Abstract
Impairment in activities of daily living (ADL) accompanies cognitive and behavioral symptoms in Alzheimer’s disease (AD). Conventionally, ADL impairment has been relegated to the stage of dementia, but instrumental ADL impairment has been shown to occur earlier at the stage of mild cognitive impairment (MCI). There are many subjective and performance-based instrumental ADL scales, some of which are useful in distinguishing between MCI and AD dementia, and even between MCI and clinically normal elderly individuals. These scales have been associated with amyloid and neurodegeneration biomarkers of AD. Clinically normal elderly individuals who have a positive AD biomarker are thought to be at the preclinical stage of AD. Scales of more complex ADL are needed to better capture individuals with preclinical AD before they start to progress to MCI.
Alzheimer’s disease (AD) dementia, which is the most common cause of dementia, is a neurodegenerative disease with an insidious onset and gradual progression, consisting of cognitive, behavioral and functional impairment. A decline in the ability to perform activities of daily living (ADL) or functional impairment is required to make a diagnosis of dementia [1] and, specifically, AD dementia [2]. Basic ADL are impaired in the moderate-to-severe stages of AD dementia, and consist of eating, dressing, grooming, bathing and toileting. Instrumental ADL typically begin to decline at the stage of mild cognitive impairment (MCI) and decline more rapidly at the transition to mild AD dementia. Instrumental ADL consist of activities such as preparing meals, performing household chores and repairs, driving or using public transportation, shopping for clothes or food and handling the finances. Amnestic MCI is widely viewed as the precursor stage to AD dementia and its diagnostic criteria allow for mild difficulties in instrumental ADL [3,4]. This particular element often leads to an arbitrary distinction between MCI and very mild AD dementia and has been a source of controversy in the literature, with some experts recommending the elimination of the MCI due to AD construct altogether [5–7]. On the other hand, there are also proponents of allowing for the presence of mild impairment in instrumental ADL in MCI [3,8,9].
In this article, a brief overview of commonly used subjective and performance-based ADL scales will be provided, evidence of ADL impairment across the AD spectrum focusing on earlier stages and the association of ADL impairment with AD biomarkers will be described. The possibility that scales of more complex ADL could demonstrate very early functional impairment in preclinical AD, as well as an association with AD biomarkers will be explored.
This focused review of the literature will help to address the question of where ADL fit in the diagnosis of AD. ADL are highly dependent on cognitive function and behavior, both of which are altered in AD well before the stage of dementia. As such, it will be demonstrated that some sensitive scales can detect instrumental ADL impairment at the MCI stage preceding dementia. The term ‘complex ADL’ will be introduced, which refers to very challenging ADL tasks, some of which are tested in instrumental ADL scales but are usually in combination with simpler ADL tasks. Therefore, there is an overlap between complex ADL and instrumental ADL. However, it is proposed that even more sensitive scales focused exclusively on complex ADL will allow us the detection of the earliest alterations in daily functioning in minimally symptomatic individuals at the stage of preclinical AD and at the transition to MCI. ADL can be viewed as the practical extension of cognition and behavior. Therefore, there should be assessments that are capable of detecting changes in ADL as soon as changes in cognition and behavior are detected.
Subjective ADL scales
In the clinical setting, information about ADL is most commonly ascertained by asking a patient’s caregiver about the patient’s daily functioning because it is thought that the patient with dementia is too impaired to accurately report about his or her current abilities. Over the years, multiple subjective informant-based ADL scales have been developed for clinical and research use. This article will focus on scales assessing primarily instrumental ADL (Table 1). The psychometric properties of most subjective instrumental ADL scales have not been studied adequately and even those scales that have been studied more thoroughly have been shown to be of moderate quality at best [10]. New scales have been periodically developed in order to help with the assessment of instrumental ADL earlier in the AD course, specifically at the stage of MCI to reduce the duration of assessment and burden on the individual being questioned, to keep up with real-world changes in order to maintain ecological validity and to improve on existing scales.
Table 1.
Representative performance on different activities of daily living scales.
| ADL scale | CN | MCI | AD dementia | CN vs MCI | CN vs AD | MCI vs AD | Ref. | |
|---|---|---|---|---|---|---|---|---|
| Score range | Time to administer (min) | |||||||
| FAQ/ADNI | [9] | |||||||
| 0–30 | 5 | 0.1 ± 0.6 | 3.8 ± 4.4 | 12.7 ± 6.7† | p < 0.001 | p < 0.001 | p < 0.001 | |
| FAQ/NACC | [7] | |||||||
| 0–30 | 5 | 0.3 ± 1.4 | 3.9 ± 4.8 | 14.4 ± 6.5†‡ | p < 0.001 | p < 0.001 | p < 0.001 | |
| ADCS-ADL | [23] | |||||||
| 0–78 | 15–20 | 72.3 ± 6.6 | 69.5 ± 6.0 | 58.3 ± 6.4§ | NS | p < 0.05 | p < 0.05 | |
| CDR-SB/ADNI | [9] | |||||||
| 0–18 | 40 | 0.0 ± 0.1 | 1.6 ± 0.9 | 4.3 ± 1.6† | p < 0.001 | p < 0.001 | p < 0.001 | |
| CDR-SB/NACC | [7] | |||||||
| 0–18 | 40 | 0.0 ± 0.1 | 1.4 ± 0.9 | 4.8 ± 1.3†‡ | p < 0.001 | p < 0.001 | p < 0.001 | |
| ECog | [17] | |||||||
| 1–4 | 20 | 1.4 ± 0.5 | 1.9 ± 0.7 | 3.1 ± 0.8§ | p < 0.05 | p < 0.05 | p < 0.05 | |
| TFLS | [22] | |||||||
| 0–52 | 15–20 | 45.4 ± 4.6 | – | 30.9 ± 12.4 | – | p < 0.001 | – | |
| UPSA | [23] | |||||||
| % correct | 30 | 73.7 ± 12.0 | 63.6 ± 11.4 | 42.1 ± 11.7§ | p < 0.05 | p < 0.05 | p < 0.05 | |
| FCI | [26] | |||||||
| 0–258 | 40 | 239.3 ± 10.9 | 219.3 ± 25.6‡ | – | p < 0.001 | – | – |
All values represent mean ± standard deviation.
