Abstract
Background
Gardner-Diamond syndrome (GDS) is also known as psychogenic purpura, autoerythrocyte sensitization syndrome and painful bruising syndrome.
Main observation
This is a case report of 27-year-old woman who presented with unexplained bruising and intramuscular hematomas after a seven year history of complex regional pain syndrome. Her evaluation consisted of hematological studies, skin and muscle biopsy; it failed to reveal an underlying coagulopathy, vasculitis or other demonstrable cause. In the absence of any other etiology, she was diagnosed as Gardner-Diamond syndrome.
Conclusions
This patient is unique because of intramuscular hematomas and the association of Complex regional pain syndrome with Gardner-Diamond syndrome.
Keywords: autoimmune, complex regional pain syndrome, Gardner-Diamond Syndrome, hematoma, pain, phosphatidylserine, purpura
Introduction
Gardner-Diamond syndrome (GDS) is also known as psychogenic purpura, autoerythrocyte sensitization syndrome and painful bruising syndrome. It is thought to primarily affect women who are undergoing an acute emotionally stressful event or have an underlying psychiatric disorder. The condition was first characterized by Gardner and Diamond in 1955 who reported four women who developed spontaneous bruising months after sustaining an initial trauma.[1] The cause of GDS is thought to be an autoimmune reaction against a component of the patient's own erythrocytes. There is no specific laboratory test for GDS. Hematological studies including coagulation parameters are generally normal. The skin biopsy often shows only non-specific changes. The diagnosis is most often made by injecting a suspension of autologous washed erythrocytes subcutaneously and is considered positive if bruising occurs at the site of injection. The antigen is thought to be phosphatidylserine, a phosphoglyceride of the red cell membrane.[2] This is a case report of a 27-year-old woman with long standing complex regional pain syndrome (CRPS) who developed repeated episodes of severe spontaneous bruising in skin and muscle consistent with GDS which has not been previously described.
CRPS is an entity defined by the Budapest Consensus Panel (2007) as having four diagnostic factors: (1) abnormalities in pain processing; (2) autoimmune dysregulation manifest by color and temperature changes; (3) neurogenic edema, vasomotor and sudomotor abnormalities and (4) motor dysfunction and trophic changes. The diagnosis is made if the patient has at least one symptom from each of these factors as well as one sign from two of the four factors. CRPS is classified as type II if a nerve lesion is causative and type I if no nerve lesion can be identified.[3]
Case Report
A 27-year-old woman developed CRPS in 2000 after a minor injury to her right hand. She subsequently developed GDS in 2007. The GDS progressed to a hematoma in her right hand that required surgical drainage. Several months later she developed a large hematoma of her right medial thigh and calf with no apparent preceding trauma.
During the first seven years of illness she exhibited the classic signs and symptoms of CRPS in her right upper extremity that included severe burning pain, thermal allodynia, neurogenic edema, erythema and hyperhidrosis.[3,4] She also developed atrophy of the musculature and dystrophic skin changes exemplified by epidermal and dermal thickening, dryness and flaking. In 2007, her symptoms of GDS developed after minor trauma to her right knee. She noted one week after the injury a dime sized bruise on the medial aspect of her right knee. This bruise then spread to cover her entire lower leg. After the first initial episode of bruising her symptoms completely resolved, but three months later she developed bruising of her medial right thigh and lower leg again without antecedent injury. Since this episode she developed spontaneous bruising of her right medial thigh and lower leg on average every three months. Her symptoms resolve in the interim periods, but a persistent discoloration of her medial right thigh and an area of swelling in the medial aspect of her right calf remain. She continues to have all of the classic symptoms and signs of CRPS in her right leg [Fig. 1].
Figure 1.

The patient’s right medial thigh during an episode of GDS exacerbation.
