Small interfering RNA (siRNA) has specific and effective gene silencing effects and has rapidly emerged as a potent strategy for cancer therapy.[1] Naked siRNA cannot readily cross cellular membranes, and the delivery of siRNA to its target remains the biggest hurdle to clinical translation.[2] Various types of synthetic nanovectors have been investigated for siRNA delivery.[3] Although many promising systems for the delivery of siRNA have been reported, clinical translation of siRNA therapeutics remains a challenge. Herein, we report a nanoparticle-based system for delivering siRNA into cells that uses a biodegradable conjugate with a high cell permeability for targeting tumors, and a universal phosphate-binding analogue for high siRNA binding affinity.
Recently, we developed a hyaluronic acid (HA) based nanoformulation for tumor therapy that is nontoxic, highly specific for targeting tumor cells, drug-loadable, and biodegradable. Importantly, this HA-based system is easy to formulate and cost-effective to manufacture.[4,5] Similar to other nanoparticle (NP) formulations, HA-NPs deliver encapsulated drugs into tumors by the enhanced permeability retention (EPR) effect. Furthermore, as HA is the main ligand for CD44,[6] which is overexpressed on the surfaces of a variety of tumor cells, HA-NPs can actively accumulate in tumor cells. Once HA-NPs accumulate in tumor cells by passive and active targeting mechanisms, the encapsulated drugs are released efficiently into the tumor cells through enzyme-triggered degradation of the NPs by the HA-degrading intracellular enzyme hyaluronidase-1 (Hyal-1).[7] Although HA-NPs are an ideal platform for new nanoformulations with small-molecule anticancer drugs, they are not adequate for siRNA delivery. This is because HA-NPs are composed of highly anionic polymer that cannot form nanosized polyelectrolyte complexes with anionic siRNA for facile delivery into cells (Scheme 1).
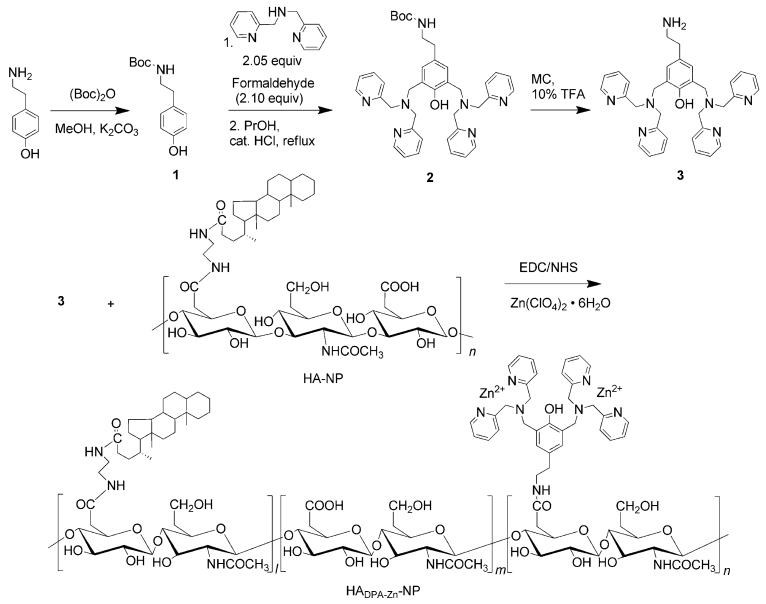
Scheme 1.
Synthesis and chemical structure of HADPA-Zn-NPs. Boc=tert-butyloxycarbonyl, MC=CH2Cl2, TFA=trifluoroacetic acid.
Previously, we reported a series of chemosensors based on ZnII–dipicolylamine complexes (Zn–DPA) that are highly selective for phosphate-containing molecules in aqueous solution. The selectivity is achieved through specific interactions between the coordinated zinc ions of the DPA and the anionic phosphate moieties.[8] Zn–DPA analogues have also been used as probes for the anionic membrane surfaces of dead or dying cells by targeting phosphatidylserine exposed at the cell membrane.[9] Although various types of Zn–DPA analogues have been developed, to our knowledge none have been applied for the delivery of therapeutic molecules that contain phosphate groups, such as siRNA. By using the principles of supramolecular chemistry, we hypothesized that synthetic receptor molecules, such as Zn–DPA, may have the ability to associate selectively with the phosphodiester groups that constitute the backbone of siRNA.
