Abstract
Purpose
The aim of this study was to make 5-aminolevulinic acid (5-ALA)-incorporated nanoparticles using methoxy polyethylene glycol/chitosan (PEG-Chito) copolymer for application in photodynamic therapy for colon cancer cells.
Methods
5-ALA-incorporated (PEG-Chito-5-ALA) nanoparticles were prepared by ion complex formation between 5-ALA and chitosan. Protoporphyrin IX accumulation in the tumor cells and phototoxicity induced by PEG-Chito-5-ALA nanoparticles were assessed using CT26 cells in vitro.
Results
PEG-Chito-5-ALA nanoparticles have spherical shapes with sizes diameters 200 nm. More specifically, microscopic observation revealed a core-shell structure of PEG-Chito-5-ALA nanoparticles. 1H NMR spectra showed that 5-ALA was incorporated in the core of the nanoparticles. In the absence of light irradiation, all components such as 5-ALA, empty nanoparticles, and PEG-Chito-5-ALA nanoparticles did not affect the viability of cells. However, 5-ALA or PEG-Chito-5-ALA nanoparticles induced tumor cell death under light irradiation, and the viability of tumor cells was dose-dependently decreased according to the increase in irradiation time. In particular, PEG-Chito-5-ALA nanoparticles induced increased phototoxicity and higher protoporphyrin IX accumulation into the tumor cells than did 5-ALA alone. Furthermore, PEG-Chito-5-ALA nanoparticles accelerated apoptosis/necrosis of tumor cells, compared to 5-ALA alone.
Conclusion
PEG-Chito-5-ALA nanoparticles showed superior delivery capacity of 5-ALA and phototoxicity against tumor cells. These results show that PEG-Chito-5-ALA nanoparticles are promising candidates for photodynamic therapy of colon cancer cells.
Keywords: 5-ALA, photosensitizer, chitosan, nanoparticles, colon cancer, protoporphyrin IX
Introduction
Palliative therapies such as photodynamic therapy (PDT), immunotherapy, gene therapy, stent displacement, modified chemotherapy, and radiation therapy have been investigated for cancer treatment because they are regarded to be effective in prolonging survivability of patients and enhancing the quality of life of patients.1–6 Among them, PDT has been spotlighted as palliative therapy for the treatment of various types of cancer cells due to its composition of nontoxic components, such as photosensitizers, light, and oxygen, as well as its minimal side effects on patients.1,6 Generally, a photosensitizer is activated only under light irradiation of a specific wavelength; ie, the photosensitizer alone is not harmful to the human body. Light irradiation in the presence of photosensitizers in tumor tissue causes energy transfer to surrounding oxygen, followed by the generation of reactive singlet oxygen (ROS) after apoptosis or necrosis of tumor cells by ROS.7,8
Various kinds of photosensitizers have been developed in the last decade.9 Among them, 5-aminolevulinic acid (5-ALA) has been extensively used as a photosensitizer due to its negligible toxicity and rapid excretion from the body.1 Basically, 5-ALA alone is not a photosensitizer; ie, it gets interconverted to a strong photosensitizer, protoporphyrin IX (PpIX), via the heme biosynthetic pathway in the mitochondria, which then functions as a photosensitizer.1,10 Successful cases of suppression of solid tumors by excessive administration of 5-ALA have been reported.1,10–12 In addition to these successful experiences, some drawbacks of 5-ALA, such as hydrophilicity and low specificity for tumor cells, have been noted to induce lower cellular uptake and low bioavailability of ALA.13 To overcome these drawbacks, various kinds of modified 5-ALA have been developed.14–16 For example, esterification of ALA, such as methyl- or hexyl-ester conjugated 5-ALA was reported to show enhanced cellular uptake and a better interconversion rate to PpIX than the parent compound.14–16 Drug delivery strategies using colloidal drug carriers such as polymeric nanoparticles and liposomes have also been studied for their enhancement of 5-ALA cellular uptake.17–20 Among them, nanoscopic vehicles such as polymeric micelles and nanoparticles have been investigated as ideal carriers for drugs, including photosensitizers.20–22 Nanoparticles are regarded as ideal vehicles for targeting solid tumors, because of their favorable biodistribution, excellent bioavailability, and reduction of the intrinsic toxicity of the drugs.22–24
In this study, we prepared 5-ALA-incorporated nanoparticles using methoxy poly(ethylene glycol)/chitosan (PEG-Chito) copolymer, and their photodynamic potential was tested using CT26 colorectal carcinoma cells. Since chitosan has cationic properties, it is an ideal macromolecule to incorporate anionic drugs.25 In this context, 5-ALA, an anionic hydrophilic photosensitizer, can be attached to the cationic group of chitosan. Furthermore, chitosan is known to enhance the trans-mucosal delivery of drugs and the targetability of vehicles. Therefore, chitosan can be used as a vehicle for 5-ALA delivery to tumor cells. The physicochemical properties and photodynamic potentials of PEG-Chito-5-ALA nanoparticles were investigated in vitro.
Materials and methods
Materials
5-ALA, potassium persulfate, dialysis membrane (molecular weight [MW] cut-off size = 12,000 g/mol), 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT), dichloromethane, and dimethyl sulfoxide were obtained from Sigma (Sigma Chemical, St Louis, MO, USA). Chitosan (MW = 15,000 g/mol) was obtained from Wako Pure Chem (Osaka, Japan). Methoxy poly(ethylene glycol)-acrylate (MPEG-acrylate, MW = 5,000 g/mol) was purchased from Sunbio (Anyang, Republic of Korea). All other chemicals used were of analytical grade.
Synthesis of PEG-Chito block copolymer
The chitosan copolymer was synthesized following the method previously reported by Ganji and Abdekhodaie.26 Chitosan (150 mg) was dissolved in 10 mL of 0.1 N HCl solution. To this solution, potassium persulfate was added and stirred for 60 minutes at 60oC under a nitrogen atmosphere. Next, a 1.5 times equivalent amount of MPEG-acrylate was added and stirred for 12 hours. The resulting solution was then introduced into the dialysis membrane and dialyzed with deionized water for 1 day, followed by lyophilization for 2 days. The lyophilized solid was precipitated into methanol and then filtered. This process was repeated three times to remove unreacted MPEG-acrylate. Finally, the filtered solid was dried in a vacuum at room temperature.
Preparation of 5-ALA-incorporated (PEG-Chito-5-ALA) nanoparticles
The 5-ALA-incorporated (PEG-Chito-5-ALA) nanoparticles were prepared by dispersing PEG-Chito copolymer in 10 mL of deionized water, followed by the addition of 5-ALA. This solution was sonicated with a bar-type sonicator and then stirred magnetically for 24 hours. The resulting solution was centrifuged at 12,000 rpm, and the supernatant was used to measure the drug content in the nanoparticles. Flakes were reconstituted in 10 mL of deionized water to analyze or lyophilize them. Empty nanoparticles were prepared in the absence of 5-ALA, using the same procedure.
