Summary
Classical lesions of diabetic nephropathy have been known for many decades, but the natural history of the disease in terms of progression of lesions as well as renal dysfunction is now understood to be more heterogeneous than initially thought. Some patients may develop significant GFR decline while still normoalbuminuric while others may be microalbuminuric and stay so or regress to normoalbuminuria without significant loss of GFR. Current models of structural functional relationships of diabetic nephropathy in patients with type 1 diabetes are mainly driven by strong correlations between morphology and renal dysfunction in the more advanced stages of the disease, while in the initial stages when the disease, and hence the lesions, are generally less severe there is significant variability among the patients precluding emergence of any robust model so far. This fact hampers our ability to predict progression of the disease early on when, in fact, lesions are more likely amenable to therapy. Incorporation of parameters other than known renal structural and routine functional measures, e.g. genetics or epigenetics and new biomarkers may be especially helpful to better understand and predict the natural history and progression of diabetic nephropathy in patients with type 1 diabetes.
Introduction
Despite improved control of hyperglycemia and hypertension in the last several decades, type 1 diabetes results in over 23,000 end stage kidney disease (ESRD) cases per year in the US [1], calling for a need for more efficient monitoring and treatment of kidney complications in these patients. This goal cannot be met without understanding the natural history of the disease. Herein, we provide an integrated overview of evolution of diabetic nephropathy lesions in patients with type 1 diabetes in relation to diabetes duration and renal function to provide a more informative perspective about the natural history of the disease. Although increased urinary albumin excretion rate (AER) is an important diabetic nephropathy biomarker and concomitant, in the end, it is the loss of glomerular filtration rate (GFR) which leads to ESRD and death and, thus, GFR loss in patients with type 1 diabetes is the primary focus of this review.
Accumulation of Extracellular Matrix, the Mainstay of Diabetic Nephropathy Lesions
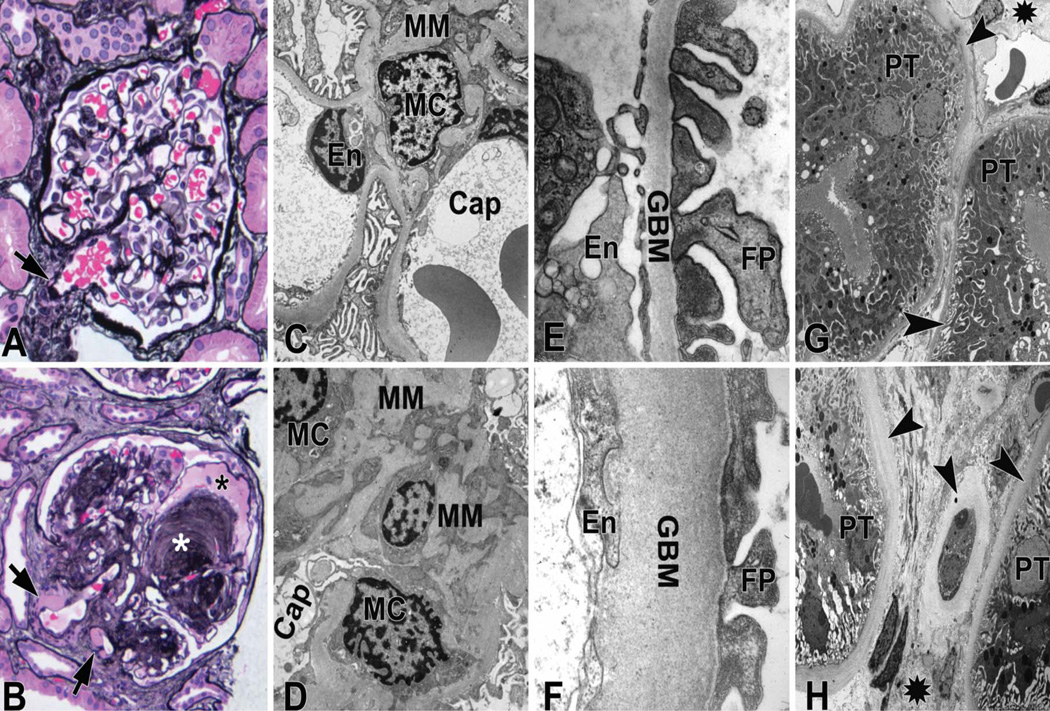
The essence of diabetic nephropathy pathology is the accumulation of basement membrane extracellular matrix (ECM) material manifesting as glomerular and tubular basement membrane thickening and increased mesangial matrix (Figure 1). The first quantifiable structural change of diabetic nephropathy is glomerular basement membrane thickening [2], which is very closely paralleled by thickening of tubular basement membranes[3, 4]. This parallelism represents a strong argument against increased glomerular capillary pressure and flow as a major cause of diabetic glomerulopathy changes and favors a primarily metabolic pathogenesis. Different from glomerular basement membrane thickening which is an early finding, increased fraction of the volume of the glomerulus occupied by the mesangium [Vv(Mes/glom)] is often only measurable 15 or more years of type 1 diabetes [5]; although it is sometimes able to be detected after only 4 to 5 years of type 1 diabetes[2]. Mesangial expansion in type 1 diabetes is primarily due to absolute and relative increases in mesangial matrix, with lesser contribution from increases in the fraction of the glomerulus occupied by mesangial cells[6]. Thus, even in cases with Vv(Mes/glom) within the normal range, the fraction of mesangium which is matrix is usually increased compared to the cellular component.