In June of 2009 during a flare of GDS, she had an intramuscular hematoma of her right lower extremity. A CT scan revealed an increased density in the rectus femoris, vastus intermedius and vastus lateralis muscles which proved to be a subacute hemorrhage superimposed on a chronic hematoma. A periosteal reaction was also noted by MRI which extended from the proximal to distal femoral diaphysis. Linear calcifications were noted in the affected muscles that suggested myositis ossificans. During this hospital stay her hemoglobin dropped from 11.5 gm/dl to 7.0 gm/dl over a 24 hour period and she was transfused 2 units of packed red blood cells. Her urine and stool were negative for blood. A retroperitoneal bleed was ruled out with a CT scan of her abdomen and pelvis. As previously tested, her CBC and coagulation studies were within normal limits. Her hemoglobin post-transfusion returned to 12.6 gm/dl, hematocrit was 21.9 - 37.6 gm/dl, white blood cell count was 10.4 -21.0 × 103/mm3 and her platelets were 335-400 × 103/mm3. The increase in her white blood cell count was thought to be secondary to steroid therapy. On this admission laboratory values revealed a prothrombin time of 12.5 seconds, partial thromboplastin time was 22.6 seconds and her international normalized ratio (INR) was 1.09. Her peripheral smear was normal. She also underwent a complete clotting battery that included Factor VIII activity, von Willebrand Factor (vWF) antigen and vWF activity which were within normal limits. She was treated with pulsed solumedrol (1 gram/day for 5 days) which produced some improvement. The only abnormalities were a low iron level of 27 Units and a low vitamin B12 of 188 meq/ml.
In 2010, she underwent an MRI of her right lower extremity that revealed two hematomas medial head of the gastrocnemius muscles. A year later in 2011, she developed an apparent collection of blood on the dorsum of her right hand. Surgical drainage revealed a chronic organized hematoma associated with profuse bleeding. A sample of tissue from the dorsum of her hand revealed granulation tissue. Later, that year she developed another exacerbation of GDS deep in her right thigh and again MRI revealed a hematoma of the quadriceps and tensor fascia lata [Fig. 2].
Figure 2.
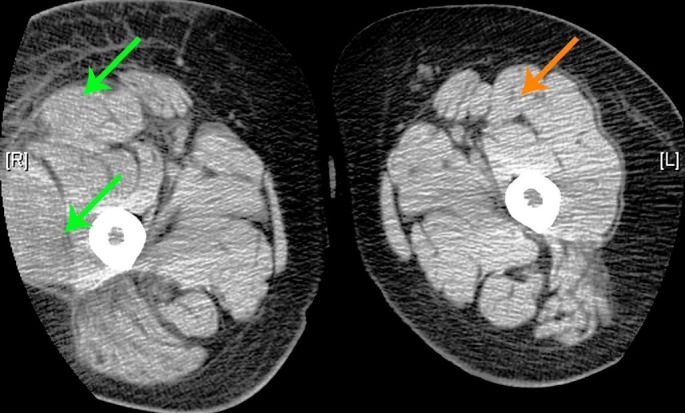
CT scan showing enlargement of the right thigh relative to the left thigh. The superior green arrow is the hematoma in right rectus femoris and it is much larger than the rectus femoris on the left (indicated by the orange arrow). The inferior green arrow is pointing to a hematoma in the right vastus lateralis muscle.
During her hospitalizations she has undergone multiple coagulation evaluations and treatments. She was treated with solumedrol 1 gm per day over 3-5 days which gave her minimal pain relief and decreased swelling. Two five day courses of IVIG were tried without benefit. She has been treated with both ketamine and lidocaine infusions for her CRPS.[5,6] The ketamine has shown benefit in terms of decreasing the diffuse erythema and swelling which occur intermittently over her entire body and face. Lidocaine infusions produce some relief of her pain. She has also had numerous hematological work ups as outlined above, all of which were normal. She underwent a skin biopsy in 2009 of her right thigh and calf. The epidermal nerve fiber density of her right calf was 6.99/mm, which is normal. The low normal range for this area is 5.4 - 5.7/mm. The epidermal nerve fiber density of her right thigh was 7.01/mm; the normal being 6.8 - 8.0/mm. Testing for vasculitis and amyloid were both negative. Overall, the skin biopsy was considered negative for a small fiber neuropathy or any other abnormality.