Herein, we demonstrate a functional, simple, and cost-effective delivery system for siRNA that works by utilizing Zn–DPA to bind siRNA, and HA-NPs as a carrier ligand to target tumor cells. Unlike many other conventional siRNA delivery systems that involve chemical or physical conjugation of the siRNA to polycations, HA-NPs that are conjugated with Zn–DPA (HADPA-Zn-NPs, Scheme 1) have a high affinity for siRNA. This affinity improves the targeting of the system for gene silencing and maintains the advantages of HA-NPs for cellular delivery of the siRNA. As siRNA and hydrophobic anticancer drugs can be simultaneously loaded onto the surface and the inner core of HADPA-Zn-NPs, this system can be used as a co-delivery carrier to maximize and synergize therapeutic effects. Polymeric NPs that are functionalized with Zn–DPA provide highly targeted small-molecule delivery and efficient intraceullular transfer of siRNA with low toxicity. This combination makes HADPA-Zn-NPs promising as safe and efficient vectors for targeted gene/anticancer drug therapy.
The HADPA-Zn-NP conjugates were prepared by covalent conjugation of the aminated bis(DPA) analogue 3 to the carboxyl groups of HA-NP by using a standard ethyl-(dimethylaminopropyl) carbodiimide/N-hydroxysuccinamide (EDC/NHS) activation reaction in a buffer solution. HA-NP and 3 were synthesized as previously reported (Scheme 1; see also Figure S1-S4 in the Supporting Information).[4b,8c] After dialysis of the mixture against distilled water, zinc ions were incorporated into the complex by the addition of excess Zn(ClO4)2·6H2O and sonication. Zn–DPA has an apparent Kd value of 70 nm.[10] The HADPA-Zn-NP conjugate was isolated as a white powder by lyophilization. Analysis by 1H NMR spectroscopy showed that HADPA-Zn-NPs were conjugated to about 32 molecules of 3 per 100 repeating units of HA (see Figure S5 in the Supporting Information). As a result of its amphiphilic structure, the HADPA-Zn-NPs self-assembled in aqueous solution and remained stable in a solution of buffer for at least two months at 4 °C. TEM images show that the conjugation of 3 does not affect the structure of the NPs (see Figure S6 in the Supporting Information). The ability of HADPA-Zn-NPs to form Zn–DPA-mediated complexes with siRNA was investigated by using a siRNA (siLuc) that targets the firefly luciferase (fluc) gene as a model compound. Different amounts of HA and NPs with (HADPA-Zn-NPs) and without zinc ions (HADPA-NPs) were mixed with 15 pmol of siLuc and analyzed in a retardation assay by agarose gel electrophoresis. As shown in Figure 1a, the HADPA-Zn-NPs were able to bind siLuc but HA and the HADPA-NPs alone did not bind to siLuc at any concentration. To confirm that this complex structure was based on coordination between the phosphate groups of the siRNA and Zn–DPA, excess sodium phosphate was added to induce decomplexation of the siLuc from the HADPA-Zn-NPs (Figure 1b). After the addition of sodium phosphate, siLuc was released from HADPA-Zn-NPs, whereas no significant change was observed after the addition of other salts. This result indicates that siLuc was bound on the HADPA-Zn-NPs by coordination with Zn–DPA. SiLuc/HADPA-Zn-NPs had a negative zeta potential of (−15.7 ± 4.0) mV and an average hydrodynamic diameter of (227.6 ± 25.4) nm (see Figure S7 in the Supporting Information). In terms of biocompatibility, in vitro cellular toxicity studies showed that the HADPA-Zn-NPs were nontoxic (Figure 1c). Prior to functional biological studies, we evaluated the cellular uptake of siRNA/HADPA-Zn-NPs in cells that were strongly positive for CD44 (4T1-fluc) and cells that were less-strongly positive for