients after temporary cessation until vesicles have resolved, and conventional anti-viral therapy (acyclovir or valaciclovir) has been done.[7] As it is known that VZV infection can have severe complications in adults, particularly immunocompromised individuals who may show an atypical clinical presentation, physicians should be extremely cautious when patients under treatment with TNF-α inhibitors present with a vesicular rash and evaluations for VZV infections should be implemented immediately. Indeed VZV reactivation may be more severe in anti-TNFα-treated patients, showing a higher % of cases affecting multiple dermatomes, of patients needing hospitalization or of subjects who may develop repeated recurrences like reported by Strangfeld et al.[3] In this study 15 of 66 (22,7%) analyzed cases of HZ occurred during anti-TNF-α treatment were multidermatomal or ophthalmic zoster and 13% of these required hospitalization: the severity and duration of HZ resulted commonly increased in patients treated with anti-TNF-α.[3] Moreover, the possibility of VZV infection with atypical presentation mimicking other conditions must always be kept in mind as highlighted by Tresch et al.[6] whereas Baek et al.[13] and Buccoliero et al.[14] showed that serious morbidity and mortality (e.g. encephalitis or vasulopathy) from VZV infection can occur in patients who received treatment with TNF-α blockers. Therefore education and close surveillance of patients on TNF-α blockers is critical for timely diagnosis and management of these potentially fatal infections. Screening recommendations for the presence of antibodies to VZV or a prophylactic vaccination in patients who do not exhibit immunity prior to biologic treatment remains a matter of discussion and more information from larger patient populations is needed before a clear conclusion can be drawn.20
Table 1. Review of the literature: main features of herpes zoster on anti TNF-α therapy.
| Adalimumab[7,13,14] | Etanercept[6,7,11] | Infliximab[5,7,10,12,15-17] | |
|---|---|---|---|
| Number of cases of HZ | 4 | 4 | 11 |
| Age, years (mean [SD]) | 38-66 (49 [11.97]) | 43-70 (57 [11.9]) | 12-83 (47 [21.26]) |
| Females, number (%) | 3 (75%) | 3 (75%) | 6 (54.5%) |
| Time on anti TNF-α therapy at HZ occurrence, months (mean[SD]) | 17-36 (23.7 [10.7]) | 6-60 (28 [25.1]) | 0.5-96 (20.7 [28.8]) |
Conclusions
TNF-α blockers are immunomodulating agents introduced for treatment of a variety of chronic inflammatory disease conditions. Adverse effects include an increased incidence of infections and viral reactivations. Clinically, these infections often have atypical presentations that may hamper prompt diagnosis. Defining the infectious complications associated with different targeted immunotherapies may provide a better understanding of the host defences required to control certain pathogens and develop more effective prophylactic and monitoring strategies. We report this case to highlight the increased risk of HZ infections in patient on anti-TNF-α therapy and to enrich the literature: there is a very limited number of studies regarding the development of HZ during adalimumab therapy[3,7,13,14] among the case reports and surveys which describe the occurrence of HZ during anti-TNF-α treatment.[3,5-17] It is very important to identify early signs and symptoms of HZ in these patients in order to start prompt efficient antiviral therapy to prevent the development of severe complications.
References
- Weger W. Current status and new developments in the treatment of psoriasis and psoriatic arthritis with biological agents. Br J Pharmacol. 2010;160:810–820. doi: 10.1111/j.1476-5381.2010.00702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domm S, Cinatl J, Mrowietz U. The impact of treatment with tumour necrosis factor-alpha antagonists on the course of chronic viral infections: a review of the literature. Br J Dermatol. 2008;159:1217–1228. doi: 10.1111/j.1365-2133.2008.08851.x. [DOI] [PubMed] [Google Scholar]
- Strangfeld A, Listing J, Herzer P, Liebhaber A, Rockwitz K, Richter C, Zink A. Risk of herpes zoster in patients with rheumatoid arthritis treated with anti-TNF-alpha agents. JAMA. 2009;301:737–744. doi: 10.1001/jama.2009.146. [DOI] [PubMed] [Google Scholar]
