Abstract
Throughout the hypothalamus there are several regions known to contain sex differences in specific cellular, neurochemical, or cell grouping characteristics. The current study examined the potential origin of sex differences in calbindin expression in the preoptic area and hypothalamus as related to sources of nitric oxide. Specific cell populations were defined by immunoreactive (ir) calbindin and neuronal nitric oxide synthase (nNOS) in the preoptic area/anterior hypothalamus (POA/AH), anteroventral periventricular nucleus (AVPv), and ventromedial nucleus of the hypothalamus (VMN). The POA/AH of adult mice was characterized by a striking sex difference in the distribution of cells with ir-calbindin. Examination of the POA/AH of androgen receptor deficient Tfm mice suggests that this pattern was in part androgen receptor dependent, since Tfm males had reduced ir-calbindin compared with wild-type males and more similar to wild-type females. At P0 ir-calbindin was more prevalent than in adulthood, with males having significantly more ir-calbindin and nNOS than have females. Cells that contained either ir-calbindin or ir-nNOS in the POA/AH were in adjacent cell groups, suggesting that NO derived from the enzymatic activity of nNOS may influence the development of ir-calbindin cells. In the region of AVPv, at P0, there was a sex difference with males having more ir-nNOS fibers than have females while ir-calbindin was not detected. In the VMN, at P0, ir-nNOS was greater in females than in males, with no significant difference in ir-calbindin. We suggest that NO as an effector molecule and calbindin as a molecular biomarker illuminate key aspects of sexual differentiation in the developing mouse brain.
Keywords: sex differences, hypothalamus, preoptic area, nitric oxide, calbindin
INTRODUCTION
Throughout the hypothalamus there are several regions known to contain sex differences in specific cellular, neurochemical, or cell grouping characteristics (reviewed Tobet and Fox, 1992). One set of primary factors that contribute to brain sexual differentiation are steroid hormones that are produced after the development of the gonads and act directly in the developing brain (Carrer and Cambiasso, 2002; Tobet, 2002; Morris et al., 2004; Simerly, 2005). In addition, the actions of genes located on the sex chromosomes are being noted with increasing frequency as potential initiating mechanisms for the differentiation of sex differences in brain (Arnold, 2004). The means by which sex differences develop may be due to differences in virtually all known neurodevelopmental mechanisms, including neurogenesis, migration, cell fate determination, or cell death/survival between males and females (reviewed in Tobet and Hanna, 1997; Tobet, 2002; Simerly, 2002).
There are several ways to categorize the molecular mechanisms that drive brain development with or without sexual differentiation. One class of molecules that control gene expression is transcription factors. For example, the transcription factor steroidogenic factor-1 (SF-1) is essential for development of the ventromedial nucleus of the hypothalamus (VMN; Ikeda et al., 1995; Davis et al., 2004) while the transcription factors SIM1 and ARNT2 are important for the development of the paraventricular nucleus of the hypothalamus (PVN; Michaud et al., 1998, 2000; Hosoya et al., 2001). The expression pattern of other transcription factors suggests roles in the differentiation of regions that are compelling, but less clear in their definition of selective cell groups (e.g., estrogen receptors (ER), Brown et al., 1999; COUP-TF1, Pereira et al., 2000; islet-1, Davis et al., 2004).
Complementary to transcription factors are another class of molecules that could be referred to as effector molecules, which control and contribute to signaling from one cell to another. Of those molecules that might be considered “effectors,” the neurotransmitter γ-aminobutyric acid (GABA) might be particularly important in hypothalamic development (McCarthy et al., 2002; McClellan et al., 2006). Another potential molecular effector of differentiation is nitric oxide (NO), which is a product of the enzymatic conversion of L-arginine to citrulline. NO plays many roles in development as well as adulthood. NO helps direct cell migration (Bicker, 2005; Bulotta et al., 2005), cell proliferation (Ciani et al., 2006), and survival (Contestabile and Ciani, 2004), which are all important factors for sexual differentiation. NO is produced by three forms of nitric oxide synthase (NOS): neuronal (nNOS), inducible (iNOS), and endothelial (eNOS) (reviewed Mungrue et al., 2003; Huang, 2004). Since NO is a gaseous molecule that is difficult to isolate in vivo, many studies evaluate NOS expression as an indicator of the location of NO production. Sex differences in the adult preoptic area/anterior hypothalamic region (POA/AH) have been found for nNOS mRNA-positive cells in the rat (Ishihara et al., 2002) and immunoreactive (ir) nNOS cells in the mouse (Scordalakes et al., 2002). In the current study we examined the distribution of ir-nNOS in developing mice to determine if they are in position to contribute to the development of sex differences in the hypothalamus.
A third class of molecules, those which might be called “biomarkers” of sexual differentiation, have undetermined functions in development. A potentially important sexually dimorphic biomarker is the calcium binding protein calbindin (a 28 kDa EF hand protein). Calbindin expression is sexually dimorphic in the preoptic area or hypothalamus of the rat (Brager et al., 2000; Sickel and McCarthy, 2000; Scallet et al., 2004; Pei et al., 2006) and rabbit (Bisenius et al., 2006). Although the exact function of calbindin remains unclear, one of the primary suggestions for the function of calbindin has been a potential involvement in cell survival or death (Lephart, 1996; Sickel and McCarthy, 2000). In the current study we examined the distribution of ir-calbindin in developing mice to determine whether this biomarker could be used to follow the development of sex differences in the hypothalamus. To determine the hormone receptor dependence of expression for this key biomarker, we examined the sex difference in the POA/AH distribution of calbindin in androgen receptor disrupted (Tfm) mice.
In the current study we focused on three regions characterized in previous studies for sex differences and neuroendocrine functions. Rostrally we examined the region of the anteroventral periventricular nucleus (AVPv) of the preoptic area (Simerly et al., 1985; Sumida et al., 1993; Lund et al., 2000) that we have previously characterized for a unique apposition of cells containing estrogen receptor alpha (ERα) versus islet-1 (Davis et al., 2004). More caudally, we examined the boundary region at the caudal POA/AH that we have previously characterized for developmental sex differences in mice for ERβand GABABR1 (Wolfe et al., 2005). Finally, in the medial basal hypothalamus, we analyzed the VMN where ongoing studies are characterizing factors that control its development (McClellan et al., 2006). The results show selective sex differences in the expression of either calbindin or nNOS in the POA/AH, AVPv region, and the VMN.
METHODS
Animals
Thy1-YFP mice generated by Feng et al. (2000) were purchased from Jackson Laboratories (Bar Harbor, ME; referred to as B6.Cg-Tg(Thy1-YFP)16Jrs/J). Mice were housed in plastic cages with bedding (autoclaved sanichips, Harlan Teklad, Madison, WI) in a 14:10 light–dark cycle (lights on 07:00). Mice were provided standard rodent chow (#8640, Harlan Teklad, Madison, WI) and water ad libitum, at Colorado State University’s laboratory animal facility. Pups taken within 24 h of birth, postnatal day 0 (P0), were anesthetized using cryoanesthesia. Sex was established by direct visual inspection of the gonads. Animals were transcardially perfused with 5 mL of 4% formaldehyde (Polysciences Inc., Warrington, PA) and 16% methanol-free formaldehyde stock in 0.1M phosphate buffer (PB, pH 7.4) using a 27-gauge needle hand-held syringe under constant observation with a dissecting scope. The heads were post-fixed overnight in 4% formaldehyde, and stored in 0.1M PB prior to sectioning in the coronal plane.
