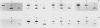Abstract
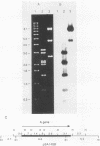
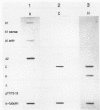
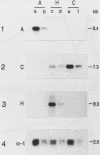
A family of genes is responsible for production of surface antigenic components of Paramecium tetraurelia. These surface proteins are expressed in a mutually exclusive manner. Individuals rarely display more than one type. However, changes in environmental conditions can cause different surface proteins which replace preexisting types to be expressed. We investigated the nature of regulation of the genes for the A, C, and H surface antigens of P. tetraurelia. A system for in vitro run-on transcription was developed from crude Paramecium extracts and used in this analysis. The genes for surface antigens A and H were controlled at the level of transcription. However, the gene for surface antigen C demonstrated both transcriptional and posttranscriptional control, depending on the serotype being expressed. When animals expressed serotype A, the gene for surface antigen C was not transcribed. However, when animals expressed serotype H, the gene for surface antigen C was actively transcribed and stable surface antigen C mRNA was present in the cells, although surface antigen C was not detectable by serotype testing or by a salt-alcohol extraction method. The kinetics of transformation from serotype H to serotype C were determined by using the in vitro transcription system and monitoring steady-state RNA levels. During the transition, serotype A transcription was detected in run-on transcription experiments, although this RNA did not accumulate. The results indicate that serotype expression is controlled at several levels and that not all serotype genes are controlled in the same manner.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bannon G. A., Perkins-Dameron R., Allen-Nash A. Structure and expression of two temperature-specific surface proteins in the ciliated protozoan Tetrahymena thermophila. Mol Cell Biol. 1986 Sep;6(9):3240–3245. doi: 10.1128/mcb.6.9.3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst P. Discontinuous transcription and antigenic variation in trypanosomes. Annu Rev Biochem. 1986;55:701–732. doi: 10.1146/annurev.bi.55.070186.003413. [DOI] [PubMed] [Google Scholar]
- Epstein L. M., Forney J. D. Mendelian and non-mendelian mutations affecting surface antigen expression in Paramecium tetraurelia. Mol Cell Biol. 1984 Aug;4(8):1583–1590. doi: 10.1128/mcb.4.8.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forney J. D., Epstein L. M., Preer L. B., Rudman B. M., Widmayer D. J., Klein W. H., Preer J. R., Jr Structure and expression of genes for surface proteins in Paramecium. Mol Cell Biol. 1983 Mar;3(3):466–474. doi: 10.1128/mcb.3.3.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilley D., Preer J. R., Jr, Aufderheide K. J., Polisky B. Autonomous replication and addition of telomerelike sequences to DNA microinjected into Paramecium tetraurelia macronuclei. Mol Cell Biol. 1988 Nov;8(11):4765–4772. doi: 10.1128/mcb.8.11.4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godiska R., Aufderheide K. J., Gilley D., Hendrie P., Fitzwater T., Preer L. B., Polisky B., Preer J. R., Jr Transformation of Paramecium by microinjection of a cloned serotype gene. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7590–7594. doi: 10.1073/pnas.84.21.7590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godiska R. Structure and sequence of the H surface protein gene of Paramecium and comparison with related genes. Mol Gen Genet. 1987 Jul;208(3):529–536. doi: 10.1007/BF00328151. [DOI] [PubMed] [Google Scholar]
- Love H. D., Jr, Allen-Nash A., Zhao Q. A., Bannon G. A. mRNA stability plays a major role in regulating the temperature-specific expression of a Tetrahymena thermophila surface protein. Mol Cell Biol. 1988 Jan;8(1):427–432. doi: 10.1128/mcb.8.1.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer T. F., Mlawer N., So M. Pilus expression in Neisseria gonorrhoeae involves chromosomal rearrangement. Cell. 1982 Aug;30(1):45–52. doi: 10.1016/0092-8674(82)90010-1. [DOI] [PubMed] [Google Scholar]
- Preer J. R., Preer L. B., Rudman B. M. mRNAs for the immobilization antigens of Paramecium. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6776–6778. doi: 10.1073/pnas.78.11.6776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldo A. T., Godoy G. A. The kinetic complexity of Paramecium macronuclear deoxyribonucleic acid. J Protozool. 1972 Nov;19(4):673–678. doi: 10.1111/j.1550-7408.1972.tb03558.x. [DOI] [PubMed] [Google Scholar]
- Van der Ploeg L. H. Control of variant surface antigen switching in trypanosomes. Cell. 1987 Oct 23;51(2):159–161. doi: 10.1016/0092-8674(87)90140-1. [DOI] [PubMed] [Google Scholar]
- Zieg J., Hilmen M., Simon M. Regulation of gene expression by site-specific inversion. Cell. 1978 Sep;15(1):237–244. doi: 10.1016/0092-8674(78)90098-3. [DOI] [PubMed] [Google Scholar]