Abstract
Much remains to be understood about the etiology of eating disorders. There is substantial evidence that reproductive hormones, specifically estrogens, play a direct role in normal food intake. Evidence is increasing that the reproductive hormones play a role in the abnormal food intake associated with eating disorders. For example, there is an inverse association between estradiol and eating disorder symptoms. Preliminary studies also suggest that hormone augmentation may be a beneficial adjunct to the standard treatment of choice for eating disorders. However, research is limited, so definitive conclusions about the benefit of hormone augmentation in treatment cannot be drawn. Future research, with a focus on translational studies, should continue to explore the role of reproductive hormones in the vulnerability to and maintenance of eating disorders.
Keywords: eating disorder, estradiol, progesterone, reproductive hormone, review, testosterone
Knowledge of the factors that play a role in the development and maintenance of eating disorders has grown substantially over the past two decades. Eating disorders are typically conceptualized as biopsychosocial disorders – culminating from biological, psychological and cultural risk factors. However, despite our increased knowledge of the vulnerability factors for eating disorders, much is still unknown about their pathophysiology. Given the increased incidence of eating disorders associated with times of reproductive axis change, such as puberty, reproductive hormones represent a potential endocrine candidate in the pathophysiology of eating disorders.
This article will provide a summary of reports addressing the association between reproductive hormones and eating disorders and their symptomatology. Reproductive hormones can influence behavior in two ways. First, these hormones can act in an organizational manner. During development, exposure to gonadal steroids organizes neural circuits to behave in a specific fashion. These effects are generally permanent, do not require the presence of the hormone to persist and often occur prenatally. Second, gonadal steroids can act in an activational manner. These effects are transient or reversible in nature and require the presence of the hormone. Initial findings suggest that reproductive hormones may influence eating disorder symptoms both in an organizational and activational manner. In this review, the authors comprehensively summarize these findings. The authors begin by providing readers with a brief summary of eating disorders and the menstrual cycle. Second, the authors provide an overview of the role of reproductive hormones in eating behavior in general (Box 1). The reports addressing the association between reproductive hormones and eating disorders are discussed, specifically highlighting the role of estradiol, progesterone and testosterone. The authors focus a majority of their discussion on women, as minimal research has addressed the association between reproductive hormones and eating disorders in men.
Box 1. Reproductive hormonal influence on food intake.
Estradiol
Inhibits food intake
Decreases meal size
May advance onset of satiety
Progesterone
Increases food intake in the presence of estrogens
Estradiol antagonist
Testosterone
Stimulates food intake
Increases the number of meals
Neonatal exposure enhances food intake in adulthood
Eating disorders
The current version of the Diagnostic and Statistical Manual (DSM-IV-TR) [1] identifies three eating disorder diagnoses: anorexia nervosa (AN), bulimia nervosa (BN) and eating disorder not otherwise specified (EDNOS). EDNOS also includes binge-eating disorder (BED), which will become a distinct diagnosis in DSM-5 [101].
AN is characterized by low bodyweight and an undue influence of weight or shape on self-evaluation. BN is characterized by binge-eating episodes followed by inappropriate compensatory behaviors such as vomiting, or laxative or diuretic abuse, as well as by an undue influence of weight and shape on self-evaluation. BED is also characterized by binge-eating episodes, but these episodes occur in the absence of regular inappropriate compensatory behaviors. Although the diagnostic criteria for BED do not explicitly include a body image criterion, it is believed that over-evaluation of weight and shape are core features of BED, similar to what is observed in BN [2]. EDNOS includes those individuals who do not meet the explicit diagnostic criteria of AN or BN.
Eating disorders afflict approximately 0.5–3% of the general population and are more common in women compared with men, with the largest sex difference estimated at 10:1 for AN, while the prevalence of BN and BED is one- to three-times higher in women compared with men [1,3]. Onset of AN and BN typically occurs during adolescence and young adulthood, with prepubertal onset being uncommon but apparently increasing [3,4]. A population-based study of adolescents found that the median ages of onset for AN, BN and subthreshold AN and BN were strikingly similar to the average age of menarche in the population [5]. Taken together, these facts have led many to postulate the role of reproductive hormones in eating disorders.
Reproductive hormones & the menstrual cycle
Estrogens, progestogens and testosterone represent the classic reproductive hormones found in humans. Estrogens are produced primarily by developing follicles in the ovaries, the corpus luteum and the placenta during pregnancy. Estradiol is the primary estrogen in childbearing women and is formed from developing ovarian follicles [6]. However, testosterone is also converted to estradiol via aromatization. Progesterone is also a steroid hormone belonging to the class of hormones called progestogens and is the major naturally occurring human progestogen [6]. Progesterone is produced in the ovaries (by the corpus luteum), the adrenal glands and, during pregnancy, in the placenta. Testosterone is a steroid hormone that is part of the androgen group. It is the principal male sex hormone and the male body produces up to eight-times more testosterone than the female body [6]. However, testosterone is secreted in women, primarily in the ovaries, with a small amount also secreted from the adrenal glands [7].
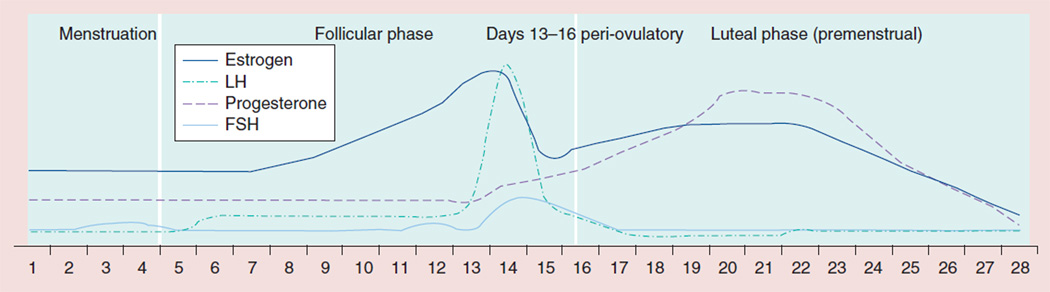
The reproductive cycle is divided into a follicular phase, an ovulatory phase and a luteal phase (Figure 1). The first day of menses is the beginning of the follicular phase, during the first half of which concentrations of estrogens and progesterone are stable and low [7]. Approximately 7–8 days before the preovulatory luteinizing hormone (LH) surge, the second half of the follicular phase begins, which is characterized by an increase in estradiol concentrations [7]. Progesterone concentrations do not increase during this period. During the ovulatory phase, there is a rapid increase in plasma LH levels eventually leading to the rupture of the mature follicle, which marks the beginning of the luteal phase [8]. Representing the hallmark of the luteal phase is an increase in progesterone levels, which reaches maximum concentrations approximately 8 days after the LH peak [8]. At this time, estradiol and progesterone concentrations increase in parallel [7]. Testosterone concentrations are low in women, but do show a cyclic pattern across the menstrual phases, being higher in the luteal phase than the follicular phase [9].
Figure 1. Changes in reproductive hormone concentrations across the menstrual cycle.
The graph is not based on actual data but is meant to provider readers with a general idea of the change in reproductive hormones that occurs across the menstrual cycle. FSH: Follicle-stimulating hormone; LH: Luteinizing hormone.
Reproductive hormones & eating behavior
Estradiol & progesterone
Estradiol plays a role in normal food intake, with data across animal species showing an inhibiting effect of estradiol [10–12]. In women, food intake decreases throughout the follicular phase of the menstrual cycle and is lowest during periovulation when estradiol concentrations are at their highest [13–16]. In rodents, food intake decreases the night following the LH surge, corresponding to the night of ovulation and estrus [13,17]. Estradiol concentrations are at their highest immediately prior to the LH surge and lowest during estrus [18]. However, the physiological effects of estradiol can last more than 12 h, so the decrease in food intake prior to the LH surge may be due to the preceding increase in estradiol [13].
