Abstract
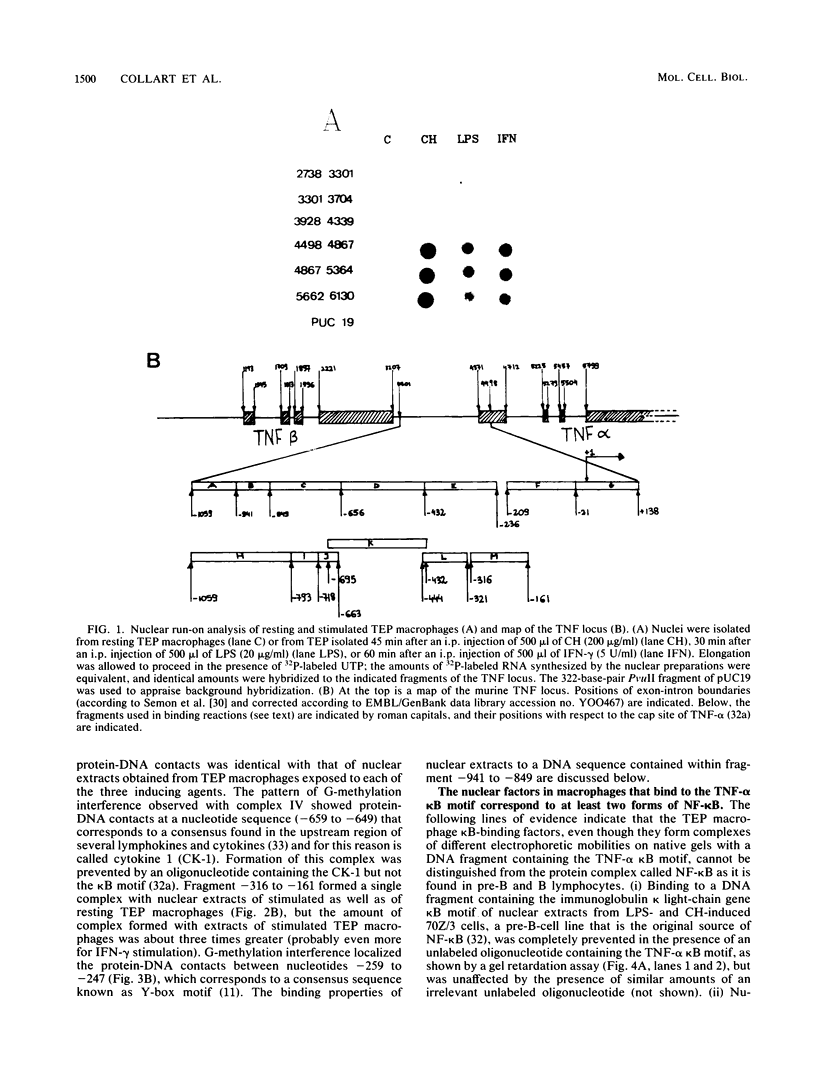
This study characterizes the interaction of murine macrophage nuclear proteins with the tumor necrosis factor alpha (TNF-alpha) promoter. Gel retardation and methylation interference assays showed that stimulation of TNF-alpha gene transcription in peritoneal exudate macrophages was accompanied by induction of DNA-binding proteins that recognized with different affinities four elements related to the kappa B consensus motif and a Y-box motif. We suggest that the basal level of TNF-alpha expression in macrophages is due to the binding of a constitutive form of NF-kappa B, present at low levels in nuclei from resting thioglycolate exudate peritoneal macrophages, to some if not all of the kappa B motifs; we postulate that this constitutive form contains only the 50-kilodalton (kDa) DNA-binding protein subunits of NF-kappa B, not the 65-kDa protein subunits (P. Baeuerle and D. Baltimore, Genes Dev. 3:1689-1698, 1989). Agents such as glucocorticoids, which decrease TNF-alpha transcription, diminished the basal level of nuclear NF-kappa B. Stimulation of Stimulation of TNF-alpha transcription in macrophages by lipopolysaccharide, gamma interferon, or cycloheximide led to an increased content of nuclear NF-kappa B. This induced factor represents a different form of NF-kappa B, since it generated protein-DNA complexes of slower mobility; we propose that this induced form of NF-kappa B contains both the 50- and 65-kDa protein subunits, the latter ones being necessary to bind NF-kappa B to its cytoplasmic inhibitor in uninduced cells (Baeuerle and Baltimore, Genes Dev., 1989). In resting cells, this inducible form of NF-kappa B was indeed detectable in the cytosol after deoxycholate treatment.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baeuerle P. A., Baltimore D. A 65-kappaD subunit of active NF-kappaB is required for inhibition of NF-kappaB by I kappaB. Genes Dev. 1989 Nov;3(11):1689–1698. doi: 10.1101/gad.3.11.1689. [DOI] [PubMed] [Google Scholar]
- Baeuerle P. A., Baltimore D. Activation of DNA-binding activity in an apparently cytoplasmic precursor of the NF-kappa B transcription factor. Cell. 1988 Apr 22;53(2):211–217. doi: 10.1016/0092-8674(88)90382-0. [DOI] [PubMed] [Google Scholar]
- Baeuerle P. A., Baltimore D. I kappa B: a specific inhibitor of the NF-kappa B transcription factor. Science. 1988 Oct 28;242(4878):540–546. doi: 10.1126/science.3140380. [DOI] [PubMed] [Google Scholar]
- Baldwin A. S., Jr, Sharp P. A. Two transcription factors, NF-kappa B and H2TF1, interact with a single regulatory sequence in the class I major histocompatibility complex promoter. Proc Natl Acad Sci U S A. 1988 Feb;85(3):723–727. doi: 10.1073/pnas.85.3.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler B., Cerami A. The history, properties, and biological effects of cachectin. Biochemistry. 1988 Oct 4;27(20):7575–7582. doi: 10.1021/bi00420a001. [DOI] [PubMed] [Google Scholar]
- Beutler B., Cerami A. The history, properties, and biological effects of cachectin. Biochemistry. 1988 Oct 4;27(20):7575–7582. doi: 10.1021/bi00420a001. [DOI] [PubMed] [Google Scholar]
