Abstract
Background
Carotid intima-media thickness (CIMT) and carotid plaques represent preclinical markers of atherosclerosis. We sought to describe predictors of CIMT and carotid plaques, including early life growth, in a young urban Indian cohort free of clinical cardiovascular disease (CVD).
Methods
In 2006-2009, we performed B-mode carotid ultrasound on 600 participants (mean [SD] age 36 [1.1] years; 45% women) from the New Delhi Birth Cohort to evaluate CIMT and carotid plaques (> 1mm). Height and weight were recorded at birth, 2 and 11 years of age. Data on CVD risk factors, anthropometry, medical history, socio-economic position, and lifestyle habits were collected in 1998-2002.
Results
Mean (SD) CIMT for men and women was 0.91 (0.12) and 0.86 (0.13) mm, respectively. Carotid plaque was present in 33% of men and 26% of women. Waist circumference in 1998-2002 was positively associated with CIMT (β coefficient 0.26 mm [0.17, 0.36] per SD) and carotid plaque (OR 1.27 [1.06,1.52] per SD) in 2006-2009. Higher triglycerides, PAI-1, insulin resistance, and diastolic blood pressure, metabolic syndrome, and lower HDL-cholesterol and physical activity predicted higher CIMT and/or plaque (p<0.05). Longer length at 2 years was associated with higher CIMT (p<0.05). These associations were attenuated after adjusting for adult waist circumference.
Conclusions
These are the first prospective data from India showing that early life growth, adult socio-demographics, and CVD risk factors predict future CIMT and /or carotid plaque. These relationships appear primarily mediated through central adiposity, highlighting the importance of maintaining a healthy weight in early adulthood to prevent CVD.
Keywords: carotid intima media thickness, carotid plaque, India, cohort, birth weight, infant and childhood growth
INTRODUCTION
Carotid intima-media thickness (CIMT) serves as a surrogate marker for atherosclerosis (1) and provides incremental information about future cardiovascular disease (CVD) risk beyond traditional risk factors in adults >40 years old (2). Non-occlusive carotid artery plaques represent preclinical atherosclerosis and demonstrate similar, if not higher, relative risks for incident CVD compared to CIMT (3-5). The American Society of Echocardiography guidelines suggest that individuals with CIMT >75th percentile (stratified by age, sex, race/ethnicity) or carotid plaque are at increased CVD risk and may require more aggressive risk reduction strategies (1).
Associations between preceding risk factors and CIMT in individuals <40 years old, such as age, male sex, BMI, smoking, low-density lipoprotein-(LDL)-cholesterol and systolic blood pressure have been established in high-income countries, such as the United States (6,7), and Finland (8). While there have been numerous cross-sectional studies in India describing associations between prevalent risk factors and CIMT (9-14), there have been no prospective studies describing the distribution and predictors of CIMT in young healthy adults in India.
In this current study, we sought: 1) to describe the distribution of CIMT in a young urban Indian cohort free of CVD, 2) to determine associations between preceding lifestyle and cardiovascular risk factors and CIMT and/or carotid plaques, and 3) to determine associations between growth in early life and CIMT and/or carotid plaques.
METHODS
Details of the study cohort have been published elsewhere (15). Briefly, during 1969-1972, married women living in a 12 km2 area of Delhi (N=20 755) participated in a study of pregnancy outcomes and childhood growth (15,16). During this period, there were 9169 pregnancies, and 8181 live births. Trained personnel recorded the babies’ weight and length within 72 hours of birth and 6-monthly until 14-21 years. Follow-up took place in phases. Phase 1 lasted from 1969-1972 and Phase 2 from 1973-1980. Gaps in funding and municipal closure of unauthorized housing interrupted measurements in 1972-1973 and 1980-1982. Data collection resumed in 1983-1990 (Phases 3 and 4).
At initial recruitment, 60% of families had incomes >50 rupees per month (national average 28 rupees) and 15% of parents were illiterate (national average 66%). Nevertheless, 43% of families lived in one room. Hindus were the majority religious group (84%), followed by Sikhs (12%), Christians (2%), Muslims (1%) and Jains (1%).
Adult Follow-up Phase 5 (April 1999 – August 2002)
Between 1999-2002, we retraced 2584 (32%) of the cohort, and 1526 (61%, or 19% of the original cohort) agreed to participate. Trained clinical research associates visited participants at their homes in order to explain the study, obtain consent, and administer a questionnaire on schooling, occupation, household possessions, alcohol consumption, tobacco use, physical activity and family history(16). Education was recorded as one of seven categories from ‘no schooling’ (category 1) through to ‘professional degree’ (e.g. – Master’s degree, PhD, medical qualification) and occupation as one of six categories, ranging from ‘unemployed’ (category 1) and ‘unskilled manual labour’ (category 2) to ‘professional’. Housewives were categorized according to their husband’s occupation. Information on material possessions was recorded as an indicator of socio-economic status. Participants were given a score of one for each of the following household items: electricity, fan, cycle, radio, motorized 2-wheel vehicle, gas stove, television, cable television, electric mixer, electric food grinder, electric air cooler, washing machine, car, air conditioner, computer, television antenna, and telephone. This information was used to create a score derived as the first principal component from a correlation matrix of the 17 binary variables and compared with national demographic data on household possessions (17).
Alcohol consumption was recorded as the frequency of intake and measure-size of spirits, beer and wine per week. These data were converted into units of alcohol (1 unit = 25 ml of spirits, 282 ml of beer, or 125 ml of wine), and categorized as none, <7 units, 7-14 units, and >14 units per week. Tobacco consumption was recorded as whether subjects smoked (cigarettes, beedis, cigars, or hookah), chewed (raw tobacco or with pan) or inhaled (snuff). Participants were categorized as current tobacco users or non-users. A score was derived as a summary estimate of daily physical activity. Work-related activity was classified on a 6-point scale ranging from ‘almost entirely sedentary’ to ‘heavy physical work’. Additional time spent per day in domestic activities (e.g. – sweeping, washing clothes, cooking) and leisure activities (e.g. – jogging, swimming, yoga) was recorded. Distances walked and cycled each day, with and without a load, were estimated, and converted into approximate periods of time spent in these activities. The time spent in all these activities was then multiplied by metabolic constants, derived from the relative energy expenditure of activities (18) and summed to derive a score, as we have previously reported (16).