CD44 (293T). CD44 expression was confirmed by flow cytometry (see Figure S8 in the Supporting Information). As shown in Figure 1d, the red fluorescence from Cy3-labeled siRNA was more intense in 4T1-fluc cells that were treated with siRNA/HADPA-Zn-NPs than in the cells that were treated with Lipofectamine 2000 (Lipo2K)/siRNA. In contrast, no fluorescence was observed in cells that were treated with HA, HADPA-NPs, and Zn–DPA (see Figure S9 in the Supporting Information). Furthermore, the uptake of siRNA/HADPA-Zn-NPs into 4T1-fluc cells was (3.7 ± 0.5)-fold higher than the uptake into 293T cells (Figure 1e). To determine whether CD44 receptors are responsible for the efficient cellular uptake of siRNA/HADPA-Zn-NPs, the CD44 receptors were blocked with excess HA or the anti-CD44 antibody HERM-1. Additionally, to investigate whether the cellular uptake of NPs is energy-dependent and proceeds by endocytosis, the active transport processes were strongly inhibited by using a low temperature or by introducing metabolic inhibitors. Each of the two cell lines were treated with siRNA/HADPA-Zn-NPs and incubated at 4 °C without a metabolic inhibitor or at 37 °C with the metabolic inhibitor sodium azide. All of the treatments clearly reduced the efficiency of siRNA/HADPA-Zn-NPs uptake into 4T1-fluc cells ((36.0 ± 6.2)%, (28.7 ± 1.5)%, (28.3 ± 3.5)%, and (26.3 ± 3.1)% for HA, HERM-1, 4°C, and sodium azide, respectively), which demonstrated that HADPA-Zn-NP/siRNA complexes enter the cells to some extent through CD44 receptor mediated endocytosis, and also in an energy-dependent manner (Figure 1e). The ability of HADPA-Zn-NPs to deliver siRNA was confirmed by treating 4T1-fluc cells with siLuc/HADPA-Zn-NPs and measuring the expression of fluc (Figure 1 f). The bioluminescence imaging (BLI) signal intensity of untreated 4T1-fluc cells was set to 100% fluc expression. In agremment with the uptake of Cy3-siRNA shown in Figure 1d, siLuc/HADPA-Zn-NPs showed a remarkably high gene silencing efficacy in a dose-dependent manner (10 μg, 20 μg, and 40 μg of siLuc/HADPA-Zn-NPs reduced the expression of fluc to (54.9 ± 5.6)%, (33.4 ± 3.5)%, and (10.5 ± 1.2)%, respectively). The HADPA-Zn-NP/siLuc complex was (2.2 ± 0.4)-fold more effective in silencing the expression of fluc than the Lipo2K formulation. To rule out the possibility that the decreased expression of fluc was caused by a reduction in cell viability because of nonspecific cytotoxicity, we performed an MTT assay to assess cell viability after the BLI experiment. No significant cytotoxicity was detected in the 4T1-fluc cells after BLI, which confirmed that the gene silencing effect was a consequence of the treatment with HADPA-Zn-NP/siLuc and was not caused by a reduced number of viable cells (see Figure S10 in the Supporting Information).
Figure 1.
In vitro characterization of siRNA/HADPA-Zn-NPs complexes. a) Electrophorectic retardation analysis of siRNA (15 pmol) binding with different carriers (0–100 μg). b) Release of siRNA from siRNA/HADPA-Zn-NPs after the addition of the salts indicated. c) Cytotoxicity of HA-NPs and HADPA-Zn-NPs in 4T1-fluc cells. d) Confocal microscopy images of 4T1-fluc cells treated with Cy3-siRNA complexed with HADPA-Zn-NPs or Lipofectamine 2000 (L2K); blue: nuclei (DAPI), red: siRNA. e) Intracellular uptake of Cy3-siRNA/HADPA-Zn-NPs (NP) by 4T1-fluc cells and 293T cells at low temperature (4 °C) or in the presence of various metabolic inhibitors at 37 °C; *p < 0.005. f) Suppression of fluc gene expression by HADPA-Zn-NPs (NP) or Lipo2K (L2K) complexed with control nontargeting siRNA or siLuc; B=buffer only *p < 0.005 versus control, **p < 0.005 versus Lipo2K/siLuc.