- Beutler B., Krochin N., Milsark I. W., Luedke C., Cerami A. Control of cachectin (tumor necrosis factor) synthesis: mechanisms of endotoxin resistance. Science. 1986 May 23;232(4753):977–980. doi: 10.1126/science.3754653. [DOI] [PubMed] [Google Scholar]
- Blanar M. A., Burkly L. C., Flavell R. A. NF-kappa B binds within a region required for B-cell-specific expression of major histocompatibility complex class II gene E alpha d. Mol Cell Biol. 1989 Feb;9(2):844–846. doi: 10.1128/mcb.9.2.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhnlein E., Lowenthal J. W., Siekevitz M., Ballard D. W., Franza B. R., Greene W. C. The same inducible nuclear proteins regulates mitogen activation of both the interleukin-2 receptor-alpha gene and type 1 HIV. Cell. 1988 Jun 3;53(5):827–836. doi: 10.1016/0092-8674(88)90099-2. [DOI] [PubMed] [Google Scholar]
- Collart M. A., Belin D., Briottet C., Thorens B., Vassalli J. D., Vassalli P. Receptor-mediated phagocytosis by macrophages induces a calcium-dependent transient increase in c-fos transcription. Oncogene. 1989 Feb;4(2):237–241. [PubMed] [Google Scholar]
- Collart M. A., Belin D., Vassalli J. D., Vassalli P. Modulations of functional activity in differentiated macrophages are accompanied by early and transient increase or decrease in c-fos gene transcription. J Immunol. 1987 Aug 1;139(3):949–955. [PubMed] [Google Scholar]
- Collart M. A., Belin D., Vassalli J. D., de Kossodo S., Vassalli P. Gamma interferon enhances macrophage transcription of the tumor necrosis factor/cachectin, interleukin 1, and urokinase genes, which are controlled by short-lived repressors. J Exp Med. 1986 Dec 1;164(6):2113–2118. doi: 10.1084/jem.164.6.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn A., Bollekens J., Staub A., Benoist C., Mathis D. A multiplicity of CCAAT box-binding proteins. Cell. 1987 Sep 11;50(6):863–872. doi: 10.1016/0092-8674(87)90513-7. [DOI] [PubMed] [Google Scholar]
- Goldfeld A. E., Maniatis T. Coordinate viral induction of tumor necrosis factor alpha and interferon beta in human B cells and monocytes. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1490–1494. doi: 10.1073/pnas.86.5.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorski K., Carneiro M., Schibler U. Tissue-specific in vitro transcription from the mouse albumin promoter. Cell. 1986 Dec 5;47(5):767–776. doi: 10.1016/0092-8674(86)90519-2. [DOI] [PubMed] [Google Scholar]
- Griffin G. E., Leung K., Folks T. M., Kunkel S., Nabel G. J. Activation of HIV gene expression during monocyte differentiation by induction of NF-kappa B. Nature. 1989 May 4;339(6219):70–73. doi: 10.1038/339070a0. [DOI] [PubMed] [Google Scholar]
- Hoyos B., Ballard D. W., Böhnlein E., Siekevitz M., Greene W. C. Kappa B-specific DNA binding proteins: role in the regulation of human interleukin-2 gene expression. Science. 1989 Apr 28;244(4903):457–460. doi: 10.1126/science.2497518. [DOI] [PubMed] [Google Scholar]