Discussion
This patient is unique because she developed intramuscular hematomas and for the association of GDS and CRPS. There are many dermatological manifestations of CRPS which most commonly include erythema and brawny edema.[7] Other dermatological manifestations are persistent livedo reticularis, hyperhidrosis of the hands and feet and generalized erythema. As the disease progresses the skin becomes atrophic, dry and thin. It can appear shiny and demonstrates abnormal hair growth which is either sparse or is too thick, curly and overgrown. Nails often become very thin, brittle and grow too quickly.[7] A subset of patients develop bullae and areas of desquamation in addition to the edema. These changes often lead to cellulitis and chronic ulcerations of the skin. Purpuric lesions have been reported in two patients who had numerous petechial eruptions that arose spontaneously. In one patient these hyperpigmented areas appeared superimposed upon edematous skin. The lesions were described as numerous petechiae that were confluent in some areas. A skin biopsy demonstrated extravasation of erythrocytes with lymphocytes and histiocytes surrounding the blood vessels consistent with Schamberg's disease.[7] Schamberg's disease is a form of pigmented purpuric dermatoses and is classically described as having a "cayenne pepper" like appearance with pinhead sized reddish puncta that coalesce to form areas of orange or brown pigmentation.[8] However, any other type of purpuric lesion or GDS associated with CRPS has not been previously reported in the literature. There has been brief mention that CRPS and GDS maybe associated conditions.[9]
In the 1955 article by Gardner and Diamond, three of the four patients described also had some symptoms suggestive of CRPS in addition to GDS. All patients suffered trauma prior to the onset of their symptoms. In the first patient vasomotor changes were noted in her right hand which were thought to be consistent with Raynaud's syndrome. The second patient had episodic syncope that was never explained which is often seen in CRPS.[10] The fourth patient had episodic abdominal pain and obstructive symptoms despite cholecystectomy and surgery for intestinal obstruction. The symptoms of vasomotor changes in a limb, unexplained syncopal episodes and associated abdominal pain are suggestive of CRPS.[11] Vasomotor changes in a limb are a well-known and classic sign in CRPS.[12] Syncope, although less recognized, can also be associated with CRPS.[10] The abdominal pain reported by the fourth patient also went unexplained despite two interventions and several evaluations. CRPS can be associated with gastroparesis and can lead to a pseudo-obstruction.[13]
This patient had episodes of spontaneous bruising which could not be explained by any coagulation disorder. Both the tissue biopsy from the evacuation of the hematoma of the dorsum of her hand and her skin biopsy for epidermal fiber density were both negative for signs of vasculitis, which has been reported as a associated feature of GDS.[14] The most common method of diagnosis for GDS is an injection into the skin of a suspension of autologous washed erythrocytes. This could not be undertaken in this patient because of her CRPS as any trauma can spread the process.