Mild dementia severity.
Data averaged from two groups in referenced study.
Mild-to-moderate dementia severity.
–: Not applicable; AD: Alzheimer’s disease; ADCS: Alzheimer’s Disease Cooperative Study; ADL: Activities of daily living; ADNI: Alzheimer’s Disease Neuroimaging Initiative; CDR-SB: Clinical Dementia Rating Sum of boxes; CN: Clinically normal elderly; ECog: Everyday cognition; FAQ: Functional activities questionnaire; FCI: Financial capacity instrument; MCI: Mild cognitive impairment; NACC: National Alzheimer’s Coordinating Center; NS: Not significant; TFLS: Texas Functional Living Scale; UPSA: University of California, San Diego Performance-Based Skills Assessment.
One of the first and most commonly used subjective informant-based instrumental ADL scales used in the clinical setting in mild-to-moderate dementia was developed by Lawton and Brody [11]. This scale looks at eight domains of instrumental ADL: telephone use, shopping, food preparation, housekeeping, laundry, transportation, responsibility for own medications and finances. The Lawton and Brody scale is quick and easy to administer and has been validated in many settings. It has primarily been used for detecting instrumental ADL impairment at the stage of dementia. The Functional Activities Questionnaire (FAQ) is another subjective instrumental ADL scale, which is primarily used as an informant-based scale in MCI and mild dementia, but has also been used as a self-report scale in clinically normal elderly (CN) individuals [12]. The FAQ has been widely used in AD observational and biomarker studies, including the National Alzheimer’s Coordinating Center and Alzheimer’s Disease Neuroimaging Initiative (ADNI) databases. The FAQ consists of ten items similar to those in the Lawton and Brody scale, but also includes items focusing on remembering appointments/occasions, keeping track of current events, and games and hobbies. The Alzheimer’s Disease Cooperative Study–ADL scale is an informant-based questionnaire that has been widely used in clinical trials of mild-to-moderate AD dementia [13]. It consists of 18–23 items and is commonly used as a co-primary outcome measure, along with a global cognitive instrument, in order to assess daily functioning. It has been shown to consistently detect instrumental ADL impairment in mild AD dementia, but less consistently in MCI, as discussed later on. The Clinical Dementia Rating (CDR) is a scale of global functioning based on informant report, as well as on an objective assessment of the patient or subject [14]. The CDR consists of six domains, three cognitive and three functional (two of which require informants to assess instrumental ADL): memory, orientation, judgment and problem solving, community affairs, home and hobbies, and personal care. The CDR has been widely used in observational studies, clinical trials, and the clinical setting to classify individuals as CN, MCI or AD dementia of varying severities, as well as to track disease progression over time. A potential disadvantage of the CDR is that it requires more time to administer (approximately 40 min) when compared with most subjective ADL scales.
More recently, two subjective scales that have identical informant-based and self-report portions were developed for the assessment of cognitive function related to everyday activities, as well as instrumental ADL: the Everyday Cognition (ECog) scale and the Structured Interview and Scoring Tool – Massachusetts Alzheimer’s Disease Research Center (SIST-M) [15,16]. The ECog consists of 39 items divided into six domains: memory, language, visuospatial/perceptual abilities, executive functioning-planning, executive functioning-organization and executive functioning-divided attention. The ECog is now starting to be used more widely in observational and biomarker studies, such as ADNI, assessing individuals across the early AD spectrum, including CN and early MCI subjects. Recently, a 12-item short form of the ECog was also validated [17]. The SIST-M consists of 60 items divided into six domains: memory, orientation, judgment and problem solving, community activities and interests, household activities and hobbies, and personal care. The SIST-M requires more time (approximately 30 min) to administer when compared with most subjective ADL scales and has not been as widely used, but is a promising scale for assessing instrumental ADL impairment in early AD.
Performance-based ADL scales
Subjective ADL scales are usually quick and easy to administer, but some have criticized these scales because they do not assess the individual’s actual ability to perform ADL. To address these concerns and to potentially provide a more objective measure of ADL, performance-based ADL scales have been developed. In these assessments, individuals are asked to simulate everyday tasks, such as arranging one’s medications for the week in a pill-box, writing a check and mailing it or preparing a simple meal. However, these performance-based scales are performed in artificial environments outside the typical routine in which individuals normally perform their ADL [18]. That said, direct observation of ADL in a real-life setting does not necessarily correlate well with cognitive function [19]. Performance-based ADL scales have mostly been administered to individuals with mild-to-moderate dementia, but more recently they have also been used in MCI (Table 1). These scales either consist of multiple different tasks providing a sampling of many ADL or focus on one aspect of ADL in more depth.