Tfm mice were generated at the University of Virginia by mating females carrying the Tfm mutation, originally purchased from Jackson Laboratory (C57BL/6J-Aw-J-Ta +/+ ArTfm), with WT males, all mice were in a C57BL/6J background. To distinguish Tfm, wild type, and carrier females, the offspring were screened by PCR amplification of tail DNA as previously described (Scordalakes and Rissman, 2004). The mice were maintained on a 12:12 h light–dark cycle (lights off at 1200 h EDT). Food (Harlan Teklad Mouse/Rat Sterilizable Diet #7012) and water were provided ad libitum. Tfm animals between the ages of 18–21 days were taken from their mother at time of weaning. Animals were anesthetized using an overdose of sodium pentobarbital. Animals were perfused with heparinized-saline solution (~10 mL) followed by 4% paraformaldehyde (40 mL). After perfusions, brains were removed and post-fixed in 4% paraformaldehyde for 1 h, then placed in 30% sucrose overnight. Cryoprotected brains were frozen in 2-methylbutane and stored at −70°C until sectioning. Tissue was cut in a coronal plane at 30 μm on a cryostat. Alternating sections were collected into vials and stored at −20°C in antifreeze until processing. All animal procedures were approved by the appropriate Institutional Animal Care and Use Committee.
Immunocytochemistry
P0 brains were dissected out of the skull, embedded in 5% agarose, and sectioned at 50 μm using a vibrating microtome (Leica VT-1000S). ir-nNOS was determined using a rabbit polyclonal C-terminal directed antiserum acquired from ImmunoStar Inc. (Hudson, WI; diluted1/10,000). ir-Calbindin was determined using a mouse monoclonal D-28K directed antiserum from Sigma-Aldrich (Saint Louis, MO; diluted 1/40,000). Controls for nNOS and calbindin immunocytochemistry included exclusion of primary antibody, in which case no reaction products were detected. Other controls included comparing antibodies from different sources. ir-Calbindin using the mouse monoclonal D-28K from Sigma-Aldrich was compared with a rabbit polyclonal affinity purified D-28K calbindin antiserum from Chemicon International (Temecula, CA; diluted 1/2500). nNOS C-terminal antiserum from Immunostar Inc. was compared with a rabbit polyclonal N-terminal nNOS antiserum from Zymed Laboratories, Inc. (San Francisco, CA; diluted 1/1000). In both cases, immunoreactivity was similar among antisera directed against the same protein. As an additional control for immunoreactive nNOS, we used brain sections from mice in which Gyurko et al. (2002) deleted exon 6 of the nNOS gene, in these mice ir-nNOS was not detected.
Immunocytochemical procedures were conducted on free-floating sections in a similar manner to those previously reported (Davis et al., 2004; Wolfe et al., 2005). In brief, the tissue sections were pretreated at 4°C with 0.1M glycine in 0.05M phosphate-buffered-saline (PBS; pH 7.5) for 30 min and 0.5% sodium borohydride in PBS for 15 min. For tissue sections assigned for ir-calbindin, an antigen retrieval procedure was used to enhance the immunoreactive signal (Dellovade et al., 2001). To perform antigen retrieval, sections were washed in 0.05M sodium citrate (pH 8.6) for 1 h at room temperature (RT) and then washed in preheated 0.05M sodium citrate for 30 min at 80°C. Sections were returned to RT and washed thrice in PBS for about 5 min. All tissue sections were subsequently washed in 5% normal goat serum (NGS) with 0.3% TritonX-100 (Tx) and 1% H202 in PBS for a minimum of 30 min. Sections were then incubated with primary antibodies diluted with 1% BSA and 0.3% Tx in PBS over 2–3 nights at 4°C.
After primary antibody incubation, tissue sections were washed at RT four times in PBS containing 1% NGS and 0.02% Tx for 15 min each and incubated in the proper secondary antibody diluted with 1% NGS and 0.32% Tx in PBS for 2 h. Donkey anti-rabbit IgG biotinylated secondary antibody from Jackson ImmunoResearch (West Grove, Pennsylvania; diluted 1/500) was used for ir-nNOS and donkey anti-mouse IgG biotinylated secondary antibody (Jackson ImmunoResearch; diluted 1/2500) was used for ir-calbindin. Sections were subsequently rinsed and incubated in peroxidase-conjugated streptavidin (Jackson ImmunoResearch; diluted 1/2500) in PBS with 0.32% Tx for 1 h. After 1 h of washes using 0.05M Tris-buffered saline (TBS, pH 7.5), sections were placed in a diaminobenzidine (DAB) solution (0.025% 3,3′-DAB, 0.2% nickel ammonium sulfate, and 0.02% hydrogen peroxide in TBS) for 5 min to produce a black reaction product. Following immunocytochemistry, tissue sections were mounted on gelatin-subbed slides, dehydrated, and coverslipped with Permount.
Analysis
Only one coronal section per animal was analyzed for each region. Tfm mice were compared with wild-type litter-mates, and analyzed only in the caudal POA/AH. The immunoreactive markers used defined unique cell subpopulations within the regions of interest. Digital mages (10×) were obtained using a Spot Insight QE camera (Diagnostic Instruments, Sterling Heights, MI) with 1600 × 1200 pixel resolution, mounted on an Olympus BH-2 photomicroscope. Adobe Photoshop (version CS) was used to optimize contrast in digital images. To ensure that digital image processing did not influence the results, all digital images were contrast adjusted following a standard procedure (levels adjustment followed by “unsharp mask”) and investigators were blind to treatment group. Since the distribution of immunoreactive cells varies relative to the angle at which the brain is cut, tissue sections were grouped by angle and referred to as angle A, B, or C (see Fig. 1). Comparisons were only made between brains at matched angles. Data are presented for the angle that most clearly demonstrated significant sex differences.
Figure 1.