The pathophysiology underlying GDS may be an autoimmune reaction against phosphatidylserine, which is a phosphoglyceride in the membranes of red blood cells.[15] Supportive evidence for this hypothesis was the induction of an immune response in vitro using homologous erythrocytes from a normal donor and then incubating them with plasma from a patient with GDS. This reaction results in a redistribution of phosphatidylserine to the surface of the donor erythrocytes. IgE antibodies to cardiolipin and to phosphatidylserine were also noted in the patient’s plasma. Although this was performed with only one GDS patient, it suggests a precise and non-invasive method of diagnosing GDS.[16]
GDS and CRPS may both have autoimmune underlying mechanisms. An increased level of TNF-α receptor type 1 is a common manifestation of CRPS. In CSF studies of CRPS patients, increased level of IL-6 and IL-1β were found.[17] Autoimmune modulating therapies may be useful for the treatment of both entities. IVIG with steroids has been successful in one third of patients with CRPS.[18] Both thalidomide and lenalidomide have also shown some benefit in the treatment of CRPS.[19,20]
Converging evidence supports the development of a sterile inflammatory response at the site of injury in CRPS patients. This is precipitated and maintained by a pro-inflammatory cytokine profile (IL-1, IL-6, TNF-α, IL-1β) and depressed anti-inflammatory cytokines (IL-4 and IL-10) derived from extravasated leukocytes and recruited immune cells.[21-26] In GDS as well as CRPS, lesions may appear at a distance from the site of injury. The only CRPS patients that have undergone autopsy reveal microglial activation throughout the spinal cord which maybe a mechanism of spread and its distant effects.[27] There is evidence in CRPS-I of microvascular damage and reduced and damaged unmyelinated C-fibers and A-delta neurites in the skin and skeletal muscle in the area of injury.[28] These abnormalities are also associated with abnormal capillary endothelial cells.[29] Experimental evidence has demonstrated that inhibition of NF-kB translocation into the nucleus of leukocytes and macrophages decreases the production of TNF-α, IL-1β and cyclooxygenase-2 which decreases hyperalgesia in neuropathic pain models.[30-32] Inhibition of NF-kB transcription in immune cells also decreases TNF- α-mediated expression of cell adhesion molecules in endothelial cells that reduces extravasation of leukocytes and their release of excitatory cytokines.[33] The extravasation of blood into muscle and skin may be a consequence of C-fiber release of Substance P (SP) and calcitonin gene related peptide. The former opens holes in capillaries and the latter may paralyze smooth muscle.[34-36] An autoimmune reaction to the extravasted blood maybe the etiology of GDS in CRPS patients.
Conclusion
Gardner-Diamond syndrome is a disease entity whose underlying mechanism in not fully understood. This case is unique in both the severity of the symptoms as well as the patient's co-morbid complex regional pain syndrome. Both processes likely have an autoimmune component. Further characterization of GDS through in vitro laboratory testing as well as its relationship to CRPS is needed.
References
- Gardner FH, Diamond LK. Autoerythrocyte sensitization; a form of purpura producing painful bruising following autosensitization to red blood cells in certain women. Blood. 1955;10:675–690. [PubMed] [Google Scholar]
- Groch GS, Finch SC, Rogoway W, Fischer DS. Studies in the pathogenesis of autoerythrocyte sensitization syndrome. Blood. 1966;28:19–33. [PubMed] [Google Scholar]