Following the home visit, participants were asked to attend a clinic after an overnight fast. Their weight, height, and waist circumference were measured using standardized techniques. Blood pressure was measured using an automated device (Omron 711, Bannockburn, IL, USA) with the participants seated after five minutes rest. The mean of two consecutive blood pressure measurements was recorded. Fasting blood samples were drawn for plasma glucose, insulin, cholesterol, triglyceride, fibrinogen, and plasminogen activator inhibitor-1 (PAI-1) concentrations, and high sensitivity C-reactive protein (hsCRP). A further sample was drawn for glucose 120 minutes after a 75 gm oral glucose load. Glucose, cholesterol, and triglyceride concentrations were analyzed by enzymatic methods using Randox kits on a Beckman autoanalyser, and HDL-cholesterol using the same method after phosphotungstate precipitation. Insulin concentrations were measured by radioimmunoassay (Coat-a-Count insulin kit, Diagnostic Products, Los Angeles, CA, USA). Impaired fasting glucose (IFG), impaired glucose tolerance (IGT) and diabetes were defined using World Health Organization (WHO) criteria (19). Metabolic syndrome was defined using National Cholesterol Education Program, Adult Treatment Panel III criteria (20). Insulin resistance was calculated according to the homeostasis model assessment (HOMA) (21). Plasma clottable fibrinogen was determined using the Clauss clotting method (22). High sensitivity C-reactive protein (hsCRP) was measured in serum using solid Phase ELISA assay kits (BioCheck Inc., Foster City, CA, USA). PAI-1 was estimated using enzyme immuno-assay kits (Diagnostica Stago, Asnières-sur-Seine, France).
Adult Follow-up Phase 6 (August 2006 – January 2009)
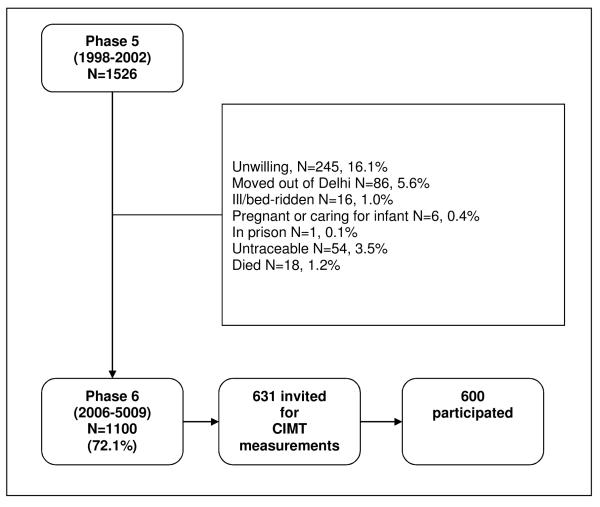
At a mean (SD) interval of 6.9 (1.0) years following the Phase 5 follow-up, all participants were invited for a repeat of the above investigations. Loss to follow-up between Phases 5 and 6 was minimized through the following algorithm until all non-responders were accounted for: telephone calls/emails, house visits, meeting with neighbours, and contact with local post office. Eighteen (1%) participants had died, and 54 (3.5%) participants could not be located. Of the surviving participants, 1100 (73%) agreed to participate in Phase 6. Figure 1 outlines the reasons for loss-to-follow-up. The methods used and risk factor measurements were identical in Phase 6 to Phase 5, except that for assessment of socio-economic status (material possessions) mobile phone was used in place of electric food grinder.
Figure 1.
Flowchart showing loss to follow up since Phase 5.
CIMT Measurements
For logistic reasons, CIMT measurements were limited to a sub-set of the participants. A sample size of 600 was chosen; ex post facto, this provided 80% power to detect a change of 0.12 SD in mean CIMT per SD change in a predictor (e.g. – waist circumference, birth weight) using a test at the 5% level. Participants were selected based on the completeness of their weight and height record in early life. A list was prepared of the 1,526 participants in Phase 5, in descending order of the number of available measurements in early life, starting with those with complete measurements at birth, 2 years, and all measurements up to the age of 17 years (a maximum of 19 measurements, N=631). Of these, 600 cohort members had CIMT measurements (362 men; 268 women).
All CIMT measurements were obtained and interpreted using a GE ultrasound machine (Model 5×3; GE Healthcare, Barrington, IL, USA) and a 5 MHz transducer by one individual (AK). The common carotid artery, proximal to the carotid bulb, was identified on both sides. The transducer was oriented longitudinally, and an area located where the intima was clearly defined and free from plaque on the far wall (1). The intima-media thickness was measured in 3 places, within one view, on each side via B-mode ultrasonography. For analysis, the following variables were derived: 1) mean (SD) of all 6 CIMT values (23), 2) the maximum CIMT value out of the 6 CIMT values, and 3) presence of carotid plaque, as defined by the presence of focal wall thickening ≥50% than that of the surrounding vessel wall or as a focal CIMT >1 mm area that protrudes into the lumen and is distinct from the adjacent boundary (1).
The All India Institute of Medical Sciences research ethics committee approved the study, and informed consent was obtained from all participants.
Statistical Analyses
The distributions of fibrinogen, hsCRP, PAI-1, triglycerides, glucose concentrations, and insulin resistance were skewed and were transformed to normality using inverse square roots (hsCRP only) or logarithms. For the analyses relating CIMT to growth in early life, data from the whole original cohort was used to derive individual SD-scores for weight, BMI and height at birth, 2 and 11 years. Interpolated values were used if measurements were made within 1 year (at age 2 years) and 2 years (at age 11 years). To assess changes in measurements (growth) in early life (e.g. from 2-11 years), we created conditional BMI and height variables for birth to 2 years of age (‘infancy’), 2-11 years of age (‘pre-pubertal childhood’) and from 11 years to adult (Phase 5). We regressed SD-scores at the end of the interval (e.g. 11 years) on SD-scores at the beginning (e.g. 2 years) and at birth, and expressed the residuals as SD-scores. This produces uncorrelated variables describing change between specific ages (‘conditional SD-scores’) (24). Associations of size and conditional growth in early life with CIMT were examined using multiple linear regression for mean and maximum CIMT, and multiple logistic regression for presence/absence of plaques. These models were adjusted for age and sex. In a further set of models we adjusted additionally for adult waist circumference.
RESULTS
We compared Phase 5 characteristics of the men and women who had CIMT measurements in Phase 6 with those who did not. Those who had CIMT measurements were slightly older (29.3 vs. 29.1 years, p=0.006), better educated (63% were graduates vs. 53%, p<0.001 across all education groups) and had more household possessions (mean 11.8 vs. 11.5 units, p<0.001), higher BMI (25.1 vs. 24.6 kg/m2, p=0.04) and waist circumference (90.7 vs. 89.9 cm in men and 81.3 vs. 78.6 cm in women, p=0.04), were less likely to be tobacco users (males: 26.8% vs. 31.9%, p=0.04) and less likely to consume >14 alcohol units per week (males: 13.5% vs. 19.7%, p=0.02 for Chi square test across all alcohol intake groups). These differences were no longer present after adjusting for education level and household possessions score. There were no differences between the two groups in terms of height, plasma lipid concentrations, blood pressure, glucose and insulin concentrations or prevalence of glucose intolerance or metabolic syndrome, PAI-1 and CRP, family history and physical exercise scores.