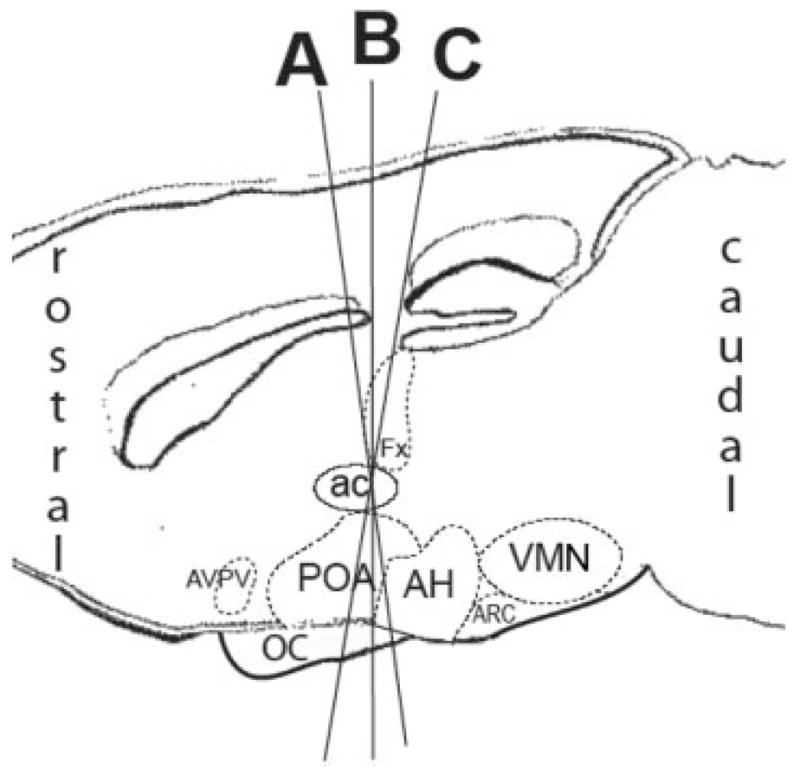
Sagittal section of the mouse brain illustrates different angles of sectioning (A, B, or C), which create alternate ventral/dorsal alignments of brain regions. In coronal plane A, the preoptic area overlays different regions of the anterior hypothalamus than in coronal plane B or C. AVPV, anteroventral periventricular nucleus of the preoptic area; POA, preoptic area; ac, anterior commisure, OC, optic chiasm; Fx, fornix; AH, anterior hypothalamus; ARC, arcuate; VMN, ventromedial nucleus of the hypothalamus.
The POA is located at the rostral tip of the hypothalamus at the base of the diencephalon and stretches dorsally to the anterior commissure (AC). Sections were analyzed only in the caudal POA at the boundary region between the POA and anterior hypothalamus, ventral to the midline crossing of the AC. Only the side with the most immunoreactivity in each POA/AH section, at angle C (see Fig. 1) was analyzed. Following contrast enhancement, images were cropped along the third ventricle and at the base of the AC. IPLab software (Scanalytics, Fairfax, VA) was used to quantify the clearly defined immunoreactive cells and fibers in the region of interest. The distributions, as well as the total area, of immunoreactive markers were analyzed using a grid system that used a reference point to create columns and rows 100 μm across (Davis et al., 2004; Wolfe et al., 2005; Bisenius et al., 2006). For POA/AH images, the reference point was the point at which the third ventricle meets the AC. The angle of section is a key element in evaluating sex differences in cell grouping in the POA/AH (Tobet and Fox, 1992). For the current study, an angle that contains the caudal aspects of the AC and rostral aspects of the optic chiasm contained the region of the POA/AH that revealed the greatest sex difference. Statistical analyses for each age and marker were based on the number of rows (dorsal–ventral) and columns (medial–lateral) needed to cover the critical region of interest. The size of the grid reflects the area of immunoreactivity analyzed. At P0, calbindin was analyzed in a 6 row × 6 column grid and nNOS was analyzed in a 7 row × 6 column grid. In adulthood, C57BL/6J animals were analyzed in a 7 row × 7 column grid, while Tfm animals and littermate controls were analyzed in a 9 row × 7 column grid. The data as immunoreactive areas were then analyzed using a sex × column (in females using a “group” × column) two-way ANOVA with column (i.e., location) analyzed as a repeated measure (JMP 5.1.2, SAS Institute). Tfm males were compared with wild-type males.
The region of AVPv lies just lateral to the opening of the third ventricle and is in the periventricular zone of the POA. After capturing the image and optimizing the contrast at angle C (see Fig. 1), each of the AVPv image was cropped along the third ventricle and the base of the brain. IPLab software was then used to circle and determine the area of the region of dense fibers, if present, in each image. The average area of the region of dense fibers was used to approximate the same sized area for all sections even when a region of dense fibers was not clearly discernible. The area of the immunoreactive fibers and cells within the circled region was obtained. The data was analyzed using a one-way ANOVA for sex effects (SPSS 11.0). ir-Calbindin was not seen in the AVPv at P0.
The VMN is positioned in the medial hypothalamus, close to the base of the diencephalon and above the arcuate nucleus and median eminence. The VMN, based on shape and position within the forebrain, has been categorized into four major planes moving from rostral to caudal (McClellan et al., 2006). Only the central most region of the VMN at angle B (see Fig. 1) was analyzed. After contrast enhancement, VMN images were adjusted so that the edge of the image was aligned along the third ventricle and at the base of the brain. In contrast to the POA/AH analysis, IPLab software and the grid system were used to define the region of interest to measure the total area of the clearly defined immunoreactive cells and fibers. The reference points for the VMN were the third ventricle and base of the brain. To be considered the first row of the grid, which is along the base of the brain, at least half of the boxes in that row had to contain tissue. In the VMN at P0 calbindin occupied a 5 row × 6 column grid and nNOS occupied a 7 row × 7 column grid. The sections were then separated by sex and analyzed in a one-way ANOVA for total area of immunoreactivity (JMP 5.1.2, SAS Institute).
RESULTS
POA/AH
A striking sex difference in ir-calbindin was seen in the POA/AH of adult mice. In adult C57BL/6J mice, males had a notable cell grouping in the medial region of the POA/AH that was not discernible in females [arrows in Fig. 2(A,B)]. Using a two-way ANOVA with location as a repeated measure there was a main effect of sex [Fig. 2(C); F(1,24) = 1.6, p < 0.05; n = 5 males, 5 females] with no significant interaction. Nonetheless, the difference was primarily due to the greater than twofold difference occurring 100–300 μm from the third ventricle. In a replication and extension using Tfm mice, there was a significant main effect of group (F(2,36) = 12.6, p < 0.001; n = 3 wild-type males, 3 wild-type females, 3 Tfm males). The majority of the immunoreactive calbindin was located within 400 μm of the third ventricle. Male mice had 3–4 times the amount of ir-calbindin than had females, and had 2–3-fold more ir-calbindin than had Tfm male mice with a disrupted androgen receptor [Fig. 2(D)].
Figure 2.
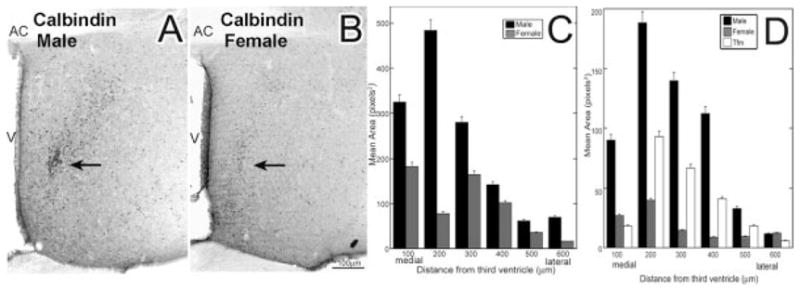
In the POA/AH, adult C57BL/6J mice, males and females differed in the total number of cells that contained immunoreactive calbindin. Males (A) had more ir-calbindin cells and fibers than females (B), p < 0.01 (data are mean ± SEM). Positional analysis shows that the sex difference was primarily due to fourfold differences in the amount of ir-calbindin at 100–200 μm away from the third ventricle (C). Similarly, in the POA/AH of weanling males, females, and Tfm mice, the areas of ir-calbindin differed significantly (D). Wild-type males had three to four times the amount of ir-calbindin than had wild-type females and had 2–3-fold more ir-calbindin than had Tfm mice, whether measured in dorsal/ventral distances away from the AC or in medial/lateral distances from the third ventricle (D), p < 0.01 (data are mean ± SEM). AC, anterior commissure; V, third ventricle.
