Abstract
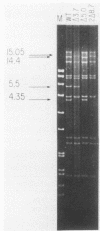
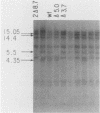
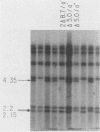
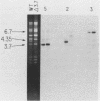
Saccharomyces cerevisiae mitochondrial DNA deletion mutants have been used to examine whether base-biased intergenic regions of the genome influence mitochondrial biogenesis. One strain (delta 5.0) lacks a 5-kilobase (kb) segment extending from the proline tRNA gene to the small rRNA gene that includes ori1, while a second strain (delta 3.7) is missing a 3.7-kb region between the genes for ATPase subunit 6 and glutamic acid tRNA that encompasses ori7 plus ori2. Growth of these strains on both fermentable and nonfermentable substrates does not differ from growth of the wild-type strain, indicating that the deletable regions of the genome do not play a direct role in the expression of mitochondrial genes. Examination of whether the 5- or 3.7-kb regions influence mitochondrial DNA transmission was undertaken by crossing strains and examining mitochondrial genotypes in zygotic colonies. In a cross between strain delta 5.0, harboring three active ori elements (ori2, ori3, and ori5), and strain delta 3.7, containing only two active ori elements (ori3 and ori5), there is a preferential recovery of the genome containing two active ori elements (37% of progeny) over that containing three active elements (20%). This unexpected result, suggesting that active ori elements do not influence transmission of respiratory-competent genomes, is interpreted to reflect a preferential conversion of the delta 5.0 genome to the wild type (41% of progeny). Supporting evidence for conversion over biased transmission is shown by preferential recovery of a nonparental genome in the progeny of a heterozygous cross in which both parental molecules can be identified by size polymorphisms.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldacci G., Chérif-Zahar B., Bernardi G. The initiation of DNA replication in the mitochondrial genome of yeast. EMBO J. 1984 Sep;3(9):2115–2120. doi: 10.1002/j.1460-2075.1984.tb02099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanc H., Dujon B. Replicator regions of the yeast mitochondrial DNA responsible for suppressiveness. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3942–3946. doi: 10.1073/pnas.77.7.3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark-Walker G. D. In vivo rearrangement of mitochondrial DNA in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8847–8851. doi: 10.1073/pnas.86.22.8847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark-Walker G. D., McArthur C. R., Sriprakash K. S. Partial duplication of the large ribosomal RNA sequence in an inverted repeat in circular mitochondrial DNA from Kloeckera africana. Implications for mechanisms of the petite mutation. J Mol Biol. 1981 Apr 15;147(3):399–415. doi: 10.1016/0022-2836(81)90492-7. [DOI] [PubMed] [Google Scholar]
- Colleaux L., D'Auriol L., Galibert F., Dujon B. Recognition and cleavage site of the intron-encoded omega transposase. Proc Natl Acad Sci U S A. 1988 Aug;85(16):6022–6026. doi: 10.1073/pnas.85.16.6022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieckmann C. L., Gandy B. Preferential recombination between GC clusters in yeast mitochondrial DNA. EMBO J. 1987 Dec 20;6(13):4197–4203. doi: 10.1002/j.1460-2075.1987.tb02767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ephrussi B., de Margerie-Hottinguer H., Roman H. SUPPRESSIVENESS: A NEW FACTOR IN THE GENETIC DETERMINISM OF THE SYNTHESIS OF RESPIRATORY ENZYMES IN YEAST. Proc Natl Acad Sci U S A. 1955 Dec 15;41(12):1065–1071. doi: 10.1073/pnas.41.12.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. J., Clark-Walker G. D. Elevated levels of petite formation in strains of Saccharomyces cerevisiae restored to respiratory competence. II. Organization of mitochondrial genomes in strains having high and moderate frequencies of petite mutant formation. Genetics. 1985 Nov;111(3):403–432. doi: 10.1093/genetics/111.3.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. J., Oakley K. M., Clark-Walker G. D. Elevated levels of petite formation in strains of Saccharomyces cerevisiae restored to respiratory competence. I. Association of both high and moderate frequencies of petite mutant formation with the presence of aberrant mitochondrial DNA. Genetics. 1985 Nov;111(3):389–402. doi: 10.1093/genetics/111.3.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faugeron-Fonty G., Goyon C. Polymorphic variations in the ori sequences from the mitochondrial genomes of different wild-type yeast strains. Curr Genet. 1985;10(4):269–282. doi: 10.1007/BF00365623. [DOI] [PubMed] [Google Scholar]