Providing more substantial evidence for the role of estrogens in food intake, animals that are ovariectomized (ovaries removed) exhibit an increase in food intake, which is reversed with the administration of estradiol [19,20]. The inhibitory effect of estradiol is only present post-pubertally [21,22], and the decrease in food intake results from changes in meal size (compared with a decrease in number of meals), suggesting that estradiol may advance the onset of satiety [11,23].
In contrast to estradiol, ovariectomized rodents and healthy women exhibit no changes in food intake when treated with progesterone alone [11,24]. During the premenstrual period, when progesterone concentrations are high, a peak in food intake is observed in women [14]. However, this occurs only in the presence of estrogens. Thus, it appears that progesterone does not have a direct effect on food intake [24,25]; rather, it is a neutral antagonist to the effects of estradiol, only increasing food intake in the presence of estradiol [12,25] by blocking its inhibitory effects. Thus, in the luteal phase, it is progesterone’s antagonism of estradiol that increases food intake rather than the luteal phase increase in progesterone per se [12].
Testosterone
In contrast to estradiol, testosterone stimulates food intake by increasing the number of meals rather than the size of the meal [13,26]. In addition, neonatal exposure to testosterone in female mice enhances food intake during adulthood [27], likely through the prenatal organization of neural circuits. However, compared with estradiol and progesterone, much less is known about testosterone’s role in stimulating food intake [13].
Reproductive hormones & eating disorders
Given the abundance of research suggesting that reproductive hormones play a critical part in normal eating behaviors and food intake, it is reasonable to postulate that these hormones also have a functional role in the dysregulated eating behaviors associated with eating disorders, with estrogens being the most promising candidate. Much of the research addressing the role of reproductive hormones in the development and maintenance of eating disorders has occurred within the past decade. However, few studies have focused on eating disorder diagnosis and/or directly obtained reproductive hormone measurements. Therefore, the authors focus their discussion on both eating disorder diagnoses and symptoms and also review studies that observed eating disorder symptomatology across periods of reproductive axis change – the menstrual cycle and pregnancy, as these periods are marked by significant fluctuation and change in reproductive hormones in women.
Estradiol & progesterone
It is well established that women with AN have decreased concentrations of estrogens and progesterone [28–30]. In fact, estradiol concentrations are similar to the concentrations observed in postmenopausal women. Amenorrhea is also currently part of the diagnostic criteria for AN (although this will change with DSM-5) [101], while women with BN also frequently experience menstrual dysfunction [31]. Importantly, estradiol and progesterone dysregulation and disruption of the menstrual cycle could also be a consequence of the eating disorder rather than a cause.
Examining the association between estradiol and progesterone and eating disorder symptoms reveals an association with diminishing estradiol and increasing progesterone. Circulating estradiol concentrations are inversely related to binge eating in women with BN [32,33], suggesting that binge eating may increase in women with BN when estradiol concentrations decrease. Estradiol concentrations may also be inversely related to disordered eating (i.e., continuous measure of eating disorder-related psychological and behavioral symptoms) in community samples [34,35]. By contrast, binge eating in women with BN and disordered eating in community samples are positively associated with progesterone concentrations [32,33]. Notably, Edler et al. reported that estradiol and progesterone together accounted for 24% of the variance in binge eating in women with BN after controlling for negative affect [32]. Moreover, the psychological symptoms of eating disorders are also associated with estradiol and progesterone. Body dissatisfaction (i.e., dissatisfaction with the size and shape of one’s body) and drive for thinness (i.e., preoccupation with weight, dieting and pursuit of thinness) both showed an inverse association with estradiol and a positive association with progesterone concentrations in a community sample [36].
Finally, animal studies provide additional evidence for the role of estradiol and progesterone in binge eating, and further implicate reproductive hormones in the etiology of eating disorders. In animal models of binge eating, ovariectomized rats consume more palatable food and engage in increased binge eating than nonovariectomized rats [37]. Fat intake in ovariectomized rats under binge-like conditions is reduced by exogenous administration of combined estradiol and progesterone [38]. Moreover, the cyclic inhibitory effect of estradiol is compromised in animals consuming large, binge-type amounts of fatty meals [38], suggesting that regular binge eating may disrupt the normal functioning of the estrogen system on food intake. This disruption may play a role in the chronicity of these disorders.
In summary, clinical research examining the association between estradiol and progesterone concentrations and eating disorder symptoms and animal models of eating disorder symptoms, provide support for the role of these hormones in the activation of the abnormal eating behaviors that occur in eating disorders. Furthermore, animal models suggest that these hormones may play a role in both the development and maintenance of binge eating-type behaviors. However, despite the significant positive association between binge-eating frequency and progesterone, a number of studies have indicated that progesterone does not have a direct effect on normal food intake, but it only increases food intake in the presence of estrogens by inhibiting the effects of estradiol. Therefore, when estradiol concentrations decrease and progesterone concentrations increase, progesterone may be at sufficiently high concentrations to exert its antagonistic role, which in turn stimulates food intake. However, without the presence of estradiol, progesterone has no direct influence on food intake. To date, no study has examined whether progesterone’s influence on eating disorder symptomatology only occurs in the presence of estradiol.
Testosterone
The majority of studies examining testosterone in eating disorders have only included acutely ill individuals. These findings indicate that circulating concentrations of testosterone are elevated in women with BN [29,39], and may be decreased in women with AN [29]. Importantly, it is unclear whether this is a cause or consequence of the disorder, so the role of circulating testosterone concentrations in the etiology of eating disorders is unclear. Interestingly, however, several psychological [40,41] (i.e., impulsivity, irritability and aggressiveness) and medical [42,43] (i.e., acne and polycystic ovary syndrome) conditions associated with elevated testosterone are more common in women with BN than in the general population. This may suggest that elevated testosterone concentrations promote bulimic behaviors via its effects on impulse control.
It has also been postulated that prenatal testosterone exposure plays an organizational role in eating disorder risk, such that it is protective against the development of an eating disorder. For example, index finger (2D): ring finger (4D) finger ratio, which is a sexually dimorphic characteristic that is highly correlated with prenatal testosterone concentrations, has been used as a proxy for prenatal testosterone exposure. A lower 2D:4D ratio is indicative of lower prenatal testosterone exposure. Low concentrations of prenatal testosterone exposure, as indexed by this 2D:4D finger ratio, are associated with increased eating disorder symptoms [34] and BN diagnosis [44]. However, in contrast to the postulation that prenatal testosterone plays a protective role in eating disorder vulnerability, high concentrations of prenatal testosterone are associated with AN diagnosis [44].
Comparing the prevalence of disordered eating in opposite- and same-sex twin pairs as an additional proxy for prenatal testosterone exposure partially confirms 2D:4D finger ratio findings. It is postulated that females from an opposite-sex twin pair are exposed to more testosterone in utero, protecting them against the later development of eating disorder symptomatology. Indeed, Culbert et al. found that same-sex female twins exhibited the highest mean concentrations of disordered eating (mean = 8.5), using a modified version of the Eating Disorder Inventory, followed by females from opposite-sex twin pairs (mean = 6.7), males from opposite-sex twin pairs (mean = 4) and finally same-sex male twins [45] (mean = 3; of note, disordered eating means estimated from figure provided in Culbert et al.). This significant linear trend was hypothesized to be attributable to prenatal testosterone exposure. However, no study has been able to replicate the findings of Culbert et al. comparing the prevalence of disordered eating and eating disorders across same- and opposite-sex twin pairs [46–48]. Given that this area of research is still in its infancy, definitive conclusions cannot yet be made concerning the meaning of these inconsistencies.