Associations between Phase 5 variables and Phase 6 CIMT
CIMT values (measured in Phase 6), and anthropometry, CVD risk factors, and lifestyle characteristics (all measured in Phase 5) are shown in Table 1. Mean CIMT was higher in men than women (Table 1) and increased with age (Table 2). All subsequent analyses were adjusted for sex and age. In these regression models, CIMT was positively related to adiposity (BMI and waist circumference) with waist circumference being the stronger predictor (Table 2). Lower HDL-cholesterol, higher plasma triglyceride concentrations, diastolic blood pressure, insulin resistance, PAI-1 concentrations, and the presence of metabolic syndrome predicted higher CIMT. There was an inverse association between total physical activity score and CIMT. Many of these associations were attenuated after adjusting for waist circumference, although those with HDL-cholesterol and physical exercise were not. There were no associations between CIMT and total cholesterol, glucose intolerance or tobacco use. Findings were similar for maximum CIMT (data not shown) and for presence of plaque (Table 3).
Table 1.
Characteristics of the cohort (age and CIMT measurements measured in Phase 6 and anthropometry, CVD risk factors and lifestyle factors measured in Phase 5).
| Men N=362 | Women N=238 | |
|---|---|---|
| Age (years) (Phase 6) | 36.3 (1.0) | 36.2 (1.0) |
| CIMT Measurements (Phase 6) | ||
| Mean CIMT (mm) | 0.91 (0.12) | 0.86 (0.13) |
| Maximum CIMT (mm) | 1.05 (0.19) | 0.98 (0.19) |
| Carotid plaque >1mm* | 33.4 | 25.6 |
| Adult anthropometry (Phase 5) | ||
| BMI (kg/m2) | 25.0 (4.1) | 25.3 (5.1) |
| Overweight (>25kg/m2)* | 49.4 | 50.0 |
| Obese (>30kg/m2) | 9.1 | 16.0 |
| Height (cm) | 169.8 (6.3) | 155.4 (5.3) |
| Waist circumference (cm) | 90.7 (11.5) | 81.3 (12.6) |
| Waist/hip ratio | 0.93 (0.06) | 0.82 (0.07) |
| CVD risk factors (Phase 5) | ||
| Total cholesterol (mg/dl) | 198.7 (42.8) | 186.7 (36.7) |
| HDL-cholesterol (mg/dl) | 45.2 (11.0) | 49.4 (12.1) |
| LDL-cholesterol (mg/dl) | 121.9 (34.4) | 116.4 (33.3) |
| Triglycerides (mg/dl)† | 128 (95, 194) | 93 (70, 126) |
| Systolic BP (mmHg) | 118.7 (10.3) | 107.2 (11.7) |
| Diastolic BP (mmHg) | 77.9 (9.4) | 73.8 (9.7) |
| Fasting glucose (mg/dl) † | 96.9 (86.9, 106.9) | 97.9 (88.9, 104.9) |
| 120-min glucose (mg/dl) † | 104.9 (89.7, 126.9) | 112.9 (97.9, 129.9) |
| Impaired fasting glucose (IFG)* | 16.5 | 13.8 |
| Impaired glucose tolerance (IGt)* | 10.2 | 9.6 |
| History/Diagnosis of Diabetes* | 5.4 | 4.8 |
| Insulin resistance (HOMA) † | 2.3 (1.4, 2.7) | 2.0 1.1, 2.6) |
| Metabolic syndrome* | 34.5 | 23.6 |
| Fibrinogen (g/dl) † | 2.6 (2.2, 3.0) | 2.8 (2.4, 3.2) |
| PAI-1 (ng/ml)† | 95 (58,148) | 80 (42, 140) |
| hs CRP (mg/l) † | 2.0 (1.0, 4.0) | 2.0 (0.9, 4.4) |
| Lifestyle factors (Phase 5) | ||
| Family history* | 67.1 | 70.6 |
| Alcohol >14 u/wk* | 13.5 | 0 |
| Tobacco* | 30.7 | 0 |
| Occupation: | ||
| Unemployed/unskilled/semi- skilled*‡ |
9.1 | 10.1 |
| Education: | ||
| Illiterate/primary/middle school | 10.8 | 5.9 |
| Possessions score | 11.9 (1.8) | 11.7 (2.0) |
Data are mean (SD) unless otherwise indicated:
median and interquartile range for skewed variables
percent for categorical variables. Family history = high blood pressure, angina, myocardial infarct, stroke or diabetes in a first-degree relative. Tobacco = any tobacco use currently or in the past.
For women who described their occupation as housewife (63%) we have categorised them here by their husband’s occupation.
Table 2.
Associations of adult anthropometry, CVD risk factors and lifestyle factors (all measured in Phase 5) with mean CIMT (per 0.1 mm) measured in Phase 6.
| Predictor variable | Regression coefficient |
95% confidence Interval |
p value | Regression coefficient |
95% confidence interval |
p value |
|---|---|---|---|---|---|---|
| Model 1 adjusted for age and sex only |
Model 2 further adjusted for adult waist circumference |
|||||
| Age (years) | 0.22 | 0.12 to 0.32 | <0.001 | 0.19 | 0.09 to 0.29 | <0.001 |
| Sex (Male=0, Female=1) | −0.54 | −0.74 to -0.34 | <0.001 | −0.54 | −0.74 to -0.34 | <0.001 |
| Adult anthropometry (Phase 5) | ||||||
| BMI (z) | 0.19 | 0.09 to 0.29 | <0.001 | |||
| Height (z) | 0.05 | −0.05 to 0.15 | 0.3 | |||
| Waist circumference (z) | 0.26 | 0.17 to 0.36 | <0.001 | |||
| CVD risk factors (Phase 5) | ||||||
| Total cholesterol (z) | 0.04 | −0.06 to 0.14 | 0.4 | −0.02 | −0.13 to 0.08 | 0.6 |
| HDL cholesterol (z) | −0.14 | −0.23 to -0.04 | 0.008 | −0.11 | −0.21 to -0.02 | 0.02 |
| LDL cholesterol (z) | 0.03 | −0.07 to 0.13 | 0.6 | −0.03 | −0.13 to 0.07 | 0.6 |
| Triglycerides (z) | 0.16 | 0.06 to 0.26 | 0.002 | 0.08 | −0.03 to 0.18 | 0.1 |
| Systolic BP (z) | 0.06 | −0.05 to 0.16 | 0.3 | −0.03 | −0.13 to 0.08 | 0.6 |
| Diastolic BP (z) | 0.11 | 0.01 to 0.21 | 0.04 | 0.00 | −0.10 to 0.11 | 1.0 |
| Fasting glucose (z) | 0.09 | −0.02 to 0.19 | 0.1 | 0.06 | −0.03 to 0.16 | 0.2 |
| 120-min glucose (z) | 0.09 | −0.01 to 0.19 | 0.09 | 0.04 | −0.06 to 0.14 | 0.5 |
| IFG (1) v normoglycaemic (0) | −0.10 | −0.38 to 0.18 | 0.5 | −0.16 | −0.44 to 0.12 | 0.3 |
| IGT (1) v normoglycaemic (0) | 0.11 | −0.23 to 0.45 | 0.5 | −0.02 | −0.36 to 0.32 | 0.9 |
| Diabetes (1) v normoglycaemic (0) |
0.15 | −0.31 to 0.61 | 0.5 | −0.02 | −0.48 to 0.44 | 0.9 |
| Any glucose tolerance (IFG, IGT or DM) |
0.04 | −0.19 to 0.27 | 0.7 | −0.04 | −0.27 to 0.18 | 0.7 |