Figure 1.
Mesangial expansion can be diffuse, i.e., more or less uniform within glomeruli or may be nodular (Kimmelstiel-Wilson nodules), characterized by round areas of marked mesangial expansion with palisading of mesangial nuclei around the periphery of the nodule often with extreme compression of the adjacent glomerular capillaries (Figure 1). About half of proteinuric type 1 diabetic patients have at least a few glomeruli with nodular lesions. Although, usually nodular mesangial expansion is a late finding and is concomitant with moderate to severe diffuse mesangial expansion, occasionally these nodules are found in biopsies with only mild diffuse mesangial expansion, suggesting that these two forms of diabetic mesangial change may, at least in part, have different pathogeneses. It is likely that mesangiolysis secondary to focal detachment of capillary wall from mesangial anchoring points to the GBM resulting in glomerular microaneurysms (Figure 1) is a precursor to these Kimmelsteil-Wilson nodules[7]. The various lesions of diabetic nephropathy may evolve and progress at differing rates within and between type 1 diabetic patients[8, 9]. Thus, while GBM thickening advances, more or less, in a linear fashion in relation to diabetes duration, the relationship of mesangial expansion to diabetes duration is nonlinear, with slow development earlier and more rapid development later in the disease[5]. Irrespective of diabetes duration, GBM width and Vv(Mes/glom) are not very highly correlated with one another either; some patients have relatively marked GBM thickening without much mesangial expansion or vice versa[8]. However, prominent GBM thickening and mesangial expansion are typically both present in most patients with type 1 diabetes and overt diabetic nephropathy[8, 9]. Among the various lesions of diabetic nephropathy, and in a wide range of renal function from normoalbuminuria to overt proteinuria, mesangial expansion is the strongest correlate with renal dysfunction in patients with type 1 diabetes [9]. Mesangial expansion correlates inversely with glomerular filtration surface density [Sv(PGBM/glom)][8], while glomerular filtration surface per glomerulus, as perhaps expected, strongly and directly correlates with GFR from hyperfiltration to renal insufficiency in patients with type 1 diabetes [10, 11]. Vv(Mes/glom) is also a strong concomitant of hypertension[8]. GBM width similarly, though weaker than Vv(Mes/glom), correlates directly with blood pressure and AER and inversely with GFR8, 9]. In linear analyses, Vv(Mes/glom) and GBM width, together, explain about 60% of AER variability in patients with type 1 diabetes with AER ranging from normoalbuminuria to proteinuria[9].
Glomerular enlargement often parallels mesangial expansion in patients with type 1 diabetes[12]. In other words, in marked contrast to animal models of diabetic nephropathy, glomerular enlargement is a relatively late finding in humans with type 1 diabetes. Bilous, et al. showed that mean glomerular volumes were greater in patients developing diabetic nephropathy after 25 years of type 1 diabetes compared to a group that developed nephropathy after only 15 years[13], suggesting that glomerular enlargement may be a compensatory phenomenon to mesangial expansion to preserve glomerular filtration surface. Glomerular volume is thought to be controlled by nephron endowment as well and can vary markedly among normal individuals and among patients with diabetes[14]. Thus, fewer glomeruli per kidney could be a risk factor for diabetic nephropathy[15]. However, studies of type 1 diabetic transplant recipients indicate that having a single kidney does not result in accelerated lesion development compared to having two kidneys[16]. Diabetic patients with very advanced renal failure have reduced numbers of glomeruli but this likely results from resorption of sclerotic glomeruli[14]. If reduced glomerular number were a risk factor, it would be predicted that type 1 diabetic patients with overt proteinuria but less severe GFR loss would have fewer glomeruli than normal, but this was not the case[14].