Mice at P0 were used to determine if sex differences arose during development. In the POA/AH, males had significantly more ir-nNOS and ir-calbindin than females (see Fig. 3). There was a significant main effect of sex for ir-nNOS with males having a significantly greater area of immunoreactivity than have females [Fig. 3(C); F(1, 78) = 4.36, p < 0.05; n = 9 males, 6 females]. There was not a significant interaction (sex × location as a repeated measure; p > 0.40). Nonetheless, the sex difference in ir-nNOS was most apparent in the medial portion of the POA/AH (~200–400 μm lateral to the third ventricle). The main variations in expression occurred dorsally, just ventral to the AC [arrowheads in Fig. 3(A,B)], and in a ventral stripe of cells [see arrows in Fig. 3(A,B)]. There was a main effect of sex for ir-calbindin with significantly more ir-calbindin in males than in females [Fig. 3(F); F(1, 48) = 7.84, p < 0.01; n = 6 males, 5 females]. Again, there was not a significant interaction (sex × location as a repeated measure; p > 0.90). Nonetheless, the cells and fibers with ir-calbindin appeared more densely localized in males between 200 and 400 μm lateral to the third ventricle [Fig. 3(D)], whereas the ir-calbindin was more spread in females [Fig. 3(E)].
Figure 3.
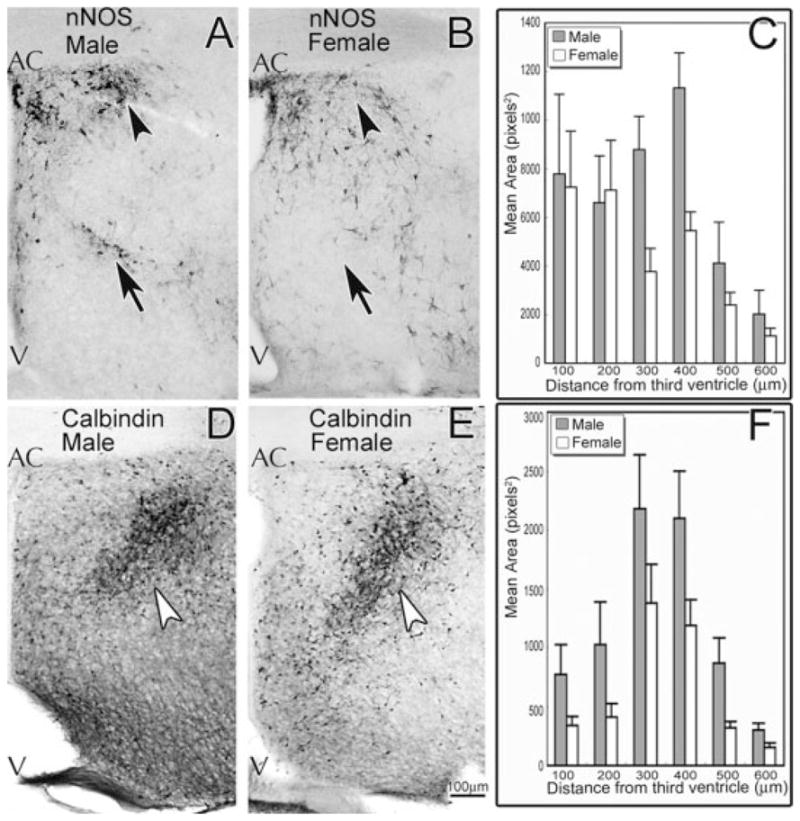
In the POA/AH at birth (P0) male mice had more immunoreactive nNOS and calbindin cells and fibers than had females. Sex differences were found approximately 200–400 μm lateral to the third ventricle (C) with more ir-nNOS in males (A) than in females (B). Differences in nNOS expression occurred dorsally, ventral to the AC (black arrowheads), and in a ventral stripe of cells that was oriented medial/lateral (black arrows). ir-Calbindin was more densely localized in males (D) compared to more spread in females (E, white arrowheads). Males and females differed in the total number of cells that contained ir-calbindin (F), p < 0.01 (data are mean ± SEM). AC, anterior commissure; V, third ventricle.
There was a striking relationship between the distribution of ir-nNOS and ir-calbindin, shown in both the pseudocolor overlay of adjacent tissue sections immunoreacted for either nNOS (red) or calbindin (green) [Fig. 4(A)], or in double-label immunofluorescence in the same section (similarly nNOS red, calbindin green) [Fig. 4(B)]. These two cell populations distinctly border each other rather than overlap. Dense nNOS-ir (red) was located adjacent to the third ventricle in the dorsal POA/AH. ir-Calbindin (green) was found just lateral and ventral to ir-nNOS. The apposition of these two cell groups formed a pattern in which ir-nNOS surrounded the more interior ir-calbindin.
Figure 4.
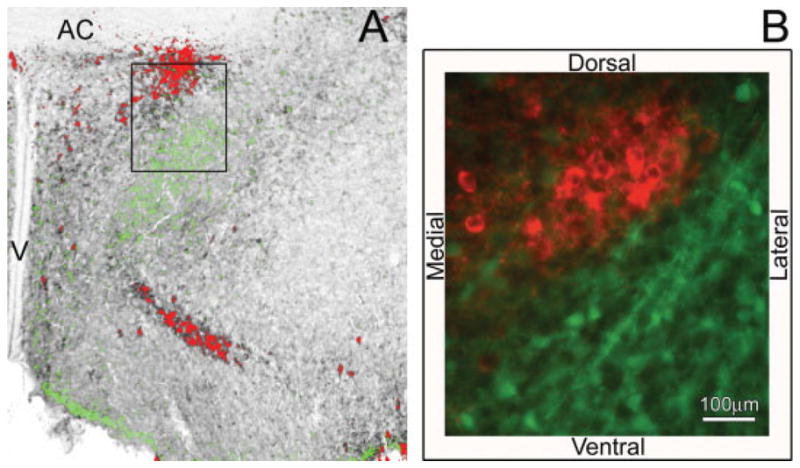
In the POA/AH, a striking relationship was seen in the apposition of cells containing immunoreactive nNOS and calbindin at P0. A pseudocolor overlay of adjacent sections containing nNOS-ir (red) or calbindin-ir (green) illustrates this apposition (A). The box in the overlay image of panel A depicts the approximate location of the boundary in the fluorescent double-label experiment (B). Dense ir-nNOS (red) was located adjacent to the third ventricle and ir-calbindin (green) was found just lateral and ventral to ir-nNOS.
AVPv
In the rostral POA, in the region of the AVPv, there was notably more ir-nNOS in males than in females (see Fig. 5), although the immunoreactivity itself was light compared to darker cells in the surrounding region. Nonetheless, the sex difference was so striking that the sex of each brain could be determined by looking for the region of dense fibers [arrows in Fig. 5(A,B)]. When quantifying differences, males had a threefold larger immunoreactive area than had females in the same relative region [Fig. 5(C); F(1, 9) = 11.47, p < 0.01; n = 6 males, 5 females]. No ir-calbindin was observed in this region at P0.