The 5-ALA content in the nanoparticles was evaluated by subtracting the 5-ALA contents from the supernatant. The 5-ALA was measured at 264 nm with a UV spectrophotometer (UV-1601 UV-VIS spectrophotometer, Shimadzu Co., Kyoto, Japan). The supernatant of empty nanoparticles was used for the blank test.
Analysis of PEG-Chito-5-ALA nanoparticles
The morphology of PEG-Chito-5-ALA nanoparticles was observed using a transmission electron microscope (TEM, JEM 2000 FX II, JEOL Ltd., Tokyo, Japan). One drop of PEG-Chito-5-ALA nanoparticle solution was placed onto a carbon film coated with a copper grid for transmission electron microscope (TEM). The observation was done at 80 kV Particle size was analyzed by Nano-ZS (Malvern, Worcestershire, UK). A sample solution prepared with the dialysis method was used to determine particle size (concentration: 0.1 wt%).
The 1H nuclear magnetic resonance (NMR) spectra of PEG-Chito-5-ALA nanoparticles were obtained using 500 MHz 1H NMR spectroscopy (Varian Unity Inova 500 Mhz NMR spectroscopy, Agilent Technologies, Inc., Santa Clara, CA, USA) in D2O with or without DCl.
Powder X-ray diffractions were employed to evaluate the crystallinity of the drug and nanoparticles (Rigaku D/Max-1200; Rigaku Americas Corporation, The Woodlands, TX, USA) with Ni-filtered CuK radiation (40 kV, 20 mA).
Cell culture
CT26 colorectal carcinoma cells were cultured in Roswell Park Memorial Institute 1640 (RPMI-1640) medium (GIBCO, Grand Island NY, USA), supplemented with 10% fetal bovine serum (Invitrogen, Grand Island NY, USA) and 1% antibiotics at 37°C in 5% CO2.
Cytotoxicity assay
The CT26 cells were seeded into 96-well plates at a density of 2 × 104 cells per well and incubated for 24 hours in 5% CO2 at 37°C. After removing the culture medium, the cells were washed with phosphate buffered saline (PBS, 0.01M, pH 7.4) buffer and then further incubated with serum-free RPMI-1640 for 18 hours. Each well was then replaced with 100 μL of fresh RPMI-1640 medium containing 0.1 mM 5-ALA or PEG-Chito-5-ALA nanoparticles. The cells were then incubated in the dark at 37°C for 24 hours. After incubation, the cells were washed with PBS twice, and the cytoxicity was measured using the MTT assay.
PpIX generation in cells
CT26 cells in 96-well plates were incubated with 100 μL of serum-free medium containing 0.1 mM 5-ALA or PEG-Chito-5-ALA nanoparticles for 24 hours. The cells were then solubilized in 100 μL lysis buffer (GenDEPOT, Barker, TX, USA) and subjected to quantitative spectrofluorometry The fluorescence signal from each well was measured using a Tecan M200 fluorescence spectrometer (Tecan, Salsberg, Austria) at an excitation/emission wavelength of 485/635 nm. The absolute amount of PpIX accumulation in the cells was corrected by protein concentration. Protein concentration was evaluated using a commercial BCA protein assay kit according to the manufacturer’s instructions (Pierce Pharmaceuticals, Rockford IL, USA).
Confocal image
The PpIX products were observed by confocal fluorescence microscopy. The microscope was equipped with a 460–480 mm excitation filter and a 610 nm filter for detection of PpIX fluorescence. The 1 × 106 CT26 cells were seeded on a cover glass in a six-well plate. The cells were then treated with 0.1 mM 5-ALA or PEG-Chito-5-ALA nanoparticles for 24 hours, after which the medium was discarded and the cells were washed with PBS. The cells on a cover glass were fixed by 4% paraformaldehyde in PBS, and these were mounted on a glass slide. The cells were observed using a confocal fluorescence microscope.
PDT
The CT26 cells seeded in a 96-well plate were cultured for 24 hours. The cells were then washed with PBS and ALA alone or PEG-Chito-5-ALA nanoparticles (ALA concentration: 0.1 mM) were added to each well, followed by incubation for 24 hours. Following this, the plates were irradiated with 635 nm red light (635 nm LED lamp module, SH system, Gwangju, South Korea) at doses of 0.6, 1.2, and 1.8 J/cm2, as measured by a photoradiometer (HD2102-1, DeltaOHM, Padova, Italy). Immediately after irradiation, the medium was discarded and washed with PBS. The 100 μL of fresh RPMI containing 10% FBS was then added and the cells were incubated for an additional 24 hours. Cell phototoxicity was determined using the MTT assay.
MTT assay
The MTT assay is based on the idea that live cells are able to cleave the tetrazolium ring to a molecule that absorbs 570 nm in active mitochondria. The 2 × 104 cells were cultured in 96-well plates. After removing the culture media, 100 μL of fresh medium with 25 μL of MTT reagent (2 mg/mL in PBS) (Sigma Chemical) was added to each well. The cells were incubated at 37°C for an additional 3 hours. The cells were then lysed with 100 μL of lysis buffer solution (10% sodium dodecyl sulfate in 0.01 N HCl) for 18 hours, and absorbance was measured at 570 nm.
Apoptosis and necrosis analysis
Flow cytometric analysis of apoptotic and necrotic cells following photodynamic therapy was performed as previously described.6 Propidium iodide (PI) and FITC-annexin V (sc-4252 FITC, Santa Cruz, CA) were used to identify, respectively, necrosis and apoptosis of CT26 cells. CT26 cells were treated with 0.1 mM ALA or PEG-Chito-5-ALA nanoparticles for 24 hours and were then exposed to light doses of 0.6, 1.2, and 1.8 J/cm2. The cells were harvested by trypsinization, and then the collected pellets were resuspended with binding buffer (10 mM HEPES pH 7.4, 150 mM NaCl, 5 mM KCl, 1 mM MgCl2, and 1.8 mM CaCl2) containing FITC-annexin V (1 μg/mL) and were further incubated for 30 minutes. Ten minutes prior to terminating incubation, PI (10 μg/mL) was added to the cells to stain necrotic cells, in darkness. The cells were then immediately analyzed with a FACScan flow cytometer (Becton Dickenson Biosciences, San Jose, CA, USA) equipped with an excitation laser line at 488 nm. PI was collected through a 575 ± 15 nm band pass filter.
Quantification of total protein
The total protein content from each sample was determined using the Pierce BCA protein assay kit (Rockford, IL, USA) following the manufacturer’s instructions. Ten μL of the sample lysate was added to 200 μL of reagent A and B (A:B = 50:1). After 30 minutes of incubation in a CO2 incubator, colorimetric detection was performed using the Tecan M200 spectrometer at a wavelength of 562 nm. The protein concentration of the samples was determined based on the bovine serum albumin standard curve.
Statistical data analysis
Results were expressed as the means of at least three parallel experiments ± standard error of the mean (SEM). Statistical data analysis was performed using the t-test, with P < 0.05 as the minimal level of significance.