Podocyte Injury, Segmental Glomerulosclerosis, Glomerulotubular Junction Abnormalities, and Nephron Loss
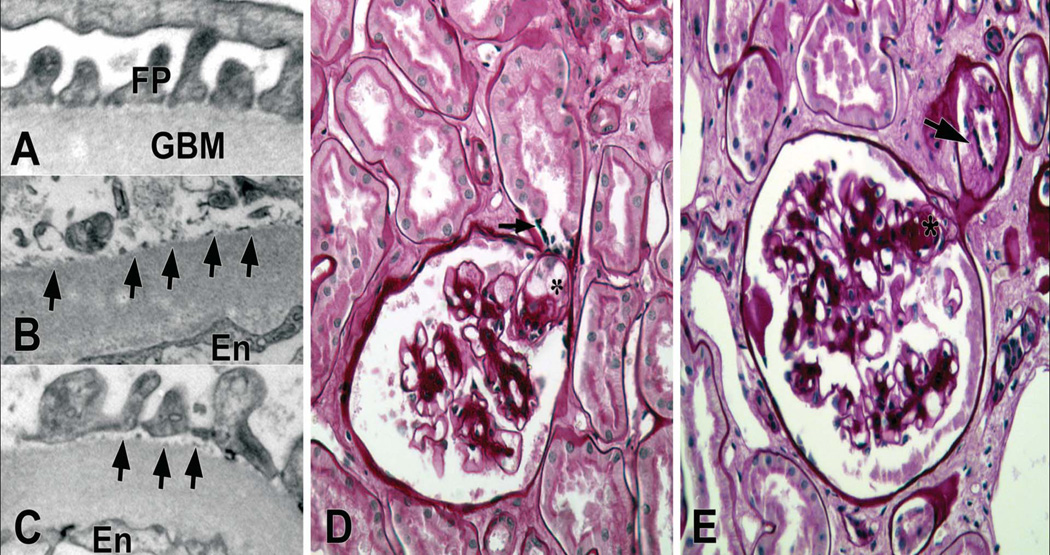
Foot process widening, a morphological counterpart of podocyte injury is detectable in normoalbuminuric type 1 diabetic patients[17]. Concordant with this morphologic finding, nephrinuria can be detected in 30% of such patients, suggesting early damage to the slit diaphragm [18]. Detachment of podocytes from glomerular basement membrane, reflecting more severe injury to these cells is also detectable in some normoalbuminuric type 1 diabetic patients, and progressively increases in microalbuminuric and proteinuric patients[19] (Figure 2). Detached podocytes are retrievable in the urine and increased urinary podocytes has been found in patients with type 2 diabetes[20]. Once lost, podocytes are difficult to replace and, if sufficiently severe, podocyte loss will lead to segmental and, ultimately, to global glomerulosclerosis. White et al. found decreased number density (number per volume) of podocytes per glomerulus in patients with diabetes [21], but this was due to increased glomerular volume rather than to decreased number of podocytes per glomerulus. These investigators showed that podocyte number density per glomerulus correlated inversely with AER in proteinuric patients[21]. Moreover, podocyte number per glomerulus was stable compared to baseline in 3-year follow up biopsies in these patients with type 1 diabetes[21]. Given that increased glomerular volume is not an early finding in diabetic nephropathy[13], reduced number density of podocytes per glomerulus due to increased glomerular volume cannot fully account for early podocyte injury as evidenced by foot process effacement, in patients with type 1 diabetes. These findings suggest that podocyte injury may start early in the process of diabetic nephropathy, and thus, may play a role in progression of the disease. However, another study using a methodology different from White et al. showed reduction in both podocyte number and number density in glomeruli in patients with type 1 diabetes[22].
Figure 2.
Podocyte detachment and loss sets the stage for adhesion of the glomerular tuft to Bowman’s capsule and segmental glomerulosclerosis, a phenomenon which is almost entirely restricted to the proteinuric stages of nephropathy in patients with type 1 diabetes. Also, there is a marked predilection for segmental glomerulosclerosis to occur at or near glomerular tubular take off, the so-called “tip lesion” [Najafian B, Mauer M, unpublished data] (Figure 2). Although a combination of podocyte injury, perhaps enhanced at the tubular pole of the glomerular tuft due to increased sheer stress[23], and tubular epithelial cell injury secondary to proteinuria has been proposed as potential mechanisms for emergence of these lesions, it is not clear why this phenomenon is so common in patients with type 1 diabetes and proteinuria[24]. Nevertheless, segmental glomerulosclerosis (secondary type) either at the glomerulotubular junction or other regions of the glomerulus, a relatively late finding in diabetic nephropathy, can eventually lead to nephron loss either through progression to obstructed or atubular glomeruli (Figure 3) or to globally sclerosed glomeruli, and in this way, ultimately contributing to the progression of GFR loss[25].