The HA-NPs were designed with the objective of creating tumor-targeting NPs for anticancer therapy. These NPs are intended to carry hydrophobic drugs in their inner cores and, once the NPs are delivered into tumor cells, release the encapsulated drug by Hyal-1-mediated degradation of the NPs. To demonstrate that HADPA-Zn-NPs can be used as a co-delivery carrier for both an anticancer drug and for siRNA, the hydrophobic and fluorescent carbocyanine 3,3′-dioctade-cyloxacarbocyanine perchlorate (DiO) was used as a model compound (see Figure S11 in the Supporting Information). As DiO is a lipophilic molecule, it was readily encapsulated in HADPA-Zn-NPs in its fluorescently quenched state. The release of DiO from the NPs can be easily monitored by measuring changes in fluorescence. The encapsulation of DiO along with siRNA in the HADPA-Zn-NPs slightly increased the mean diameter of the HADPA-Zn-NPs to (290.6 ± 31.7) nm (see Figure S7 in the Supporting Information). After the siRNA/DiO/HADPA-Zn-NPs complex was exposed to a solution of buffer that contained Hyal-1 (120 units mL−1), the fluorescence intensity increased significantly and was saturated within 30 min as a result of the release of DiO from the HADPA-Zn-NPs (Figure 2a). To monitor the release of DiO in cells, 4T1-fluc and 293T cells were treated with siRNA/DiO/HADPA-Zn-NPs, and the fluorescence intensity was recorded by a fluorescence microplate reader. As shown in Figure 2b, a gradual increase in the fluorescence occurred as early as 10 min after the cells were treated with siRNA/DiO/HADPA-Zn-NPs. The maximum fluorescence signal was detected after 30 min in 4T1-fluc cells. This implies that 4T1-fluc cells are highly permeable to the HADPA-Zn-NPs and that the HADPA-Zn-NPs are highly susceptible to intracellular Hyal-1, which readily destroys the structural integrity of the HADPA-Zn-NPs and leads to rapid release of the payload. To verify if the intracellular release of DiO is Hyal-1-dependent, the release of DiO was also tested in NIH3T3 cells, which have a lower level of intracellular Hyal-1. The results demonstrate that the release of DiO was significantly lower in NIH3T3 cells relative to 4T1-fluc cells (see Figure S12 in the Supporting Information). The finding that HADPA-Zn-NPs enhance cellular uptake of both encapsulated drugs and siRNAs that are coordinated to Zn–DPA is supported by confocal microscopy images of 4T1-fluc cells after treatment with siRNA/DiO/HADPA-Zn-NPs (Figure 2c).
Figure 2.
In vitro enzyme-triggered release of DiO from DiO/HADPA-Zn-NPs. Fluorescence quantification of DiO/HADPA-Zn-NPs after incubation a) with or without Hyal-1 enzyme (120 units mL−1) in acetate buffer at 37°C and b) with 4T1-fluc and 293T cells; *p < 0.005 versus 293T cells. c) Intracellular uptake of siRNA/DiO/HADPA-Zn-NPs in 4T1-fluc cells; blue: nuclei (DAPI), green: DiO, and red: siRNA.
To demonstrate the use of the siLuc/HADPA-Zn-NPs in vivo, we investigated the gene silencing effect of siLuc/HADPA-Zn-NPs in a 4T1-fluc xenograft mouse model. Four groups of Balb/C mice (n = 5 per group) with subcutaneous 4T1-fluc tumors were treated with an intratumor injection of 80 μL of a buffer solution that contained siLuc/HADPA-Zn-NPs (100 pmol/200 μg), HADPA-Zn-NPs (200 μg), naked siLuc (100 pmol), or phosphate-buffered saline (PBS). The silencing of the fluc gene was measured by in vivo BLI. Figure 3 a shows a series of typical optical images of fluc-expressing tumors after the administration of each formulation. After 72 hours, the level of fluc expression was significantly reduced in the tumor that was treated with siLuc/HADPA-Zn-NPs; however, the expression of fluc did not decrease in the other control groups. Quantitative analysis showed that the relative level of fluc expression was suppressed at 48 hours after the injection of siLuc/HADPA-Zn-NPs and was significantly inhibited to (−6.2 ± 3.7)% at 72 hours. In contrast the control groups had increased levels of fluc expression ((30.0 ± 4.1)%, (38.2 ± 3.9)%, and (39.2 ± 5.3)%, for HADPA-Zn-NPs, naked siRNA, and PBS, respectively, Figure 3b). The tumors from each group were harvested and weighed after BLI to determine whether the apparent gene silencing effect was a result of a reduction in the volume of the tumors as a consequence of the toxicity of the formulation (see Figure S13 in the Supporting Information). As shown by the in vitro testing, there was no significant difference in the neoplastic weights of the tumors between the various groups. This result implies that the downregulation of the fluc gene was selectively induced by the gene silencing mechanism.
Figure 3.
a) In vivo optical images and b) quantitative analysis of fluc-expressing tumor after intratumor injection of siLuc/HADPA-Zn-NPs(NP), HADPA-Zn-NPs, naked siLuc, or PBS (n = 5 per group). *p < 0.005 versus control groups.
