Abstract
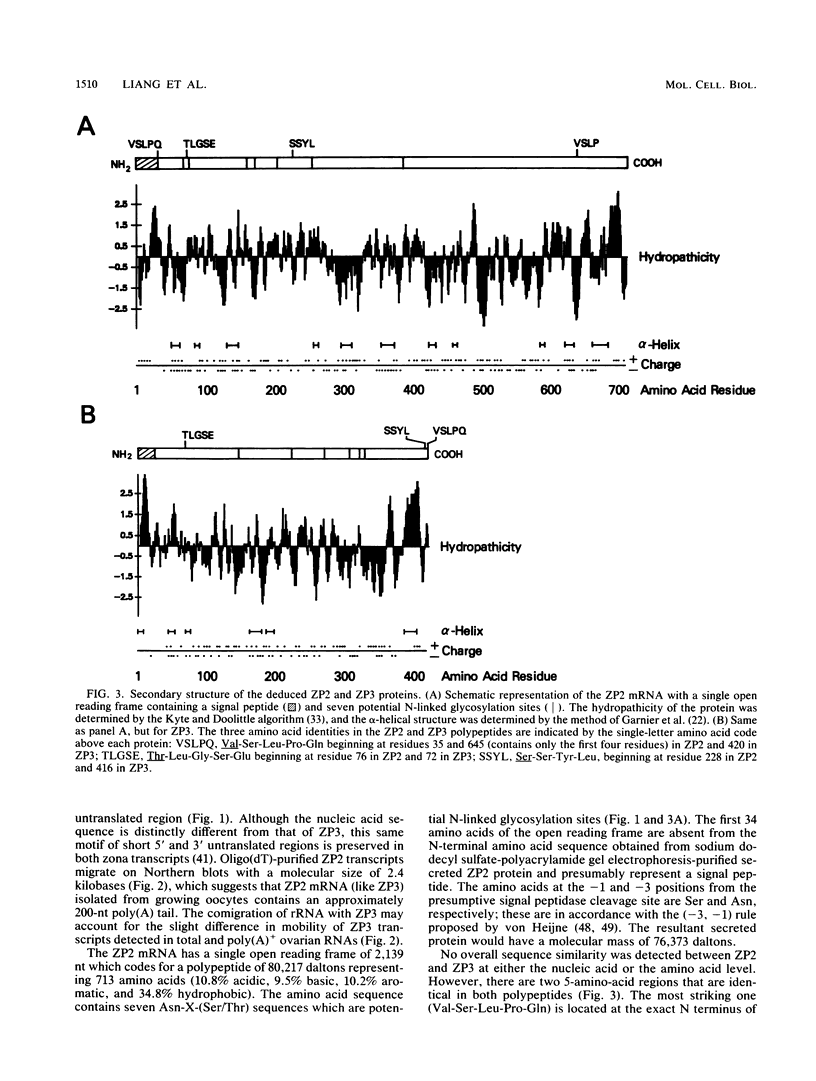
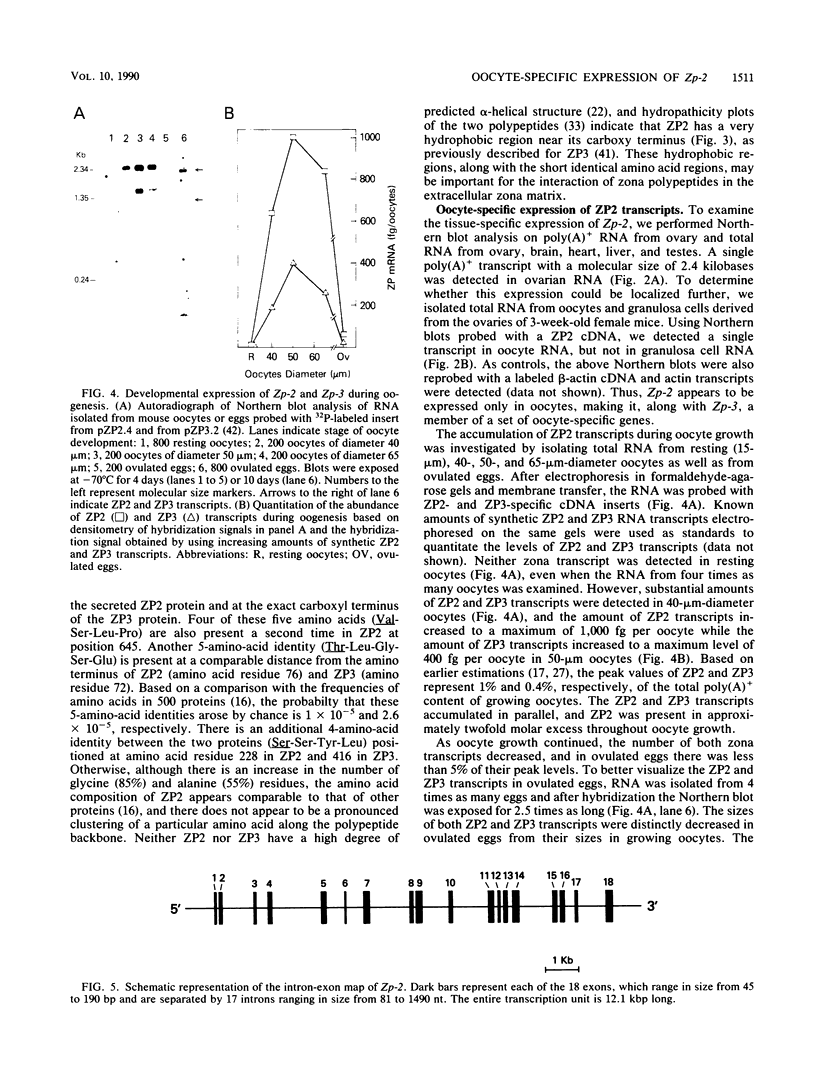
The zona pellucida surrounds all mammalian oocytes and plays a vital role at fertilization and in early development. The genes that code for two of the mouse zona proteins (ZP2 and ZP3) represent a developmentally regulated set of genes whose expression serves as markers of mouse oocyte growth and differentiation. We previously characterized the single-copy Zp-3 gene and showed that its expression is oocyte specific and restricted to a narrow window of oocyte development. We now define the Zp-2 gene transcript and show that it is coordinately expressed with Zp-3 only during the 2-week growth phase of oogenesis that occurs prior to ovulation. Like Zp-3, the expression of Zp-2 is restricted to oocytes, and, although not detectable in resting oocytes, both ZP2 and ZP3 transcripts accumulate to become very abundant messengers in 50-microns-diameter oocytes. Ovulated eggs contain ZP2 and ZP3 transcripts which are 200 nucleotides shorter than those found in growing oocytes and have an abundance of less than 5% of the peak levels. In an attempt to understand the molecular details associated with the developmentally regulated, tissue-specific gene expression of the zona genes, the Zp-2 genetic locus has been characterized and its 5' flanking sequences have been compared with those of Zp-3. Both genes contain three short (8- to 12-base-pair) DNA sequences of 80 to 88% identity located within 250 base pairs of their transcription start sites.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachvarova R. Gene expression during oogenesis and oocyte development in mammals. Dev Biol (N Y 1985) 1985;1:453–524. doi: 10.1007/978-1-4615-6814-8_11. [DOI] [PubMed] [Google Scholar]
- Bishop D. F., Calhoun D. H., Bernstein H. S., Hantzopoulos P., Quinn M., Desnick R. J. Human alpha-galactosidase A: nucleotide sequence of a cDNA clone encoding the mature enzyme. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4859–4863. doi: 10.1073/pnas.83.13.4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleil J. D., Beall C. F., Wassarman P. M. Mammalian sperm-egg interaction: fertilization of mouse eggs triggers modification of the major zona pellucida glycoprotein, ZP2. Dev Biol. 1981 Aug;86(1):189–197. doi: 10.1016/0012-1606(81)90329-8. [DOI] [PubMed] [Google Scholar]