Figure 3.
Interstitial Fibrosis, Tubular Atrophy and Vascular Lesions of Diabetic Nephropathy
Progressive interstitial expansion due to fibrosis is often closely linked to GFR decline in chronic kidney disease. In fact, some have argued that renal dysfunction in diabetes is primarily consequent to interstitial rather than glomerular lesions[26, 27]. However, the conclusion that the interstitium is more closely related to renal dysfunction in diabetes than glomerular changes has derived from studies where most, if not all, patients already have serum creatinine values above 2.0mg/dl and where the interstitial expansion is carefully quantitated by point counting but the glomerular structures are only subjectively scored[26, 27]. In fact, during most of the natural history of diabetic nephropathy, glomerular parameters are far more important determinants of renal dysfunction, whereas interstitial changes in patients with type 1 diabetes may become a stronger determinant of the rate of progression from established renal insufficiency to terminal uremia[28]. Over a wide range of renal function, from hyperfiltration to a GFR ≥45ml/min/1.73m2, the major portion of the variability observed in AER and GFR in patients with type 1 diabetes is best explained by glomerular lesions alone (see below). In fact, in the first decade of diabetes, fraction of the cortex which is interstitium [Vv(Int/cortex)] is decreased, perhaps secondary to tubular hypertrophy in these enlarged kidneys[29], while Vv(Mes/glom) and GBM width are already increased. Moreover, early interstitial expansion in type 1 diabetes is mainly due to expansion of its cellular component, while increased interstitial fibrillar collagen becomes apparent in patients whose GFR, on average, is already reduced. Thus, while the early changes in glomeruli in patients with type 1 diabetes are largely consequent to GBM and mesangial ECM accumulation, early interstitial expansion is primarily cellular. These and other findings suggest that the interstitial and glomerular changes in type 1 diabetes have somewhat different pathogenetic mechanisms or different local tissue responses to similar drivers of injury, and that progressive interstitial fibrosis generally follows the establishment of relatively advanced glomerular lesions in type 1 diabetes.
Exudative lesions of diabetic nephropathy include arteriolar hyalinosis, glomerular capillary subendothelial hyaline accumulation (hyaline caps) and capsular drops (hyaline material between the parietal epithelium and Bowman’s capsule); the more advanced lesions of arteriolar hyalinosis, where at least 75% of the arteriolar wall smooth muscle is replaced by hyaline material, correlates with global glomerulosclerosis[30]. Hyalinosis of glomerular afferent and efferent arterioles, virtually diagnostic of diabetic lesions, can be seen as early as 3 to 5 years following the onset of type 1 diabetes[31]. In addition to hyalinosis, the fraction of afferent and efferent arteriole walls occupied by ECM increases[32].
The importance of vascular lesions in nephron loss in diabetic nephropathy may be underappreciated due to biopsy sampling limitations in studying vascular lesions. Although, the sequence of glomerular events described above plays major role in nephron loss in diabetic nephropathy, study of nephrectomy specimens in patients with type 1 diabetes and advanced renal insufficiency showed that globally sclerosed glomeruli, more often than by chance, occur in clusters oriented in planes vertical to the renal capsule, this suggesting a vaso-occlusive pathophysiology in medium sized (interlobular) renal arteries[33].
Structural-Functional Relationships of Diabetic Nephropathy
Although through much of the natural history of diabetic nephropathy lesions develop in complete clinical silence, when microalbuminuria and, especially, proteinuria initially manifest lesions are often far advanced and loss of GFR may then progress relatively rapidly toward end-stage renal disease. This typical clinical story is best mirrored by non-linear analyses of structural-functional relationships[25]. Using piecewise regression models in a relatively small number of patients with type 1 diabetes, glomerular structural variables alone [Vv(Mes/glom), GBM width, and total filtration surface per glomerulus or TFS] explained 95% of variability in AER ranging from normoalbuminuria to proteinuria, thus leaving little room for improvement by adding non-glomerular structural variables to this model. These same glomerular structures, however, explained only 78% of GFR variability, while addition of indices of glomerulotubular junction abnormalities and Vv(Int/cortex) increased this to 92%[25]. These smaller number patients could lead to overfitting of these structure-function models. However, evaluation of glomerular structural-functional relationships in a much larger cohort of patients with type 1 diabetes with a wide range of AER yielded similar, albeit with somewhat less strong, overall correlations (unpublished data). Importantly, the breakpoints of piecewise-linear regression analyses, where a steep change in the slope of relationships between glomerular structural parameters and renal function occurs, were in the microalbuminuric (52 µg/min) and normo-GFR (110 ml/min) ranges, indicating that significant glomerular lesions are already in place while patients are microalbuminuric and GFR is preserved, and arguing against the sensitivity of these two biomarkers to monitor early progression of diabetic nephropathy in patients with type 1 diabetes. In other words, these studies suggest that an ideal biomarker of the disease progression should be a reliable predictor when measured in pre-breakpoint zone. It is also noteworthy that the GFR breakpoint was well within the normal range. At least 75% of patients hyperfilter (GFR >135 ml/min/1.73m2) in the earlier years of type 1 diabetes. The above findings argue that the decline of GFR from hyperfiltration to the normal range (i.e., GFR <110 ml/min/1.73m2) may, in fact, reflect progression of underlying diabetic nephropathy lesions. It is also important to note that these findings were in type 1 diabetic patients with normal to increased GFR. GFR estimating equations in the ranges from hyperfiltration to about 90 ml/min/1.73m2 are probably far too imprecise for these structural-functional relationships to emerge.