Figure 5.
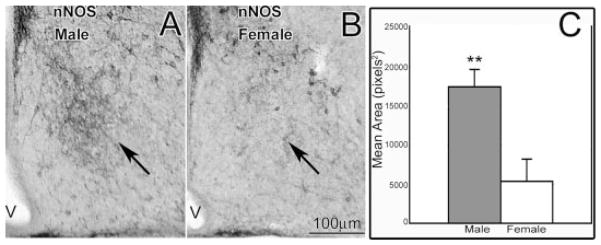
In the region of AVPv at birth (P0), males (A) and females (B) differed in the amount of fibers containing immunoreactive nNOS. Males had 300% more ir-nNOS than females (C, **p < 0.01; data are mean ± SEM). V, third ventricle.
VMN
In the more caudal hypothalamus, in the region of the VMN, there was significantly more ir-nNOS cells and fibers in females [Fig. 6(B)] when compared with males [Fig. 6(A); F(1, 15) = 6.61, p < 0.05; n = 10 males, 7 females]. Unlike the POA/AH, the sex difference in the VMN could not be localized to a particular subregion (see Fig. 6). There was not a significant difference in the cells and fibers containing ir-calbindin in the VMN [Fig. 6(F); p > 0.50; n = 4 males, 5 females].
Figure 6.
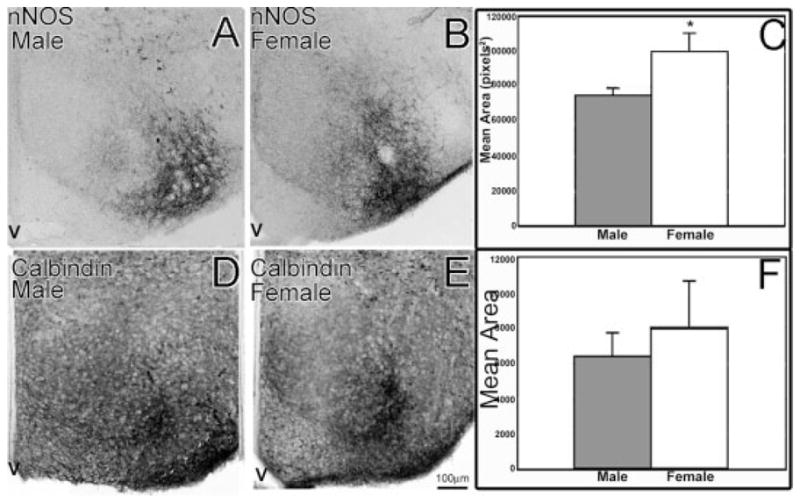
In the VMN at birth (P0) males and females differed in the amount of cells and fibers containing ir-nNOS, but not ir-calbindin. Females (B) had more ir-nNOS than had males (A) as illustrated in the graph of mean immunoreactive areas (C, *p < 0.05; data are mean ± SEM). There was no significant difference in the amount of ir-calbindin between males (D) and females (E) as depicted by the graph (F). V, third ventricle.
DISCUSSION
Sex differences in morphology have been described in regions of the hypothalamus and preoptic area of a number of species (Tobet and Fox, 1992). Some of these sex differences are relatable to particular cell phenotypes (e.g., galanin in ferrets; Park et al., 1997 or vasopressin in a number of species; De Vries and Panzica, 2006). We have recently shown that the positions of identified cells at E17 may be one aspect of sex-dependent characteristics during development with potential consequences for longer-term sex differences (particularly ERβ expression; Wolfe et al., 2005). In the current study we examined two additional markers for sex differences in development. Calbindin, serves as a potential biomarker for sex differences in brain structure across several species (Sickel and McCarthy, 2000; Bisenius et al., 2006). The other, nNOS, is an enzyme responsible for the synthesis of NO, which is likely bioactive in the developing brain. Based on the sex-dependent distribution of nNOS and calbindin, NO may be an effector molecule in the determination of selected sex differences in development. Using calbindin as a biomarker for sexual differentiation in the POA/AH, the data in the current study suggests significant androgen actions in the mouse brain based on decreases in Tfm mice. This contrasts with a previous examination of immunoreactive calbindin in the POA/AH of ERαKO mice that did not show differences from wild-type (Hall et al., 2000).
POA/AH
Sex differences in the brain morphology of adult animals often arise as sex differences in development. The sex difference in ir-calbindin in the POA/AH in the current study fits this pattern. However, sex differences in development can sometimes carry forward with a different appearance in adulthood. In both the current study and in a companion study of older mice (Majdic et al., 2006), a much tighter distribution of ir-calbindin cells was a striking characteristic found in adult males relative to adult females. From the perspective of developmental relationship of the cell groups containing ir-calbindin and ir-nNOS, it is important to consider that nNOS generates NO that may function to help create sex differences in other characteristics such as cells containing ir-calbindin (current study) or ERβ (Wolfe et al., 2005). Steroid hormones, acting through ER-α and androgen receptors, play crucial roles in regulating nNOS in adults (Scordalakes et al., 2002). Gonadal steroid hormones may influence sex differences in ir-calbindin by either altering nNOS or by acting directly on cells containing ir-calbindin. We found that androgen receptor defective Tfm male mice had significantly reduced ir-calbindin expression compared to wild-type mice suggesting that androgen receptors may be necessary for the normal male pattern of ir-calbindin in the POA/AH, in agreement with some earlier work in rats (Watson et al., 1998). Since there is more ir-calbindin in the Tfm male than in the wild-type female, other factors must also influence ir-calbindin. Other previous work in rats had suggested that calbindin expression in the rat sexually dimorphic nucleus (SDN) of the POA was fully estrogen dependent (Sickel and McCarthy, 2000). The current result in mice suggests that there may be synergies between androgens and estrogens in the murine POA/AH. An alternative in addition to hormones, may be sex chromosome complement that might also affect sex differences in neural differentiation. For example there is direct evidence for effects of sex chromosome complement on ir-AVP in the laternal septum (Gatewood et al., 2006; De Vries et al., 2002) and for nNOS in the POA (Budefeld et al., Sex differences in brain morphology in agonadal adult steroidogenic factor-1 knockout mice, submitted for publication). Given that the Tfm mice are XY, yet lack functional androgen receptors their ir-calbindin, which is intermediate between WT males and females may be influenced by both hormone-dependent and -independent factors.
The POA/AH is important for regulating homeostatic, neuroendocrine, and behavioral functions. It is a region where hormones dramatically influence development and where hormone-secreting cells regulate physiology and behavior. This region of the mammalian forebrain is where sex differences are most common (reviewed Tobet and Fox, 1992). Sex differences in peptides, receptors, and protein content have been repeatedly shown in the peripubertal and adult POA/AH (Scouten et al., 1985; Simerly et al., 1985; Micevych et al., 1987; Bloch et al., 1992; 1993; Dubois-Dauphin et al., 1996; Grattan and Selmanoff, 1997; Segarra et al., 1998). Nonetheless, sex differences in the mouse POA/AH have been less well characterized, possibly because some sex differences in mice may be strain dependent (Brown et al., 1999). Unlike the rat, the mouse has not been shown to have a specific sexually dimorphic nuclear group in the POA (Brown et al., 1999). However, the current study demonstrates that ir-calbindin in C57BL/6J mice occupies a similar location in the POA/AH to the rat SDN. The current study shows significantly more of both immunoreactive nNOS and calbindin in male mice compared to females in the POA/AH for animals on a C57BL/6J background.