The Direct Assessment of Functional Status (DAFS) is one of the first performance-based scales to assess a wide variety of basic and instrumental ADL [20,21]. It takes approximately 40 min to administer and consists of seven domains: dressing/grooming skills, feeding abilities, orientation, communication abilities, financial skills and shopping skills. The DAFS is helpful in staging dementia severity but is not sensitive enough to detect ADL impairment at the stage of MCI. The Texas Functional Living Scale (TFLS) is similar to the DAFS but takes less time to administer (15–20 min), focuses on a variety of instrumental ADL and includes only two basic ADL items in order to avoid floor effects in dementia [22]. The TFLS consists of 21 items divided into five subscales: dressing, memory, time, money and communication. Similar to the DAFS, the TFLS is useful at the dementia stage, but not at the stage of MCI. However, since the TFLS takes less time to administer, it can be used more widely in the clinical setting.
Recently, performance-based ADL scales have tried to capture instrumental ADL impairment in MCI. The University of California, San Diego Performance-Based Skills Assessment (UPSA) has been modified to increase sensitivity to functional impairment in early AD [23,24]. The UPSA consists of 27 items in four domains: comprehension and planning, communication, mobility and financial procedures. The UPSA takes approximately 30 min to administer, but is much more sensitive in detecting early ADL impairment, possibly even in CN individuals. The Financial Capacity Instrument (FCI) focuses on one particular element of instrumental ADL in greater detail [25,26]. The FCI assesses an individual’s ability to carry out the finances at three levels: specific abilities (across 20 tasks), broader activities (across seven to nine domains) and overall capacity (global scores). The FCI is a well-validated and ecologically accurate tool assessing a challenging aspect of ADL. However, the FCI is not sensitive enough to detect changes in ADL in CN individuals at risk of AD.
ADL impairment in MCI & mild AD dementia
Table 1 provides typical performances by CN, MCI and mild-to-moderate AD dementia individuals on seven commonly used subjective and performance-based ADL scales, primarily focused on instrumental ADL. Most of these scales are useful for differentiating between individuals with MCI and AD dementia and also between CN and MCI individuals. Mild impairment in instrumental ADL is noted at the stage of MCI, but CN individuals score almost perfectly with little variability in scores on these scales.
In two similar, large multicenter studies at mostly academic sites, the ADNI and National Alzheimer’s Coordinating Center, a subjective informant-based scale, the FAQ and a global functioning scale, the CDR, clearly differentiated between CN, MCI and AD dementia subjects [7,9]. Similarly, the more recently developed ECog has been shown to differentiate well between all three groups [17]. On the other hand, one version of the Alzheimer’s Disease Cooperative Study–ADL was only useful in differentiating between CN and AD dementia or MCI and AD dementia, but not between CN and MCI subjects [23], while another version was useful in differentiating between CN and MCI subjects [27]. The performance-based ADL scales, UPSA and FCI, have also been shown to be helpful in differentiating between CN, MCI and AD dementia individuals [23,26]. As illustrated by Table 1 and the information above, the FAQ, CDR, ECog, UPSA and FCI are all useful in detecting instrumental ADL impairment in MCI. Moreover, the ECog and UPSA also appear to detect slight instrumental ADL changes in CN individuals. However, more sensitive scales are needed to consistently detect changes in complex ADL in CN individuals at risk of AD.
Impairment in instrumental ADL in MCI and AD dementia has been associated with global cognitive impairment; it has also been frequently, but not consistently, associated with individual cognitive domains, including certain aspects of executive dysfunction, memory impairment and slowing of processing speed [9,18,28–30]. Executive dysfunction has also been shown to predict future instrumental ADL impairment in CN individuals [31,32], while instrumental ADL impairment has in turn been shown to predict progression from MCI to AD dementia [33,34]. Although the Disability Assessment for Dementia is not a sensitive scale for detecting instrumental ADL impairment in MCI, most of its items can be broken down into three components relevant to this discussion: an individual’s ability to initiate (opposite of apathy), plan (executive function) and perform (final ADL outcome) activities [35]. Accordingly, lack of initiative manifesting as apathy has also been shown to be associated with instrumental ADL impairment and executive dysfunction in MCI and AD dementia [9,36,37]. Thus, a new ADL scale that is more sensitive to functional impairment in MCI, or even earlier, with a similar three component structure as the Disability Assessment for Dementia may prove to be particularly useful in the future.
ADL impairment & AD biomarkers
As ADL impairment is a requirement for making a diagnosis of AD dementia [2], it is not surprising that it has been shown to be associated with the hallmark pathological changes of AD dementia at post-mortem: amyloid plaques and neurofibrillary tangles [38,39]. The studies demonstrating this association were assessing individuals with moderate-to-severe AD dementia. In an effort to move earlier in the disease process, worsening impairment in instrumental ADL over time has been shown to be associated with lower baseline cerebrospinal fluid amyloid-β1–42 and higher total and phospho-tau in CN and MCI [40]. Similarly, greater instrumental ADL impairment has been associated with greater in vivo amyloid burden in MCI using Pittsburgh Compound B-PET [41]. A composite of temporal, lateral parietal and posterior cingulate hypometabolism, a neurodegenerative pattern typically seen in AD, has been associated with worsening instrumental ADL over time in MCI and mild AD dementia using 18F-fluorodeoxyglucose (FDG)-PET [42]. Moreover, global brain atrophy, also suggestive of neurodegeneration, visualized on MRI has been shown to be associated with worsening instrumental ADL over time in MCI [43].