As discussed above, the relationships between renal structural parameters and renal function in patients with type 1 diabetes are largely driven by more advanced lesions associated with increased slope of progression after these breakpoints. In fact, renal structural parameters are highly variable (from virtually none to moderate severity) in patients without apparent renal functional abnormalities. Thus, it is possible that the observation of two distinctly different slopes of structural-functional relationships is because the intrinsic inter-individual variability in renal structure (e.g., glomerular number), and perhaps, less so, in renal function, masks these relationships in early stages of diabetic nephropathy.
Not only does the pace of progression of increasing AER and decreasing GFR vary among patients with type 1 diabetes but , it also appears that they often do not follow a similar pattern of relationships between renal structural and function. For example, some normoalbuminuric patients with long-standing type 1 diabetes (particularly females with retinopathy and/or hypertension), have reduced measured GFR defined as <90 ml/min/1.73m2, and this is associated with worse diabetic glomerulopathy lesions than in patients with similar duration and normal to increased GFR, suggesting that careful GFR measurements may be indicated in normoalbuminuric patients, especially females with hypertension and retinopathy[34, 35]. Although the large majority of subjects in these studies were not receiving drugs which block the renin angiotensin system (RASB), this potentially confusing constellation of clinical and laboratory findings is likely to be even more prevalent in patients on such drugs as they may lower AER and, in fact, may even mask AER progression [36].
Persistent microalbuminuria is a predictor of the development of clinical nephropathy, whereas the absence of microalbuminuria in patients with long-standing type 1 diabetes indicates a lower nephropathy risk. Normoalbuminuric type 1 diabetic patients with greater GBM width are more likely to progress to microalbuminuria[37]. However, merely based on presence or absence of microalbuminuria alone, one could not reliably predict progression to overt diabetic nephropathy. The older paradigm that the vast majority of patients with type 1 diabetes and microalbuminuria are doomed to progress to proteinuria and end stage kidney disease may not be true, since AER in about 60% of microalbuminuric patients may regress to the normoalbuminuric range or remain persistent with no progression over a long time[38]. Whether this phenomenon is associated with a halt on progression or reversal of diabetic nephropathy lesions is a very important question which remains to be answered. While reversal of diabetic nephropathy lesions has been documented after a long period (10 years) of normoglycemia following pancreas transplantation[39, 40], currently there is no evidence of reversal of lesions in presence of a persistent diabetic milieu.