| Insulin resistance (HOMA) (z) | 0.13 | 0.03 to 0.23 | 0.01 | 0.02 | −0.09 to 0.13 | 0.7 |
| Metabolic syndrome | 0.41 | 0.20 to 0.63 | <0.001 | 0.20 | −0.04 to 0.43 | 0.1 |
| Fibrinogen (z) | 0.04 | −0.06 to 0.14 | 0.5 | 0.03 | −0.07 to 0.12 | 0.6 |
| PAI-1 (z) | 0.11 | 0.01 to 0.21 | 0.03 | 0.08 | −0.02 to 0.18 | 0.1 |
| hs CRP (z) | 0.07 | −0.03 to 0.17 | 0.2 | −0.03 | −0.13 to 0.08 | 0.6 |
| Lifestyle factors (Phase 5)* | ||||||
| Family history | 0.07 | −0.14 to 0.28 | 0.5 | −0.02 | −0.23 to 0.19 | 0.8 |
| Alcohol | 0.04 | −0.08 to 0.16 | 0.5 | 0.02 | −0.09 to 0.14 | 0.7 |
| Tobacco | 0.04 | −0.10 to 0.19 | 0.6 | 0.04 | −0.10 to 0.19 | 0.5 |
| Occupation | 0.06 | −0.04 t0 0.16 | 0.2 | 0.03 | −0.07 to 0.12 | 0.6 |
| Education | −0.04 | −0.12 to 0.05 | 0.4 | −0.05 | −0.13 to 0.03 | 0.2 |
| Possessions | 0.01 | −0.09 to 0.11 | 0.8 | −0.03 | −0.13 to 0.08 | 0.6 |
| Total exercise score | −0.17 | −0.27 to -0.07 | 0.001 | −0.14 | −0.24 to -0.05 | 0.004 |
z=standard deviation score.
Family history of high blood pressure, angina, myocardial infarction, stroke or diabetes in a first degree relative; Alcohol consumption (four levels from none to heavy); Tobacco use (any type of tobacco, categorised into current user vs. non-user); Total exercise score derived as a summary estimate of daily physical activity (work-related activity classified on a 6-point scale ranging from ‘almost entirely sedentary’ to ‘heavy physical work’; additional time spent per day in domestic activities (e.g. – sweeping, washing clothes, cooking) and leisure activities (e.g. – jogging, swimming, yoga); distances walked and cycled each day, with and without a load; converted into approximate periods of time spent in these activities, then multiplied by metabolic constants and summed; Occupation as one of six categories, ranging from ‘unemployed’ (category 1) and ‘unskilled manual labour’ (category 2) to ‘professional’; housewives categorized according to their husband’s occupation; Education: one of seven categories from ‘no schooling’ (category 1) through to ‘professional degree’ (e.g. – Masters degree, PhD, medical qualification); Material possessions: score of one for each of the following household items: electricity, fan, cycle, radio, motorized 2-wheeler vehicle, gas stove, television, cable television, electric mixer, electric grinder, electric air cooler, washing machine, car, air conditioner, computer, television antenna, and telephone; this information was used to create a score derived as the first principal component from a correlation matrix of the 17 binary variables.
Table 3.
Associations of adult anthropometry, CVD risk factors and lifestyle factors (all measured in Phase 5) with the presence of carotid plaque measured in Phase 6.
| Predictor variable | OR | 95% confidence interval |
p value | OR | 95% confidence interval |
p value |
|---|---|---|---|---|---|---|
| Model 1 adjusted for age and sex only |
Model 2 further adjusted for adult waist circumference |
|||||
| Age (years) | 1.20 | 1.00 to 1.44 | 0.05 | 1.17 | 0.97 to 1.41 | 0.1 |
| Sex (Male=0, Female=1) | 0.69 | 0.48 to 0.99 | 0.04 | 0.69 | 0.48 to 0.99 | 0.04 |
| Adult anthropometry (Phase 5) | ||||||
| BMI (z) | 1.14 | 0.95 to 1.36 | 0.1 | |||
| Height (z) | 1.09 | 0.91 to 1.30 | 0.4 | |||
| Waist circumference (z) | 1.27 | 1.06 to 1.52 | 0.01 | |||
| CVD risk factors (Phase 5) | ||||||
| Total cholesterol (z) | 1.02 | 0.85 to 1.22 | 0.8 | 0.96 | 0.80 to 1.16 | 0.7 |
| HDL cholesterol (z) | 0.84 | 0.70 to 1.00 | 0.05 | 0.85 | 0.71 to 1.02 | 0.08 |
| LDL cholesterol (z) | 1.03 | 0.86 to 1.22 | 0.8 | 0.98 | 0.82 to 1.18 | 0.8 |
| Triglycerides (z) | 1.16 | 0.97 to 1.38 | 0.1 | 1.08 | 0.90 to 1.31 | 0.4 |
| Systolic BP (z) | 1.16 | 0.97 to 1.39 | 0.1 | 1.09 | 0.91 to 1.32 | 0.4 |
| Diastolic BP (z) | 1.21 | 1.01 to 1.44 | 0.04 | 1.12 | 0.92 to 1.36 | 0.3 |
| Fasting glucose (z) | 1.05 | 0.88 to 1.25 | 0.6 | 1.03 | 0.86 to 1.23 | 0.8 |
| 120-min glucose (z) | 1.12 | 0.94 to 1.35 | 0.2 | 1.07 | 0.89 to 1.29 | 0.5 |
| IFG (1) v normoglycaemic (0) | 0.97 | 0.59 to 1.59 | 0.9 | 0.90 | 0.54 to 1.49 | 0.7 |
| IGT (1) v normoglycaemic (0) | 1.00 | 0.55 to 1.83 | 1.0 | 0.88 | 0.47 to 1.62 | 0.7 |
| Diabetes (1) v normoglycaemic (0) | 0.55 | 0.22 to 1.40 | 0.2 | 0.44 | 0.17 to 1.13 | 0.09 |
| Any glucose tolerance (IFG, IGT or DM) | 0.92 | 0.61 to 1.38 | 0.7 | 0.84 | 0.56 to 1.27 | 0.4 |
| Insulin resistance (HOMA) (z) | 1.13 | 0.95 to 1.35 | 0.2 | 1.03 | 0.85 to 1.26 | 0.8 |
| Metabolic syndrome | 1.75 | 1.20 to 2.55 | 0.003 | 1.54 | 1.01 to 2.35 | 0.04 |
| Fibrinogen (z) | 1.04 | 0.87 to 1.24 | 0.7 | 1.03 | 0.86 to 1.23 | 0.8 |
| PAI-1 (z) | 0.98 | 0.82 to 1.17 | 0.8 | 0.95 | 0.79 to 1.14 | 0.6 |
| hs CRP (z) | 1.03 | 0.86 to 1.23 | 0.7 | 0.95 | 0.78 to 1.16 | 0.6 |
| Lifestyle factors (Phase 5) * | ||||||
| Family history | 0.98 | 0.67 to 1.43 | 0.9 | 0.90 | 0.62 to 1.33 | 0.6 |
| Alcohol | 0.87 | 0.70 to 1.07 | 0.2 | 0.85 | 0.69 to 1.05 | 0.1 |
| Tobacco | 0.92 | 0.71 to 1.18 | 0.5 | 0.92 | 0.71 to 1.18 | 0.5 |
| Occupation | 1.07 | 0.89 to 1.28 | 0.5 | 1.04 | 0.86 to 1.24 | 0.7 |
| Education | 0.88 | 0.77 to 1.02 | 0.09 | 0.87 | 0.75 to 1.00 | 0.06 |
| Possessions | 0.88 | 0.74 to 1.06 | 0.2 | 0.85 | 0.71 to 1.03 | 0.09 |
| Total exercise score | 0.70 | 0.58 to 0.84 | <0.001 | 0.72 | 0.60 to 0.86 | <0.001 |
z=standard deviation score.