- Israël A., Kimura A., Kieran M., Yano O., Kanellopoulos J., Le Bail O., Kourilsky P. A common positive trans-acting factor binds to enhancer sequences in the promoters of mouse H-2 and beta 2-microglobulin genes. Proc Natl Acad Sci U S A. 1987 May;84(9):2653–2657. doi: 10.1073/pnas.84.9.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami K., Scheidereit C., Roeder R. G. Identification and purification of a human immunoglobulin-enhancer-binding protein (NF-kappa B) that activates transcription from a human immunodeficiency virus type 1 promoter in vitro. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4700–4704. doi: 10.1073/pnas.85.13.4700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenardo M. J., Fan C. M., Maniatis T., Baltimore D. The involvement of NF-kappa B in beta-interferon gene regulation reveals its role as widely inducible mediator of signal transduction. Cell. 1989 Apr 21;57(2):287–294. doi: 10.1016/0092-8674(89)90966-5. [DOI] [PubMed] [Google Scholar]
- Lenardo M. J., Kuang A., Gifford A., Baltimore D. NF-kappa B protein purification from bovine spleen: nucleotide stimulation and binding site specificity. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8825–8829. doi: 10.1073/pnas.85.23.8825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenardo M., Pierce J. W., Baltimore D. Protein-binding sites in Ig gene enhancers determine transcriptional activity and inducibility. Science. 1987 Jun 19;236(4808):1573–1577. doi: 10.1126/science.3109035. [DOI] [PubMed] [Google Scholar]
- Leung K., Nabel G. J. HTLV-1 transactivator induces interleukin-2 receptor expression through an NF-kappa B-like factor. Nature. 1988 Jun 23;333(6175):776–778. doi: 10.1038/333776a0. [DOI] [PubMed] [Google Scholar]
- Lin H. S., Stewart C. C. Peritoneal exudate cells. I. Growth requirement of cells capable of forming colonies in soft agar. J Cell Physiol. 1974 Jun;83(3):369–378. doi: 10.1002/jcp.1040830307. [DOI] [PubMed] [Google Scholar]
- Lowenthal J. W., Ballard D. W., Böhnlein E., Greene W. C. Tumor necrosis factor alpha induces proteins that bind specifically to kappa B-like enhancer elements and regulate interleukin 2 receptor alpha-chain gene expression in primary human T lymphocytes. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2331–2335. doi: 10.1073/pnas.86.7.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Nabel G., Baltimore D. An inducible transcription factor activates expression of human immunodeficiency virus in T cells. Nature. 1987 Apr 16;326(6114):711–713. doi: 10.1038/326711a0. [DOI] [PubMed] [Google Scholar]
- Nedwin G. E., Svedersky L. P., Bringman T. S., Palladino M. A., Jr, Goeddel D. V. Effect of interleukin 2, interferon-gamma, and mitogens on the production of tumor necrosis factors alpha and beta. J Immunol. 1985 Oct;135(4):2492–2497. [PubMed] [Google Scholar]