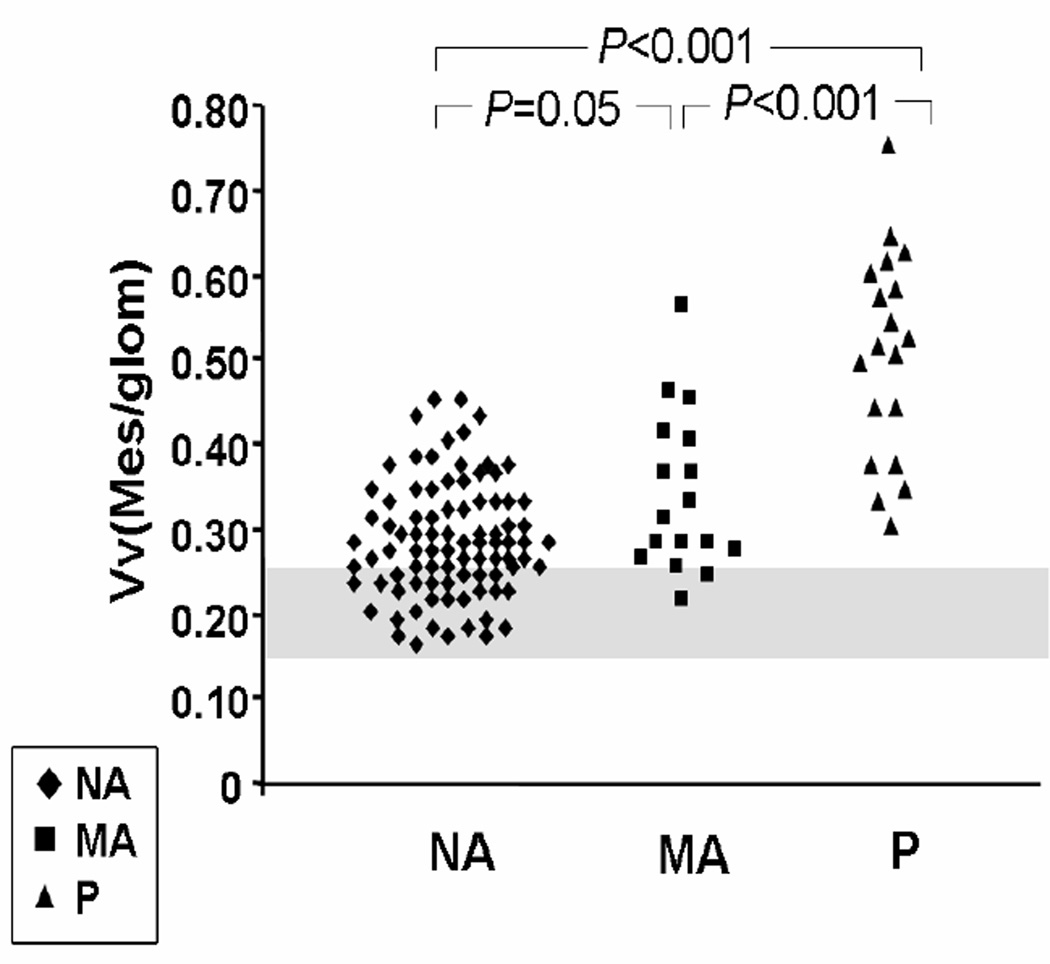
As outlined above, structural-functional relationships of diabetic nephropathy in normoalbuminuric patients are often not strong enough to provide robust models with reasonable predictive value for progression of the disease. Over 30% of normoalbuminuric patients with longstanding type 1 diabetes have GBM width and Vv(Mes/glom) overlapping with non-diabetic control subjects[9] (Figure 4). This may, at least in part, be partially explained by differences in baseline values within the normal range at the onset of diabetes. In a study of identical twin pairs discordant for type 1 diabetes[3], in every pair studied, the diabetic twin had higher values for GBM and TBM width and Vv(Mes/glom) than the non diabetic twin. Several diabetic twins had values for GBM width and Vv(Mes/glom) that were within the range of normal and had “lesions” only in comparison with their no diabetic twin[3].
Figure 4.
Supplementary Material
Acknowledgements
We thank Ms. Patricia L. Erickson for help in preparation of this manuscript. Work reported here was, in part, supported by NIH (DK013083) and grants previously awarded by NIH and JDFR. We especially thank the patients who volunteered for the renal biopsy studies that emanated from the University of Minnesota and the many research fellows who performed much of this work.