Finally, as previously mentioned, the majority of research to date has focused on the role of reproductive hormones in the vulnerability to eating disorders in females. However, one report has directly examined the association between testosterone concentrations and disordered eating in males. The authors reported a significant inverse association between postpubertal testosterone concentrations and disordered eating, controlling for mood, anxiety and BMI [49].
The results addressing testosterone’s role in eating disorder vulnerability are inconsistent and difficult to interpret given the lack of studies examining circulating testosterone concentrations pre- and post-illness. Notably, testosterone is also converted to estradiol through aromatization in both sexes and is the primary mechanism responsible for estradiol concentrations in men. Therefore, findings about testosterone may further implicate estradiol in the role of eating disorders, as this conversion could affect the amount and flux of estradiol present in the body at any given time. Thus, the influence of testosterone on disordered eating may be mediated through estradiol. It will be important for research to continue to build knowledge in this area before empirically supported hypotheses about the role of testosterone in eating disorder risk can be made.
Reproductive stage & eating disorders
Menstrual cycle
Similar to the patterns noted previously with regard to the fluctuations of normal food intake across the menstrual cycle and associations with clinical assessments of estradiol and progesterone concentrations, severity of eating disorder symptomatology, specifically binge eating, body dissatisfaction and drive for thinness, also fluctuates across the menstrual cycle.
Studies comparing binge-eating frequency in women with BN across menstrual cycle phases show that both binge-eating frequency and bulimic symptoms are significantly elevated in the luteal (premenstrual) phase compared with the follicular and ovulatory phases [32,50–52]. Specifically, one report showed a 60% increase in binge-eating frequency in the premenstrual phase [52]. The mean number of binges during the premenstrual period was 6.65, and in the menstrual period frequency decreased to 4.41 [52]. Moreover, it has been suggested that exacerbation of binge eating is more consistently present across all women with BN the week preceding menses compared with exacerbation during the mid-luteal phase, during which time only some women show exacerbation in binge eating [50]. Similar premenstrual exacerbations are suggested for purging behaviors [32,50] and continuous measures of binge eating in community samples [33].
Furthermore, body dissatisfaction and drive for thinness in nonclinical samples show similar patterns across the menstrual cycle. Body dissatisfaction and drive for thinness are highest during the luteal phase of the menstrual cycle compared with the follicular/ovulatory phase [36]. However, these findings have not been consistent. Some reports only revealed differences in body dissatisfaction across the menstrual cycle when the premenstrual and menstrual phases were combined [53,54].
Findings for the psychological symptoms of eating disorders (i.e., body dissatisfaction and drive for thinness) may be less robust than findings related to the behavioral symptoms (e.g., binge eating) because psychological symptoms are more subjective in nature, rendering them more difficult to assess and quantify. Furthermore, the biological underpinnings of eating disorders may be more closely linked with the behavioral symptoms than the psychological symptoms. This interpretation is supported by genetic data that indicate a moderately strong heritability of binge eating [55,56] and vomiting as a compensatory behavior [56]. Notably, however, the psychological symptoms of eating disorders also demonstrate heritability [57,58], although the estimates are less consistent than heritability estimates of the behavioral symptoms. It appears that the more interpretation that is involved, the lower the heritability estimates [58].
Change in eating disorder symptomatology across the menstrual cycle may have important implications for treatment. Contingency plans can be made in order to cope with the increase in symptomatology and urges that may arise during the premenstrual phase. In addition, psychiatric hospitalizations, suicidal behaviors and depressed mood are also more frequent during the premenstrual phase [59–61]. The menstrual cycle influence on eating disorder and mood symptomatology would be important for a clinician treating an individual with an eating disorder to have on their radar and assess for.
Although the weight of the evidence strongly suggests that changes in the reproductive hormonal milieu across the female menstrual cycle influence eating disorder symptoms in vulnerable women, methodological limitations may contribute to inconsistencies in the literature. For example, owing to significant between-subject differences in absolute concentrations of gonadal steroid hormones over the menstrual cycle [62], a within-subjects design is methodologically important in accurately testing for menstrual cycle effects. Within-person, individual change also provides the most meaningful results clinically – whether binge-eating frequency is high or low across the menstrual cycle relative to other individuals is not as functionally significant as change relative to self [51]. In addition, because normal menstrual cycle lengths are variable (22–36 days), and there is a lack of correspondence between self-reported cycle length and actual cycle length [62], it is important to obtain hormonal verification of menstrual cycle phase. Finally, results are difficult to compare across studies because menstrual cycle phase definitions are not consistent across differing reports.
Pregnancy
During pregnancy, both estradiol and progesterone concentrations progressively increase until childbirth, with a sharp increase in estradiol after the first trimester [63]. Both hormones abruptly decrease postpartum [63]. Initial retrospective reports of clinical samples found that some women with AN and BN report improvement and remission of eating disorder symptomatology during pregnancy [64,65]. One such study showed that the presence of bulimic symptomatology decreased sequentially during each trimester, with 75% of women exhibiting no bulimic symptomatology by the third trimester [65]. This sequential decrease may correspond to the progressive increase of estradiol across pregnancy. However, a resurgence of symptoms often occurs postpartum [64,65].
Larger-scale prospective reports have corroborated these findings, describing eating disorder remission rates between 29 and 78% during pregnancy [66]. Moreover, body dissatisfaction also decreases during pregnancy in some women with a current eating disorder [67]. These findings are similar to what is observed across the menstrual cycle such that eating disorder symptoms increase when estradiol concentrations are low (i.e., postpartum) and decrease when estradiol concentrations are high (i.e., during pregnancy).
Although pregnancy may be a period of improvement and remission for some women, some studies have observed that women who have recovered from an eating disorder experience a relapse during pregnancy. For example, a prospective report following a small sample of women with a past history of AN found that 33% experienced a relapse during pregnancy [68]. Pregnancy also appears to be a specific vulnerability period for new-onset BED [66]. An increase in body dissatisfaction has also been observed during pregnancy in women with a history of an eating disorder [69].
Taken together, there are substantial changes in eating disorder symptoms across the menstrual cycle and during pregnancy, which suggest that gonadal steroids may play an activational role in eating disorder symptoms. However, findings are somewhat inconsistent with regard to pregnancy. It appears that the impact of pregnancy on eating disorder symptoms varies across women, representing a window of remission, relapse and new onset for BED. However, to date, studies have not examined the separate phases of pregnancy to determine in which phase risk and remission typically occurs, or whether it is the hormone concentration or flux in estradiol and progesterone that may serve as the critical window for risk or remission. In addition to any role that reproductive hormones may play in eating disorder symptoms around pregnancy, pregnancy can also be a significant time of change and stress in a woman’s life, and these changes may contribute to a woman’s vulnerability to the impact of hormonal change on eating disorders during pregnancy.
Vulnerability to reproductive hormone change
As posited above, women with eating disorders may have increased sensitivity to fluctuation, or change, in reproductive hormone concentrations. This hypothesis is supported by the fact that significant changes are observed in eating disorder symptomatology during periods of substantial reproductive hormone change (i.e., puberty and pregnancy) and across the menstrual cycle.
Although the authors will not comprehensively review findings here, it is well established that puberty is a potent vulnerability period for eating disorder onset and related symptomatology [70]. Since estradiol plays an important role in gene transcription, it is hypothesized that estradiol may be involved in the ‘activation’ of some genetic factors that influence eating disorders [34]. For example, comparing the genetic effects on disordered eating in girls with low versus high concentrations of estradiol, as measured through salivary samples, shows that the genetic effects on disordered eating in girls with low concentrations of estradiol are minimal, whereas the genetic effects on disordered eating in girls with high concentrations of estradiol are substantial [71]. Furthermore, examining whether there were individual differences in binge-eating proneness in rats before, during and after pubertal onset revealed a dramatic increase in binge-eating proneness across puberty, such that there was minimal evidence for differences in binge-eating proneness prior to puberty, but significant differences in binge-eating proneness after puberty [72].