Results
Characterization of PEG-Chito-5-ALA nanoparticles
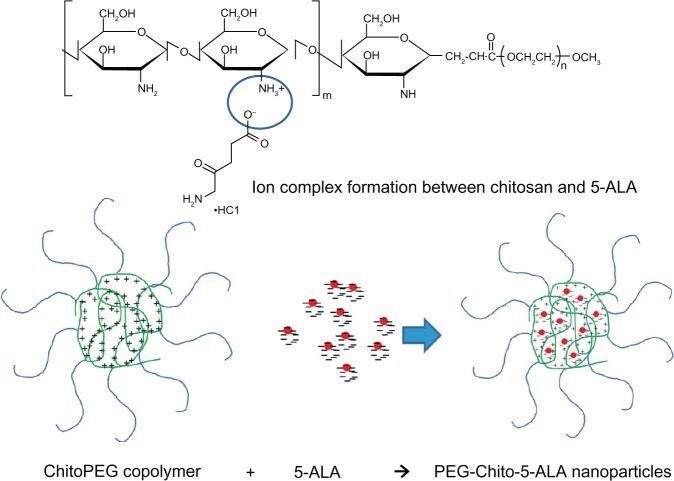
A block copolymer composed of chitosan and MPEG was synthesized as reported previously (Figure 1).26 The yield was approximately 68% (w/w). Since chitosan has cationic properties, it can complex with anionic molecules such as DNA and anionic drugs.25,27,28 Because 5-ALA also has anionic properties, it can be conjoined with chitosan to form nanoparticles, as shown in Figure 1.
Figure 1.
Chemical structure of ChitoPEG copolymer and formation of 5-ALA-incorporated nanoparticles.
Abbreviations: 5-ALA, 5-aminolevulinic acid; PEG-Chito-5-ALA NP, Polyethylene glycol-Chito-5-ALA nanoparticles.
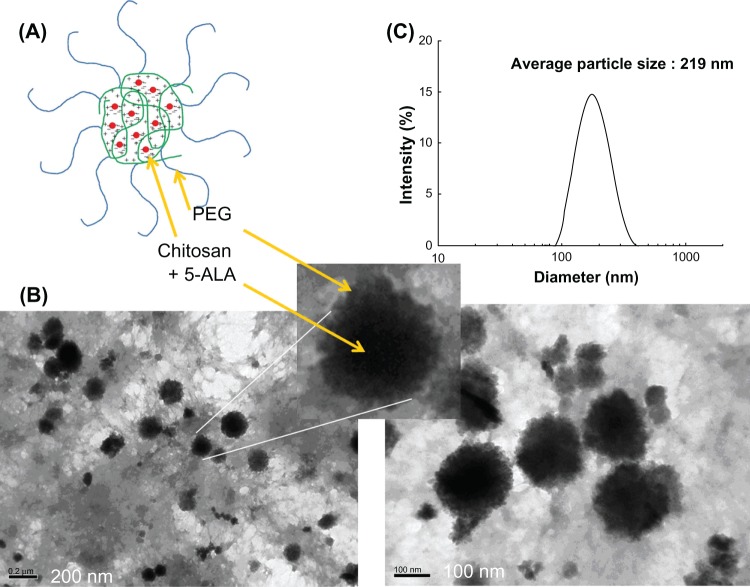
Figure 2 shows the typical particle-size distribution and morphology of PEG-Chito-5-ALA nanoparticles. The nanoparticles have a spherical shape and a diameter of about 200 nm. The characteristics of PEG-Chito-5-ALA nanoparticles are summarized in Table 1. As shown in Table 1, a higher feeding amount of ALA induced a higher drug content in the nanoparticles. However, the experimental 5-ALA contents in nanoparticles were significantly lower than the theoretical value. Particle sizes were approximately 200 nm, and the sizes were not significantly changed by 5-ALA incorporation. Especially, in Figure 2, a discrete region was observed in the TEM photo; nanoparticles are seen formed with a dark core region, with their surroundings a grey color.
Figure 2.
A schematic illustration of polyelectrolyte complex formation of 5-ALA and PEG-Chito copoylmer (A). TEM observation of PEG-Chito-5-ALA nanoparticles (B). Arrows indicated that dark region in the center of the nanoparticles is identified as a inner-core composed of chitosan and 5-ALA while PEG outershell reveals grey color. Typical particle size distribution (C).
Table 1.
Characterization of PEG-Chito-5-ALA nanoparticles
| Polymer/5-ALA weight ratio (mg/mg) |
Drug contents (%, w/w)
|
Particle size (nm) |
||
|---|---|---|---|---|
| Theoretical | Experimental | |||
| Empty NPs | 100/0 | 0 | 0.0 | 191 |
| PEG-Chito-5-ALA NPs | 90/10 | 10 | 2.8 | 194 |
| 80/20 | 20 | 5.6 | 211 | |
| 60/40 | 40 | 9.3 | 219 | |
Abbreviations: 5-ALA, 5-aminolevulinic acid; PEG-Chito-5-ALA NP, Polyethylene glycol-Chito-5-ALA nanoparticles; empty NPs, empty nanoparticles.
Figure 3 shows the 1H NMR characterization of PEG-Chito-5-ALA nanoparticles. When nanoparticles were in DCl solution, specific peaks of 5-ALA appeared, while they disappeared at D2O. These results indicate that 5-ALA was incorporated into the core region of the nanoparticles.
Figure 3.
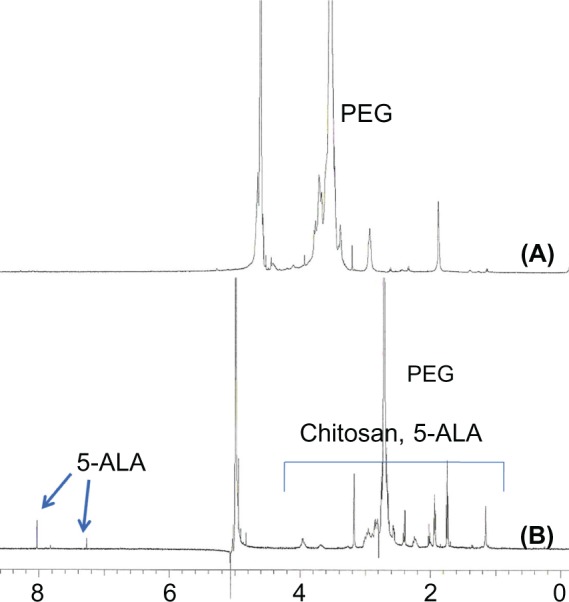
1H NMR of PEG-Chito-5-ALA nanoparticles. PEG-Chito-5-ALA nanoparticles in deuterium oxide (D2O) (A) and deuterium chloride (DCl) (0.1 N) (B).
Abbreviations: PEG-Chito-5-ALA NP, Polyethylene glycol-Chito-5-Aminolevulinic acid nanoparticles.