The development of biocompatible, efficient, and clinically relevant delivery systems remains an important challenge for the clinical applications of siRNA-based therapeutics. In this study we demonstrated a siRNA delivery platform that is based on DPA coordinated to ZnII, which is an artificial receptor for phosphate anions. A combination of self-assembled, tumor-targeting NPs and a Zn–DPA analogue conferred high siRNA binding affinity on the complex, which is based on the multivalent interactions between the NPs and the siRNAs. This binding affinity enables specific, intracellular siRNA delivery and maintains the enhanced cell-penetrating and tumor-targeting properties of HA-based NPs. HADPA-Zn-NPs are inexpensive to produce on a large scale and are easy to formulate to contain small-molecule drugs and siRNA by simple sonication and subsequent mixing. This delivery system may prevent the need for further chemical modification of drugs that have potentially limited clinical translation. Furthermore, unlike most reported siRNA delivery systems, our formulation does not need cationic derivatives to complex with siRNAs; therefore, we expect lower toxicity and nonspecific accumulation than is typically associated with polycationic-based formulations. Currently, the formation of siRNA/HADPA-Zn-NPs complexes (for example, the binding constant of NPs with zinc ions and siRNA, the effects of dinuclear Zn–DPA on hydrolysis,[11] and the enhanced-delivery of DNA/RNA[12]) as well as their transportation and transfection process in cells are under evaluation. Although a detailed mechanism for the interaction of these therapeutic delivery systems with cells needs to be verified, this strategy may open up exciting new opportunities in gene delivery and nanomedicine.
Supplementary Material
Footnotes
This work was supported by the Intramural Research Program of the NIBIB, NIH and partially supported by an NIH Pathway to Independence (K99/R00) Award, the National Natural Science Foundation of China (NSFC, grant nos. 81101101 and 2011JQ0032), the International Cooperative Program of the NSFC (grant no. 81028009), and NRF grant 2009-0080734 from the MEST (Korea).
Supporting information for this article is available on the WWW under http://dx.doi.org/10.1002/anie.201105565.
Contributor Information
Dr. Gang Liu, Laboratory of Molecular Imaging and Nanomedicine (LOMIN), National Institute of Biomedical Imaging and Bioengineering (NIBIB), National Institutes of Health (USA); Sichuan Key Laboratory of Medical Imaging, North Sichuan Medical College (China).
Dr. Ki Young Choi, Laboratory of Molecular Imaging and Nanomedicine (LOMIN), National Institute of Biomedical Imaging and Bioengineering (NIBIB), National Institutes of Health (USA).
Dr. Ashwinkumar Bhirde, Laboratory of Molecular Imaging and Nanomedicine (LOMIN), National Institute of Biomedical Imaging and Bioengineering (NIBIB), National Institutes of Health (USA)
Magdalena Swierczewska, Laboratory of Molecular Imaging and Nanomedicine (LOMIN), National Institute of Biomedical Imaging and Bioengineering (NIBIB), National Institutes of Health (USA).
Dr. Juan Yin, National Cancer Institute (NCI), National Institutes of Health (USA)
Sang Wook Lee, Department of Polymer Science and Engineering, Sungkyunkwan University (Korea).
Prof. Jae Hyung Park, Department of Polymer Science and Engineering, Sungkyunkwan University (Korea)
Prof. Jong In Hong, Department of Chemistry and Bio-Imaging Research Center University of Georgia (USA)
Prof. Jin Xie, Department of Chemistry and Bio-Imaging Research Center, University of Georgia (USA)
Dr. Gang Niu, Laboratory of Molecular Imaging and Nanomedicine (LOMIN), National Institute of Biomedical Imaging and Bioengineering (NIBIB), National Institutes of Health (USA)
Dr. Dale O. Kiesewetter, Laboratory of Molecular Imaging and Nanomedicine (LOMIN), National Institute of Biomedical Imaging and Bioengineering (NIBIB), National Institutes of Health (USA)
Dr. Seulki Lee, Laboratory of Molecular Imaging and Nanomedicine (LOMIN), National Institute of Biomedical Imaging and Bioengineering (NIBIB), National Institutes of Health (USA).
Dr. Xiaoyuan Chen, Laboratory of Molecular Imaging and Nanomedicine (LOMIN), National Institute of Biomedical Imaging and Bioengineering (NIBIB), National Institutes of Health (USA).