Similarly, studies have also addressed whether there are genetic associations between aspects of puberty and eating disorder symptoms. Twin studies, which allow for the proportion of the genetic and environmental influences on a trait to be delineated, have established that eating disorders have a moderately strong genetic component, but specific genes that influence risk for eating disorders have not yet been identified [73]. Comparing the genetic influences on eating disorder symptomatology in girls pre- and post-puberty indicates that the genetic effects for eating disorder symptomatology in prepubertal girls were near 0% but rose markedly to approximately 60% in postpubertal girls and young adults [74,75]. It is hypothesized that the increase in estrogens that occurs during puberty accounts for the substantial increase in the genetic effects of disordered eating postpuberty. Thus, estrogens would moderate the genetic influences on disordered eating. It is also suggested that aspects of puberty and eating disorder symptoms share genetic vulnerability factors. For example, two additional twin studies indicate moderate overlap in the genetic factors responsible for age at menarche and disordered eating and dieting [76,77]. Thus, genetic factors specifically involved in the underlying regulation and functioning of the estrogen system, such as those involved in gene transcription and estrogen receptor signaling, may underlie the shared genetic risk observed between aspects of puberty and eating disorder symptoms.
Evidence exists for an activational role of gonadal steroids in eating disorder symptoms. Specifically, findings suggest that estradiol may play a role in the genetic vulnerability to eating disorder symptomatology. This underlying genetic vulnerability may be involved in an increased sensitivity to hormonal fluctuation – increasing substantially at puberty via mechanisms related to estradiol.
Reproductive hormones in the treatment of eating disorders
Estradiol & progesterone
To date, research examining the efficacy of estrogens in the treatment of eating disorders has either focused on estradiol replacement’s impact on bone mass and density in women with AN or on the efficacy of oral contraceptives in improving bulimic symptomatology. Importantly, hormone augmentation alone is not an adequate treatment for an eating disorder, so this should be applied only in conjunction with additional forms of treatment, such as cognitive–behavioral therapy – which is often the treatment of choice for eating disorders, specifically BN and BED.
High-dose estradiol in the form of an oral contraceptive does not improve bone mass density in patients with AN [78,79]. However, physiological doses of estradiol show promising results for improving spine and hip bone mass density in girls with AN [80]. Patients received incremental, low-dose, oral ethinylestradiol for 18 months in order to replicate pubertal increases in estradiol, and those receiving the incremental estradiol treatment showed improvement in spine and hip bone mass density compared with girls receiving placebo. However, the impact of estradiol on additional AN symptomatology is not clear as this was not addressed in the report. The authors concluded that the administration of small, incremental doses of oral estradiol in girls 12–18 years of age with AN is effective in increasing spine and hip bone mass density [80]. Thus, it appears that high-dose estradiol in concentrations that do not replicate normal pubertal physiology are not beneficial in improving bone mass density in girls with AN, but that treatment mimicking normal pubertal development could be a beneficial adjunct to psychological treatment for AN.
Examining the impact of the oral contraceptive Yasmin® (drospirenone 3 mg, estradiol 0.03 mg; Schering, AG, Bergkamen, Germany) on bulimic symptomatology after a 3-month treatment cycle showed a reduction in compensatory behaviors, meal-related hunger and gastric distention in women with BN [81]. Twenty eight percent of women in the study displayed a reduction in bulimic symptomatology and 15% no longer met diagnostic criteria for BN at the end of treatment [81]. Of note, treatment responders had significantly higher baseline concentrations of testosterone, and the reduction in bulimic behavior was related to a decline in testosterone concentrations. This finding suggests that oral contraceptives or estradiol and progesterone may provide a beneficial pharmacological addition to cognitive–behavioral therapy, for example for a subgroup of BN women with hyperandrogenic symptoms [81].
Testosterone
Very few studies have examined the efficacy of testosterone in eating disorder treatment. Case studies suggest that the administration of a testosterone receptor antagonist decreases bulimic symptomatology in women with BN [82,83], while the administration of low-dose testosterone improves depressive symptoms and spatial cognition in women with AN [84]. However, more research is needed to delineate whether testosterone may be a potential pharmacological treatment for AN and BN.
Expert commentary
In summary, an increase in the attention paid to the role of reproductive hormones in eating disorder development in the past decade has important implications for the pathophysiology of eating disorders. Taken together, it appears that reproductive hormones may play an activational role in the risk for and chronicity of an eating disorder – with estradiol and progesterone (in the presence of estradiol) being the key players. Currently, whether these hormones play an organizational role in eating disorder risk is unclear.
There are two main possibilities in which estradiol and progesterone may influence eating disorders. First, the absolute concentrations of these hormones may directly influence eating disorder symptoms. As discussed, bulimic symptomatology tends to increase when estradiol concentrations decrease and progesterone concentrations increase. Therefore, this increase in bulimic symptomatology could be directly related to absolute concentrations of estradiol and progesterone such that the combination of the increase in estradiol and decrease in progesterone increases binge-eating frequency.
An additional possibility is that some women in the population may be more sensitive to changes in reproductive hormones. Specifically, women with eating disorders may represent a small subpopulation of women who are highly sensitive to fluctuations in estradiol or react strongly to changing estradiol concentrations [85]. Of relevance to this proposition, women with AN exhibit increased sensitivity to sensory experience (i.e., visual, auditory, touch, taste, smell, vestibular and kinesthetic) compared with healthy controls, suggesting that they may actually be hypersensitive to interoceptive change and visceral sensations [Zucker N, Pers. Comm.]. Therefore, it is reasonable to postulate that women with eating disorders may also exhibit heightened sensitivity to hormonal fluctuations. Moreover, it is frequently observed that puberty is a marked vulnerability period for an eating disorder and that the genetic influences on eating disorder symptoms are null prior to puberty yet substantial after puberty. This suggests that there may be genetic, individual differences determining the response to hormonal fluctuations, and that this sensitivity to hormone fluctuation may be triggered at puberty (or other periods of reproductive hormone change) with increasing estradiol concentrations, in turn substantially increasing the vulnerability to an eating disorder. Thus, change, rather than absolute level, in reproductive hormones may be a catalyst towards eating disorder symptoms in genetically vulnerable women.
Sensitivity to change in reproductive hormones as a catalyst towards eating disorder symptoms is additionally supported by the fact that changes in eating disorder symptoms are observed during pregnancy in both directions: remission in those with a current eating disorder and relapse in those with a history of an eating disorder. We postulate that women with a current eating disorder during pregnancy are benefited by the progressive increases (and reduced fluctuations) in estradiol and progesterone concentrations that occur during pregnancy. Hormonal fluctuations in the postpartum could represent another period during which women with eating disorders experience hypersensivity to hormonal change, which could activate relapse. However, it will also be important to examine additional contextual factors (e.g., negative life events, personality characteristics and stress) that may predict remission, relapse, or a new-onset eating disorder during pregnancy, as well as to examine if there are specific periods during pregnancy that are associated with increased risk of developing symptoms or remitting.
Furthermore, limited work suggests that eating disorder symptoms affect the normal functioning of the estrogen system such that increased consumption of binge-type fatty meals decreases the inhibitory effect of estradiol. This may play a role in the chronicity that is often observed in eating disorders. The underlying individual differences in the sensitivity to hormonal fluctuations may increase vulnerability to eating disorder symptoms, while engaging in the eating disorder symptoms themselves (i.e., binge eating) also serves to maintain the eating disorder.
Currently, the treatment of choice for BN and BED is cognitive–behavioral therapy. Treatment of choice for AN first includes weight restoration and family-based therapy for adolescents. However, data are limited on how to best treat adults with AN, with some evidence for the efficacy of cognitive–behavioral therapy after weight restoration [86]. Very little research has examined the influence of hormone augmentation on eating disorder symptomatology, especially in regard to AN, so definitive conclusions and recommendations cannot be made at this time. However, it appears that, at least for some individuals with BN, oral contraceptives and testosterone antagonists may be a beneficial adjunct to cognitive–behavioral therapy.