A few studies have attempted to localize instrumental ADL impairment in the brain of individuals with AD dementia and MCI utilizing FDG-PET and MRI. One study found that general instrumental ADL impairment was associated with inferior parietal, inferior temporal and superior occipital hypometabolism in mild-to-moderate AD dementia [44], while another study found an association with medial frontal and temporoparietal atrophy in mild AD dementia [45]. A study focusing on financial capacity in MCI found an association with angular gyrus (lateral parietal) atrophy [46]. Some of the regions associated with ADL impairment mentioned above are typical of the neurodegenerative distribution seen in AD (temporal and parietal), while others are not (occipital and frontal), suggesting that some aspects of ADL impairment might result from distinct pathologic changes in AD. However, since ADL depend on, or are associated with, many cognitive domains, it is not easy to localize them to one region in the brain. Since ADL impairment tracks well with disease progression, it makes sense that scales of more general ADL performance are associated with multiple regions in the brain or with diffuse pathologic changes that are typically seen in AD and that one particular ADL task (e.g., financial capacity) is associated with only one region.
ADL in preclinical AD
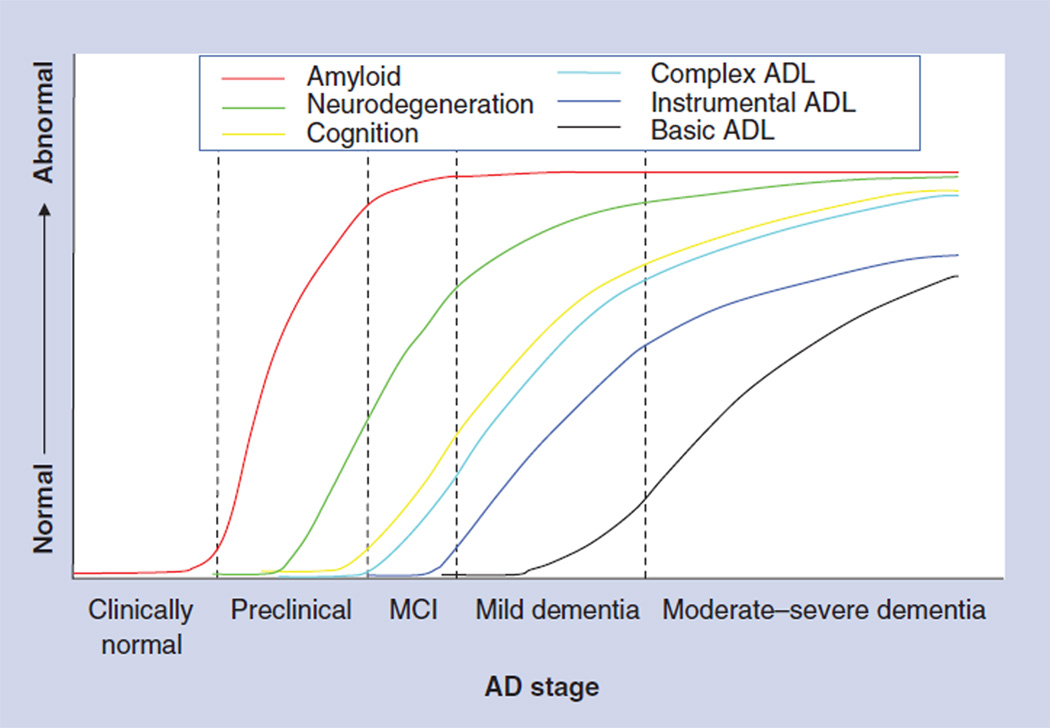
The recently proposed, revised AD criteria included a new stage of preclinical AD, aiming to capture individuals who are asymptomatic or only minimally symptomatic, but are at risk of progression to MCI and AD dementia based on the presence of positive biomarkers [47]. The construct of preclinical AD is currently meant to be used only in the research setting until more longitudinal data on these individuals are available and the long-term implications are better understood. Jack et al. proposed a dynamic model of biomarker progression in AD, with amyloid burden building up at the preclinical stage, followed by neurodegeneration visualized by hypometabolism on FDG-PET and atrophy on MRI, followed by cognitive impairment, and finally ADL impairment [48]. Building on this model, the progression of ADL impairment is illustrated in greater detail (Figure 1).
Figure 1. Hypothetical model of timing of the development of activities of daily living impairment along the Alzheimer’s disease spectrum compared with cognitive impairment and pathologic changes.
Instrumental ADL impairment has conventionally been thought to appear in the transition from MCI to mild AD dementia. The authors suggest that more complex ADL impairment appears earlier in the transition from preclinical AD (in which individuals are asymptomatic or minimally symptomatic) to MCI, similarly to the earliest signs of cognitive impairment.
AD: Alzheimer’s disease; ADL: Activities of daily living; MCI: Mild cognitive impairment.
Adapted with permission from [48].