Family history of high blood pressure, angina, myocardial infarction, stroke or diabetes in a first degree relative; Alcohol consumption (four levels from none to heavy); Tobacco use (any type of tobacco, categorised into current user vs. non-user); Total exercise score derived as a summary estimate of daily physical activity (work-related activity classified on a 6-point scale ranging from ‘almost entirely sedentary’ to ‘heavy physical work’; additional time spent per day in domestic activities (e.g. – sweeping, washing clothes, cooking) and leisure activities (e.g. – jogging, swimming, yoga); distances walked and cycled each day, with and without a load; converted into approximate periods of time spent in these activities, then multiplied by metabolic constants and summed; Occupation as one of six categories, ranging from ‘unemployed’ (category 1) and ‘unskilled manual labour’ (category 2) to ‘professional’; housewives categorized according to their husband’s occupation; Education: one of seven categories from ‘no schooling’ (category 1) through to ‘professional degree’ (e.g. – Masters degree, PhD, medical qualification); Material possessions: score of one for each of the following household items: electricity, fan, cycle, radio, motorized 2-wheeler vehicle, gas stove, television, cable television, electric mixer, electric grinder, electric air cooler, washing machine, car, air conditioner, computer, television antenna, and telephone; this information was used to create a score derived as the first principal component from a correlation matrix of the 17 binary variables.
Associations between growth in early life and CIMT
Longer length at the age of 2 years and faster length growth between birth and 2 years were positively associated with mean CIMT (Table 4). Greater conditional BMI gain between 11 years and adulthood was also associated with higher mean CIMT. These associations were attenuated after adjusting for adult waist circumference (Table 4). There were no other associations of BMI or height at any age from birth through to 11 years, nor of conditional BMI or height growth, with mean CIMT. The findings were similar if maximum CIMT or presence of plaque were the outcomes, rather than mean CIMT (data not shown).
Table 4.
Associations between size and growth in early life and mean CIMT (per 0.1mm) measured in Phase 6.
| Size and growth in early life | Regression coefficient |
95% confidence interval |
p value | Regression coefficient |
95% confidence interval |
p value |
|---|---|---|---|---|---|---|
| Model 1 adjusted for age and sex only |
Model 2 further adjusted for adult waist circumference |
|||||
| Size variables | ||||||
| Birth weight (z) | 0.01 | −0.10 to 0.11 | 0.9 | −0.03 | −0.13 to 0.07 | 0.5 |
| Birth length (z) | 0.00 | −0.10 to 0.10 | 1.0 | −0.03 | −0.13 to 0.07 | 0.6 |
| Birth BMI (z) | 0.01 | −0.10 to 0.11 | 0.9 | −0.03 | −0.13 to 0.07 | 0.6 |
| 2 year height (z) | 0.12 | 0.02 to 0.22 | 0.02 | 0.05 | −0.05 to 0.15 | 0.3 |
| 2 year BMI (z) | 0.06 | −0.04 to 0.16 | 0.2 | 0.00 | −0.10 to 0.10 | 1.0 |
| 11 year height (z) | 0.06 | −0.04 to 0.16 | 0.2 | −0.02 | −0.13 to 0.08 | 0.7 |
| 11 year BMI (z) | 0.06 | −0.04 to 0.16 | 0.2 | −0.10 | −0.22 to 0.01 | 0.08 |
| Growth variables | ||||||
| Conditional height (0-2 y) | 0.13 | 0.03 to 0.23 | 0.01 | 0.06 | −0.04 to 0.16 | 0.3 |
| Conditional BMI (0-2 y) | 0.06 | −0.04 to 0.16 | 0.3 | 0.00 | −0.10 to 0.11 | 0.9 |
| Conditional height (2-11 y) | −0.02 | −0.12 to 0.08 | 0.7 | −0.08 | −0.18 to 0.03 | 0.2 |
| Conditional BMI (2-11 y) | 0.04 | −0.06 to 0.14 | 0.4 | −0.11 | −0.23 to 0.00 | 0.05 |
| Conditional height (11 y-adult) | −0.05 | −0.15 to 0.06 | 0.4 | −0.05 | −0.15 to 0.06 | 0.4 |
| Conditional BMI (11 y-adult) | 0.22 | 0.11 to 0.32 | <0.001 | 0.03 | −0.12 to 0.18 | 0.7 |
Z=standard deviation score. Data were analysed using linear regression. Each row represents a separate regression for each model.
DISCUSSION
Summary of main findings
We have measured carotid intima-media thickness (CIMT) and have assessed the presence of carotid plaque in a large sample of men and women aged 33-38 years in the city of New Delhi, India. We related CIMT to cardio-metabolic risk factors measured approximately 7 years earlier, and to the cohort members’ size and trajectory of BMI and height growth during infancy and pre-pubertal childhood. CIMT values and carotid plaque were positively associated with traditional cardiovascular risk factors including adiposity, blood pressure, plasma triglyceride, biomarkers such as insulin and PAI-1 concentrations, and negatively associated with HDL-cholesterol concentration and physical activity. These associations appeared to be mediated, at least partly, by adiposity. Of the early growth measurements, only height at the age of 2 years and conditional height gain in the first two years of life were positively associated with CIMT; this effect also appeared to be mediated by adult adiposity.