Five-year view
The next 5 years are likely to bring about new and exciting findings in the pathophysiology and treatment of eating disorders, specifically with regard to the role of reproductive hormones. Translational studies will need to be at the forefront of this research. Much can be gleaned from animal models that cannot be accomplished in human samples as hormonal manipulation in humans is difficult. The findings from these animal models must then be examined in human populations, which will then inform future animal models. In order to fully characterize the role of reproductive hormones in eating disorder vulnerability and maintenance, researchers examining animal populations and researchers examining human populations will need to work closely together.
There are four specific areas we believe future research should home in on. First, it will be important to tease apart the role of estradiol versus progesterone. It appears that estradiol has a direct role in normal food intake while the role of progesterone is indirect. However, it is currently unclear whether this is also true for eating disorder symptomatology, specifically binge eating.
Second, it will be important to address individual factors that may influence vulnerability to reproductive hormone change. Indeed, one report suggests that negative urgency (i.e., rash action in response to negative affect) moderates the association between ovarian hormones (i.e., estradiol and progesterone) and binge eating, such that the influence of ovarian hormones on binge eating is stronger in individuals with high concentrations of negative urgency [35]. It will also be important to consider how culture and society may fit in, given the importance that Western society often places on an unrealistic standard of thinness. Exposure to this cultural thin ideal and the departure from it that typically happens during puberty (and other periods of reproductive axis change) may interact with an underlying genetic sensitivity to hormonal fluctuations. For example, when puberty occurs, internalization of the cultural thin ideal may have a more significant impact on those girls with an underlying sensitivity to hormonal fluctuations. There are probably many important, confounding, individual differences influencing sensitivity to reproductive hormone fluctuation.
Third, exogenous administration of reproductive hormones may have important implications for eating disorder treatment and the chronicity often observed in these disorders. Compared with other psychiatric disorders, the knowledge about beneficial pharmacological treatments for eating disorders is minimal. For AN specifically, pharmacological treatments often show no benefit until the patient becomes weight-restored. One hypothesis for this is that the hormonal dysregulation often observed in women with eating disorders influences the efficacy of psychotropic treatments. For example, it has been postulated that estrogen supplementation will improve the efficacy of selective serotonin reuptake inhibitors (SSRIs) in women with AN [87], which are often used in the treatment of BN in an effort to decrease bulimic symptomatology. Specifically, an initial case report suggests that estradiol facilitates the antidepressant effect of fluoxetine in menopausal women with major depression and is superior to antidepressant or estradiol treatment alone [88]. However, case reports have been inconsistent with reference to the benefit of augmenting antidepressants with estradiol and the authors are not aware of any randomized clinical trials [89]. Notably, women with AN often exhibit estradiol concentrations similar to those observed in menopausal women, suggesting that this decrease in estradiol may hinder the effectiveness of SSRI treatments. If the dysregulation in estradiol often observed in eating disorders, especially AN, is decreasing the efficacy of our standard treatment approaches, this would help to explain the chronic course and high frequency of relapse often observed.
Paradigms such as this could lead to more individualized treatment approaches – women who are experiencing hormonal dysregulation along with their eating disorder may receive the most benefit from hormonal augmentation in conjunction with typical pharmacological and therapeutic treatments. Moreover, much further down the line, paradigms such as this may provide insight into a neuroendocrine profile of women at risk for an eating disorder.
Fourth, it will be important to explore additional populations to further our knowledge of the role of reproductive hormones in eating disorder vulnerability and maintenance. It would be of great interest to examine other times of substantial reproductive axis change, such as the menopause transition. During the menopause transition, estradiol and progesterone concentrations not only decrease substantially, but also fluctuate significantly on a day-to-day basis. Consistent with what has been observed during puberty and pregnancy, the menopause transition may be an additional vulnerability period for eating disorder symptoms. If, in fact, the menopause transition is an additional reproductive axis period of vulnerability for an eating disorder, this provides support for the hypothesis that it may be hormonal fluctuation rather than absolute concentrations of reproductive hormones that increase risk. We would then expect that those women who are susceptible during puberty and pregnancy to also be susceptible during the menopause transition.
In line with this hypothesis, examining a continuous measure of disordered eating across the lifespan showed that eating disorder symptoms decrease after age 24 years. However, there was a slow increase again, with scores peaking during the 45–54 years age range, which is around the menopause transition [90]. Exploring this additional period of reproductive axis change would inform our understanding of reproductive hormones as pathophysiological triggers. Moreover, it will be important for future research to include males and continue exploring male dominant reproductive hormones (i.e., testosterone) in order to obtain a full picture of neuroendocrine risk.
Finally, future studies should attempt to develop an integrated model of eating disorders. For example, estrogens influence many additional psychological and physiological factors that are associated with eating disorders: depression, stress reactivity, anxiety and the secretion of appetite hormones – all of which could play a small, yet important role in the culmination of risk for an eating disorder. Currently, findings are most robust for the impact of estradiol on binge eating, indicating an ideal starting point from which to initiate this work.
Key issues.
There is significant evidence that reproductive hormones play a role in normal food intake. However, much less research has addressed the role that reproductive hormones may play in abnormal food intake, such as that observed in eating disorders.
Estradiol inhibits food intake while testosterone stimulates food intake. It appears that progesterone does not have a direct role in food intake, but is an estradiol antagonist.
Pregnancy appears to be a high-risk period of relapse for women with a history of an eating disorder and a possible period of remission for some women with a current eating disorder.
Pregnancy is a specific period of vulnerability for new-onset binge-eating disorder.
Eating disorder symptoms, specifically binge eating and body dissatisfaction, show an inverse association with estradiol and positive association with progesterone.
Prenatal testosterone may play a protective role in the later development of an eating disorder.
Clinical research studies are needed to explore the impact of hormone augmentation, in conjunction with cognitive–behavioral therapy, in treatment outcomes for anorexia nervosa.
Future research should focus on building translational models from animal and human study findings and developing an integrated model of risk for eating disorders.
Acknowledgments
JH Baker was supported by NIH grant T32MH076694 (Principal Investigator: CM Bulik).
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition Text Revision. DC, USA: American Psychiatric Press; 2000. [Google Scholar]
- 2.Goldschmidt AB, Hilbert A, Manwaring JL, et al. The significance of overvaluation of shape and weight in binge eating disorder. Behav. Res. Ther. 2010;48(3):187–193. doi: 10.1016/j.brat.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hudson JI, Hiripi E, Pope HG, Jr, Kessler RC. The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biol. Psychiatry. 2007;61(3):348–358. doi: 10.1016/j.biopsych.2006.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gowers SG, Crisp AH, Joughin N, Bhat A. Premenarcheal anorexia nervosa. J. Child Psychol. Psychiatry. 1991;32(3):515–524. doi: 10.1111/j.1469-7610.1991.tb00328.x. [DOI] [PubMed] [Google Scholar]
- 5.Swanson SA, Crow SJ, Le Grange D, Swendsen J, Merikangas KR. Prevalence and correlates of eating disorders in adolescents. Results from the National Comorbidity Survey Replication Adolescent Supplement. Arch. Gen. Psychiatry. 2011;68(7):714–723. doi: 10.1001/archgenpsychiatry.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Norman AW. Hormones. CA, USA: Academic Press; 1997. [Google Scholar]
- 7.Farage MA, Neill S, MacLean AB. Physiological changes associated with the menstrual cycle: a review. Obstet. Gynecol. Surv. 2009;64(1):58–72. doi: 10.1097/OGX.0b013e3181932a37. [DOI] [PubMed] [Google Scholar]
- 8.Girdler SS, Light KC. Reproductive hormones and stages of life in women: moderators of mood and cardiovascular health. In: Steptoe A, editor. Handbook of Behavioral Medicine. Paris, France: Springer; 2010. pp. 585–602. [Google Scholar]
- 9.Oka K, Hirano T, Noguchi M. Changes in the concentration of testosterone in serum during the menstrual cycle, as determined by liquid chromatography. Clin. Chem. 1988;34(3):557–560. [PubMed] [Google Scholar]
- 10. Brown LM, Clegg DJ. Central effects of estradiol in the regulation of food intake, body weight, and adiposity. J. Steroid Biochem. Mol. Biol. 2010;122(1–3):65–73. doi: 10.1016/j.jsbmb.2009.12.005. • Provides readers with a general overview of the influence of estradiol on food intake.