- Faugeron-Fonty G., Le Van Kim C., de Zamaroczy M., Goursot R., Bernardi G. A comparative study of the ori sequences from the mitochondrial genomes of twenty wild-type yeast strains. Gene. 1984 Dec;32(3):459–473. doi: 10.1016/0378-1119(84)90020-9. [DOI] [PubMed] [Google Scholar]
- Gaillard C., Strauss F., Bernardi G. Excision sequences in the mitochondrial genome of yeast. Nature. 1980 Jan 10;283(5743):218–220. doi: 10.1038/283218a0. [DOI] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Macino G., Tzagoloff A. Assembly of the mitochondrial membrane system: sequence analysis of a yeast mitochondrial ATPase gene containing the oli-2 and oli-4 loci. Cell. 1980 Jun;20(2):507–517. doi: 10.1016/0092-8674(80)90637-6. [DOI] [PubMed] [Google Scholar]
- Maxwell R. J., Devenish R. J., Nagley P. The nucleotide sequence of the mitochondrial DNA genome of an abundant petite mutant of Saccharomyces cerevisiae carrying the ori1 replication origin. Biochem Int. 1986 Jul;13(1):101–107. [PubMed] [Google Scholar]
- McArthur A. J., Monteith J. L. Air movement and heat loss from sheep. II. Thermal insulation of fleece in wind. Proc R Soc Lond B Biol Sci. 1980 Aug 13;209(1175):209–217. doi: 10.1098/rspb.1980.0091. [DOI] [PubMed] [Google Scholar]
- McClelland M., Hanish J., Nelson M., Patel Y. KGB: a single buffer for all restriction endonucleases. Nucleic Acids Res. 1988 Jan 11;16(1):364–364. doi: 10.1093/nar/16.1.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piskur J. A 5 kb intergenic region containing ori1 in the mitochondrial DNA of Saccharomyces cerevisiae is dispensable for expression of the respiratory phenotype. FEBS Lett. 1988 Feb 29;229(1):145–149. doi: 10.1016/0014-5793(88)80815-9. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strausberg R. L., Butow R. A. Gene conversion at the var1 locus on yeast mitochondrial DNA. Proc Natl Acad Sci U S A. 1981 Jan;78(1):494–498. doi: 10.1073/pnas.78.1.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Séraphin B., Simon M., Faye G. The mitochondrial reading frame RF3 is a functional gene in Saccharomyces uvarum. J Biol Chem. 1987 Jul 25;262(21):10146–10153. [PubMed] [Google Scholar]
- Weiller G., Schueller C. M., Schweyen R. J. Putative target sites for mobile G + C rich clusters in yeast mitochondrial DNA: single elements and tandem arrays. Mol Gen Genet. 1989 Aug;218(2):272–283. doi: 10.1007/BF00331278. [DOI] [PubMed] [Google Scholar]
- Wenzlau J. M., Saldanha R. J., Butow R. A., Perlman P. S. A latent intron-encoded maturase is also an endonuclease needed for intron mobility. Cell. 1989 Feb 10;56(3):421–430. doi: 10.1016/0092-8674(89)90245-6. [DOI] [PubMed] [Google Scholar]
- Zinn A. R., Butow R. A. Nonreciprocal exchange between alleles of the yeast mitochondrial 21S rRNA gene: kinetics and the involvement of a double-strand break. Cell. 1985 Apr;40(4):887–895. doi: 10.1016/0092-8674(85)90348-4. [DOI] [PubMed] [Google Scholar]
- Zinn A. R., Pohlman J. K., Perlman P. S., Butow R. A. In vivo double-strand breaks occur at recombinogenic G + C-rich sequences in the yeast mitochondrial genome. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2686–2690. doi: 10.1073/pnas.85.8.2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Zamaroczy M., Bernardi G. Sequence organization of the mitochondrial genome of yeast--a review. Gene. 1985;37(1-3):1–17. doi: 10.1016/0378-1119(85)90252-5. [DOI] [PubMed] [Google Scholar]
- de Zamaroczy M., Bernardi G. The GC clusters of the mitochondrial genome of yeast and their evolutionary origin. Gene. 1986;41(1):1–22. doi: 10.1016/0378-1119(86)90262-3. [DOI] [PubMed] [Google Scholar]
- de Zamaroczy M., Bernardi G. The primary structure of the mitochondrial genome of Saccharomyces cerevisiae--a review. Gene. 1986;47(2-3):155–177. doi: 10.1016/0378-1119(86)90060-0. [DOI] [PubMed] [Google Scholar]
- de Zamaroczy M., Faugeron-Fonty G., Baldacci G., Goursot R., Bernardi G. The ori sequences of the mitochondrial genome of a wild-type yeast strain: number, location, orientation and structure. Gene. 1984 Dec;32(3):439–457. doi: 10.1016/0378-1119(84)90019-2. [DOI] [PubMed] [Google Scholar]
- de Zamaroczy M., Faugeron-Fonty G., Bernardi G. Excision sequences in the mitochondrial genome of yeast. Gene. 1983 Mar;21(3):193–202. doi: 10.1016/0378-1119(83)90002-1. [DOI] [PubMed] [Google Scholar]
- de Zamaroczy M., Marotta R., Faugeron-Fonty G., Goursot R., Mangin M., Baldacci G., Bernardi G. The origins of replication of the yeast mitochondrial genome and the phenomenon of suppressivity. Nature. 1981 Jul 2;292(5818):75–78. doi: 10.1038/292075a0. [DOI] [PubMed] [Google Scholar]