Figure 4 shows the crystallization properties of PEG-Chito-5-ALA nanoparticles. As shown in Figure 4, 5-ALA showed intrinsic crystalline peaks, while empty PEG-Chito nanoparticles displayed broad peak properties. Furthermore, PEG-Chito-5-ALA nanoparticles also showed broad peak characteristics that were similar to those of empty nanoparticles, while the physical mixture of 5-ALA and empty nanoparticles showed both sharp peaks of 5-ALA and broad peaks of empty nanoparticles. These results also indicate that 5-ALA was incorporated into the core of the nanoparticles and complexed with chitosan.
Figure 4.
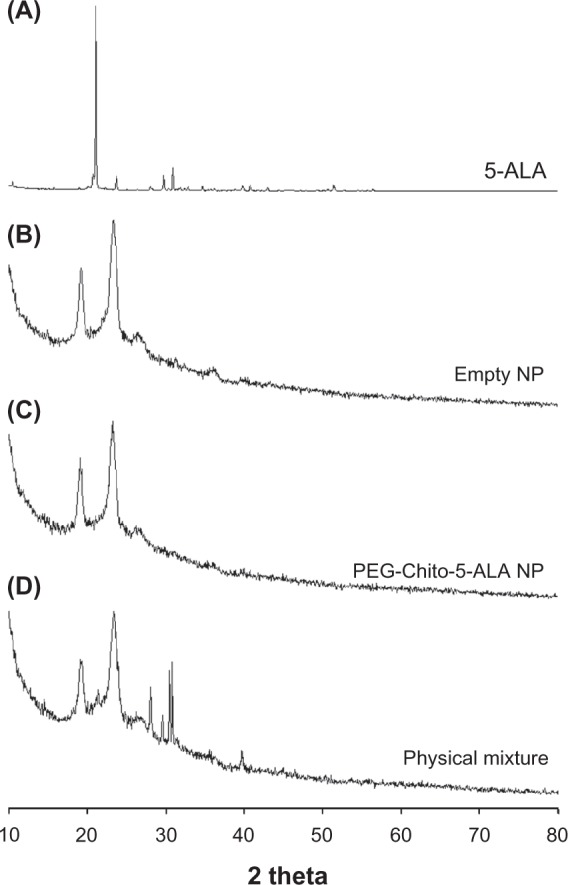
Powder X-ray diffraction spectra of PEG-Chito-5-ALA nanoparticles. 5-ALA (A); empty nanoparticles (B); PEG-Chito-5-ALA nanoparticles (C); physical mixture (D) of 5-ALA and empty nanoparticles.
Abbreviations: 5-ALA, 5-aminolevulinic acid; PEG-Chito-5-ALA NP, Polyethylene glycol-Chito-5-ALA nanoparticles; Empty NP, empty nanoparticles.
Figure 5.
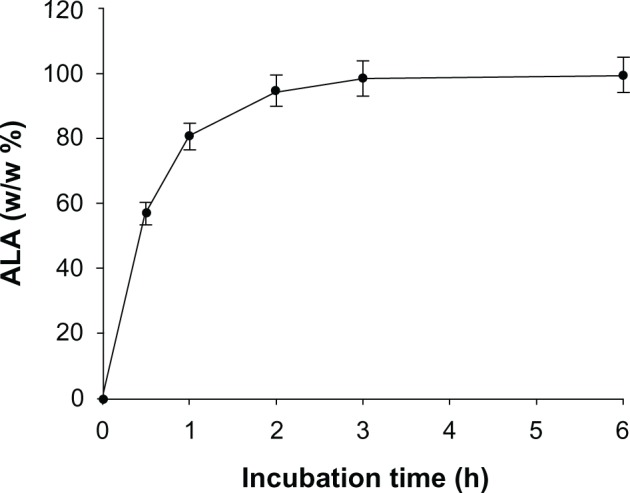
5-ALA release from PEG-Chito-5-ALA NP.
Abbreviations: 5-ALA, 5-aminolevulinic acid; PEG-Chito-5-ALA NP, Polyethylene glycol-Chito-5-ALA nanoparticles.
Figure 5 shows the time course of 5-ALA release from nanoparticles. This result indicated that 5-ALA was complexed within the core of the nanoparticles and its release kinetics continued for 6 hours.
PpIX formation in tumor cells
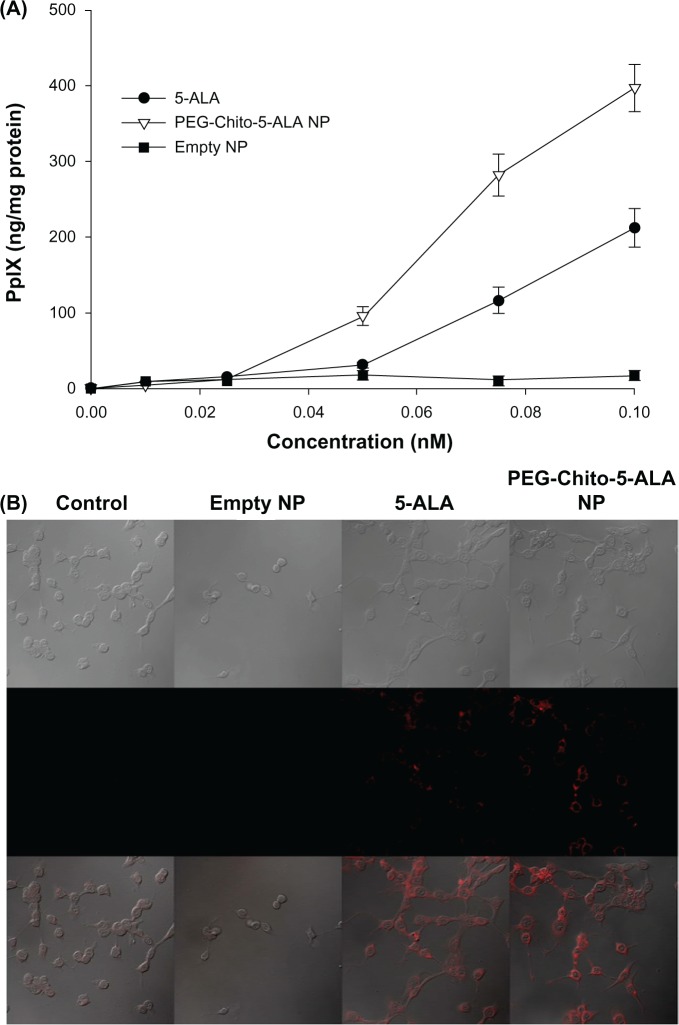
The effect of PEG-Chito-5-ALA nanoparticles on PpIX generation in CT26 cells was investigated in vitro, as shown in Figure 6. The tumor cells were treated with a 0.1 mM equivalent amount of 5-ALA alone or PEG-Chito-5-ALA nanoparticles for 24 hours; the interconversion of 5-ALA to PpIX was then evaluated. PpIX accumulation in the tumor cells was gradually increased dose-dependently after all treatments (Figure 6A). In particular, the PpIX content in the tumor cells after treatment with PEG-Chito-5-ALA nanoparticles was relatively higher than that after 5-ALA treatment. At 0.1 mM 5-ALA, the generated PpIX content in the tumor cells was highest after treatment with PEG-Chito-5-ALA nanoparticles, and the difference in value between 5-ALA and PEG-Chito-5-ALA nanoparticles was also the highest; ie, approximately two times the amount of PpIX was accumulated in the tumor cells after treatment with PEG-Chito-5-ALA nanoparticles. Empty nanoparticles were also tested with tumor cells to examine whether chitosan alone had an effect on PpIX generation in CT26 cells. The results indicated that no interconverted PpIX accumulation was observed with empty nanoparticle treatment (Figure 6A). Fluorescence images of tumor cells also showed increased fluorescence intensity after treatment with PEG-Chito-5-ALA nanoparticles, indicating that PEG-Chito-5-ALA nanoparticles induced increased PpIX accumulation in the tumor cells (Figure 6B). In other cases, no fluorescence intensity was observed after treatment with empty nanoparticles. These results indicate that PEG-Chito copolymer alone did not affect the accumulation of PpIX in the tumor cells and that interconverted PpIX must be accelerated by nanoparticle formation with 5-ALA.