- 11.Butera PC. Estradiol and the control of food intake. Physiol. Behav. 2010;99(2):175–180. doi: 10.1016/j.physbeh.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eckel LA. The ovarian hormone estradiol plays a crucial role in the control of food intake in females. Physiol. Behav. 2011;104(4):517–524. doi: 10.1016/j.physbeh.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Asarian L, Geary N. Modulation of appetite by gonadal steroid hormones. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2006;361(1471):1251–1263. doi: 10.1098/rstb.2006.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buffenstein R, Poppitt SD, McDevitt RM, Prentice AM. Food intake and the menstrual cycle: a retrospective analysis, with implications for appetite research. Physiol. Behav. 1995;58(6):1067–1077. doi: 10.1016/0031-9384(95)02003-9. [DOI] [PubMed] [Google Scholar]
- 15.Lyons PM, Truswell AS, Mira M, Vizzard J, Abraham SF. Reduction of food intake in the ovulatory phase of the menstrual cycle. Am. J. Clin. Nutr. 1989;49(6):1164–1168. doi: 10.1093/ajcn/49.6.1164. [DOI] [PubMed] [Google Scholar]
- 16.Gong EJ, Garrel D, Calloway DH. Menstrual cycle and voluntary food intake. Am. J. Clin. Nutr. 1989;49(2):252–258. doi: 10.1093/ajcn/49.2.252. [DOI] [PubMed] [Google Scholar]
- 17.Geary N. Estradiol, CCK and satiation. Peptides. 2001;22(8):1251–1263. doi: 10.1016/s0196-9781(01)00449-1. [DOI] [PubMed] [Google Scholar]
- 18.Becker JB, Arnold AP, Berkley KJ, et al. Strategies and methods for research on sex differences in brain and behavior. Endocrinology. 2005;146(4):1650–1673. doi: 10.1210/en.2004-1142. [DOI] [PubMed] [Google Scholar]
- 19.Geary N, Asarian L. Cyclic estradiol treatment normalizes body weight and test meal size in ovariectomized rats. Physiol. Behav. 1999;67(1):141–147. doi: 10.1016/s0031-9384(99)00060-8. [DOI] [PubMed] [Google Scholar]
- 20.Asarian L, Geary N. Cyclic estradiol treatment normalizes body weight and restores physiological patterns of spontaneous feeding and sexual receptivity in ovariectomized rats. Horm. Behav. 2002;42(4):461–471. doi: 10.1006/hbeh.2002.1835. [DOI] [PubMed] [Google Scholar]
- 21.Wade GN, Zucker I. Development of hormonal control over food intake and body weight in female rats. J. Comp. Physiol. Psychol. 1970;70(2):213–220. doi: 10.1037/h0028713. [DOI] [PubMed] [Google Scholar]
- 22.Wade GN. Interaction between estradiol-17 beta and growth hormone in control of food intake in weanling rats. J. Comp. Physiol. Psychol. 1974;86(2):359–362. doi: 10.1037/h0035945. [DOI] [PubMed] [Google Scholar]
- 23.Eckel LA. Estradiol: a rhythmic, inhibitory, indirect control of meal size. Physiol. Behav. 2004;82(1):35–41. doi: 10.1016/j.physbeh.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 24.Pelkman CL, Chow M, Heinbach RA, Rolls BJ. Short-term effects of a progestational contraceptive drug on food intake, resting energy expenditure, and body weight in young women. Am. J. Clin. Nutr. 2001;73(1):19–26. doi: 10.1093/ajcn/73.1.19. [DOI] [PubMed] [Google Scholar]
- 25.Wade GN. Some effects of ovarian hormones on food intake and body weight in female rats. J. Comp. Physiol. Psychol. 1975;88(1):183–193. doi: 10.1037/h0076186. [DOI] [PubMed] [Google Scholar]
- 26.Chai JK, Blaha V, Meguid MM, Laviano A, Yang ZJ, Varma M. Use of orchiectomy and testosterone replacement to explore meal number-to-meal size relationship in male rats. Am. J. Physiol. 1999;276(5 Pt 2):R1366–R1373. doi: 10.1152/ajpregu.1999.276.5.R1366. [DOI] [PubMed] [Google Scholar]
- 27.Nohara K, Zhang Y, Waraich RS, et al. Early-life exposure to testosterone programs the hypothalamic melanocortin system. Endocrinology. 2011;152(4):1661–1669. doi: 10.1210/en.2010-1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Himmerich H, Schönknecht P, Heitmann S, Sheldrick AJ. Laboratory parameters and appetite regulators in patients with anorexia nervosa. J. Psychiatr. Pract. 2010;16(2):82–92. doi: 10.1097/01.pra.0000369969.87779.1c. • Helpful resource for physicians on the expected laboratory parameters in women with anorexia nervosa.
- 29.Monteleone P, Luisi M, Colurcio B, et al. Plasma levels of neuroactive steroids are increased in untreated women with anorexia nervosa or bulimia nervosa. Psychosom. Med. 2001;63(1):62–68. doi: 10.1097/00006842-200101000-00008. [DOI] [PubMed] [Google Scholar]
- 30.Autry JH, Stover ES, Reatig N, Casper R. Anorexia nervosa and bulimia. Annu. Rev. Public Health. 1986;7:535–543. doi: 10.1146/annurev.pu.07.050186.002535. [DOI] [PubMed] [Google Scholar]
- 31.Poyastro Pinheiro A, Thornton LM, Plotonicov KH, et al. Patterns of menstrual disturbance in eating disorders. Int. J. Eat. Disord. 2007;40(5):424–434. doi: 10.1002/eat.20388. [DOI] [PubMed] [Google Scholar]
- 32. Edler C, Lipson SF, Keel PK. Ovarian hormones and binge eating in bulimia nervosa. Psychol. Med. 2007;37(1):131–141. doi: 10.1017/S0033291706008956. •• Research paper exploring the change in bulimic symptomatology across the menstrual cycle and associations with estradiol and progesterone concentrations.
- 33.Klump KL, Keel PK, Culbert KM, Edler C. Ovarian hormones and binge eating: exploring associations in community samples. Psychol. Med. 2008;38(12):1749–1757. doi: 10.1017/S0033291708002997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klump KL, Gobrogge KL, Perkins PS, Thorne D, Sisk CL, Breedlove SM. Preliminary evidence that gonadal hormones organize and activate disordered eating. Psychol. Med. 2006;36(4):539–546. doi: 10.1017/S0033291705006653. [DOI] [PubMed] [Google Scholar]
- 35.Racine SE, Keel P, Burt SA, et al. Impulsivity as a moderator of associations between ovarian hormones and binge eating across the menstrual cycle; Presented at: The 2012 International Conference on Eating Disorders; 3–5 May 2012; Austin, TX. [Google Scholar]
- 36.Racine SE, Culbert KM, Keel PK, Sisk CL, Burt SA, Klump KL. Differential associations between ovarian hormones and disordered eating symptoms across the menstrual cycle in women. Int. J. Eat. Disord. 2012;45(3):333–344. doi: 10.1002/eat.20941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klump KL, Suisman JL, Culbert KM, Kashy DA, Keel PK, Sisk CL. The effects of ovariectomy on binge eating proneness in adult female rats. Horm. Behav. 2011;59(4):585–593. doi: 10.1016/j.yhbeh.2011.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yu Z, Geary N, Corwin RL. Ovarian hormones inhibit fat intake under binge-type conditions in ovariectomized rats. Physiol. Behav. 2008;95(3):501–507. doi: 10.1016/j.physbeh.2008.07.021. • Research report indicating that ovariectomized rats engage in increased binge eating-type behaviors and that engaging in binge eating-type behaviors disrupts the estrogen system.