- Bleil J. D., Greve J. M., Wassarman P. M. Identification of a secondary sperm receptor in the mouse egg zona pellucida: role in maintenance of binding of acrosome-reacted sperm to eggs. Dev Biol. 1988 Aug;128(2):376–385. doi: 10.1016/0012-1606(88)90299-0. [DOI] [PubMed] [Google Scholar]
- Bleil J. D., Wassarman P. M. Galactose at the nonreducing terminus of O-linked oligosaccharides of mouse egg zona pellucida glycoprotein ZP3 is essential for the glycoprotein's sperm receptor activity. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6778–6782. doi: 10.1073/pnas.85.18.6778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleil J. D., Wassarman P. M. Mammalian sperm-egg interaction: identification of a glycoprotein in mouse egg zonae pellucidae possessing receptor activity for sperm. Cell. 1980 Jul;20(3):873–882. doi: 10.1016/0092-8674(80)90334-7. [DOI] [PubMed] [Google Scholar]
- Bleil J. D., Wassarman P. M. Structure and function of the zona pellucida: identification and characterization of the proteins of the mouse oocyte's zona pellucida. Dev Biol. 1980 Apr;76(1):185–202. doi: 10.1016/0012-1606(80)90371-1. [DOI] [PubMed] [Google Scholar]
- Bleil J. D., Wassarman P. M. Synthesis of zona pellucida proteins by denuded and follicle-enclosed mouse oocytes during culture in vitro. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1029–1033. doi: 10.1073/pnas.77.2.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Calzone F. J., Britten R. J., Davidson E. H. Mapping of gene transcripts by nuclease protection assays and cDNA primer extension. Methods Enzymol. 1987;152:611–632. doi: 10.1016/0076-6879(87)52069-9. [DOI] [PubMed] [Google Scholar]
- Chamberlin M. E., Dean J. Genomic organization of a sex specific gene: the primary sperm receptor of the mouse zona pellucida. Dev Biol. 1989 Jan;131(1):207–214. doi: 10.1016/s0012-1606(89)80052-1. [DOI] [PubMed] [Google Scholar]
- Chien Y. H., Gascoigne N. R., Kavaler J., Lee N. E., Davis M. M. Somatic recombination in a murine T-cell receptor gene. Nature. 1984 May 24;309(5966):322–326. doi: 10.1038/309322a0. [DOI] [PubMed] [Google Scholar]
- Dayhoff M. O., Orcutt B. C. Methods for identifying proteins by using partial sequences. Proc Natl Acad Sci U S A. 1979 May;76(5):2170–2174. doi: 10.1073/pnas.76.5.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Leon V., Johnson A., Bachvarova R. Half-lives and relative amounts of stored and polysomal ribosomes and poly(A) + RNA in mouse oocytes. Dev Biol. 1983 Aug;98(2):400–408. doi: 10.1016/0012-1606(83)90369-x. [DOI] [PubMed] [Google Scholar]