Figure 6.
PpIX generation in CT26 colorectal carcinoma cells. Tumor cells were treated with 5-ALA, PEG-Chito-5-ALA nanoparticles, or empty nanoparticles for 24 hours (A). The PpIX concentration in the cells was corrected by protein content. The intracellular PpIX in CT26 cells was observed using a confocal laser scanning microscope (B).
Notes: CT26 cells were treated with 0.1 mM ALA, PEG-Chito-5-ALA nanoparticles or empty nanoparticles for 24 hours. The cells were then washed with PBS (×600).
Abbreviations: 5-ALA, 5-aminolevulinic acid; PEG-Chito-5-ALA NP, Polyethylene glycol-Chito-5-ALA nanoparticles; Empty NP, empty nanoparticles.
Phototoxicity of ALA and PEG-Chito-5-ALA nanoparticles
The dark toxicity of 5-ALA and PEG-Chito-5-ALA nanoparticles was studied against CT26 cells to examine whether 5-ALA or empty nanoparticles affected the viability of cells. After starvation with serum-free media for 18 hours, the cells were exposed to 0.1 mM ALA, PEG-Chito-5-ALA nanoparticles, or empty nanoparticles in serum-free media for 24 hours. As shown in Figure 6A, none of the treatments affected the survivability of tumor cells. However, the viability of tumor cells was gradually decreased according to the irradiation time, and in particular, PEG-Chito-5-ALA nanoparticles showed higher phototoxicity (Figure 7B). These results indicate that cell death was induced by irradiation in the presence of 5-ALA, and that the nanoparticles accelerated cell death. Furthermore, the results shown in Figure 7 support the results shown in Figure 6; ie, nanoparticles induced a higher entrance of ALA into the tumor cells, higher PpIX accumulation, and higher phototoxicity than 5-ALA. From the results of Figures 6 and 7, ALA or PEG-Chito-5-ALA nanoparticles did not have cytotoxicity in the absence of irradiation, and cell death occurred only after irradiation treatment.
Figure 7.
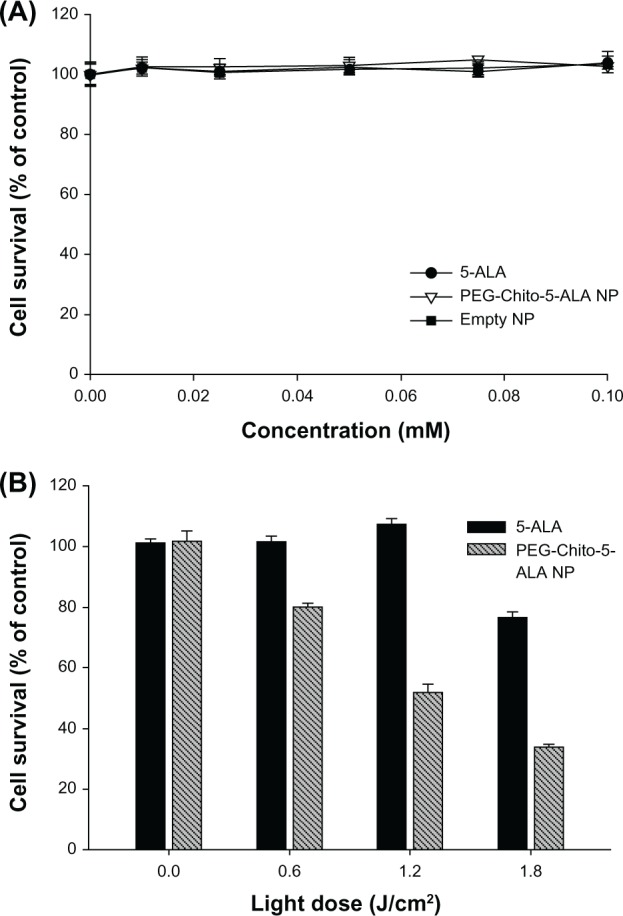
Intrinsic cytotoxicity of 5-ALA, PEG-Chito-5-ALA nanoparticles, or empty nanoparticles. Intrinsic cytotoxicity of 5-ALA, PEG-Chito-5-ALA nanoparticles, and empty nanoparticles, respectively, against CT26 cells (A). Dark- and photo-toxicity of 5-ALA, PEG-Chito-5-ALA nanoparticles, or empty nanoparticles (B).
Notes: CT26 cells were incubated for 24 hours in the presence of 0.1 mM ALA in the dark and were irradiated under different light intensities (0.0–1.8 J/cm2). Cell survival was expressed as the percentage of the control, which was not treated with drug or carrier with exposure to light.
Abbreviations: 5-ALA, 5-aminolevulinic acid; PEG-Chito-5-ALA NP, Polyethylene glycol-Chito-5-ALA nanoparticles.
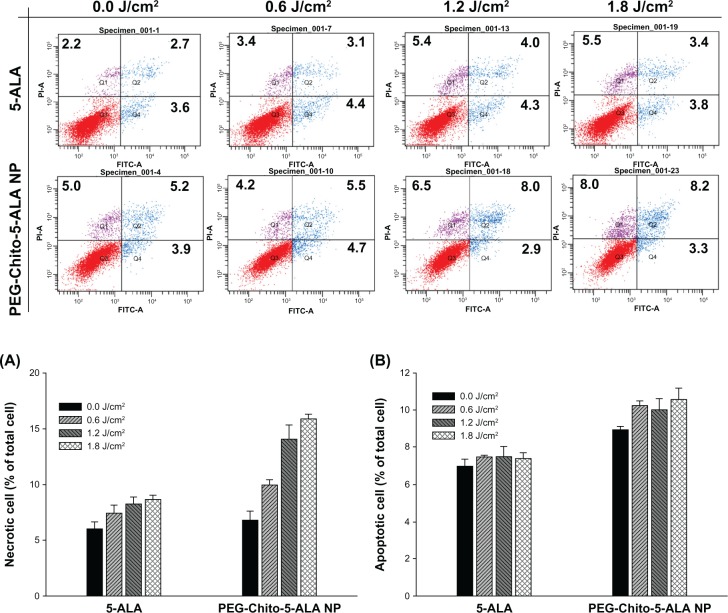
The mechanism of cell death after treatment of 5-ALA or PEG-Chito-5-ALA nanoparticles was investigated. Twenty-four hours after treatment of CT26 cells with 0.1 mM 5-ALA or PEG-Chito-5-ALA nanoparticles, the cells were irradiated under different light intensities. The cells were immediately stained with FITC-annexin V and PI for apoptosis and necrosis analysis, respectively. As shown in Figure 8, apoptotic cells were not significantly increased in the ALA-treated group, regardless of irradiation dose, while PEG-Chito-5-ALA nanoparticles gradually increased the apoptosis of tumor cells according to the irradiation dose. Necrotic cell number was markedly increased after both ALA and PEG-Chito-5-ALA nanoparticle treatment. More specifically, PEG-Chito-5-ALA nanoparticles significantly increased the necrotic cells according to the irradiation power; ie, the highest necrotic cell number was obtained at 1.8 J/cm2 light intensity.
