Abstract
We have isolated cDNA clones complementary to a truncated immunoglobulin heavy-chain C epsilon RNA transcript previously found to be induced in B lymphoid cells by treatment with lipopolysaccharide (LPS) combined with interleukin-4 (IL-4). We demonstrate that this transcript initiates from a promoter upstream of the germ line epsilon class-switch recombination region (S epsilon region). The major germ line C epsilon transcript contains a small 5' exon contributed by sequences upstream of the S epsilon region spliced to the normal C epsilon exons. Treatment of splenic B lymphoid cells with LPS plus IL-4 induces the expression of transcripts from the germ line epsilon transcription unit followed by expression of normal immunoglobulin epsilon heavy-chain mRNA. Furthermore, we demonstrate that similar treatment of transformed precursor B cell lines induces the expression of germ line epsilon transcripts followed by class switching to epsilon expression in these lines. This is the first demonstration of switching to epsilon in cells of the pre-B stage. The general structure of the germ line epsilon transcript and transcription unit is similar to that previously characterized for germ line gamma 2b transcripts. However, expression of these two germ line transcription units in B-lineage cells is inversely regulated by IL-4 (plus LPS) treatment, correlating with the effects of these treatments on switching to these loci.
Full text
PDF

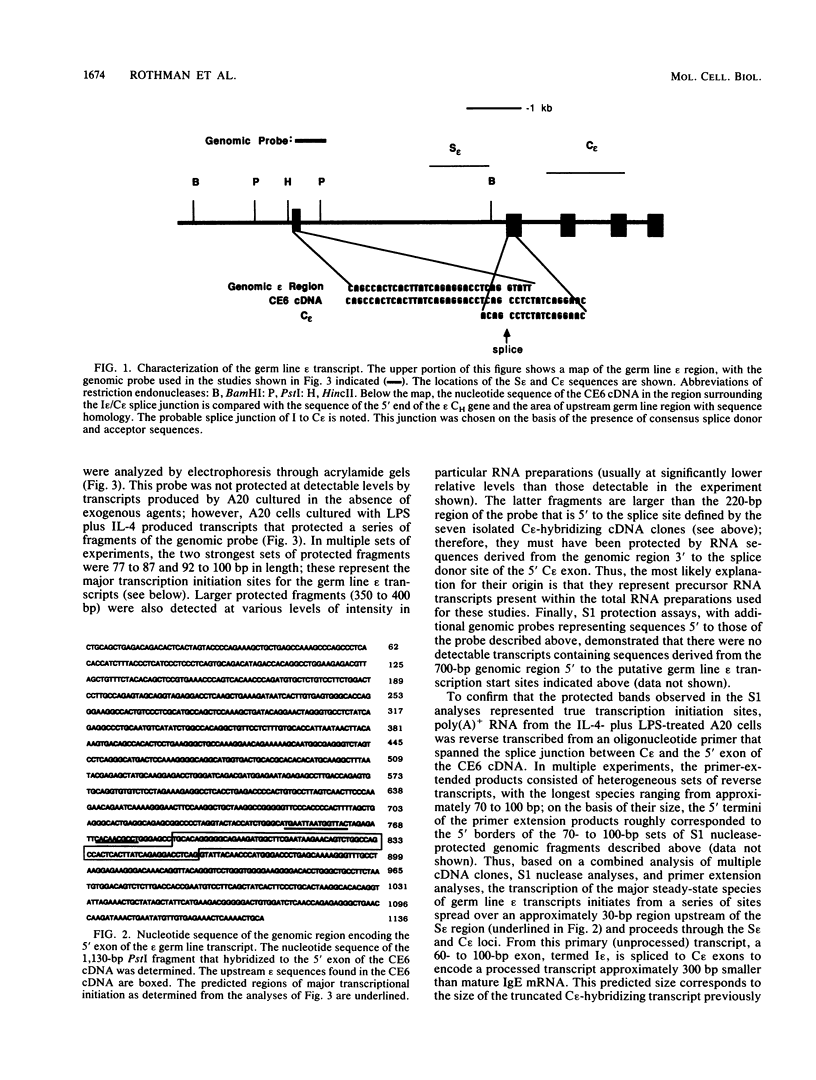
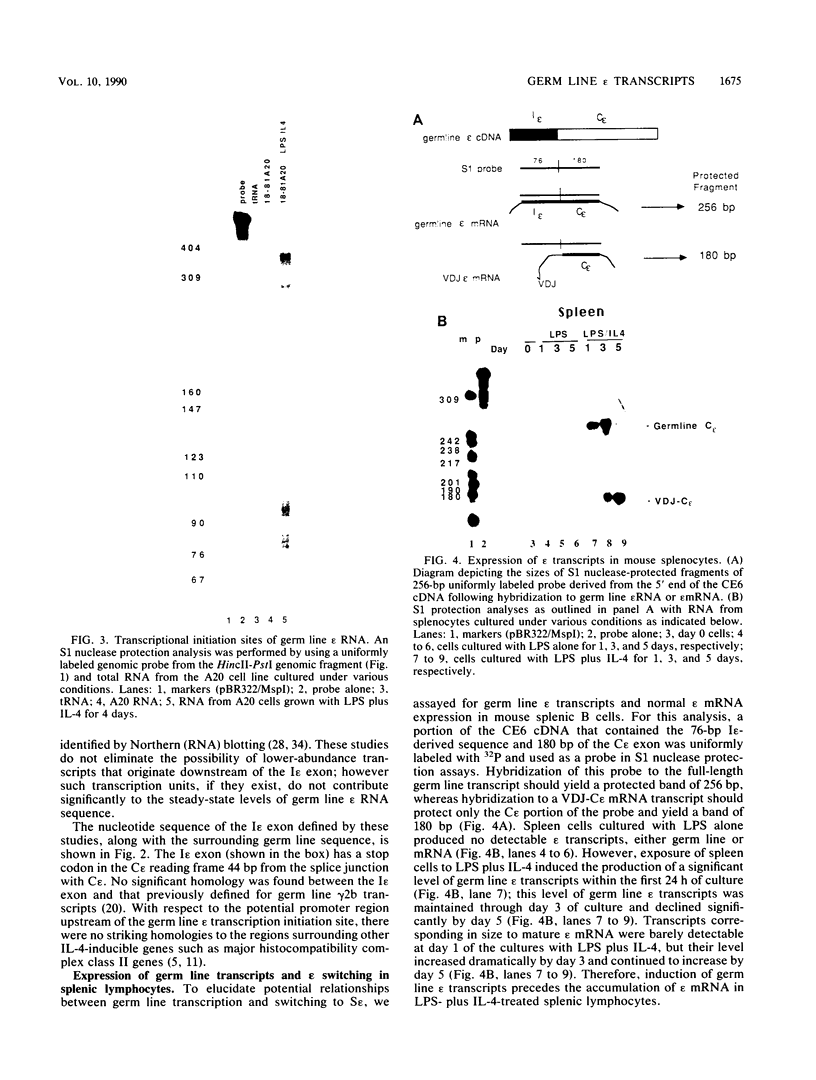



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt F. W., Rosenberg N., Casanova R. J., Thomas E., Baltimore D. Immunoglobulin heavy-chain expression and class switching in a murine leukaemia cell line. Nature. 1982 Mar 25;296(5855):325–331. doi: 10.1038/296325a0. [DOI] [PubMed] [Google Scholar]
- Alt F. W., Rosenberg N., Enea V., Siden E., Baltimore D. Multiple immunoglobulin heavy-chain gene transcripts in Abelson murine leukemia virus-transformed lymphoid cell lines. Mol Cell Biol. 1982 Apr;2(4):386–400. doi: 10.1128/mcb.2.4.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach M. K. Mediators of anaphylaxis and inflammation. Annu Rev Microbiol. 1982;36:371–413. doi: 10.1146/annurev.mi.36.100182.002103. [DOI] [PubMed] [Google Scholar]
- