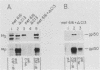Abstract
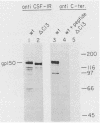
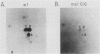
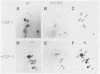
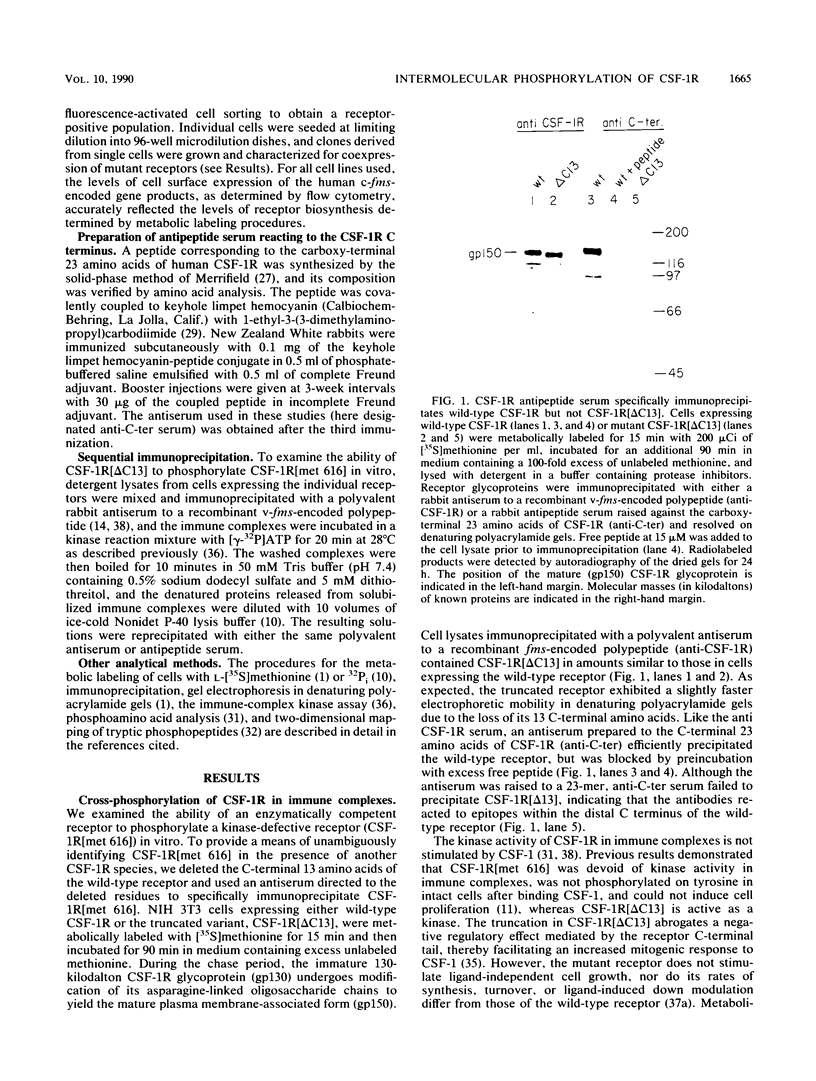
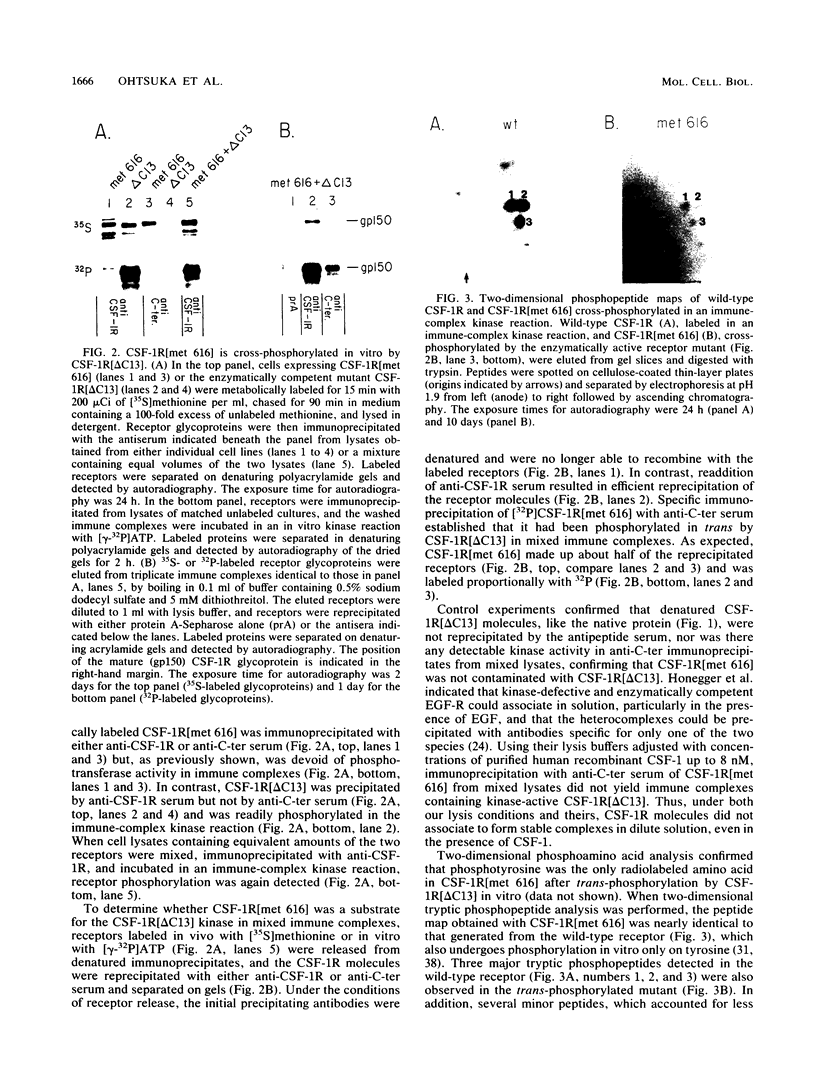
Ligand-induced tyrosine phosphorylation of the human colony-stimulating factor 1 receptor (CSF-1R) could involve either an intra- or intermolecular mechanism. We therefore examined the ability of a CSF-1R carboxy-terminal truncation mutant to phosphorylate a kinase-defective receptor, CSF-1R[met 616], that contains a methionine-for-lysine substitution at its ATP-binding site. By using an antipeptide serum that specifically reacts with epitopes deleted from the enzymatically competent truncation mutant, cross-phosphorylation of CSF-1R[met 616] on tyrosine was demonstrated, both in immune-complex kinase reactions and in intact cells stimulated with CSF-1. Both in vitro and in vivo, CSF-1R[met 616] was phosphorylated on tryptic peptides identical to those derived from wild-type CSF-1R, suggesting that receptor phosphorylation on tyrosine can proceed via an intermolecular interaction between receptor monomers. When expressed alone, CSF-1R[met 616] did not undergo ligand-induced down modulation, but its phosphorylation in cells coexpressing the kinase-active truncation mutant accelerated its degradation.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S. J., Gonda M. A., Rettenmier C. W., Sherr C. J. Subcellular localization of glycoproteins encoded by the viral oncogene v-fms. J Virol. 1984 Sep;51(3):730–741. doi: 10.1128/jvi.51.3.730-741.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashmun R. A., Look A. T., Roberts W. M., Roussel M. F., Seremetis S., Ohtsuka M., Sherr C. J. Monoclonal antibodies to the human CSF-1 receptor (c-fms proto-oncogene product) detect epitopes on normal mononuclear phagocytes and on human myeloid leukemic blast cells. Blood. 1989 Feb 15;73(3):827–837. [PubMed] [Google Scholar]
- Bertics P. J., Chen W. S., Hubler L., Lazar C. S., Rosenfeld M. G., Gill G. N. Alteration of epidermal growth factor receptor activity by mutation of its primary carboxyl-terminal site of tyrosine self-phosphorylation. J Biol Chem. 1988 Mar 15;263(8):3610–3617. [PubMed] [Google Scholar]
- Bertics P. J., Gill G. N. Self-phosphorylation enhances the protein-tyrosine kinase activity of the epidermal growth factor receptor. J Biol Chem. 1985 Nov 25;260(27):14642–14647. [PubMed] [Google Scholar]