Figure 8.
Necrosis (A) and apoptosis (B) analysis of CT26 cells 24 hours after treatment with 5-ALA, PEG-Chito-5-ALA nanoparticles, or empty nanoparticles with irradiation. Necrosis and apoptosis analysis were repeated three times and results were expressed as mean ± standard deviation. Top figures: typical apoptosis and necrosis analysis of CT26 cells after treatment of 5-ALA or PEG-Chito-5-ALA nanoparticles.
Notes: Cells were preincubated with 0.1 mM 5-ALA or PEG-Chito-5-ALA nanoparticles for 24 hours, and then the cells were exposed to red light (1.8 J/cm2). The cells were stained with FITC-annexinV and PI for flow cytometric analysis.
Abbreviations: 5-ALA, 5-aminolevulinic acid; PEG-Chito-5-ALA NP, Polyethylene glycol-Chito-5-ALA nanoparticles.
Discussion
We know that 5-ALA-based PDTs have been extensively investigated for clinical and biomedical use in cancer treatment, due to their safety.1–3,6,12,13,17,29–32 Since PDTs based on 5-ALA cause minimal unwanted side effects, and because photosensitizers are only activated under light irradiation of specific wavelengths, 5-ALA is increasingly used in malignant and nonmalignant disease.1–3,6,29–32 Wang et al reported the successful treatment of skin cancer and precancer using 5-ALA-based PDT; ie, photosensitizers are not harmful to the human body.29 Tierney et al suggested that PDT is a promising candidate for the treatment of various dermatological diseases, such as actinic keratoses, basal cell carcinoma, squamous cell carcinoma, and photoaging.31 Furthermore, 5-ALA-based PDT can be used to detect and take part in the therapy of cancers.12 Friesen et al discussed potential application of ALA-induced pPIX as a photosensitizing agent in the detection and treatment of brain tumors.12 Otherwise, the high aqueous solubility of 5-ALA and its low bioavailability have frequently been discussed as drawbacks to its clinical application.1,2,6,10 To overcome these problems, chemical modification of 5-ALA, 5-ALA-incorporated nanocolloid, patch-based delivery system, or liposomal carriers were tried, in order to improve delivery of 5-ALA.14,15,18,19
Chitosan has been extensively investigated as a gene-or drug-delivery carrier.25–28,33–35 In particular, chitosan is known to enhance mucosal transport or delivery of bioactive agents.34,35 Therefore, chitosan is regarded as a promising vehicle for transmucosal or oral delivery of drugs. Due to its cationic properties, chitosan is appropriate for the incorporation of anionic drugs. We previously reported that all-trans retinoic acid (ATRA) was simply incorporated into glycol chitosan nanoparticles through the ion complex formation between ATRA and glycol chitosan.36 Furthermore, methotrexate, which is relatively less hydrophobic than ATRA, can also be incorporated into MPEG-grafted chitosan.25 Therefore, chitosan can be used for the delivery of 5-ALA.
In the present study, we prepared 5-ALA-incorporated nanoparticles using PEG-Chito copolymer, as shown in Figures 1 and 2. The 5-ALA was incorporated into the core of the nanoparticles through ion complex formation with chitosan; the core-shell structure of this nanoparticle can be seen in Figure 2. Since chitosan has cationic properties, it can form ionic complexes with anionic molecules. Experimental values of the loading efficiency of 5-ALA were significantly lower than those of the theoretical values, as shown in Table 1, indicating that the hydrophilic property of 5-ALA and its weak ionic interaction with chitosan backbone account for its low loading efficiency. Interestingly, a discrete region was observed in TEM photo in Figure 2: PEG-Chito-5-ALA nanoparticles are formed with a dark core region, and the surroundings are expressed in a grey color; ie, these nanoparticles have core-shell structures. Since PEG-Chito copolymer is composed of the hydrophilic MPEG domain and cationic chitosan domain, chitosan and 5-ALA can form the core of the nanoparticles while MPEG forms the boundary layer of the nanoparticles, as shown in Figures 2B and 3. There is no excess free drug in the PEG-Chito-5-ALA nanoparticles, as shown in Figure 4. Furthermore, specific peaks of 5-ALA in the nanoparticles were found in the DCl solution, while the peaks disappeared in D2O, indicating that 5-ALA was incorporated into the core of the nanoparticles.
PEG-Chito-5-ALA nanoparticles induced enhanced delivery of 5-ALA into the tumor cells, increased PpIX accumulation, and resulted in higher cell death, compared to 5-ALA alone. In a preliminary study, we observed that the accumulation of pPIX was increased time-dependently, and the incubation time after treatment of 5-ALA or PEG-Chito-5-ALA nanoparticles was determined to 224 hours (data not shown). In another report, 3-hour incubation of 5-ALA was known to be sufficient to produce a high amount of pPIX in several cell lines and in clinical applications. However, longer incubation times with 5-ALA or PEG-Chito-5-ALA nanoparticles were beneficial to increasing the pPIX accumulation and phototoxicity of CT26 colon cancer cells.
Although these differences are not understood at present, possible reasons for our results are our use of a different type of cancer cells and the delayed released of PEG-Chito-5-ALA nanoparticles. In our previous report, pPIX accumulation and phototoxicity were time-dependently increased when 5-ALA was used to treat cancer cells.35 Furthermore, other researchers have reported that chitosan hydrogels or folic acid-conjugated chitosan can be an efficient carrier for delivering 5-ALA to tumor cells.37,38 Yang et al also observed time-dependent accumulation of pPIX in the tumor cells and pPIX accumulation was highest at 112 hours of incubation.38 Our results revealed that PEG-Chito-5-ALA nanoparticles also induce enhanced phototoxicity against CT26 colon cancer cells and then accelerate the apoptosis or necrosis of the cells, as shown in Figure 8.