- Derman E., Krauter K., Walling L., Weinberger C., Ray M., Darnell J. E., Jr Transcriptional control in the production of liver-specific mRNAs. Cell. 1981 Mar;23(3):731–739. doi: 10.1016/0092-8674(81)90436-0. [DOI] [PubMed] [Google Scholar]
- Drummond D. R., Armstrong J., Colman A. The effect of capping and polyadenylation on the stability, movement and translation of synthetic messenger RNAs in Xenopus oocytes. Nucleic Acids Res. 1985 Oct 25;13(20):7375–7394. doi: 10.1093/nar/13.20.7375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- East I. J., Dean J. Monoclonal antibodies as probes of the distribution of ZP-2, the major sulfated glycoprotein of the murine zona pellucida. J Cell Biol. 1984 Mar;98(3):795–800. doi: 10.1083/jcb.98.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florman H. M., Wassarman P. M. O-linked oligosaccharides of mouse egg ZP3 account for its sperm receptor activity. Cell. 1985 May;41(1):313–324. doi: 10.1016/0092-8674(85)90084-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnier J., Osguthorpe D. J., Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- Greve J. M., Salzmann G. S., Roller R. J., Wassarman P. M. Biosynthesis of the major zona pellucida glycoprotein secreted by oocytes during mammalian oogenesis. Cell. 1982 Dec;31(3 Pt 2):749–759. doi: 10.1016/0092-8674(82)90329-4. [DOI] [PubMed] [Google Scholar]
- Greve J. M., Wassarman P. M. Mouse egg extracellular coat is a matrix of interconnected filaments possessing a structural repeat. J Mol Biol. 1985 Jan 20;181(2):253–264. doi: 10.1016/0022-2836(85)90089-0. [DOI] [PubMed] [Google Scholar]
- Huarte J., Belin D., Vassalli A., Strickland S., Vassalli J. D. Meiotic maturation of mouse oocytes triggers the translation and polyadenylation of dormant tissue-type plasminogen activator mRNA. Genes Dev. 1987 Dec;1(10):1201–1211. doi: 10.1101/gad.1.10.1201. [DOI] [PubMed] [Google Scholar]
- JONES E. C., KROHN P. L. The relationships between age, numbers of ocytes and fertility in virgin and multiparous mice. J Endocrinol. 1961 Feb;21:469–495. doi: 10.1677/joe.0.0210469. [DOI] [PubMed] [Google Scholar]
- Jahn C. L., Baran M. M., Bachvarova R. Stability of RNA synthesized by the mouse oocyte during its major growth phase. J Exp Zool. 1976 Aug;197(2):161–171. doi: 10.1002/jez.1401970202. [DOI] [PubMed] [Google Scholar]