- Lin J, Ziring D, Desai S, Kim S, Wong M, Korin Y, Braun J, Reed E, Gjertson D, Singh RR. TNFalpha blockade in human diseases: an overview of efficacy and safety. Clin Immunol. 2008;126:13–30. doi: 10.1016/j.clim.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz MJ, Baudrier T, Ferreira O, Azevedo F. Herp zoster at the site of infliximab infusion: case report. Cutan Ocul Toxicol. 2011;30:236–238. doi: 10.3109/15569527.2010.551302. [DOI] [PubMed] [Google Scholar]
- Tresch S, Trueb RM, Kamarachev J, French LE, Hofbauer GF. Disseminated herpes zoster mimicking rheumatoid vasculitis in a rheumatoid arthritis patient on etanercept. Dermatology. 2009;219:347–349. doi: 10.1159/000232389. [DOI] [PubMed] [Google Scholar]
- Wendling D, Streit G, Toussirot E, Prati C. Herpes zoster in patients taking TNFalpha antagonists for chronic inflammatory joint disease. Joint Bone Spine. 2008;75:540–543. doi: 10.1016/j.jbspin.2007.10.011. [DOI] [PubMed] [Google Scholar]
- McDonald JR, Zeringue AL, Caplan L, Ranganathan P, Xian H, Burroughs TE, Fraser VJ, Cunningham F, Eisen SA. Herpes zoster risk factors in a national cohort of veterans with rheumatoid arthritis. Clin Infect Dis. 2009;48:1364–1371. doi: 10.1086/598331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreiher J, Kresch FS, Comaneshter D, Cohen AD. Risk of Herpes zoster in patients with psoriasis treated with biologic drugs. J Eur Acad Dermatol Venereol. 2012;26:1127–1132. doi: 10.1111/j.1468-3083.2011.04230.x. [DOI] [PubMed] [Google Scholar]
- Kinder A, Stephens S, Mortimer N, Sheldon P. Severe herpes zoster after infliximab infusion. Postgrad Med J. 2004;80:26. doi: 10.1136/pmj.2003.014373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi A. Varicella zoster virus infection in patients taking the TNF-alpha inhibitor, etanercept: coincidence or causal? Hawaii Med J. 2009;68:277–278. [PubMed] [Google Scholar]
- Failla V, Castronovo C, Meex C, Nikkels AF. Protracted herpes zoster and severe postherpetic neuralgia after inadvertant infliximab administration. Eur J Dermatol. 2011;21:782–783. doi: 10.1684/ejd.2011.1424. [DOI] [PubMed] [Google Scholar]
- Baek W, Lee SG, Kim YS, Kim JH, Jun JB, Kim HY. Fatal varicella-zoster virus vasculopathy associated with adalimumab therapy. Arch Neurol. 2012;69:1193–1196. doi: 10.1001/archneurol.2011.3741. [DOI] [PubMed] [Google Scholar]
- Buccoliero G, Lonero G, Romanelli C, Loperfido P, Resta F. Varicella zoster virus encephalitis during treatment with anti-tumor necrosis factor-alpha agent in a psoriatic arthritis patient. New Microbiol. 2010;33:271–274. [PubMed] [Google Scholar]
- Baumgart DC, Dignass AU. Shingles following infliximab infusion. Ann Rheum Dis. 2002;61:661. doi: 10.1136/ard.61.7.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobile S, Catassi C, Felici L. Herpes zoster infection followed by Henoch-Schönlein purpura in a girl receiving infliximab for ulcerative colitis. J Clin Rheumatol. 2009;15:101. doi: 10.1097/RHU.0b013e31819bca9e. [DOI] [PubMed] [Google Scholar]
- Garcia-Vidal C, Rodríguez-Fernández S, Teijón S, Esteve M, Rodríguez-Carballeira M, Lacasa JM, Salvador G, Garau J. Risk factors for opportunistic infections in infliximab-treated patients: the importance of screening in prevention. Eur J Clin Microbiol Infect Dis. 2009;28:331–337. doi: 10.1007/s10096-008-0628-x. [DOI] [PubMed] [Google Scholar]
- Ito M, Nakano T, Kamiya T, Kitamura K, Ihara T, Kamiya H, Sakurai M. Effects of tumor necrosis factor alpha on replication of varicella-zoster virus. Antiviral Res. 1991;15:183–192. doi: 10.1016/0166-3542(91)90065-y. [DOI] [PubMed] [Google Scholar]
- Yawn BP, Saddier P, Wollan PC, St Sauver JL, Kurland MJ, Sy LS. A population-based study of the incidence and complications of herpes zoster before zoster vaccine introduction. Mayo Clin Proc. 2007;82:1341–1349. doi: 10.4065/82.11.1341. [DOI] [PubMed] [Google Scholar]
- Zhang J, Delzell ES, Xie F, Baddley JW, Spettell C, McMahan RM, Fernandes J, Chen L, Winthrop K, Curtis JR. The use, safety and effectiveness of herpes zoster vaccination in individuals with inflammatory and autoimmune diseases: a longitudinal observational study. Arthritis Res Ther. 2011;13:R174. doi: 10.1186/ar3497. [DOI] [PMC free article] [PubMed] [Google Scholar]