AVPv
The hypothalamus is comprised of a highly interconnected and integrated series of nuclear groups and scattered neuroendocrine effector cells. Sex differences in one component are often mirrored by sex differences in another (Simerly, 1998; Segovia et al., 1999). For example, the AVPv projects to several regions including the preoptic area and the medial hypothalamus. The POA receives projections from the amygdala and the VMN and projects extensively including to regions thought to aid in neuroendocrine responses. The VMN also has extensive projections to and from the amygdala. Sex differences in various aspects of morphology have been reported in all of these regions. In the region of AVPv, the results of the current study indicated that males had an immunoreactive region of dense fibers three times larger than females. The AVPv has been extensively characterized as sexually dimorphic (Simerly et al., 1985; Sumida et al., 1993; Lund et al., 2000; Lephart et al., 2001; Orikasa et al., 2002; Rubin et al., 2006). While some data suggests late development for aspects of AVPv sexual dimorphism (Davis et al., 1996; Hutton et al., 1998), other evidence, now including the current study, indicates that sex differences are also detectable during perinatal development (Arai et al., 1994; Sumida et al., 1993).
VMN
The VMN plays an important role in regulating female sexual behavior, energy balance, and feeding behavior (reviewed McClellan et al., 2006). The VMN has been described as sexually dimorphic for specific cell types in a number of studies (Ikeda et al., 2003; Sá and Madeira, 2005). The current study found that females have significantly more ir-nNOS when compared with males while there were no apparent differences in the amount of immunoreactive calbindin in the VMN. Interestingly, our study in older mice found sex differences in wild-type mice in the VMN for calbindin with females having more immunoreactive cells than have males (Majdic et al., 2006). Thus, NO may contribute to the development of sex differences in VMN characteristics that only become apparent later in life.
In summary, evidence from a number of sources suggests that the expression of nNOS and calbindin is regulated by steroid hormones. The current study suggests that androgen receptor action is one factor that helps determine calbindin expression in the POA/AH. In the POA/AH, there is an intriguing spatial pattern between cells that contain immunoreactive nNOS and calbindin. As a developmental effector, NO derived from the enzymatic activity of nNOS may thereby influence the development of cells containing ir-calbindin. In the AVPv region, where males have three times the amount of ir-nNOS in fibers compared to females, late developing sex differences in tyrosine hydroxylase (Tobet and Hanna, 1997) or incoming fibers (Hutton et al., 1998) may be influenced. In the VMN where females have significantly more ir-nNOS when compared to males, VMN characteristics at later ages may be influenced by developmental sex differences in the action of NO.
Acknowledgments
ME and CW should be considered co-first authors. Special thanks to Brandon Wadas and Kristy McClellan for technical assistance with the mice during the project. This research was supported by MH61376 (SAT) and MH057759 (EFR).
References
- Arai V, Murakami S, Nishizuka M. Androgen enhances neuronal degeneration in the developing preoptic area: apoptosis in the anteroventral periventricular nucleus (AVPvN-POA) Horm Behav. 1994;28:313–319. doi: 10.1006/hbeh.1994.1027. [DOI] [PubMed] [Google Scholar]
- Arnold AP. Sex chromosomes and brain gender. Nat Rev Neurosci. 2004;5:701–708. doi: 10.1038/nrn1494. [DOI] [PubMed] [Google Scholar]
- Bicker G. STOP and GO with NO: Nitric oxide as a regulator of cell motility in simple brains. Bioessays. 2005;27:495–505. doi: 10.1002/bies.20221. [DOI] [PubMed] [Google Scholar]
- Bisenius ES, Veeramachaneni DN, Sammonds GE, Tobet S. Sex differences and the development of the rabbit brain: Effects of vinclozolin. Biol Reprod. 2006:75. doi: 10.1095/biolreprod.106.052795. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Bloch GJ, Eckersell C, Mills R. Distribution of galanin-immunoreactive cells within sexually dimorphic components of the medial preoptic area of the male and remale rat. Brain Res. 1993;620:259–268. doi: 10.1016/0006-8993(93)90164-i. [DOI] [PubMed] [Google Scholar]
- Bloch GJ, Kurth SM, Akesson TR, Micevych PE. Estrogen-concentrating cells within cell groups of the medial preoptic area: Sex differences and co-localization with galanin-immunoreactive cells. Brain Res. 1992;595:301–308. doi: 10.1016/0006-8993(92)91064-l. [DOI] [PubMed] [Google Scholar]
- Brager DH, Sickel MJ, McCarthy MM. Developmental sex differences in calbindin-D(28K) and calretinin immunoreactivity in the neonatal rat hypothalamus. J Neurobiol. 2000;42:315–322. [PubMed] [Google Scholar]
- Brown AE, Mani S, Tobet SA. The preoptic area/anterior hypothalamus of different strains of mice: sex differences and development. Dev Brain Res. 1999;115:171–182. doi: 10.1016/s0165-3806(99)00061-9. [DOI] [PubMed] [Google Scholar]
- Bulotta S, Perrotta C, Cerullo A, De Palma C, Clementi E, Borgese N. A cellular system to study the role of nitric oxide in cell death, survival, and migration. Neurotoxicology. 2005;26:841–845. doi: 10.1016/j.neuro.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Carrer HF, Cambiasso MJ. Sexual differentiation of the brain: genes, estrogen, and neurotrophic factors. Cell Mol Neurobiol. 2002;22:479–500. doi: 10.1023/A:1021825317546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciani E, Calvanese V, Crochemore C, Bartesaghi R, Contestabile A. Proliferation of cerebellar precursor cells is negatively regulated by nitric oxide in newborn rat. J Cell Sci. 2006;119:3161–3170. doi: 10.1242/jcs.03042. [DOI] [PubMed] [Google Scholar]
- Contestabile A, Ciani E. Role of nitric oxide in the regulation of neuronal proliferation, survival and differentiation. Neurochem Int. 2004;45:903–914. doi: 10.1016/j.neuint.2004.03.021. [DOI] [PubMed] [Google Scholar]
- Davis AM, Seney ML, Walker HJ, Tobet SA. Differential colocalization of Islet-1 and estrogen receptor alpha in the murine preoptic area and hypothalamus during development. Endocrinology. 2004;145:360–366. doi: 10.1210/en.2003-0996. [DOI] [PubMed] [Google Scholar]