- Jenh C. H., Deng T. L., Li D. W., DeWille J., Johnson L. F. Mouse thymidylate synthase messenger RNA lacks a 3' untranslated region. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8482–8486. doi: 10.1073/pnas.83.22.8482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley D. E., Coleclough C., Perry R. P. Functional significance and evolutionary development of the 5'-terminal regions of immunoglobulin variable-region genes. Cell. 1982 Jun;29(2):681–689. doi: 10.1016/0092-8674(82)90184-2. [DOI] [PubMed] [Google Scholar]
- Kinloch R. A., Roller R. J., Fimiani C. M., Wassarman D. A., Wassarman P. M. Primary structure of the mouse sperm receptor polypeptide determined by genomic cloning. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6409–6413. doi: 10.1073/pnas.85.17.6409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausner R. D. Sorting and traffic in the central vacuolar system. Cell. 1989 Jun 2;57(5):703–706. doi: 10.1016/0092-8674(89)90783-6. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lunsford R. D., Jenkins N. A., Kozak C. A., Liang L. F., Silan C. M., Copeland N. G., Dean J. Genomic mapping of murine Zp-2 and Zp-3, two oocyte-specific loci encoding zona pellucida proteins. Genomics. 1990 Jan;6(1):184–187. doi: 10.1016/0888-7543(90)90465-7. [DOI] [PubMed] [Google Scholar]
- McGrew L. L., Dworkin-Rastl E., Dworkin M. B., Richter J. D. Poly(A) elongation during Xenopus oocyte maturation is required for translational recruitment and is mediated by a short sequence element. Genes Dev. 1989 Jun;3(6):803–815. doi: 10.1101/gad.3.6.803. [DOI] [PubMed] [Google Scholar]
- Moller C. C., Wassarman P. M. Characterization of a proteinase that cleaves zona pellucida glycoprotein ZP2 following activation of mouse eggs. Dev Biol. 1989 Mar;132(1):103–112. doi: 10.1016/0012-1606(89)90209-1. [DOI] [PubMed] [Google Scholar]
- Paynton B. V., Rempel R., Bachvarova R. Changes in state of adenylation and time course of degradation of maternal mRNAs during oocyte maturation and early embryonic development in the mouse. Dev Biol. 1988 Oct;129(2):304–314. doi: 10.1016/0012-1606(88)90377-6. [DOI] [PubMed] [Google Scholar]
- Peterson C. A., Piatigorsky J. Preferential conservation of the globular domains of the beta A3/A1-crystallin polypeptide of the chicken eye lens. Gene. 1986;45(2):139–147. doi: 10.1016/0378-1119(86)90248-9. [DOI] [PubMed] [Google Scholar]
- Phillips D. M., Shalgi R. Surface architecture of the mouse and hamster zona pellucida and oocyte. J Ultrastruct Res. 1980 Jul;72(1):1–12. doi: 10.1016/s0022-5320(80)90129-x. [DOI] [PubMed] [Google Scholar]