- Böni-Schnetzler M., Pilch P. F. Mechanism of epidermal growth factor receptor autophosphorylation and high-affinity binding. Proc Natl Acad Sci U S A. 1987 Nov;84(22):7832–7836. doi: 10.1073/pnas.84.22.7832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochet C., Kashles O., Chambaz E. M., Borrello I., King C. R., Schlessinger J. Demonstration of epidermal growth factor-induced receptor dimerization in living cells using a chemical covalent cross-linking agent. J Biol Chem. 1988 Mar 5;263(7):3290–3295. [PubMed] [Google Scholar]
- Cooper J. A., MacAuley A. Potential positive and negative autoregulation of p60c-src by intermolecular autophosphorylation. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4232–4236. doi: 10.1073/pnas.85.12.4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens L., Van Beveren C., Smith D., Chen E., Mitchell R. L., Isacke C. M., Verma I. M., Ullrich A. Structural alteration of viral homologue of receptor proto-oncogene fms at carboxyl terminus. Nature. 1986 Mar 20;320(6059):277–280. doi: 10.1038/320277a0. [DOI] [PubMed] [Google Scholar]
- Downing J. R., Rettenmier C. W., Sherr C. J. Ligand-induced tyrosine kinase activity of the colony-stimulating factor 1 receptor in a murine macrophage cell line. Mol Cell Biol. 1988 Apr;8(4):1795–1799. doi: 10.1128/mcb.8.4.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downing J. R., Roussel M. F., Sherr C. J. Ligand and protein kinase C downmodulate the colony-stimulating factor 1 receptor by independent mechanisms. Mol Cell Biol. 1989 Jul;9(7):2890–2896. doi: 10.1128/mcb.9.7.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis L., Clauser E., Morgan D. O., Edery M., Roth R. A., Rutter W. J. Replacement of insulin receptor tyrosine residues 1162 and 1163 compromises insulin-stimulated kinase activity and uptake of 2-deoxyglucose. Cell. 1986 Jun 6;45(5):721–732. doi: 10.1016/0092-8674(86)90786-5. [DOI] [PubMed] [Google Scholar]
- Fanger B. O., Stephens J. E., Staros J. V. High-yield trapping of EGF-induced receptor dimers by chemical cross-linking. FASEB J. 1989 Jan;3(1):71–75. doi: 10.1096/fasebj.3.1.2783412. [DOI] [PubMed] [Google Scholar]
- Furman W. L., Rettenmier C. W., Chen J. H., Roussel M. F., Quinn C. O., Sherr C. J. Antibodies to distal carboxyl terminal epitopes in the v-fms-coded glycoprotein do not cross-react with the c-fms gene product. Virology. 1986 Jul 30;152(2):432–445. doi: 10.1016/0042-6822(86)90145-5. [DOI] [PubMed] [Google Scholar]
- Glenney J. R., Jr, Chen W. S., Lazar C. S., Walton G. M., Zokas L. M., Rosenfeld M. G., Gill G. N. Ligand-induced endocytosis of the EGF receptor is blocked by mutational inactivation and by microinjection of anti-phosphotyrosine antibodies. Cell. 1988 Mar 11;52(5):675–684. doi: 10.1016/0092-8674(88)90405-9. [DOI] [PubMed] [Google Scholar]
- Guilbert L. J., Stanley E. R. Specific interaction of murine colony-stimulating factor with mononuclear phagocytic cells. J Cell Biol. 1980 Apr;85(1):153–159. doi: 10.1083/jcb.85.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilbert L. J., Stanley E. R. The interaction of 125I-colony-stimulating factor-1 with bone marrow-derived macrophages. J Biol Chem. 1986 Mar 25;261(9):4024–4032. [PubMed] [Google Scholar]
- Heldin C. H., Bäckström G., Ostman A., Hammacher A., Rönnstrand L., Rubin K., Nistér M., Westermark B. Binding of different dimeric forms of PDGF to human fibroblasts: evidence for two separate receptor types. EMBO J. 1988 May;7(5):1387–1393. doi: 10.1002/j.1460-2075.1988.tb02955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldin C. H., Ernlund A., Rorsman C., Rönnstrand L. Dimerization of B-type platelet-derived growth factor receptors occurs after ligand binding and is closely associated with receptor kinase activation. J Biol Chem. 1989 May 25;264(15):8905–8912. [PubMed] [Google Scholar]
- Herrera R., Rosen O. M. Autophosphorylation of the insulin receptor in vitro. Designation of phosphorylation sites and correlation with receptor kinase activation. J Biol Chem. 1986 Sep 15;261(26):11980–11985. [PubMed] [Google Scholar]
- Honegger A. M., Dull T. J., Felder S., Van Obberghen E., Bellot F., Szapary D., Schmidt A., Ullrich A., Schlessinger J. Point mutation at the ATP binding site of EGF receptor abolishes protein-tyrosine kinase activity and alters cellular routing. Cell. 1987 Oct 23;51(2):199–209. doi: 10.1016/0092-8674(87)90147-4. [DOI] [PubMed] [Google Scholar]
- Honegger A. M., Kris R. M., Ullrich A., Schlessinger J. Evidence that autophosphorylation of solubilized receptors for epidermal growth factor is mediated by intermolecular cross-phosphorylation. Proc Natl Acad Sci U S A. 1989 Feb;86(3):925–929. doi: 10.1073/pnas.86.3.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honegger A., Dull T. J., Bellot F., Van Obberghen E., Szapary D., Schmidt A., Ullrich A., Schlessinger J. Biological activities of EGF-receptor mutants with individually altered autophosphorylation sites. EMBO J. 1988 Oct;7(10):3045–3052. doi: 10.1002/j.1460-2075.1988.tb03169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honegger A., Dull T. J., Szapary D., Komoriya A., Kris R., Ullrich A., Schlessinger J. Kinetic parameters of the protein tyrosine kinase activity of EGF-receptor mutants with individually altered autophosphorylation sites. EMBO J. 1988 Oct;7(10):3053–3060. doi: 10.1002/j.1460-2075.1988.tb03170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kmiecik T. E., Shalloway D. Activation and suppression of pp60c-src transforming ability by mutation of its primary sites of tyrosine phosphorylation. Cell. 1987 Apr 10;49(1):65–73. doi: 10.1016/0092-8674(87)90756-2. [DOI] [PubMed] [Google Scholar]
- Meckling-Hansen K., Nelson R., Branton P., Pawson T. Enzymatic activation of Fujinami sarcoma virus gag-fps transforming proteins by autophosphorylation at tyrosine. EMBO J. 1987 Mar;6(3):659–666. doi: 10.1002/j.1460-2075.1987.tb04805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan C. J., Stanley E. R. Chemical crosslinking of the mononuclear phagocyte specific growth factor CSF-1 to its receptor at the cell surface. Biochem Biophys Res Commun. 1984 Feb 29;119(1):35–41. doi: 10.1016/0006-291x(84)91614-0. [DOI] [PubMed] [Google Scholar]
- Ohtsuka M., Ihara S., Ogawa R., Watanabe T., Watanabe Y. Preparation and characterization of antibodies to O-phosphotyrosine and their use for identification of phosphotyrosine-containing proteins. Int J Cancer. 1984 Dec 15;34(6):855–861. doi: 10.1002/ijc.2910340618. [DOI] [PubMed] [Google Scholar]
- Piwnica-Worms H., Saunders K. B., Roberts T. M., Smith A. E., Cheng S. H. Tyrosine phosphorylation regulates the biochemical and biological properties of pp60c-src. Cell. 1987 Apr 10;49(1):75–82. doi: 10.1016/0092-8674(87)90757-4. [DOI] [PubMed] [Google Scholar]
- Rettenmier C. W., Chen J. H., Roussel M. F., Sherr C. J. The product of the c-fms proto-oncogene: a glycoprotein with associated tyrosine kinase activity. Science. 1985 Apr 19;228(4697):320–322. doi: 10.1126/science.2580348. [DOI] [PubMed] [Google Scholar]
- Rettenmier C. W., Sacca R., Furman W. L., Roussel M. F., Holt J. T., Nienhuis A. W., Stanley E. R., Sherr C. J. Expression of the human c-fms proto-oncogene product (colony-stimulating factor-1 receptor) on peripheral blood mononuclear cells and choriocarcinoma cell lines. J Clin Invest. 1986 Jun;77(6):1740–1746. doi: 10.1172/JCI112496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen O. M., Herrera R., Olowe Y., Petruzzelli L. M., Cobb M. H. Phosphorylation activates the insulin receptor tyrosine protein kinase. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3237–3240. doi: 10.1073/pnas.80.11.3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussel M. F., Downing J. R., Rettenmier C. W., Sherr C. J. A point mutation in the extracellular domain of the human CSF-1 receptor (c-fms proto-oncogene product) activates its transforming potential. Cell. 1988 Dec 23;55(6):979–988. doi: 10.1016/0092-8674(88)90243-7. [DOI] [PubMed] [Google Scholar]