References
- [1].a) Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Nature. 2001;411:494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]; b) de Fougerolles A, Vornlocher HP, Maraganore J, Lieberman J. Nat. Rev. Drug Discovery. 2007;6:443–453. doi: 10.1038/nrd2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Whitehead KA, Langer R, Anderson DG. Nat. Rev. Drug Discovery. 2009;8:129–138. doi: 10.1038/nrd2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].a) Tseng YC, Mozumdar S, Huang L. Adv. Drug Delivery Rev. 2009;61:721–731. doi: 10.1016/j.addr.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Yoo JW, Irvine DJ, Discher DE, Mitragotri S. Nat. Rev. Drug Discovery. 2011;10:521–535. doi: 10.1038/nrd3499. [DOI] [PubMed] [Google Scholar]; c) Endoh T, Ohtsuki T. Adv. Drug Delivery Rev. 2009;61:704–709. doi: 10.1016/j.addr.2009.04.005. [DOI] [PubMed] [Google Scholar]; d) Liu G, Swierczewska M, Lee S, Chen X. Nano Today. 2011;5:524–539. doi: 10.1016/j.nantod.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].a) Choi KY, Min KH, Na JH, Choi K, Kim K, Park JH, Kwon IC, Jeong SY. J. Mater. Chem. 2009;19:4102–4107. [Google Scholar]; b) Choi KY, Chung H, Min KH, Yoon HY, Kim K, Park JH, Kwon IC, Jeong SY. Biomaterials. 2010;31:106–114. doi: 10.1016/j.biomaterials.2009.09.030. [DOI] [PubMed] [Google Scholar]; c) Choi KY, Min KH, Yoon HY, Kim K, Park JH, Kwon IC, Choi K, Jeong SY. Biomaterials. 2011;32:1880–1889. doi: 10.1016/j.biomaterials.2010.11.010. [DOI] [PubMed] [Google Scholar]; d) Han S-Y, Han HS, Lee SC, Kang YM, Kim I-S, Park JH. J. Mater. Chem. 2011;21:7996–8001. [Google Scholar]
- [5].Toole BP. Nat. Rev. Cancer. 2004;4:528–539. doi: 10.1038/nrc1391. [DOI] [PubMed] [Google Scholar]
- [6].Platt VM, Szoka FC., Jr. Mol. Pharm. 2008;5:474–486. doi: 10.1021/mp800024g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Stern R, Jedrzejas MJ. Chem. Rev. 2006;106:818–839. doi: 10.1021/cr050247k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].a) Rhee HW, Choi SJ, Yoo SH, Jang YO, Park HH, Pinto RM, Cameselle JC, Sandoval FJ, Roje S, Han K, Chung DS, Suh J, Hong JI. J. Am. Chem. Soc. 2009;131:10107–10112. doi: 10.1021/ja9018012. [DOI] [PubMed] [Google Scholar]; b) Rhee HW, Lee SH, Shin IS, Choi SJ, Park HH, Han K, Park TH, Hong JI. Angew. Chem. 2010;122:5039–5043. doi: 10.1002/anie.201000879. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2010;49:4919–4923. doi: 10.1002/anie.201000879. [DOI] [PubMed] [Google Scholar]; c) Kwon TH, Kim HJ, Hong JI. Chem. Eur. J. 2008;14:9613–9619. doi: 10.1002/chem.200801260. [DOI] [PubMed] [Google Scholar]; d) Lee JH, Jeong AR, Jung JH, Park CM, Hong JI. J. Org. Chem. 2011;76:417–423. doi: 10.1021/jo1017102. [DOI] [PubMed] [Google Scholar]
- [9].a) Smith BA, Akers WJ, Leevy WM, Lampkins AJ, Xiao S, Wolter W, Suckow MA, Achilefu S, Smith BD. J. Am. Chem. Soc. 2010;132:67–69. doi: 10.1021/ja908467y. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Bae SW, Cho MS, Jeong AR, Choi BR, Kim DE, Yeo WS, Hong JI. Small. 2010;6:1499–1503. doi: 10.1002/smll.201000564. [DOI] [PubMed] [Google Scholar]
- [10].Walkup GK, Burdette SC, Lippard SJ, Tsien RY. J. Am. Chem. Soc. 2000;122:5644–5645. doi: 10.1021/ja010059l. [DOI] [PubMed] [Google Scholar]
- [11].Molenveld P, Kapsabelis S, Engbersen JFJ, Reinhoudt DN. J. Am. Chem. Soc. 1997;119:2948–2949. [Google Scholar]
- [12].a) Liu L, Zhang H, Meng X, Yin J, Li D, Liu C. Biomaterials. 2010;31:1380–1391. doi: 10.1016/j.biomaterials.2009.10.056. [DOI] [PubMed] [Google Scholar]; b) Pichon C, Guerin B, Refreqiers M, Goncalves C, Vigny P, Midoux P. J. Gene Med. 2002;4:548–559. doi: 10.1002/jgm.303. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.