Socio-demographics, traditional cardiovascular risk factors, and biomarkers as predictors of CIMT
These are the first prospective data from India showing that socio-demographics, traditional cardiovascular risk factors predict later CIMT and presence of carotid artery plaque and the largest CIMT study in India to date. Previous studies in India have only demonstrated associations with concurrent risk markers, and have shown that age, metabolic syndrome and diabetes are the risk factors most strongly related to CIMT (9,13,25). Additionally, waist-hip ratio, systolic blood pressure, total and LDL-cholesterol, oxidised LDL, insulin concentrations and insulin resistance, and C-reactive protein are correlated with CIMT in Indian populations (11,12,26). Mean CIMT values in the New Delhi Birth Cohort participants are generally higher than mean CIMT values smaller studies of individuals from North and West India (26, 27), but mean body weight, blood glucose, and total cholesterol were all higher in the New Delhi Birth Cohort participants. Mean CIMT values were higher in older, North Indian participants with diabetes (1.3 mm [0.3]) from another study (11) but lower than older individuals from South India (12) and South Asian immigrants to the United Kingdom (28).
Our results, showing that many of these risk factors predict future CIMT, were broadly similar to those found in young adults from other populations. We did not find an association between smoking and CIMT, unlike prior literature (29), but this association has been shown to be attenuated when controlling for adiposity as measured by BMI (30). Some studies have suggested different strength of associations between risk factors and CIMT in South Asian compared to white Caucasian populations; for example total cholesterol, HDL-cholesterol and diabetes were more strongly related to CIMT in Indian compared with Australian adults (10).
Studies in white Caucasian and US black populations have also shown that risk factors measured in childhood predict CIMT. For example, the International Childhood Cardiovascular Cohort (i3C) Consortium, which includes data from the Cardiovascular Risk in Young Finns Study, the Childhood Determinants of Adult Health, the Bogalusa Heart Study, and the Muscatine Study have demonstrated positive associations between adiposity as measured by BMI, total cholesterol, triglycerides, and blood pressure in childhood and CIMT in young adulthood (31).
In our data, much of the association between risk markers and CIMT appeared to be mediated by adiposity, especially central adiposity as indicated by waist circumference. Adiposity as measured by BMI at age 18 was the strongest predictor of future CIMT in iC3, with a beta coefficient (0.143) similar to our data (0.19). These data support the concept that lifestyle changes that prevent excessive adiposity in early adulthood are also likely important for the prevention CIMT progression and subsequent cardiovascular disease events.
Growth in early life and CIMT
Apart from positive associations of CIMT with length measured at the age of 2 years and gain in height between birth and 2 years, we found no associations of BMI or height at any age from birth to 11 years, or of conditional BMI or height gain between birth-2 years or 2-11 years with young adult CIMT. These results are in marked contrast to a number of studies showing that lower birth weight and/or weight in infancy, and rapid weight gain after infancy, are associated with an increased risk of adult cardiovascular disease or mortality (32-34). Data from iC3 suggest that measures of early life and childhood adiposity are associated with CIMT, but these relationships are weaker than later measures of adiposity. Our CIMT results are, however, consistent with many other cohort studies in a variety of populations. For example, four studies of adults in the UK or Europe showed either no association or a weak positive association between birth weight and CIMT (35-37). Adults who were exposed to the Dutch Famine while in utero had lower CIMT values than those not exposed to famine, despite having a higher risk of CVD events (38). This apparent paradox suggests that the well-known association of small size in very early life with or without accelerated weight gain during childhood, with cardiovascular mortality in later life cannot be attributed to changes in CIMT. Our findings may indicate that the environmental factors that produce early growth failure and/or rapid childhood weight gain influence CVD mortality through changes in endothelial function, inflammatory/immune reactivity (39, 40), or thrombosis/haemostasis, rather than through thickening of the vascular media, as measured by CIMT, or through atherosclerosis, as measured by the presence of carotid plaques.
CIMT values in New Delhi compared to elsewhere
Mean CIMT values in our study were considerably higher, and indeed the whole CIMT distribution was right-shifted, compared with those obtained in studies of young adults in the USA (29) and France (41) (Table 5). Reasons for these differences may include higher body weight, fasting plasma glucose, and total cholesterol in the New Delhi Birth Cohort participants compared with other cohorts, despite similar ages (26, 42); differences in transducer frequency among studies (5 MHz vs. 7.5 MHz); operator or reader bias; or chance.
Table 5.
Comparison of right and left common CIMT values measured in Phase 6 with large US and European cohorts with similar age ranges.
| US | Bogalusa Heart Study (27) - 35 years old | |||
|---|---|---|---|---|
| RIGHT | White Male | White Female | Black Male | Black Female |
| 25th | 0.662 | 0.611 | 0.712 | 0.685 |
| 50th | 0.740 | 0.676 | 0.793 | 0.754 |
| 75th | 0.845 | 0.754 | 0.929 | 0.837 |
| LEFT | White Male | White Female | Black Male | Black Female |
| 25th | 0.658 | 0.618 | 0.713 | 0.67 |
| 50th | 0.76 | 0.693 | 0.812 | 0.736 |
| 75th | 0.864 | 0.766 | 0.894 | 0.822 |
|
| ||||
| EUROPE | AXA Study (26) - 31-40 years old | |||
|
| ||||
| RIGHT | Male | Female | ||
| 25th | 0.42 | 0.42 | ||
| 50th | 0.46 | 0.45 | ||
| 75th | 0.5 | 0.49 | ||
| LEFT | Male | Female | ||
| 25th | 0.44 | 0.44 | ||
| 50th | 0.47 | 0.47 | ||
| 75th | 0.57 | 0.51 | ||
|
| ||||
| INDIA | New Delhi Birth Cohort – 36 years old | |||
|
| ||||
| RIGHT | Male | Female | ||
| 25th | 0.90 | 0.80 | ||
| 50th | 1.00 | 0.90 | ||
| 75th | 1.10 | 1.00 | ||
| LEFT | Male | Female | ||
| 25th | 0.80 | 0.80 | ||
| 50th | 1.00 | 0.90 | ||
| 75th | 1.10 | 1.00 | ||
Strengths and limitations of the study
Strengths of the study were CIMT measurements by a single operator on a large population-based cohort of young adults using a standard protocol, combined with high quality measurements of body size, made by trained researchers, frequently through infancy and childhood, and measurements of a range of traditional CVD risk markers 7 years previously. Our study also has limitations. First, by design, only 39% of the Phase 5 participants (7.3% of the original cohort) were included, which may bias our results. Participants who had CIMT measurements were slightly older and had higher socio-economic status than the members of the cohort who did not have these measurements, but these differences were small. Second, we used a lower threshold (1 mm) a priori to define carotid plaque than others have previously used (1.5 mm), which may partially explain the relatively high prevalence of carotid plaque in this cohort. Third, we did not evaluate intra-operator or -observer variability, which may bias our results.