References
- 1.USRDS annual data report. Volume 2. Atlas ESRD. Incidence, prevalence, patients characteristics, and modalities. 2011 [Google Scholar]
- 2.osterby R. Early phases in the development of diabetic glomerulopathy. Acta Med Scand Suppl. 1974;574:3–82. [PubMed] [Google Scholar]
- 3.Steffes MW, et al. Studies of kidney and muscle biopsy specimens from identical twins discordant for type I diabetes mellitus. N Engl J Med. 1985;312(20):1282–7. doi: 10.1056/NEJM198505163122003. [DOI] [PubMed] [Google Scholar]
- 4.Brito PL, et al. Proximal tubular basement membrane width in insulin-dependent diabetes mellitus. Kidney Int. 1998;53(3):754–61. doi: 10.1046/j.1523-1755.1998.00809.x. [DOI] [PubMed] [Google Scholar]
- 5.Steffes MW, et al. Cell and matrix components of the glomerular mesangium in type I diabetes. Diabetes. 1992;41(6):679–684. doi: 10.2337/diab.41.6.679. [DOI] [PubMed] [Google Scholar]
- 6.Drummond K, Mauer M. The early natural history of nephropathy in type 1 diabetes: II. Early renal structural changes in type 1 diabetes. Diabetes. 2002;51(5):1580–1587. doi: 10.2337/diabetes.51.5.1580. [DOI] [PubMed] [Google Scholar]
- 7.Saito Y, et al. Mesangiolysis in diabetic glomeruli: its role in the formation of nodular lesions. Kidney Int. 1988;34(3):389–96. doi: 10.1038/ki.1988.193. [DOI] [PubMed] [Google Scholar]
- 8.Mauer SM, et al. Structural-functional relationships in diabetic nephropathy. J Clin Invest. 1984;74(4):1143–55. doi: 10.1172/JCI111523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caramori ML, et al. Cellular basis of diabetic nephropathy: 1. Study design and renal structural-functional relationships in patients with long-standing type 1 diabetes. Diabetes. 2002;51(2):506–513. doi: 10.2337/diabetes.51.2.506. [DOI] [PubMed] [Google Scholar]
- 10.Ellis EN, et al. Glomerular filtration surface in type I diabetes mellitus. Kidney Int. 1986;29(4):889–894. doi: 10.1038/ki.1986.82. [DOI] [PubMed] [Google Scholar]
- 11.Hirose K, et al. A strong correlation between glomerular filtration rate and filtration surface in diabetic kidney hyperfunction. Lab Invest. 1980;43(5):434–437. [PubMed] [Google Scholar]
- 12.Osterby R, et al. Glomerular volume and the glomerular vascular pole area in patients with insulin-dependent diabetes mellitus. Virchows Arch. 1997;431(5):351–357. doi: 10.1007/s004280050110. [DOI] [PubMed] [Google Scholar]
- 13.Bilous RW, et al. Mean glomerular volume and rate of development of diabetic nephropathy. Diabetes. 1989;38(9):1142–1147. doi: 10.2337/diab.38.9.1142. [DOI] [PubMed] [Google Scholar]
- 14.Bendtsen TF, Nyengaard JR. The number of glomeruli in type 1 (insulin-dependent) and type 2 (non-insulin-dependent) diabetic patients. Diabetologia. 1992;35(9):844–850. doi: 10.1007/BF00399930. [DOI] [PubMed] [Google Scholar]
- 15.Brenner BM, Garcia DL, Anderson S. Glomeruli and blood pressure. Less of one, more the other? Am J Hypertens. 1988;1(4 Pt 1):335–347. doi: 10.1093/ajh/1.4.335. [DOI] [PubMed] [Google Scholar]
- 16.Chang S, et al. Having one kidney does not accelerate the rate of development of diabetic nephropathy lesions in type 1 diabetic patients. Diabetes. 2008;57(6):1707–11. doi: 10.2337/db07-1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perrin NE, et al. The course of diabetic glomerulopathy in patients with type I diabetes: a 6-year follow-up with serial biopsies. Kidney Int. 2006;69(4):699–705. doi: 10.1038/sj.ki.5000146. [DOI] [PubMed] [Google Scholar]
- 18.Patari A, et al. Nephrinuria in diabetic nephropathy of type 1 diabetes. Diabetes. 2003;52(12):2969–2974. doi: 10.2337/diabetes.52.12.2969. [DOI] [PubMed] [Google Scholar]
- 19.Toyoda M, et al. Podocyte detachment and reduced glomerular capillary endothelial fenestration in human type 1 diabetic nephropathy. Diabetes. 2007;56(8):2155–2160. doi: 10.2337/db07-0019. [DOI] [PubMed] [Google Scholar]
- 20.Nakamura T, et al. Urinary excretion of podocytes in patients with diabetic nephropathy. Nephrol Dial Transplant. 2000;15(9):1379–1383. doi: 10.1093/ndt/15.9.1379. [DOI] [PubMed] [Google Scholar]
- 21.White KE, et al. Podocyte number in normotensive type 1 diabetic patients with albuminuria. Diabetes. 2002;51(10):3083–3089. doi: 10.2337/diabetes.51.10.3083. [DOI] [PubMed] [Google Scholar]