- Harden RN, Bruehl S, Perez RS, Birklein F, Marinus J, Maihofner C, Lubenow T, Buvanendran A, Mackey S, Graciosa J, Mogilevski M, Ramsden C, Chont M, Vatine JJ. Validation of proposed diagnostic criteria (the "Budapest Criteria") for Complex regional pain syndrome. Pain. 2010;150:268–274. doi: 10.1016/j.pain.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruehl S, Harden RN, Galer BS, Saltz S, Bertram M, Backonja M, Gayles R, Rudin N, Bhugra MK, Stanton-Hicks M. External validation of IASP diagnostic criteria for Complex Regional Pain Syndrome and proposed research diagnostic criteria. International Association for the Study of Pain. Pain. 1999;81:147–154. doi: 10.1016/s0304-3959(99)00011-1. [DOI] [PubMed] [Google Scholar]
- Schwartzman RJ, Alexander GM, Grothusen JR, Paylor T, Reichenberger E, Perreault M. Outpatient intravenous ketamine for the treatment of complex regional pain syndrome: a double-blind placebo controlled study. Pain. 2009;147:107–115. doi: 10.1016/j.pain.2009.08.015. [DOI] [PubMed] [Google Scholar]
- Schwartzman RJ, Patel M, Grothusen JR, Alexander GM. fficacy of 5-day continuous lidocaine infusion for the treatment of refractory complex regional pain syndrome. Pain Med. 2009;10:401–412. doi: 10.1111/j.1526-4637.2009.00573.x. [DOI] [PubMed] [Google Scholar]
- Webster GF, Schwartzman RJ, Jacoby RA, Knobler RL, Uitto JJ. Reflex sympathetic dystrophy. Occurrence of inflammatory skin lesions in patients with stages II and III disease. Arch Dermatol. 1991;127:1541–1544. doi: 10.1001/archderm.127.10.1541. [DOI] [PubMed] [Google Scholar]
- Sardana K, Sarkar R, Sehgal VN. Pigmented purpuric dermatoses: an overview. Int J Dermatol. 2004;43:482–488. doi: 10.1111/j.1365-4632.2004.02213.x. [DOI] [PubMed] [Google Scholar]
- Bennett DS, Brookoff D. Complex regional pain syndromes (reflex sympathetic dystrophy and causalgia) and spinal cord stimulation. Pain Medicine. 2006;7:S64–S96. [Google Scholar]
- Smith JA, Karalis DG, Rosso AL, Grothusen JR, Hessen SE, Schwartzman RJ. Syncope in complex regional pain syndrome. Clin Cardiol. 2011;34:222–225. doi: 10.1002/clc.20879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzman R Medical Complications of Complex regional pain syndrome. In: Ghosh S, (ed.). Pain Medicine Rijeka, Croatia: InTech; 2012, in press. [Google Scholar]
- Maleki J, LeBel AA, Bennett GJ, Schwartzman RJ. Patterns of spread in complex regional pain syndrome, type I (reflex sympathetic dystrophy) Pain. 2000;88:259–266. doi: 10.1016/S0304-3959(00)00332-8. [DOI] [PubMed] [Google Scholar]
- Schwartzman RJ, Erwin KL, Alexander GM. The natural history of complex regional pain syndrome. Clin J Pain. 2009;25:273–280. doi: 10.1097/AJP.0b013e31818ecea5. [DOI] [PubMed] [Google Scholar]
- Cansu D, Kaşifoğlu T, Paşaoğlu O, Korkmaz C. Autoerythrocyte sensitization syndrome (Gardner-Diamond syndrome) associated with cutaneous vasculitis. Joint Bone Spine. 2008;75:721–724. doi: 10.1016/j.jbspin.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Ivanov OL, Lvov AN, Michenko AV, Künzel J, Mayser P, Gieler U. Autoerythrocyte sensitization syndrome (Gardner-Diamond syndrome): review of the literature. J Eur Acad Dermatol Venereol. 2009;23:499–504. doi: 10.1111/j.1468-3083.2009.03096.x. [DOI] [PubMed] [Google Scholar]
- Strunecká A, Krpejsová L, Palecek J, Mácha J, Maturová M, Rosa L, Palecková A. Transbilayer redistribution of phosphatidylserine in erythrocytes of a patient with autoerythrocyte sensitization syndrome (psychogenic purpura) Folia Haematol Int Mag Klin Morphol Blutforsch. 1990;117:829–841. [PubMed] [Google Scholar]
- Schwartzman RJ, Alexander GM, Grothusen J. Pathophysiology of complex regional pain syndrome. Expert Rev Neurother. 2006;6:669–681. doi: 10.1586/14737175.6.5.669. [DOI] [PubMed] [Google Scholar]
- Goebel A, Baranowski A, Maurer K, Ghiai A, McCabe C, Ambler G. Intravenous immunoglobulin treatment of the complex regional pain syndrome: a randomized trial. Ann Intern Med. 2010;152:152–158. doi: 10.7326/0003-4819-152-3-201002020-00006. [DOI] [PubMed] [Google Scholar]
- Schwartzman RJ Chevlen E Bengtson K Thalidomide has activity in treating complex regional pain syndrome Arch Intern Med 2003; 163:1487-1488; author reply 8. [DOI] [PubMed] [Google Scholar]