More sensitive cognitive measures are being developed to try to capture the at-risk group of preclinical AD. A particularly sensitive memory measure has been recently shown to be associated with in vivo amyloid burden in CN subjects using Pittsburgh Compound B-PET [49]. Similarly, a more sensitive measure of complex ADL that can capture the earliest functional impairment in preclinical AD is required.
Currently, subjective and performance-based scales assessing instrumental ADL often include relatively simpler tasks, such as heating water (for tea or coffee), along with more challenging tasks, such as doing the taxes. This allows for a wider range of performance and helps to capture the transition from MCI to mild dementia. However, it dilutes the signal of the more challenging tasks when assessing ADL in minimally symptomatic individuals at the stage of preclinical AD. At that stage, a subjective or performance-based scale focused only on complex ADL is more likely to successfully detect early alterations in ADL. Such a scale could assess performance at work (if not retired), ability to hold positions of leadership in organizations, navigation of an automated phone menu, use of automated teller services, and capability of using smart phones, computer software, email and internet. Of note, some of these complex tasks are assessed by existing instrumental ADL scales, but, as mentioned above, they are intermixed with simpler tasks.
The ADL prevention instrument was designed to detect early ADL impairment in dementia prevention trials of CN individuals [27]. The ADL prevention instrument is a subjective self-report and informant-based scale consisting of 15 items. It has been shown to distinguish well between CN and MCI individuals and to be associated with future cognitive decline in CN individuals. However, like other scales discussed earlier, it too exhibits ceiling effects in CN individuals. Moreover, the correlation between self-report and informant-based data is far from perfect. Other studies have also shown a discrepancy between self-report and informant-report of some aspects of instrumental ADL, such as financial capacity and driving ability, in individuals with MCI [50]. On the other hand, a study using a more general subjective instrumental ADL scale demonstrated agreement between self-report and informant-report in individuals with MCI similar to that seen in CN individuals, as opposed to demented individuals who significantly under-reported their functional deficits when compared with their informants’ reports [51].
This highlights an important complication in the tracking of early complex ADL in preclinical AD using subjective scales; as an individual becomes more impaired and transitions from preclinical AD to MCI and then AD dementia, that individual is less likely to provide a reliable self-report of his or her daily functioning, thus necessitating an informant-report. However, before MCI sets in, the individual with preclinical AD may be more attune to early functional difficulties than an informant. Therefore, at different stages of AD, either self-report or informant-based subjective ADL scales might be more reliable, suggesting that both are required at least in preclinical AD and at the transition to MCI.
Early complaints of alterations in complex ADL by patients and their family members in the clinic often indicate that the individual is still capable of independently performing the task, but that he or she does so with some difficulty or it takes longer than it used to. This important nuance is often missed in subjective instrumental ADL scales and needs to be captured in both self-report and informant-report subjective complex ADL scales.
One potential way to avoid the duplication of effort in self- and informant-report scales is to develop a performance-based complex ADL scale that will more objectively assess individuals during the preclinical stages of AD and track decline towards MCI and AD dementia. Examples of challenging ADL tasks with ecological validity that can be tested are navigating an automated phone menu to address health insurance queries or using automated teller services to withdraw or deposit money.
Conclusion & future perspective
Using ADL impairment to draw the line between MCI and dementia has been debated by experts in the field for many years. As described above, depending on how one defines ADL impairment, it can be detected at different stages of AD: basic ADL impairment is detected in the transition from mild dementia to moderate dementia and beyond, while instrumental ADL impairment is detected at the transition from MCI to dementia and even at earlier stages of MCI. Since ADL are intrinsically linked to cognition and behavior, it is likely that changes in ADL will track closely with changes in cognition and behavior. Therefore, the artificial lag between impairments in ADL and impairments in cognition and behavioral manifestations could simply be a byproduct of the tools used to assess ADL and the type of ADL being assessed. We propose that alterations in complex ADL may already be present when an individual is at the stage of preclinical AD and is minimally symptomatic.
With more than 5 million people in the USA carrying a diagnosis of AD dementia, and 20 million more being at risk for developing dementia over the next 30 years, early detection and treatment is desperately needed [52]. The field is moving towards finding more sensitive clinical measures for early diagnosis that are associated with biomarkers of AD, but are easy and inexpensive to administer by primary care physicians, who perform the initial medical evaluation of the majority of these patients. Such clinical measures can be used as screening tests and, if positive, followed-up with a biomarker test such as an MRI, amyloid PET, or cerebrospinal fluid amyloid-β1–42 and tau. Impairments in ADL are the everyday manifestation of cognitive and behavioral deficits in AD that relentlessly change the lives of patients and their caregivers. Currently, there are reliable, subjective and performance-based scales for instrumental ADL at the stage of MCI and AD dementia that have been linked to AD biomarkers, but there are very few scales that have the potential to detect the earliest functional deficits in preclinical AD and its transition to MCI. Therefore, developing more sensitive, ecologically valid scales for complex ADL is vital to the goal of improving the detection of preclinical AD and the prediction of progression to MCI and AD dementia. This in turn will help lead to earlier treatment once disease-modifying drugs are available.
Practice Points.
-
▪
Impairment in activities of daily living (ADL) is a required element for the diagnosis of dementia.
-
▪
Impairment in instrumental ADL has conventionally been thought to appear at the transition from mild cognitive impairment (MCI) to mild Alzheimer’s disease (AD) dementia.
-
▪
Some sensitive scales of instrumental ADL have detected significant functional impairment in individuals with MCI when compared with clinically normal elderly individuals.
-
▪
Most scales of ADL are subjective and informant-based. Subjective self-report scales or performance-based scales are not used as widely.
-
▪
Impairment in ADL has been associated with biomarkers of AD at the stage of dementia and MCI, but not in clinically normal elderly.
-
▪
The recently proposed revised criteria for AD included a new stage of preclinical AD, consisting of clinically normal individuals at risk for AD based on the presence of a positive biomarker (this construct is currently meant to be used only in the research setting).
-
▪
It is possible that more challenging scales of ADL will demonstrate very early functional impairment in preclinical AD, as well as an association with biomarkers of AD.
Acknowledgments
This study was supported by R01 AG027435, K23 AG033634, K24 AG035007, the Rosalinde and Arthur Gilbert Foundation/AFAR New Investigator Awards in Alzheimer’s Disease, the Alzheimer’s Association (IIRG-08-90934), the Massachusetts Alzheimer’s Disease Research Center (P50 AG005134) and the Harvard Aging Brain Study (P01 AGO36694). The authors have received research salary support from Janssen Alzheimer Immunotherapy (DM Rentz, GA Marshall and RE Amariglio), Wyeth/Pfizer Pharmaceuticals (DM Rentz, GA Marshall and RE Amariglio), and Bristol-Myers-Squibb (RA Sperling).
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, (4th Edition, Text Revision) Washington, DC, USA: American Psychiatric Association; 2000. [Google Scholar]
- 2.Mckhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albert MS, Dekosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch. Neurol. 1999;56(3):303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 5.Dubois B, Feldman HH, Jacova C, et al. Revising the definition of Alzheimer’s disease: a new lexicon. Lancet Neurol. 2010;9(11):1118–1127. doi: 10.1016/S1474-4422(10)70223-4. [DOI] [PubMed] [Google Scholar]
- 6.Dubois B, Feldman HH, Jacova C, et al. Research criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 2007;6(8):734–746. doi: 10.1016/S1474-4422(07)70178-3. [DOI] [PubMed] [Google Scholar]
- 7. Morris JC. Revised criteria for mild cognitive impairment may compromise the diagnosis of Alzheimer disease dementia. Arch. Neurol. 2012;69(6):700–708. doi: 10.1001/archneurol.2011.3152. ▪ Demonstrates the presence of instrumental activities of daily living (ADL) impairment at the stage of mild cognitive impairment (MCI) using two common scales in a large, multicenter study.
- 8.Brown PJ, Devanand DP, Liu X, Caccappolo E. Functional impairment in elderly patients with mild cognitive impairment and mild Alzheimer disease. Arch. Gen. Psychiatry. 2011;68(6):617–626. doi: 10.1001/archgenpsychiatry.2011.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Marshall GA, Rentz DM, Frey MT, et al. Executive function and instrumental activities of daily living in mild cognitive impairment and Alzheimer’s disease. Alzheimers Dement. 2011;7(3):300–308. doi: 10.1016/j.jalz.2010.04.005. ▪ Demonstrates the relationship between instrumental ADL impairment and executive dysfunction across the Alzheimer’s disease (AD) spectrum.
- 10. Sikkes SA, De Lange-De Klerk ES, Pijnenburg YA, Scheltens P, Uitdehaag BM. A systematic review of instrumental activities of daily living scales in dementia: room for improvement. J. Neurol. Neurosurg. Psychiatry. 2009;80(1):7–12. doi: 10.1136/jnnp.2008.155838. ▪ Discusses the strengths and weaknesses of commonly used instrumental ADL impairment scales in dementia.
- 11.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3):179–186. [PubMed] [Google Scholar]
- 12.Pfeffer RI, Kurosaki TT, Harrah CH, Jr, Chance JM, Filos S. Measurement of functional activities in older adults in the community. J. Gerontol. 1982;37(3):323–329. doi: 10.1093/geronj/37.3.323. [DOI] [PubMed] [Google Scholar]
- 13.Galasko D, Bennett D, Sano M, et al. An inventory to assess activities of daily living for clinical trials in Alzheimer’s disease. The Alzheimer’s Disease Cooperative Study. Alzheimer Dis. Assoc. Disord. 1997;11(Suppl. 2):S33–S39. [PubMed] [Google Scholar]
- 14.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 15.Farias ST, Mungas D, Reed BR, et al. The measurement of everyday cognition (ECog): scale development and psychometric properties. Neuropsychology. 2008;22(4):531–544. doi: 10.1037/0894-4105.22.4.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okereke OI, Copeland M, Hyman BT, Wanggaard T, Albert MS, Blacker D. The Structured Interview & Scoring Tool-Massachusetts Alzheimer’s Disease Research Center (SIST-M): development, reliability, and cross-sectional validation of a brief structured clinical dementia rating interview. Arch. Neurol. 2011;68(3):343–350. doi: 10.1001/archneurol.2010.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farias ST, Mungas D, Harvey DJ, Simmons A, Reed BR, Decarli C. The measurement of everyday cognition: development and validation of a short form of the everyday cognition scales. Alzheimers Dement. 2011;7:593–601. doi: 10.1016/j.jalz.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gold DA. An examination of instrumental activities of daily living assessment in older adults and mild cognitive impairment. J. Clin. Exp. Neuropsychol. 2012;34(1):11–34. doi: 10.1080/13803395.2011.614598. ▪ Discusses the relationship between instrumental ADL impairment and cognitive impairment in MCI across different types of scales.