- Davis EC, Shryne JE, Gorski RA. Structural sexual dimorphisms in the anteroventral periventricular nucleus of the rat hypothalamus are sensitive to gonadal steroids perinatally, but develop peripubertally. Neuroendocrinology. 1996;63:142–148. doi: 10.1159/000126950. [DOI] [PubMed] [Google Scholar]
- Dellovade TL, Davis AM, Ferguson C, Sieghart W, Homanics GE, Tobet SA. GABA influences the development of the ventromedial nucleus of the hypothalamus. J Neurobiol. 2001;49:264–276. doi: 10.1002/neu.10011. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Panzica GC. Sexual differentiation of central vasopressin and vasotocin systems in vertebrates: Different mechanisms, similar endpoints. Neuroscience. 2006;138:947–955. doi: 10.1016/j.neuroscience.2005.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries GJ, Rissman EF, Simerly RB, Yang LY, Scordalakes EM, Auger CJ, Swain A, Lovell-Badge R, Burgoyne PS, Arnold AP. A model system for study of sex chromosome effects on sexually dimorphic neural and behavioral traits. J Neurosci. 2002;22:9005–9014. doi: 10.1523/JNEUROSCI.22-20-09005.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois-Dauphin M, Barberis C, de Bilbao F. Vasopressin receptors in the mouse (Mus musculus) brain: Sex-related expression in the medial preoptic area and hypothalamus. Brain Res. 1996;743:32–39. doi: 10.1016/s0006-8993(96)01019-0. [DOI] [PubMed] [Google Scholar]
- Feng G, Mellor RH, Bernstein M, Keller-Peck C, Nguyen QT, Wallace M, Nerbonne JM, Lichtman JW, Sanes JR. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron. 2000;28:41–51. doi: 10.1016/s0896-6273(00)00084-2. [DOI] [PubMed] [Google Scholar]
- Gatewood JD, Wills A, Shetty S, Xu J, Arnold AP, Burgoyne PS, Rissman EF. Sex chromosome complement and gonadal sex influence aggressive and parental behaviors in mice. J Neurosci. 2006;26:2335–2342. doi: 10.1523/JNEUROSCI.3743-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grattan DR, Selmanoff M. Sex differences in the activity of gamma-aminobutyric acidergic neurons in the rat hypothalamus. Brain Res. 1997;775:244–249. doi: 10.1016/s0006-8993(97)01069-x. [DOI] [PubMed] [Google Scholar]
- Gyurko R, Leupen S, Huang PL. Deletion of exon 6 of the neuronal nitric oxide synthase gene in mice results in hypogonadism and infertility. Endocrinology. 2002;143:2767–2774. doi: 10.1210/endo.143.7.8921. [DOI] [PubMed] [Google Scholar]
- Hall JH, Dellovade TL, Rissman EF, Tobet SA. Hormone receptor dependence of calbindin expression in the murine preoptic area/anterior hypothalamus. Soc Neurosci Abstr. 2000;26:922. [Google Scholar]
- Hosoya T, Oda Y, Takahashi S, Morita M, Kawauchi S, Ema M, Yamamoto M, Fujii-Kuriyama Y. Defective development of secretory neurones in the hypothalamus of Arnt2-knockout mice. Genes Cells. 2001;6:361–374. doi: 10.1046/j.1365-2443.2001.00421.x. [DOI] [PubMed] [Google Scholar]
- Huang PL. Nitric oxide and cerebral ischemic preconditioning. Cell Calcium. 2004;36:323–329. doi: 10.1016/j.ceca.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Hutton LA, Gu G, Simerly RB. Development of a sexually dimorphic projection from the bed nuclei of the stria terminalis to the anteroventral periventricular nucleus in the rat. J Neurosci. 1998;18:3003–3013. doi: 10.1523/JNEUROSCI.18-08-03003.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda Y, Luo X, Abbud R, Nilson JH, Parker KL. The nuclear receptor steroidogenic factor 1 is essential for the formation of the ventromedial hypothalamic nucleus. Mol Endocrinol. 1995;9:478–486. doi: 10.1210/mend.9.4.7659091. [DOI] [PubMed] [Google Scholar]
- Ikeda Y, Nagai A, Ikeda M, Hayashi S. Sexually dimorphic and estrogen-dependent expression of estrogen receptor beta in the ventromedial hypothalamus during rat postnatal development. Endocrinology. 2003;144:5098–5104. doi: 10.1210/en.2003-0267. [DOI] [PubMed] [Google Scholar]
- Ishihara T, Orikasa C, Araki T, Sakuma Y. Sex difference in the expression and regulation of nitric oxide synthase gene in the rat preoptic area. Neurosci Res. 2002;43:147–154. doi: 10.1016/s0168-0102(02)00025-1. [DOI] [PubMed] [Google Scholar]
- Lephart ED. Dimorphic expression of calindin-D28K in the medial basal hypothalamus from perinatal male and female rats. Brain Res Dev Brain Res. 1996;96:281–284. doi: 10.1016/0165-3806(96)00100-9. [DOI] [PubMed] [Google Scholar]
- Lephart ED, Lund TD, Horvath TL. Brain androgen and progesterone metabolizing enzymes: biosynthesis, distribution and function. Brain Res Rev. 2001;37:25–37. doi: 10.1016/s0165-0173(01)00111-4. [DOI] [PubMed] [Google Scholar]
- Lund TD, Salyer DL, Fleming DE, Lephart ED. Pre-or postnatal testosterone and flutamide effects on sexually dimorphic nuclei of the rat hypothalamus. Brain Res Dev Brain Res. 2000;120:261–266. doi: 10.1016/s0165-3806(00)00013-4. [DOI] [PubMed] [Google Scholar]
- Majdic G, Budefeld T, Grgurevic N, Rissman E, Tobet S. A model for hormone independent development of sex differences in brain and behavior. Front Neuroendo. 2006;27:96–97. [Google Scholar]
- McCarthy MM, Auger AP, Perrot-Sinal TS. Getting excited about GABA and sex differences in the brain. Trends Neurosci. 2002;25:307–312. doi: 10.1016/s0166-2236(02)02182-3. [DOI] [PubMed] [Google Scholar]
- McClellan KM, Parker KL, Tobet S. Development of the ventromedial nucleus of the hypothalamus. Front Neuroendocrinol. 2006;27:193–209. doi: 10.1016/j.yfrne.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Micevych PE, Park SS, Adesson TR, Elde R. Distribution of cholecystokinin-immunoreactive cell bodies in the male and female rat: I. Hypothalamus J Comp Neurol. 1987;255:124–136. doi: 10.1002/cne.902550110. [DOI] [PubMed] [Google Scholar]
- Michaud JL, DeRossi C, May NR, Holdener BC, Fan CM. ARNT2 acts as the dimerization partner of SIM1 for the development of the hypothalamus. Mech Dev. 2000;90:253–261. doi: 10.1016/s0925-4773(99)00328-7. [DOI] [PubMed] [Google Scholar]
- Michaud JL, Rosenquist T, May NR, Fan CM. Development of neuroendocrine lineages requires the bHLH-PAS transcription factor SIM1. Genes Dev. 1998;12:3264–3275. doi: 10.1101/gad.12.20.3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JA, Jordan CL, Breedlove SM. Sexual differentiation of the vertebrate nervous system. Nat Neurosci. 2004;7:1034–1039. doi: 10.1038/nn1325. [DOI] [PubMed] [Google Scholar]