- Osborn L., Kunkel S., Nabel G. J. Tumor necrosis factor alpha and interleukin 1 stimulate the human immunodeficiency virus enhancer by activation of the nuclear factor kappa B. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2336–2340. doi: 10.1073/pnas.86.7.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce J. W., Lenardo M., Baltimore D. Oligonucleotide that binds nuclear factor NF-kappa B acts as a lymphoid-specific and inducible enhancer element. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1482–1486. doi: 10.1073/pnas.85.5.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruben S., Poteat H., Tan T. H., Kawakami K., Roeder R., Haseltine W., Rosen C. A. Cellular transcription factors and regulation of IL-2 receptor gene expression by HTLV-I tax gene product. Science. 1988 Jul 1;241(4861):89–92. doi: 10.1126/science.2838905. [DOI] [PubMed] [Google Scholar]
- Semon D., Kawashima E., Jongeneel C. V., Shakhov A. N., Nedospasov S. A. Nucleotide sequence of the murine TNF locus, including the TNF-alpha (tumor necrosis factor) and TNF-beta (lymphotoxin) genes. Nucleic Acids Res. 1987 Nov 11;15(21):9083–9084. doi: 10.1093/nar/15.21.9083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen R., Baltimore D. Inducibility of kappa immunoglobulin enhancer-binding protein Nf-kappa B by a posttranslational mechanism. Cell. 1986 Dec 26;47(6):921–928. doi: 10.1016/0092-8674(86)90807-x. [DOI] [PubMed] [Google Scholar]
- Sen R., Baltimore D. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell. 1986 Aug 29;46(5):705–716. doi: 10.1016/0092-8674(86)90346-6. [DOI] [PubMed] [Google Scholar]
- Shakhov A. N., Collart M. A., Vassalli P., Nedospasov S. A., Jongeneel C. V. Kappa B-type enhancers are involved in lipopolysaccharide-mediated transcriptional activation of the tumor necrosis factor alpha gene in primary macrophages. J Exp Med. 1990 Jan 1;171(1):35–47. doi: 10.1084/jem.171.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon M. F., Gamble J. R., Vadas M. A. Nuclear proteins interacting with the promoter region of the human granulocyte/macrophage colony-stimulating factor gene. Proc Natl Acad Sci U S A. 1988 Feb;85(3):674–678. doi: 10.1073/pnas.85.3.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirakawa F., Chedid M., Suttles J., Pollok B. A., Mizel S. B. Interleukin 1 and cyclic AMP induce kappa immunoglobulin light-chain expression via activation of an NF-kappa B-like DNA-binding protein. Mol Cell Biol. 1989 Mar;9(3):959–964. doi: 10.1128/mcb.9.3.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirakawa F., Mizel S. B. In vitro activation and nuclear translocation of NF-kappa B catalyzed by cyclic AMP-dependent protein kinase and protein kinase C. Mol Cell Biol. 1989 Jun;9(6):2424–2430. doi: 10.1128/mcb.9.6.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visvanathan K. V., Goodbourn S. Double-stranded RNA activates binding of NF-kappa B to an inducible element in the human beta-interferon promoter. EMBO J. 1989 Apr;8(4):1129–1138. doi: 10.1002/j.1460-2075.1989.tb03483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ewijk W., Ron Y., Monaco J., Kappler J., Marrack P., Le Meur M., Gerlinger P., Durand B., Benoist C., Mathis D. Compartmentalization of MHC class II gene expression in transgenic mice. Cell. 1988 May 6;53(3):357–370. doi: 10.1016/0092-8674(88)90156-0. [DOI] [PubMed] [Google Scholar]