After administering light irradiation to 5-ALA, PpIX was accumulated in the tumor cells, followed by production of ROS. This excessive ROS in the tumor cells induced apoptosis or necrosis of the cells, and then destroyed the tumor cells.35 Although 5-ALA is distributed throughout the whole body, local irradiation at a specific tumor site increases ROS generation in cancer cells, but not in normal cells and tissues. PDT with 5-ALA can then selectively inhibit the viability of tumor cells and enhance the survivability of the patient.1,39–41 However, even though 5-ALA preferentially targets cancer cells rather than normal cells, its drawbacks still include low permeability into the mucosal layer or tumor tissue, and low bioavailability. Furthermore, the targetability of 5-ALA is not very specific for cancer cells, and causes 5-ALA to be distributed throughout the whole body. Since chitosan is known to increase mucosal delivery, chitosan-based nanophotosensitizers can be considered ideal delivery vehicles. For example, local treatment of colon cancer using endoscopic equipment enables avoidance of unwanted side effects and specific accumulation in the administration site.
Our results demonstrate that chitosan-based nanoparticles enhance the delivery of 5-ALA and accelerate apoptotic cell death. We suggest that PEG-Chito-5-ALA nanoparticles are promising candidates for PDT using 5-ALA.
Conclusion
PEG-Chito copolymer was synthesized to make 5-ALA-incorporated nanoparticles for PDT. PEG-Chito-5-ALA nanoparticles have spherical shapes with small diameters of less than 250 nm. The 5-ALA was incorporated into the core of the nanoparticles. The PDT effect of 5-ALA or PEG-Chito-5-ALA nanoparticles was assessed using murine colon carcinoma CT26 cells. PEG-Chito-5-ALA nanoparticles enhanced the uptake of ALA into the tumor cells and then increased the intracellular PpIX accumulation, compared to 5-ALA alone. The 5-ALA, empty nanoparticles, and PEG-Chito-5-ALA nanoparticles had no dark toxicity against CT26 cells, while the viability of tumor cells was decreased according to the increase of irradiation time. Furthermore, PEG-Chito-5-ALA nanoparticles accelerated the apoptosis/ necrosis of tumor cells. PEG-Chito-5-ALA nanoparticles can be considered an effective vehicle for delivering 5-ALA to tumor cells.
Acknowledgments
This study was supported by a grant of the Korea Healthcare Technology R & D Project, Ministry for Health Welfare and Family Affairs, Republic of Korea (A091047).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Peng Q, Warloe T, Berg K, et al. 5-Aminolevulinic acid-based photodynamic therapy. Clinical research and future challenges. Cancer. 1997;79:2282–2308. doi: 10.1002/(sici)1097-0142(19970615)79:12<2282::aid-cncr2>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 2.Brunner TB, Eccles CL. Radiotherapy and chemotherapy as therapeutic strategies in extrahepatic biliary duct carcinoma. Strahlenther Onkol. 2010;186:672–680. doi: 10.1007/s00066-010-2161-y. [DOI] [PubMed] [Google Scholar]
- 3.Morse M, Langer L, Starodub A, Hobeika A, Clay T, Lyerly HK. Current immunotherapeutic strategies in colon cancer. Surg Oncol Clin N Am. 2007;16:873–900. doi: 10.1016/j.soc.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 4.Touchefeu Y, Harrington KJ, Galmiche JP, Vassaux G. Review article: gene therapy, recent developments and future prospects in gastrointestinal oncology. Aliment Pharmacol Ther. 2010;32:953–968. doi: 10.1111/j.1365-2036.2010.04424.x. [DOI] [PubMed] [Google Scholar]
- 5.Lee JH, Kang DH, Kim JY, et al. Endoscopic bilateral metal stent placement for advanced hilar cholangiocarcinoma: a pilot study of a newly designed Y stent. Gastrointest Endosc. 2007;66:364–369. doi: 10.1016/j.gie.2006.12.061. [DOI] [PubMed] [Google Scholar]
- 6.Yoo JJ, Kim C, Chung CW, Jeong YI, Kang DH. 5-aminolevulinic acid-incorporated poly(vinyl alcohol) nanofiber-coated metal stent for application in photodynamic therapy. Int J Nanomedicine. 2012;7:1997–2005. doi: 10.2147/IJN.S30298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Almeida RD, Manadas BJ, Carvalho AP, Duarte CB. Intracellular signaling mechanisms in photodynamic therapy. Biochim Biophys Acta. 2004;1704:59–86. doi: 10.1016/j.bbcan.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 8.Buytaert E, Dewaele M, Agostinis P. Molecular effectors of multiple cell death pathways initiated by photodynamic therapy. Biochim Biophys Acta. 2007;1776:86–107. doi: 10.1016/j.bbcan.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Agostinis P, Berg K, Cengel KA, et al. Photodynamic therapy of cancer: an update. CA Cancer J Clin. 2011;61:250–281. doi: 10.3322/caac.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zoepf T, Jakobs R, Rosenbaum A, Apel D, Arnold JC, Riemann JF. Photodynamic therapy with 5-aminolevulinic acid is not effective in bile duct cancer. Gastrointest Endosc. 2001;54:763–766. doi: 10.1067/mge.2001.119605. [DOI] [PubMed] [Google Scholar]
- 11.Peng Q, Warloe T, Moan J, et al. Antitumor effect of 5-aminolevulinic acid-mediated photodynamic therapy can be enhanced by the use of a low dose of photofrin in human tumor xenografts. Cancer Res. 2001;61:5824–5832. [PubMed] [Google Scholar]
- 12.Friesen SA, Hjortland GO, Madsen SJ, et al. 5-Aminolevulinic acid-based photodynamic detection and therapy of brain tumors. Int J Oncol. 2002;21:577–582. [PubMed] [Google Scholar]
- 13.Peng Q, Berg K, Moan J, Kongshaug M, Nesland JM. 5-Aminolevulinic acid-based photodynamic therapy: principles and experimental research. Photochem Photobiol. 1997;65:235–251. doi: 10.1111/j.1751-1097.1997.tb08549.x. [DOI] [PubMed] [Google Scholar]
- 14.Gaullier JM, Berg K, Peng Q, et al. Use of 5-aminolevulinic acid esters to improve photodynamic therapy on cells in culture. Cancer Res. 1997;57:1481–1486. [PubMed] [Google Scholar]