Conclusions
In conclusion, we have demonstrated the association between socio-demographics, traditional cardiovascular risk factors, biomarkers such as insulin and PAI-1 and future CIMT and carotid artery plaque for the first time in India. These relationships appear primarily mediated through central adiposity, highlighting the importance of maintaining a healthy weight in early adulthood to prevent or postpone the onset of cardiovascular disease.
Key Messages.
These are the first prospective data from India showing that socio-demographics, traditional cardiovascular risk factors predict later CIMT and presence of carotid artery plaque.
Carotid intima-media thickness (CIMT) and carotid plaque were positively associated with traditional cardiovascular risk factors including adiposity, blood pressure, plasma triglyceride, biomarkers such as insulin and PAI-1 concentrations, and negatively associated with HDL-cholesterol concentration and physical activity measured 7 years earlier in the 600 participants from the New Delhi Birth Cohort.
These associations appeared to be mediated, at least partly, by adiposity.
Of the early growth measurements, only height at the age of 2 years and conditional height gain in the first two years of life were positively associated with CIMT; both appeared to be mediated by adult adiposity.
Acknowledgements
We acknowledge the assistance of Ms. Rajeshwari Verma, Mr. Bhaskar Singh, Dr. K.D. Gupta, Dr. Dinesh Mishra, Ms. Arti Mishra, and Mr. Dileep Gupta, who have confirmed their acknowledgment.
Funding: This work was originally supported by the National Centre for Health Statistics and the Indian Council of Medical Research and the current phase was supported by the British Heart Foundation. The study sponsor did not participate in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication. MDH was supported by the NIH/NHLBI T32 HL069771-08 institutional fellowship in cardiovascular epidemiology through the Department of Preventive Medicine at Northwestern University Feinberg School of Medicine. DP receives research support from NIH Fogarty International Center, NIH/NHLBI, UnitedHealth, Wellcome Trust, Canadian Institute of Health Research, Indian Council of Medical Research, Department of Science and Technology (Government of India), Duke Clinical Research Institute.
Footnotes
Conflicts of interest: The authors have no conflicts of interest to disclose.
MeSH headings: carotid atherosclerosis, India, cohort studies, birth weight
REFERENCES
- 1.Stein JH, Korcarz CE, Hurst RT, et al. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Endorsed by the Society for Vascular Medicine. J Am Soc Echocardiogr. 2008;21:93–111. doi: 10.1016/j.echo.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 2.Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation. 2007;30(115):459–67. doi: 10.1161/CIRCULATIONAHA.106.628875. [DOI] [PubMed] [Google Scholar]
- 3.Belcaro G, Nicolaides AN, Ramaswami G, et al. Carotid and femoral ultrasound morphology screening and cardiovascular events in low risk subjects: a 10-year follow-up study (the CAFES-CAVE study(1)) Atherosclerosis. 2001;156:379–87. doi: 10.1016/s0021-9150(00)00665-1. [DOI] [PubMed] [Google Scholar]
- 4.Spence JD, Eliasziw M, DiCicco M, Hackam DG, Galil R, Lohmann T. Carotid plaque area: a tool for targeting and evaluating vascular preventive therapy. Stroke. 2002;33:2916–22. doi: 10.1161/01.str.0000042207.16156.b9. [DOI] [PubMed] [Google Scholar]
- 5.Stork S, van den Beld AW, von Schacky C, et al. Carotid artery plaque burden, stiffness, and mortality risk in elderly men: a prospective, population-based cohort study. Circulation. 2004;110:344–8. doi: 10.1161/01.CIR.0000134966.10793.C9. [DOI] [PubMed] [Google Scholar]
- 6.Li S, Chen W, Srinivasan SR, et al. Childhood cardiovascular risk factors and carotid vascular changes in adulthood: the Bogalusa Heart Study. JAMA. 2003;290:2271–6. doi: 10.1001/jama.290.17.2271. [DOI] [PubMed] [Google Scholar]
- 7.Davis PH, Dawson JD, Riley WA, Lauer RM. Carotid intimal-medial thickness is related to cardiovascular risk factors measured from childhood through middle age: The Muscatine Study. Circulation. 2001;104:2815–9. doi: 10.1161/hc4601.099486. [DOI] [PubMed] [Google Scholar]
- 8.Raitakari OT, Juonala M, Kahonen M, et al. Cardiovascular risk factors in childhood and carotid artery intima-media thickness in adulthood: the Cardiovascular Risk in Young Finns Study. JAMA. 2003;290:2277–83. doi: 10.1001/jama.290.17.2277. [DOI] [PubMed] [Google Scholar]
- 9.Mohan V, Gokulakrishnan K, Sandeep S, Srivastava BK, Ravikumar R, Deepa R. Intimal media thickness, glucose intolerance and metabolic syndrome in Asian Indians--the Chennai Urban Rural Epidemiology Study (CURES -22) Diabet Med. 2006;23:845–50. doi: 10.1111/j.1464-5491.2006.01898.x. [DOI] [PubMed] [Google Scholar]
- 10.Chow CK, McQuillan B, Raju PK, et al. Greater adverse effects of cholesterol and diabetes on carotid intima-media thickness in South Asian Indians: comparison of risk factor-IMT associations in two population-based surveys. Atherosclerosis. 2008;199:116–22. doi: 10.1016/j.atherosclerosis.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 11.Ahmad J, Ahmned F, Siddiqui MA, et al. Inflammatory markers, insulin resistance and carotid intima-media thickness in North-Indian type 2 diabetic subjects. J Assoc Physicians India. 2007;55:693–9. [PubMed] [Google Scholar]
- 12.Gokulakrishnan K, Deepa R, Velmurugan K, Ravikumar R, Karkuzhali K, Mohan V. Oxidized low-density lipoprotein and intimal medial thickness in subjects with glucose intolerance: the Chennai Urban Rural Epidemiology Study-25. Metabolism. 2007;56:245–50. doi: 10.1016/j.metabol.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 13.Ahmad J, Hameed B, Das G, Siddiqui MA, Ahmad I. Postprandial hypertriglyceridemia and carotid intima-media thickness in north Indian type 2 diabetic subjects. Diabetes Res Clin Pract. 2005;69:142–50. doi: 10.1016/j.diabres.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 14.Kasliwal RR, Bansal M, Gupta H, Agrawal S. Association of carotid intima-media thickness with left main coronary artery disease. Indian Heart J. 2007;59:50–5. [PubMed] [Google Scholar]