- Roussel M. F., Downing J. R., Sherr C. J. Transforming activities of human CSF-1 receptors with different point mutations at codon 301 in their extracellular domains. Oncogene. 1990 Jan;5(1):25–30. [PubMed] [Google Scholar]
- Roussel M. F., Dull T. J., Rettenmier C. W., Ralph P., Ullrich A., Sherr C. J. Transforming potential of the c-fms proto-oncogene (CSF-1 receptor). Nature. 1987 Feb 5;325(6104):549–552. doi: 10.1038/325549a0. [DOI] [PubMed] [Google Scholar]
- Roussel M. F., Rettenmier C. W., Look A. T., Sherr C. J. Cell surface expression of v-fms-coded glycoproteins is required for transformation. Mol Cell Biol. 1984 Oct;4(10):1999–2009. doi: 10.1128/mcb.4.10.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert R. A., Hart C. E., Phillips P. E., Forstrom J. W., Ross R., Murray M. J., Bowen-Pope D. F. Two different subunits associate to create isoform-specific platelet-derived growth factor receptors. J Biol Chem. 1989 May 25;264(15):8771–8778. [PubMed] [Google Scholar]
- Sherr C. J. Colony-stimulating factor-1 receptor. Blood. 1990 Jan 1;75(1):1–12. [PubMed] [Google Scholar]
- Sherr C. J., Rettenmier C. W., Sacca R., Roussel M. F., Look A. T., Stanley E. R. The c-fms proto-oncogene product is related to the receptor for the mononuclear phagocyte growth factor, CSF-1. Cell. 1985 Jul;41(3):665–676. doi: 10.1016/s0092-8674(85)80047-7. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Weinmaster G., Zoller M. J., Smith M., Hinze E., Pawson T. Mutagenesis of Fujinami sarcoma virus: evidence that tyrosine phosphorylation of P130gag-fps modulates its biological activity. Cell. 1984 Jun;37(2):559–568. doi: 10.1016/0092-8674(84)90386-6. [DOI] [PubMed] [Google Scholar]
- Yarden Y., Schlessinger J. Epidermal growth factor induces rapid, reversible aggregation of the purified epidermal growth factor receptor. Biochemistry. 1987 Mar 10;26(5):1443–1451. doi: 10.1021/bi00379a035. [DOI] [PubMed] [Google Scholar]
- Yu K. T., Czech M. P. Tyrosine phosphorylation of the insulin receptor beta subunit activates the receptor-associated tyrosine kinase activity. J Biol Chem. 1984 Apr 25;259(8):5277–5286. [PubMed] [Google Scholar]