- 15.Casas A, Batlle AM, Butler AR, et al. Comparative effect of ALA derivatives on protoporphyrin IX production in human and rat skin organ cultures. Br J Cancer. 1999;80:1525–1532. doi: 10.1038/sj.bjc.6690556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perotti C, Fukuda H, DiVenosa G, MacRobert AJ, Batlle A, Casas A. Porphyrin synthesis from ALA derivatives for photodynamic therapy. In vitro and in vivo studies. Br J Cancer. 2004;90:1660–1665. doi: 10.1038/sj.bjc.6601722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hürlimann AF, Hänggi G, Panizzon RG. Photodynamic therapy of superficial basal cell carcinomas using topical 5-aminolevulinic acid in a nanocolloid lotion. Dermatology. 1998;197:248–254. doi: 10.1159/000018006. [DOI] [PubMed] [Google Scholar]
- 18.Morrow DI, McCarron PA, Woolfson AD, et al. Novel patch-based systems for the localised delivery of ALA-esters. J Photochem Photobiol B. 2010;101:59–69. doi: 10.1016/j.jphotobiol.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 19.Casas A, Batlle A. Aminolevulinic acid derivatives and liposome delivery as strategies for improving 5-aminolevulinic acid-mediated photodynamic therapy. Curr Med Chem. 2006;13:1157–1168. doi: 10.2174/092986706776360888. [DOI] [PubMed] [Google Scholar]
- 20.Oo MK, Yang X, Du H, Wang H. 5-aminolevulinic acid-conjugated gold nanoparticles for photodynamic therapy of cancer. Nanomedicine (Lond) 2008;3:777–786. doi: 10.2217/17435889.3.6.777. [DOI] [PubMed] [Google Scholar]
- 21.Ding H, Sumer BD, Kessinger CW, et al. Nanoscopic micelle delivery improves the photophysical properties and efficacy of photodynamic therapy of protoporphyrin IX. J Control Release. 2011;151:271–277. doi: 10.1016/j.jconrel.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCarthy JR, Perez JM, Brückner C, Weissleder R. Polymeric nanoparticle preparation that eradicates tumors. Nano Lett. 2005;5:2552–2556. doi: 10.1021/nl0519229. [DOI] [PubMed] [Google Scholar]
- 23.Gref R, Minamitake Y, Peracchia MT, Trubetskoy V, Torchilin V, Langer R. Biodegradable long-circulating polymeric nanospheres. Science. 1994;263:1600–1603. doi: 10.1126/science.8128245. [DOI] [PubMed] [Google Scholar]
- 24.Nishiyama N, Okazaki S, Cabral H, et al. Novel cisplatin-incorporated polymeric micelles can eradicate solid tumors in mice. Cancer Res. 2003;63:8977–8983. [PubMed] [Google Scholar]
- 25.Seo DH, Jeong YI, Kim DG, Jang MJ, Jang MK, Nah JW. Methotrexate-incorporated polymeric nanoparticles of methoxy poly(ethylene glycol)-grafted chitosan. Colloids Surf B Biointerfaces. 2009;69:157–163. doi: 10.1016/j.colsurfb.2008.10.020. [DOI] [PubMed] [Google Scholar]
- 26.Ganji F, Abdekhodaie MJ. Synthesis and characterization of a new thermosensitive chitosan–PEG diblock copolymer. Carbohydrate Polym. 2008;74:435–441. [Google Scholar]
- 27.Wang JJ, Zeng ZW, Xiao RZ, et al. Recent advances of chitosan nanoparticles as drug carriers. Int J Nanomedicine. 2011;6:765–774. doi: 10.2147/IJN.S17296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoo HS, Lee JE, Chung H, Kwon IC, Jeong SY. Self-assembled nanoparticles containing hydrophobically modified glycol chitosan for gene delivery. J Control Release. 2005;103:235–243. doi: 10.1016/j.jconrel.2004.11.033. [DOI] [PubMed] [Google Scholar]
- 29.Wang XL, Wang HW, Guo MX, Xu SZ. Treatment of skin cancer and pre-cancer using topical ALA-PDT – a single hospital experience. Photodiagnosis Photodyn Ther. 2008;5:127–133. doi: 10.1016/j.pdpdt.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 30.Yang J, Xia Y, Liu X, Jiang S, Xiong L. Desferrioxamine shows different potentials for enhancing 5-aminolevulinic acid-based photodynamic therapy in several cutaneous cell lines. Lasers Med Sci. 2010;25:251–257. doi: 10.1007/s10103-009-0721-0. [DOI] [PubMed] [Google Scholar]
- 31.Tierney E, Barker A, Ahdout J, Hanke CW, Moy RL, Kouba DJ. Photodynamic therapy for the treatment of cutaneous neoplasia, inflammatory disorders, and photoaging. Dermatol Surg. 2009;35:725–746. doi: 10.1111/j.1524-4725.2009.01117.x. [DOI] [PubMed] [Google Scholar]
- 32.Kosaka S, Akilov OE, O’Riordan K, Hasan T. A mechanistic study of delta-aminolevulinic acid-based photodynamic therapy for cutaneous leishmaniasis. J Invest Dermatol. 2007;127:1546–1549. doi: 10.1038/sj.jid.5700719. [DOI] [PubMed] [Google Scholar]
- 33.Yang SJ, Lin FH, Tsai KC, et al. Folic acid-conjugated chitosan nanoparticles enhanced protoporphyrin IX accumulation in colorectal cancer cells. Bioconjugate Chem. 2010;21:679–689. doi: 10.1021/bc9004798. [DOI] [PubMed] [Google Scholar]
- 34.Sharma S, Kulkarni J, Pawar AP. Permeation enhancers in the trans-mucosal delivery of macromolecules. Pharmazie. 2006;61:495–504. [PubMed] [Google Scholar]
- 35.Takeuchi H, Matsui Y, Yamamoto H, Kawashima Y. Mucoadhesive properties of carbopol or chitosan-coated liposomes and their effectiveness in the oral administration of calcitonin to rats. J Control Release. 2003;86:235–242. doi: 10.1016/s0168-3659(02)00411-x. [DOI] [PubMed] [Google Scholar]
- 36.Chung KD, Jeong YI, Chung CW, Kim do H, Kang DH. Anti-tumor activity of all-trans retinoic acid-incorporated glycol chitosan nanoparticles against HuCC-T1 human cholangiocarcinoma cells. Int J Pharm. 2012;422:454–461. doi: 10.1016/j.ijpharm.2011.10.057. [DOI] [PubMed] [Google Scholar]
- 37.Filip A, Clichici S, Muresan A, et al. Effects of PDT with 5-aminolevulinic acid and chitosan on Walker carcinosarcoma. Exp Oncol. 2008;30(3):212–219. [PubMed] [Google Scholar]
- 38.Yang SJ, Lin FH, Tsai KC, et al. Folic acid-conjugated chitosan nanoparticles enhanced protoporphyrin IX accumulation in colorectal cancer cells. Bioconjug Chem. 2010;21:679–689. doi: 10.1021/bc9004798. [DOI] [PubMed] [Google Scholar]
- 39.Kim CH, Chung CW, Choi KH, et al. Effect of 5-aminolevulinic acid-based photodynamic therapy via reactive oxygen species in human cholangiocarcinoma cells. Int J Nanomedicine. 2011;6:1357–1363. doi: 10.2147/IJN.S21395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grimm S, Mvondo D, Grune T, Breusing N. The outcome of 5-ALA mediated photodynamic treatment in melanoma cells is influenced by vitamin C and heme oxygenase-1. Biofactors. 2011;37(1):17–24. doi: 10.1002/biof.129. [DOI] [PubMed] [Google Scholar]
- 41.Moon YH, Park JH, Kim SA, Lee JB, Ahn SG, Yoon JH. Anticancer effect of photodynamic therapy with hexenyl ester of 5-aminolevulinic acid in oral squamous cell carcinoma. Head Neck. 2010;32(9):1136–1142. doi: 10.1002/hed.21301. [DOI] [PubMed] [Google Scholar]