- 19.Schmitter-Edgecombe M, Parsey C, Cook DJ. Cognitive correlates of functional performance in older adults: comparison of self-report, direct observation, and performance-based measures. J. Int. Neuropsychol. Soc. 2011;17(5):853–864. doi: 10.1017/S1355617711000865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loewenstein DA, Amigo E, Duara R, et al. A new scale for the assessment of functional status in Alzheimer’s disease and related disorders. J. Gerontol. 1989;44(4):P114–P121. doi: 10.1093/geronj/44.4.p114. [DOI] [PubMed] [Google Scholar]
- 21.Zanetti O, Frisoni GB, Rozzini L, Bianchetti A, Trabucchi M. Validity of direct assessment of functional status as a tool for measuring Alzheimer’s disease severity. Age Ageing. 1998;27(5):615–622. doi: 10.1093/ageing/27.5.615. [DOI] [PubMed] [Google Scholar]
- 22.Cullum CM, Saine K, Chan LD, Martin-Cook K, Gray KF, Weiner MF. Performance-based instrument to assess functional capacity in dementia: the Texas Functional Living Scale. Neuropsychiatry Neuropsychol. Behav. Neurol. 2001;14(2):103–108. [PubMed] [Google Scholar]
- 23.Goldberg TE, Koppel J, Keehlisen L, et al. Performance-based measures of everyday function in mild cognitive impairment. Am. J. Psychiatry. 2010;167(7):845–853. doi: 10.1176/appi.ajp.2010.09050692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gomar JJ, Harvey PD, Bobes-Bascaran MT, Davies P, Goldberg TE. Development and cross-validation of the UPSA short form for the performance-based functional assessment of patients with mild cognitive impairment and Alzheimer disease. Am. J. Geriatr. Psychiatry. 2011;19(11):915–922. doi: 10.1097/JGP.0b013e3182011846. [DOI] [PubMed] [Google Scholar]
- 25.Marson DC, Sawrie SM, Snyder S, et al. Assessing financial capacity in patients with Alzheimer disease: a conceptual model and prototype instrument. Arch. Neurol. 2000;57(6):877–884. doi: 10.1001/archneur.57.6.877. [DOI] [PubMed] [Google Scholar]
- 26.Triebel KL, Martin R, Griffith HR, et al. Declining financial capacity in mild cognitive impairment: a 1-year longitudinal study. Neurology. 2009;73(12):928–934. doi: 10.1212/WNL.0b013e3181b87971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Galasko D, Bennett DA, Sano M, Marson D, Kaye J, Edland SD. ADCS Prevention Instrument Project: assessment of instrumental activities of daily living for community-dwelling elderly individuals in dementia prevention clinical trials. Alzheimer Dis. Assoc. Disord. 2006;20(4) Suppl. 3:S152–S169. doi: 10.1097/01.wad.0000213873.25053.2b. ▪ Introduces a new and more sensitive scale of instrumental ADL geared toward prevention trials in clinically normal elderly individuals.
- 28.Royall DR, Lauterbach EC, Kaufer D, et al. The cognitive correlates of functional status: a review from the Committee on Research of the American Neuropsychiatric Association. J. Neuropsychiatry Clin. Neurosci. 2007;19(3):249–265. doi: 10.1176/jnp.2007.19.3.249. [DOI] [PubMed] [Google Scholar]
- 29.Pereira FS, Yassuda MS, Oliveira AM, Forlenza OV. Executive dysfunction correlates with impaired functional status in older adults with varying degrees of cognitive impairment. Int. Psychogeriatr. 2008;20(6):1104–1115. doi: 10.1017/S1041610208007631. [DOI] [PubMed] [Google Scholar]
- 30.Razani J, Casas R, Wong JT, Lu P, Alessi C, Josephson K. Relationship between executive functioning and activities of daily living in patients with relatively mild dementia. Appl. Neuropsychol. 2007;14(3):208–214. doi: 10.1080/09084280701509125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cahn-Weiner DA, Boyle PA, Malloy PF. Tests of executive function predict instrumental activities of daily living in community-dwelling older individuals. Appl. Neuropsychol. 2002;9(3):187–191. doi: 10.1207/S15324826AN0903_8. [DOI] [PubMed] [Google Scholar]
- 32.Royall DR, Palmer R, Chiodo LK, Polk MJ. Declining executive control in normal aging predicts change in functional status: the Freedom House Study. J. Am. Geriatr. Soc. 2004;52(3):346–352. doi: 10.1111/j.1532-5415.2004.52104.x. [DOI] [PubMed] [Google Scholar]
- 33.Tabert MH, Albert SM, Borukhova-Milov L, et al. Functional deficits in patients with mild cognitive impairment: prediction of AD. Neurology. 2002;58(5):758–764. doi: 10.1212/wnl.58.5.758. [DOI] [PubMed] [Google Scholar]
- 34.Luck T, Luppa M, Angermeyer MC, Villringer A, Konig HH, Riedel-Heller SG. Impact of impairment in instrumental activities of daily living and mild cognitive impairment on time to incident dementia: results of the Leipzig Longitudinal Study of the Aged. Psychol. Med. 2011;41(5):1087–1097. doi: 10.1017/S003329171000142X. [DOI] [PubMed] [Google Scholar]