- 15.Bhargava SK, Sachdev HS, Fall CH, et al. Relation of serial changes in childhood body-mass index to impaired glucose tolerance in young adulthood. N Engl J Med. 2004;350:865–75. doi: 10.1056/NEJMoa035698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sachdev HS, Fall CH, Osmond C, et al. Anthropometric indicators of body composition in young adults: relation to size at birth and serial measurements of body mass index in childhood in the New Delhi birth cohort. Am J Clin Nutr. 2005;82:456–66. doi: 10.1093/ajcn.82.2.456. [DOI] [PubMed] [Google Scholar]
- 17.Rutstein SO, Johnson K. The DHS Wealth Index. DHS comparative reports 6. ORC Macro; Calverton, MD: 2004. [Google Scholar]
- 18.Food and Agriculture Organization of the United Nations . Human energy requirements: report of a Joint FAO/WHO/UNU expert consultation. Rome: 2004. [Google Scholar]
- 19.World Health Organization . Definition, Diagnosis and Classification of Diabetes Mellitus and its Complications. Report of a WHO Consultation. World Health Organization; Geneva: 1999. WHO/NCD/NCS/99.2. [Google Scholar]
- 20.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–52. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 21.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 22.Clauss A. Rapid physiological coagulation method in determination of fibrinogen. Acta Haematol. 1957;17:237–46. doi: 10.1159/000205234. [DOI] [PubMed] [Google Scholar]
- 23.Stein JH, Fraizer MC, Aeschlimann SE, Nelson-Worel J, McBride PE, Douglas PS. Vascular age: integrating carotid intima-media thickness measurements with global coronary risk assessment. Clin Cardiol. 2004;27:388–92. doi: 10.1002/clc.4960270704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gale CR, O’Callaghan FO, Bredow M, Martyn CN, Avon Longitudinal Study of Parents and Children Study team The influence of head growth in fetal life, infancy, and childhood from the ages of 4 and 8 years. Pediatrics. 2006;118:1486–92. doi: 10.1542/peds.2005-2629. [DOI] [PubMed] [Google Scholar]
- 25.Mohan V, Ravikumar R, Shanthi Rani S, Deepa R. Intimal medial thickness of the carotid artery in South Indian diabetic and non-diabetic subjects: the Chennai Urban Population Study (CUPS) Diabetologia. 2000;43:494–9. doi: 10.1007/s001250051334. [DOI] [PubMed] [Google Scholar]
- 26.Ahmad J, Ahmed F, Siddiqui MA, Hameed B, Ahmad I. Inflammation, insulin resistance and carotid IMT in first degree relatives of north Indian type 2 diabetic subjects. Diabetes Res Clin Pract. 2006;73:205–10. doi: 10.1016/j.diabres.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 27.Yaknik CS, Joglekar CV, Chinchwadkar MC, et al. Conventional and novel cardiovascular risk factors and markers of vascular damage in rural and urban Indian men. Int J Cardiol. 2011 Sep 16; doi: 10.1016/j.ijcard.2011.08.053. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 28.Bennett PC, Gill PS, Silverman S, Blann AD, Lip GY. Ethnic differences in common carotid intima-media thickness, and the relationship to cardiovascular risk factors and peripheral arterial disease: the Ethnic-Echocardiographic Heart of England Screening Study. QJM. 2011;104:245–54. doi: 10.1093/qjmed/hcq187. [DOI] [PubMed] [Google Scholar]
- 29.Tzou WS, Douglas PS, Srinivasan SR, et al. Distribution and predictors of carotid intima-media thickness in young adults. Prev Cardiol. 2007;10:181–9. doi: 10.1111/j.1520-037x.2007.06450.x. [DOI] [PubMed] [Google Scholar]
- 30.Oren A, Vos LE, Uiterwaal CS, Grobbee DE, Bots ML. Cardiovascular risk factors and increased carotid intima-media thickness in healthy young adults: the Atherosclerosis Risk in Young Adults (ARYA) Study. Arch Int Med. 2003;163:1787–92. doi: 10.1001/archinte.163.15.1787. [DOI] [PubMed] [Google Scholar]
- 31.Juonala M, Magnussen CG, Venn A, et al. Influence of age on associations between childhood risk factors and carotid intima-media thickness in adulthood. Circulation. 2010;122:2514–20. doi: 10.1161/CIRCULATIONAHA.110.966465. [DOI] [PubMed] [Google Scholar]
- 32.Barker DJP, Osmond C, Winter PDW, Margetts B, Simmonds SJ. Weight in infancy and death from ischaemic heart disease. Lancet. 1989;2:577–80. doi: 10.1016/s0140-6736(89)90710-1. [DOI] [PubMed] [Google Scholar]
- 33.Eriksson JG, Forsen T, Tuomilehto J, Winter PD, Osmond C, Barker DJP. Catch-up growth in childhood and death from coronary heart disease: longitudinal study. BMJ. 1999;318:427–31. doi: 10.1136/bmj.318.7181.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huxley R, Owen CG, Whincup PH, et al. Is birth weight a risk factor for ischemic heart disease in later life? Am J Clin Nutr. 2007;85:1244–50. doi: 10.1093/ajcn/85.5.1244. [DOI] [PubMed] [Google Scholar]
- 35.Lamont D, Parker L, White M, et al. Risk of cardiovascular disease measured by carotid intima-media thickness at age 49-51: lifecourse study. BMJ. 2000;320:273–8. doi: 10.1136/bmj.320.7230.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gale CR, Ashurst HE, Hall NF, MacCallum PK, Martyn CN. Size at birth and carotid atherosclerosis in later life. Atherosclerosis. 2002;163:141–7. doi: 10.1016/s0021-9150(01)00760-2. [DOI] [PubMed] [Google Scholar]
- 37.Tilling K, Smith GD, Chambless L, et al. The relation between birth weight and intima-media thickness in middle-aged adults. Epidemiology. 2004;15:557–64. doi: 10.1097/01.ede.0000135172.67293.60. [DOI] [PubMed] [Google Scholar]
- 38.Painter RC, de Rooij SR, Hutten BA, et al. Reduced intima media thickness in adults after prenatal exposure to the Dutch famine. Atherosclerosis. 2007;193:421–7. doi: 10.1016/j.atherosclerosis.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 39.Knoflach M, Kiechl S, Kind M, et al. Cardiovascular risk factors and atherosclerosis in young males: Atherosclerosis Risk-Factors in Male Youngsters (ARMY) Circulation. 2003;108:1064–9. doi: 10.1161/01.CIR.0000085996.95532.FF. [DOI] [PubMed] [Google Scholar]
- 40.Lakshmy R, Fall CHD, Sachdev HS, et al. Childhood body mass index and adult pro-inflammatory and pro-thrombotic risk factors: data from the New Delhi birth cohort. Int J Epidemiol. 2011;40:102–11. doi: 10.1093/ije/dyq121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Denarie N, Gariepy J, Chironi G, et al. Distribution of ultrasonographically-assessed dimensions of common carotid arteries in healthy adults of both sexes. Atherosclerosis. 2000;148:297–302. doi: 10.1016/s0021-9150(99)00276-2. [DOI] [PubMed] [Google Scholar]
- 42.Huffman MD, Prabhakaran D, Osmond C, et al. Incidence of cardiovascular risk factors in an Indian urban cohort results from the New Delhi birth cohort. J Am Coll Cardiol. 2011;57:1765–74. doi: 10.1016/j.jacc.2010.09.083. [DOI] [PMC free article] [PubMed] [Google Scholar]