- Mungrue IN, Bredt DS, Stewart DJ, Husain M. From molecules to mammals: What’s NOS got to do with it? Act Physiol Scan. 2003;179:123–135. doi: 10.1046/j.1365-201X.2003.01182.x. [DOI] [PubMed] [Google Scholar]
- Orikasa C, Kondo Y, Hayashi S, McEwen BS, Sakuma Y. Sexually dimorphic expression of estrogen receptor beta in the anteroventral periventricular nucleus of the rat preoptic area: Implication in luteinizing hormone surge. Proc Natl Acad Sci USA. 2002;99:3306–3311. doi: 10.1073/pnas.052707299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JJ, Baum MJ, Tobet SA. Sex difference and steroidal stimulation of galanin immunoreactivity in the ferret’s dorsal preoptic area/anterior hypothalamus. J Comp Neurol. 1997;389:277–288. [PubMed] [Google Scholar]
- Pei M, Matsuda K, Sakamoto H, Kawata M. Intrauterine proximity to male fetuses affects the morphology of the sexually dimorphic nucleus of the preoptic area in the adult rat brain. Eur J Neurosci. 2006;23:1234–1240. doi: 10.1111/j.1460-9568.2006.04661.x. [DOI] [PubMed] [Google Scholar]
- Pereira FA, Tsai MJ, Tsai SY. COUP-TF orphan nuclear receptors in development and differentiation. Cell Mol Life Sci. 2000;57:1388–1398. doi: 10.1007/PL00000624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin BS, Lenkowski JR, Schaeberle CM, Vandenberg LN, Ronsheim PM, Soto AM. Evidence of altered brain sexual differentiation in mice exposed perinatally to low, environmentally relevant levels of bisphenol A. Endocrinology. 2006;147:3681–3691. doi: 10.1210/en.2006-0189. [DOI] [PubMed] [Google Scholar]
- Sá SI, Madeira MD. Neuronal organelles and nuclear pores of hypothalamic ventromedial neurons are sexually dimorphic and change during the estrus cycle in the rat. Neuroscience. 2005;133:919–924. doi: 10.1016/j.neuroscience.2005.02.033. [DOI] [PubMed] [Google Scholar]
- Scallet AC, Divine RL, Newbold RR, Delcos KB. Increased volume of the calbindin D28k-labeled sexually dimorphic hypothalamus in genistein and nonylphenol-treated male rats. Toxicol Sci. 2004;82:570–576. doi: 10.1093/toxsci/kfh297. [DOI] [PubMed] [Google Scholar]
- Scordalakes EM, Rissman EF. Aggression and arginine vasopressin immunoreactivity regulation by androgen receptor and estrogen receptor alpha. Genes Brain Behav. 2004;3:20–26. doi: 10.1111/j.1601-183x.2004.00036.x. [DOI] [PubMed] [Google Scholar]
- Scordalakes EM, Shetty SJ, Rissman EF. Roles of estrogen receptor alpha and androgen receptor in the regulation of neuronal nitric oxide synthase. J Comp Neurol. 2002;453:336–344. doi: 10.1002/cne.10413. [DOI] [PubMed] [Google Scholar]
- Scouten CW, Heydorn WE, Creed GJ, Malsbury CW, Jacobowitz DM. Proteins regulated by gonadal steroids in the medial preoptic and ventromedial hypothalamic nucleic of male and female rats. Neuroendocrinology. 1985;41:237–245. doi: 10.1159/000124183. [DOI] [PubMed] [Google Scholar]
- Segarra AC, Acosta AM, Gonzalez JL, Angulo JA, McEwen BS. Sex differences in estrogenic regulation of preproenkephalin mRNA levels in the medial preoptic area of prepubertal rats. Brain Res Mol Brain Res. 1998;60:133–139. doi: 10.1016/s0169-328x(98)00160-0. [DOI] [PubMed] [Google Scholar]
- Segovia S, Guillamon A, del Cerro MC, Ortega E, Perez-Laso C, Rodriguez-Zafra M, Beyer C. The development of brain sex differences: A multisignaling process. Behav Brain Res. 1999;105:69–80. doi: 10.1016/s0166-4328(99)00083-2. [DOI] [PubMed] [Google Scholar]
- Sickel MJ, McCarthy MM. Calbindin-D28k immunoreactivity is a marker for a subdivision of the sexually dimorphic nucleus of the preoptic area of the rat: developmental profile and gonadal steroid modulation. J Neuroendocrinol. 2000;12:397–402. doi: 10.1046/j.1365-2826.2000.00474.x. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Swanson LW, Gorski RA. The distribution of monoaminergic cells and fibers in a periventricular preoptic nucleus involved in the control of gonadotropin release: Immunohistochemical evidence for a dopaminergic sexual dimorphism. Brain Res. 1985;330:55–64. doi: 10.1016/0006-8993(85)90007-1. [DOI] [PubMed] [Google Scholar]
- Simerly RB. Organization and regulation of sexually dimorphic neuroendocrine pathways. Behav Brain Res. 1998;92:195–203. doi: 10.1016/s0166-4328(97)00191-5. [DOI] [PubMed] [Google Scholar]
- Simerly RB. Wired for reproduction: Organization and development of sexually dimorphic circuits in the mammalian forebrain. Annu Rev Neurosci. 2002;25:507–536. doi: 10.1146/annurev.neuro.25.112701.142745. [DOI] [PubMed] [Google Scholar]
- Simerly RB. Wired on hormones: Endocrine regulation of hypothalamic development. Curr Opin Neurobiol. 2005;15:81–85. doi: 10.1016/j.conb.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Sumida H, Nishizuka M, Kano Y, Arai Y. Sex differences in the anteroventral periventral nucleus of the preoptic area and in the related effects of androgen in prenatal rats. Neurosci Lett. 1993;151:41–44. doi: 10.1016/0304-3940(93)90040-r. [DOI] [PubMed] [Google Scholar]
- Tobet SA. Genes controlling hypothalamic development and sexual differentiation. Eur J Neurosci. 2002;16:373–376. doi: 10.1046/j.1460-9568.2002.02105.x. [DOI] [PubMed] [Google Scholar]
- Tobet SA, Dellovade TL, Parker K, Homanics G. Positioning estrogen receptor alpha-containing cells during hypothalamic development. In: Handa RJ, Hayashi S, Terasawa E, Kawata M, editors. Neuroplasticity, Development and Steroid Hormone Action. CRC Press; Florida: 2002. pp. 59–72. [Google Scholar]
- Tobet SA, Fox TO. Sex differences in neural morphology influenced hormonally throughout life. In: Gerall AA, Moltz H, Ward IL, editors. Sexual Differentiation: A Lifespan Approach. Handbook of Behavioral Neurobiology. Vol. 11. New York: Plenum Press; 1992. pp. 41–83. [Google Scholar]
- Tobet SA, Hanna IK. Ontogeny of sex differences in the mammalian hypothalamus and preoptic area. Cell Mol Neurobiol. 1997;17:565–601. doi: 10.1023/A:1022529918810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson MA, Taylor H, Lephart ED. Androgen-dependent modulation of calbindin-D28K in hypothalamic tissue during prenatal development. Neurosci Res. 1998;32:97–101. doi: 10.1016/s0168-0102(98)00068-6. [DOI] [PubMed] [Google Scholar]
- Wolfe CA, Van Doren M, Walker HJ, Seney ML, McClellan KM, Tobet SA. Sex differences in the location of immunochemically defined cell populations in the mouse preoptic area/anterior hypothalamus. Brain Res Dev Brain Res. 2005;157:34–41. doi: 10.1016/j.devbrainres.2005.03.001. [DOI] [PubMed] [Google Scholar]