- 35.Gelinas G. Assessment of functional disability in Alzheimer’s disease. Can. J. Occup. Ther. 1995;62:15. [Google Scholar]
- 36.Boyle PA, Malloy PF, Salloway S, Cahn-Weiner DA, Cohen R, Cummings JL. Executive dysfunction and apathy predict functional impairment in Alzheimer disease. Am. J. Geriatr. Psychiatry. 2003;11(2):214–221. [PubMed] [Google Scholar]
- 37.Chen ST, Sultzer DL, Hinkin CH, Mahler ME, Cummings JL. Executive dysfunction in Alzheimer’s disease: association with neuropsychiatric symptoms and functional impairment. J. Neuropsychiatry Clin. Neurosci. 1998;10(4):426–432. doi: 10.1176/jnp.10.4.426. [DOI] [PubMed] [Google Scholar]
- 38.Marshall GA, Fairbanks LA, Tekin S, Vinters HV, Cummings JL. Neuropathologic correlates of activities of daily living in Alzheimer’s disease. Alzheimer Dis. Assoc. Disord. 2006;20(1):56–59. doi: 10.1097/01.wad.0000201852.60330.16. [DOI] [PubMed] [Google Scholar]
- 39.Roth M, Tomlinson BE, Blessed G. Correlation between scores for dementia and counts of ‘senile plaques’ in cerebral grey matter of elderly subjects. Nature. 1966;209(18):109–110. doi: 10.1038/209109a0. [DOI] [PubMed] [Google Scholar]
- 40.Okonkwo OC, Alosco ML, Griffith HR, et al. Cerebrospinal fluid abnormalities and rate of decline in everyday function across the dementia spectrum: normal aging, mild cognitive impairment, and Alzheimer disease. Arch. Neurol. 2010;67(6):688–696. doi: 10.1001/archneurol.2010.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marshall GA, Olson LE, Frey MT, et al. Instrumental activities of daily living impairment is associated with increased amyloid burden. Dement. Geriatr. Cogn. Disord. 2011;31(6):443–450. doi: 10.1159/000329543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Landau SM, Harvey D, Madison CM, et al. Associations between cognitive, functional, and FDG-PET measures of decline in AD and MCI. Neurobiol. Aging. 2011;32(7):1207–1218. doi: 10.1016/j.neurobiolaging.2009.07.002. ▪ Demonstrates the relationship between instrumental ADL impairment and neurodegeneration, assessed by 18F-fluorodeoxyglucose PET, in MCI and AD dementia.
- 43.Okonkwo OC, Alosco ML, Jerskey BA, Sweet LH, Ott BR, Tremont G. Cerebral atrophy, apolipoprotein E varepsilon4, and rate of decline in everyday function among patients with amnestic mild cognitive impairment. Alzheimers Dement. 2010;6(5):404–411. doi: 10.1016/j.jalz.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Salmon E, Lespagnard S, Marique P, et al. Cerebral metabolic correlates of four dementia scales in Alzheimer’s disease. J. Neurol. 2005;252(3):283–290. doi: 10.1007/s00415-005-0551-3. erratum in J. Neurol. 252(9), 1138 (2005). [DOI] [PubMed] [Google Scholar]
- 45.Vidoni ED, Honea RA, Burns JM. Neural correlates of impaired functional independence in early Alzheimer’s disease. J. Alzheimers Dis. 2010;19(2):517–527. doi: 10.3233/JAD-2010-1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Griffith HR, Stewart CC, Stoeckel LE, et al. Magnetic resonance imaging volume of the angular gyri predicts financial skill deficits in people with amnestic mild cognitive impairment. J. Am. Geriatr. Soc. 2010;58(2):265–274. doi: 10.1111/j.1532-5415.2009.02679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):280–292. doi: 10.1016/j.jalz.2011.03.003. ▪▪ Introduces the newly proposed criteria for preclinical AD.
- 48. Jack CR, Jr, Knopman DS, Jagust WJ, et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010;9(1):119–128. doi: 10.1016/S1474-4422(09)70299-6. ▪ Introduces a unifying model of a dynamic cascade of biomarkers predicting clinical decline in AD.
- 49.Rentz DM, Amariglio RE, Becker JA, et al. Face-name associative memory performance is related to amyloid burden in normal elderly. Neuropsychologia. 2011;49(9):2776–2783. doi: 10.1016/j.neuropsychologia.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Okonkwo OC, Griffith HR, Vance DE, Marson DC, Ball KK, Wadley VG. Awareness of functional difficulties in mild cognitive impairment: a multidomain assessment approach. J. Am. Geriatr. Soc. 2009;57(6):978–984. doi: 10.1111/j.1532-5415.2009.02261.x. ▪ Illustrates the complication of a lack of awareness of deficits when using subjective self-report questionnaires in individuals at the stage of MCI.
- 51.Farias ST, Mungas D, Jagust W. Degree of discrepancy between self and other-reported everyday functioning by cognitive status: dementia, mild cognitive impairment, and healthy elders. Int. J. Geriatr. Psychiatry. 2005;20(9):827–834. doi: 10.1002/gps.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thies W, Bleiler L. Alzheimer’s Association. 2012 Alzheimer’s disease facts and figures. Alzheimers Dement. 2012;8:131–168. doi: 10.1016/j.jalz.2012.02.001. [DOI] [PubMed] [Google Scholar]