- 39.Sundblad C, Bergman L, Eriksson E. High levels of free testosterone in women with bulimia nervosa. Acta Psychiatr. Scand. 1994;90(5):397–398. doi: 10.1111/j.1600-0447.1994.tb01613.x. [DOI] [PubMed] [Google Scholar]
- 40.Cotrufo P, Monteleone P, d’Istria M, Fuschino A, Serino I, Maj M. Aggressive behavioral characteristics and endogenous hormones in women with bulimia nervosa. Neuropsychobiology. 2000;42(2):58–61. doi: 10.1159/000026673. [DOI] [PubMed] [Google Scholar]
- 41.Cassin SE, von Ranson KM. Personality and eating disorders: a decade in review. Clin. Psychol. Rev. 2005;25(7):895–916. doi: 10.1016/j.cpr.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 42.Jahanfar S, Eden JA, Nguyent TV. Bulimia nervosa and polycystic ovary syndrome. Gynecol. Endocrinol. 1995;9(2):113–117. doi: 10.3109/09513599509160199. [DOI] [PubMed] [Google Scholar]
- 43.McSherry J. Bulimia nervosa and acne. Can. J. Psychiatry. 1992;37(10):731–732. doi: 10.1177/070674379203701015. [DOI] [PubMed] [Google Scholar]
- 44.Quinton SJ, Smith AR, Joiner T. The 2 to 4 digit ratio (2D:4D) and eating disorder diagnosis in women. Pers. Individ. Dif. 2011;51(4):402–405. doi: 10.1016/j.paid.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Culbert KM, Breedlove SM, Burt SA, Klump KL. Prenatal hormone exposure and risk for eating disorders: a comparison of opposite-sex and same-sex twins. Arch. Gen. Psychiatry. 2008;65(3):329–336. doi: 10.1001/archgenpsychiatry.2007.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baker JH, Lichtenstein P, Kendler KS. Intrauterine testosterone exposure and risk for disordered eating. Br. J. Psychiatry. 2009;194(4):375–376. doi: 10.1192/bjp.bp.108.054692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lydecker JA, Pisetsky EM, Mitchell KS, et al. Association between co-twin sex and eating disorders in opposite sex twin pairs: evaluations in North American, Norwegian, and Swedish samples. J. Psychosom. Res. 2012;72(1):73–77. doi: 10.1016/j.jpsychores.2011.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pisetsky EM. Unpublished Master’s thesis. NC, USA: University of North Carolina; 2010. Eating Disorders, Psychopathology, and Temperament in Opposite Sex and Same Sex Twins. [Google Scholar]
- 49.Culbert KM, Burt SA, Sisk CL, Nigg JT, Klump KL. The effects of puberty and circulating testosterone on differential risk for disordered eating in adolescent males; Presented at: The 2012 International Conference on Eating Disorders; 3–5 May 2012; Austin, TX, USA. [Google Scholar]
- 50.Lester NA, Keel PK, Lipson SF. Symptom fluctuation in bulimia nervosa: relation to menstrual-cycle phase and cortisol levels. Psychol. Med. 2003;33(1):51–60. doi: 10.1017/s0033291702006815. [DOI] [PubMed] [Google Scholar]
- 51.Gladis MM, Walsh BT. Premenstrual exacerbation of binge eating in bulimia. Am. J. Psychiatry. 1987;144(12):1592–1595. doi: 10.1176/ajp.144.12.1592. [DOI] [PubMed] [Google Scholar]
- 52.Price WA, Torem MS, DiMarzio LR. Premenstrual exacerbation of bulimia. Psychosomatics. 1987;28(7):378–379. doi: 10.1016/s0033-3182(87)72511-0. [DOI] [PubMed] [Google Scholar]
- 53.Jappe LM, Gardner RM. Body-image perception and dissatisfaction throughout phases of the female menstrual cycle. Percept. Mot. Skills. 2009;108(1):74–80. doi: 10.2466/PMS.108.1.74-80. [DOI] [PubMed] [Google Scholar]
- 54.Altabe M. Ethnicity and body image: quantitative and qualitative analysis. Int. J. Eat. Disord. 1998;23(2):153–159. doi: 10.1002/(sici)1098-108x(199803)23:2<153::aid-eat5>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 55.Bulik CM, Sullivan PF, Kendler KS. Heritability of binge-eating and broadly defined bulimia nervosa. Biol. Psychiatry. 1998;44(12):1210–1218. doi: 10.1016/s0006-3223(98)00280-7. [DOI] [PubMed] [Google Scholar]
- 56.Mazzeo SE, Mitchell KS, Bulik CM, Aggen SH, Kendler KS, Neale MC. A twin study of specific bulimia nervosa symptoms. Psychol. Med. 2010;40(7):1203–1213. doi: 10.1017/S003329170999122X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Keski-Rahkonen A, Bulik CM, Neale BM, Rose RJ, Rissanen A, Kaprio J. Body dissatisfaction and drive for thinness in young adult twins. Int. J. Eat. Disord. 2005;37(3):188–199. doi: 10.1002/eat.20138. [DOI] [PubMed] [Google Scholar]
- 58.Wade TD, Wilkinson J, Ben-Tovim D. The genetic epidemiology of body attitudes, the attitudinal component of body image in women. Psychol. Med. 2003;33(8):1395–1405. doi: 10.1017/s0033291703008572. [DOI] [PubMed] [Google Scholar]
- 59.Saunders KE, Hawton K. Suicidal behaviour and the menstrual cycle. Psychol. Med. 2006;36(7):901–912. doi: 10.1017/S0033291706007392. [DOI] [PubMed] [Google Scholar]
- 60.Tonks CM, Rack PH, Rose MJ. Attempted suicide and the menstrual cycle. J. Psychosom. Res. 1968;11(4):319–323. doi: 10.1016/0022-3999(68)90028-7. [DOI] [PubMed] [Google Scholar]
- 61.Glass GS, Heninger GR, Lansky M, Talan K. Psychiatric emergency related to the menstrual cycle. Am. J. Psychiatry. 1971;128(6):705–711. doi: 10.1176/ajp.128.6.705. [DOI] [PubMed] [Google Scholar]
- 62.Howards PP, Schisterman EF, Wactawski-Wende J, Reschke JE, Frazer AA, Hovey KM. Timing clinic visits to phases of the menstrual cycle by using a fertility monitor: the BioCycle Study. Am. J. Epidemiol. 2009;169(1):105–112. doi: 10.1093/aje/kwn287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hendrick V, Altshuler LL, Suri R. Hormonal changes in the postpartum and implications for postpartum depression. Psychosomatics. 1998;39(2):93–101. doi: 10.1016/S0033-3182(98)71355-6. [DOI] [PubMed] [Google Scholar]
- 64.Lemberg R, Phillips J. The impact of pregnancy on anorexia nervosa and bulimia. Int. J. Eat. Disord. 1989;8:285–295. [Google Scholar]
- 65.Lacey JH, Smith G. Bulimia nervosa. The impact of pregnancy on mother and baby. Br. J. Psychiatry. 1987;150:777–781. doi: 10.1192/bjp.150.6.777. [DOI] [PubMed] [Google Scholar]
- 66. Bulik CM, Von Holle A, Hamer R, et al. Patterns of remission, continuation and incidence of broadly defined eating disorders during early pregnancy in the Norwegian Mother and Child Cohort Study (MoBa) Psychol. Med. 2007;37(8):1109–1118. doi: 10.1017/S0033291707000724. • Exploration of change in eating disorder symptoms during pregnancy.