- 22.Steffes MW, et al. Glomerular cell number in normal subjects and in type 1 diabetic patients. Kidney Int. 2001;59(6):2104–2113. doi: 10.1046/j.1523-1755.2001.00725.x. [DOI] [PubMed] [Google Scholar]
- 23.Friedrich C, et al. Podocytes are sensitive to fluid shear stress in vitro. Am J Physiol Renal Physiol. 2006;291(4):F856–F865. doi: 10.1152/ajprenal.00196.2005. [DOI] [PubMed] [Google Scholar]
- 24.Najafian B, et al. Atubular glomeruli and glomerulotubular junction abnormalities in diabetic nephropathy. J Am Soc Nephrol. 2003;14(4):908–917. doi: 10.1097/01.asn.0000057854.32413.81. [DOI] [PubMed] [Google Scholar]
- 25.Najafian B, et al. Glomerulotubular junction abnormalities are associated with proteinuria in type 1 diabetes. J Am Soc Nephrol. 2006;17(4 Suppl 2):S53–S60. doi: 10.1681/ASN.2005121342. [DOI] [PubMed] [Google Scholar]
- 26.Thomsen OF, et al. Renal changes in long-term type 1 (insulin-dependent) diabetic patients with and without clinical nephropathy: a light microscopic, morphometric study of autopsy material. Diabetologia. 1984;26(5):361–365. doi: 10.1007/BF00266037. [DOI] [PubMed] [Google Scholar]
- 27.Bohle A, et al. The pathogenesis of chronic renal failure in diabetic nephropathy. Investigation of 488 cases of diabetic glomerulosclerosis. Pathol Res Pract. 1991;187(2–3):251–259. doi: 10.1016/s0344-0338(11)80780-6. [DOI] [PubMed] [Google Scholar]
- 28.Taft JL, et al. Clinical and histological correlations of decline in renal function in diabetic patients with proteinuria. Diabetes. 1994;43(8):1046–1051. doi: 10.2337/diab.43.8.1046. [DOI] [PubMed] [Google Scholar]
- 29.Katz A, et al. An increase in the cell component of the cortical interstitium antedates interstitial fibrosis in type 1 diabetic patients. Kidney Int. 2002;61(6):2058–2066. doi: 10.1046/j.1523-1755.2002.00370.x. [DOI] [PubMed] [Google Scholar]
- 30.Harris RD, et al. Global glomerular sclerosis and glomerular arteriolar hyalinosis in insulin dependent diabetes. Kidney Int. 1991;40(1):107–114. doi: 10.1038/ki.1991.187. [DOI] [PubMed] [Google Scholar]
- 31.Mauer SM, et al. Development of diabetic vascular lesions in normal kidneys transplanted into patients with diabetes mellitus. N Engl J Med. 1976;295(17):916–920. doi: 10.1056/NEJM197610212951703. [DOI] [PubMed] [Google Scholar]
- 32.Drummond KN, et al. Effects of duration and age at onset of type 1 diabetes on preclinical manifestations of nephropathy. Diabetes. 2003;52(7):1818–1824. doi: 10.2337/diabetes.52.7.1818. [DOI] [PubMed] [Google Scholar]
- 33.Horlyck A, Gundersen HJ, Osterby R. The cortical distribution pattern of diabetic glomerulopathy. Diabetologia. 1986;29(3):146–150. doi: 10.1007/BF02427084. [DOI] [PubMed] [Google Scholar]
- 34.Caramori ML, Fioretto P, Mauer M. Low glomerular filtration rate in normoalbuminuric type 1 diabetic patients: an indicator of more advanced glomerular lesions. Diabetes. 2003;52(4):1036–1040. doi: 10.2337/diabetes.52.4.1036. [DOI] [PubMed] [Google Scholar]
- 35.Caramori ML, Fioretto P, Mauer M. Enhancing the predictive value of urinary albumin for diabetic nephropathy. J Am Soc Nephrol. 2006;17(2):339–352. doi: 10.1681/ASN.2005101075. [DOI] [PubMed] [Google Scholar]
- 36.Mathiesen ER, et al. Efficacy of captopril in postponing nephropathy in normotensive insulin dependent diabetic patients with microalbuminuria. BMJ. 1991;303(6794):81–87. doi: 10.1136/bmj.303.6794.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steinke JM, et al. The early natural history of nephropathy in Type 1 Diabetes: III. Predictors of 5-year urinary albumin excretion rate patterns in initially normoalbuminuric patients. Diabetes. 2005;54(7):2164–2171. doi: 10.2337/diabetes.54.7.2164. [DOI] [PubMed] [Google Scholar]
- 38.Perkins BA, et al. Regression of microalbuminuria in type 1 diabetes. N Engl J Med. 2003;348(23):2285–2293. doi: 10.1056/NEJMoa021835. [DOI] [PubMed] [Google Scholar]
- 39.Fioretto P, et al. Reversal of lesions of diabetic nephropathy after pancreas transplantation. N Engl J Med. 1998;339(2):69–75. doi: 10.1056/NEJM199807093390202. [DOI] [PubMed] [Google Scholar]
- 40.Fioretto P, et al. Remodeling of renal interstitial and tubular lesions in pancreas transplant recipients. Kidney Int. 2006;69(5):907–912. doi: 10.1038/sj.ki.5000153. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.