- Schwartzman RJ, Irving G, Wallace M. A multicenter, open-label, 12-week study with extension to evaluate the safety and efficacy of lenalidomide (CC-5013) in the treatment of complex regional pain syndrome type-1. 11th World Congress on Pain; 2005; Seattle: IASP Press, p. 580. [Google Scholar]
- Uçeyler N, Eberle T, Rolke R, Birklein F, Sommer C. Differential expression patterns of cytokines in complex regional pain syndrome. Pain. 2007;132:195–205. doi: 10.1016/j.pain.2007.07.031. [DOI] [PubMed] [Google Scholar]
- Üçeyler N, Sommer C. Cytokine-Induced Pain: Basic Science and Clinical Implications. Reviews in Analgesia. 2007;9:87–103. [Google Scholar]
- Alexander GM, Perreault MJ, Reichenberger ER, Schwartzman RJ. Changes in immune and glial markers in the CSF of patients with Complex Regional Pain Syndrome. Brain Behav Immun. 2007;21:668–676. doi: 10.1016/j.bbi.2006.10.009. [DOI] [PubMed] [Google Scholar]
- Maihöfner C, Handwerker HO, Neundörfer B, Birklein F. Mechanical hyperalgesia in complex regional pain syndrome: a role for TNF-alpha? Neurology. 2005;65:311–313. doi: 10.1212/01.wnl.0000168866.62086.8f. [DOI] [PubMed] [Google Scholar]
- Schinkel C, Gaertner A, Zaspel J, Zedler S, Faist E, Schuermann M. Inflammatory mediators are altered in the acute phase of posttraumatic complex regional pain syndrome. Clin J Pain. 2006;22:235–239. doi: 10.1097/01.ajp.0000169669.70523.f0. [DOI] [PubMed] [Google Scholar]
- Alexander GM, van Rijn MA, van Hilten JJ, Perreault MJ, Schwartzman RJ. Changes in cerebrospinal fluid levels of pro-inflammatory cytokines in CRPS. Pain. 2005;116:213–219. doi: 10.1016/j.pain.2005.04.013. [DOI] [PubMed] [Google Scholar]
- Del Valle L, Schwartzman RJ, Alexander G. Spinal cord histopathological alterations in a patient with longstanding complex regional pain syndrome. Brain Behav Immun. 2009;23:85–91. doi: 10.1016/j.bbi.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Oaklander AL, Fields HL. Is reflex sympathetic dystrophy/complex regional pain syndrome type I a small-fiber neuropathy? Ann Neurol. 2009;65:629–638. doi: 10.1002/ana.21692. [DOI] [PubMed] [Google Scholar]
- Coderre TJ. 26 An Animal Model Suggests Microvascular Dysfunction And Chronic Tissue Ischemia Contribute To CRPS-I. Eur J Pain Suppl. 2010;4:8. [Google Scholar]
- Tegeder I, Niederberger E, Schmidt R, Kunz S, Gühring H, Ritzeler O, Michaelis M, Geisslinger G. Specific Inhibition of IkappaB kinase reduces hyperalgesia in inflammatory and neuropathic pain models in rats. J Neurosci. 2004;24:1637–1645. doi: 10.1523/JNEUROSCI.3118-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas-Ballina M, Tracey KJ. The neurology of the immune system: neural reflexes regulate immunity. Neuron. 2009;64:28–32. doi: 10.1016/j.neuron.2009.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey KJ. Reflex control of immunity. Nat Rev Immunol. 2009;9:418–428. doi: 10.1038/nri2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan S, Ye J, Bai S, Huang F, Guo YL. NF-kappaB, but not p38 MAP kinase, is required for TNF-alpha-induced expression of cell adhesion molecules in endothelial cells. J Cell Biochem. 2008;105:477–486. doi: 10.1002/jcb.21845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leis S, Weber M, Isselmann A, Schmelz M, Birklein F. Substance-P-induced protein extravasation is bilaterally increased in complex regional pain syndrome. Exp Neurol. 2003;183:197–204. doi: 10.1016/s0014-4886(03)00163-8. [DOI] [PubMed] [Google Scholar]
- Weber M, Birklein F, Neundörfer B, Schmelz M. Facilitated neurogenic inflammation in complex regional pain syndrome. Pain. 2001;91:251–257. doi: 10.1016/S0304-3959(00)00445-0. [DOI] [PubMed] [Google Scholar]
- Birklein F. Complex regional pain syndrome. J Neurol. 2005;252:131–138. doi: 10.1007/s00415-005-0737-8. [DOI] [PubMed] [Google Scholar]