- Philpott C. C., Ringuette M. J., Dean J. Oocyte-specific expression and developmental regulation of ZP3, the sperm receptor of the mouse zona pellucida. Dev Biol. 1987 Jun;121(2):568–575. doi: 10.1016/0012-1606(87)90192-8. [DOI] [PubMed] [Google Scholar]
- Ringuette M. J., Chamberlin M. E., Baur A. W., Sobieski D. A., Dean J. Molecular analysis of cDNA coding for ZP3, a sperm binding protein of the mouse zona pellucida. Dev Biol. 1988 Jun;127(2):287–295. doi: 10.1016/0012-1606(88)90315-6. [DOI] [PubMed] [Google Scholar]
- Ringuette M. J., Sobieski D. A., Chamow S. M., Dean J. Oocyte-specific gene expression: molecular characterization of a cDNA coding for ZP-3, the sperm receptor of the mouse zona pellucida. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4341–4345. doi: 10.1073/pnas.83.12.4341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roller R. J., Kinloch R. A., Hiraoka B. Y., Li S. S., Wassarman P. M. Gene expression during mammalian oogenesis and early embryogenesis: quantification of three messenger RNAs abundant in fully grown mouse oocytes. Development. 1989 Jun;106(2):251–261. doi: 10.1242/dev.106.2.251. [DOI] [PubMed] [Google Scholar]
- Rosenthal E. T., Ruderman J. V. Widespread changes in the translation and adenylation of maternal messenger RNAs following fertilization of Spisula oocytes. Dev Biol. 1987 May;121(1):237–246. doi: 10.1016/0012-1606(87)90155-2. [DOI] [PubMed] [Google Scholar]
- Shimizu S., Tsuji M., Dean J. In vitro biosynthesis of three sulfated glycoproteins of murine zonae pellucidae by oocytes grown in follicle culture. J Biol Chem. 1983 May 10;258(9):5858–5863. [PubMed] [Google Scholar]
- Tempst P., Hunkapiller M. W., Hood L. E. Separation of peptides by reverse-phase high-performance liquid chromatography using propyl- and cyanopropylsilyl supports. Anal Biochem. 1984 Feb;137(1):188–195. doi: 10.1016/0003-2697(84)90369-5. [DOI] [PubMed] [Google Scholar]
- Verner K., Schatz G. Protein translocation across membranes. Science. 1988 Sep 9;241(4871):1307–1313. doi: 10.1126/science.2842866. [DOI] [PubMed] [Google Scholar]
- Wassarman P. M. Zona pellucida glycoproteins. Annu Rev Biochem. 1988;57:415–442. doi: 10.1146/annurev.bi.57.070188.002215. [DOI] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. Signal sequences. The limits of variation. J Mol Biol. 1985 Jul 5;184(1):99–105. doi: 10.1016/0022-2836(85)90046-4. [DOI] [PubMed] [Google Scholar]