- 67.Crow SJ, Agras WS, Crosby R, Halmi K, Mitchell JE. Eating disorder symptoms in pregnancy: a prospective study. Int. J. Eat. Disord. 2008;41(3):277–279. doi: 10.1002/eat.20496. [DOI] [PubMed] [Google Scholar]
- 68.Koubaa S, Kouba S, Hällström T, Lindholm C, Hirschberg AL. Pregnancy and neonatal outcomes in women with eating disorders. Obstet. Gynecol. 2005;105(2):255–260. doi: 10.1097/01.AOG.0000148265.90984.c3. [DOI] [PubMed] [Google Scholar]
- 69.Micali N, Treasure J, Simonoff E. Eating disorders symptoms in pregnancy: a longitudinal study of women with recent and past eating disorders and obesity. J. Psychosom. Res. 2007;63(3):297–303. doi: 10.1016/j.jpsychores.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 70. Jacobi C, Hayward C, de Zwaan M, Kraemer HC, Agras WS. Coming to terms with risk factors for eating disorders: application of risk terminology and suggestions for a general taxonomy. Psychol. Bull. 2004;130(1):19–65. doi: 10.1037/0033-2909.130.1.19. •• Helpful summary for readers of the potential risk factors for eating disorders.
- 71.Klump KL, Keel PK, Sisk C, Burt SA. Preliminary evidence that estradiol moderates genetic influences on disordered eating attitudes and behaviors during puberty. Psychol. Med. 2010;40(10):1745–1753. doi: 10.1017/S0033291709992236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Klump KL, Suisman JL, Culbert KM, Kashy DA, Sisk CL. Binge eating proneness emerges during puberty in female rats: a longitudinal study. J. Abnorm. Psychol. 2011;120(4):948–955. doi: 10.1037/a0023600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bulik CM, Sullivan PF, Wade TD, Kendler KS. Twin studies of eating disorders: a review. Int. J. Eat. Disord. 2000;27(1):1–20. doi: 10.1002/(sici)1098-108x(200001)27:1<1::aid-eat1>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 74.Culbert KM, Burt SA, McGue M, Iacono WG, Klump KL. Puberty and the genetic diathesis of disordered eating attitudes and behaviors. J. Abnorm. Psychol. 2009;118(4):788–796. doi: 10.1037/a0017207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Klump KL, McGue M, Iacono WG. Differential heritability of eating attitudes and behaviors in prepubertal versus pubertal twins. Int. J. Eat. Disord. 2003;33(3):287–292. doi: 10.1002/eat.10151. [DOI] [PubMed] [Google Scholar]
- 76.Harden KP, Mendle J, Kretsch N. Environmental and genetic pathways between early pubertal timing and dieting in adolescence: distinguishing between objective and subjective timing. Psychol. Med. 2012;42(1):183–193. doi: 10.1017/S0033291711000961. [DOI] [PubMed] [Google Scholar]
- 77.Baker JH, Thornton LM, Bulik CM, Kendler KS, Lichtenstein P. Genetic covariation between age at menarche and disordered eating. J. Adolesc. Health. 2012;51:491–496. doi: 10.1016/j.jadohealth.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Golden NH, Lanzkowsky L, Schebendach J, Palestro CJ, Jacobson MS, Shenker IR. The effect of estrogen–progestin treatment on bone mineral density in anorexia nervosa. J. Pediatr. Adolesc. Gynecol. 2002;15(3):135–143. doi: 10.1016/s1083-3188(02)00145-6. [DOI] [PubMed] [Google Scholar]
- 79.Muñoz MT, Morandé G, García-Centenera JA, Hervás F, Pozo J, Argente J. The effects of estrogen administration on bone mineral density in adolescents with anorexia nervosa. Eur. J. Endocrinol. 2002;146(1):45–50. doi: 10.1530/eje.0.1460045. [DOI] [PubMed] [Google Scholar]
- 80.Misra M, Katzman D, Miller KK, et al. Physiologic estrogen replacement increases bone density in adolescent girls with anorexia nervosa. J. Bone Miner. Res. 2011;26(10):2430–2438. doi: 10.1002/jbmr.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Naessén S, Carlström K, Byström B, Pierre Y, Hirschberg AL. Effects of an antiandrogenic oral contraceptive on appetite and eating behavior in bulimic women. Psychoneuroendocrinology. 2007;32(5):548–554. doi: 10.1016/j.psyneuen.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 82.Bergman L, Eriksson E. Marked symptom reduction in two women with bulimia nervosa treated with the testosterone receptor antagonist flutamide. Acta Psychiatr. Scand. 1996;94(2):137–139. doi: 10.1111/j.1600-0447.1996.tb09838.x. [DOI] [PubMed] [Google Scholar]
- 83.Sundblad C, Landén M, Eriksson T, Bergman L, Eriksson E. Effects of the androgen antagonist flutamide and the serotonin reuptake inhibitor citalopram in bulimia nervosa: a placebo-controlled pilot study. J. Clin. Psychopharmacol. 2005;25(1):85–88. doi: 10.1097/01.jcp.0000150222.31007.a9. [DOI] [PubMed] [Google Scholar]
- 84.Miller KK, Grieco KA, Klibanski A. Testosterone administration in women with anorexia nervosa. J. Clin. Endocrinol. Metab. 2005;90(3):1428–1433. doi: 10.1210/jc.2004-1181. [DOI] [PubMed] [Google Scholar]
- 85. Young JK. Estrogen and the etiology of anorexia nervosa. Neurosci. Biobehav. Rev. 1991;15(3):327–331. doi: 10.1016/s0149-7634(05)80025-9. • Review paper specifically describing the possible role of estrogens in the pathophysiology of anorexia nervosa.
- 86.Pike KM, Walsh BT, Vitousek K, Wilson GT, Bauer J. Cognitive behavior therapy in the posthospitalization treatment of anorexia nervosa. Am. J. Psychiatry. 2003;160(11):2046–2049. doi: 10.1176/appi.ajp.160.11.2046. [DOI] [PubMed] [Google Scholar]
- 87. Keating C, Tilbrook A, Kulkarni J. Oestrogen: an overlooked mediator in the neuropsychopharmacology of treatment response? Int. J. Neuropsychopharmacol. 2011;14(4):553–566. doi: 10.1017/S1461145710000982. •• Review paper describing the possible role of estrogen as an adjunct to typical pharmacological treatments.
- 88.Westlund Tam L, Parry BL. Does estrogen enhance the antidepressant effects of fluoxetine? J. Affect. Disord. 2003;77(1):87–92. doi: 10.1016/s0165-0327(02)00357-9. [DOI] [PubMed] [Google Scholar]
- 89.Grigoriadis S, Kennedy SH. Role of estrogen in the treatment of depression. Am. J. Ther. 2002;9(6):503–509. doi: 10.1097/00045391-200211000-00008. [DOI] [PubMed] [Google Scholar]
- 90.Hilbert A, de Zwaan M, Braehler E. How frequent are eating disturbances in the population? Norms of the eating disorder examination-questionnaire. PLoS ONE. 2012;7(1):e29125. doi: 10.1371/journal.pone.0029125. [DOI] [PMC free article] [PubMed] [Google Scholar]
Website
- 101.American Psychiatric Association. DSM-5: the future of psychiatric diagnosis. [Accessed 8 May 2012]; www.dsm5.org/Pages/Default.aspx.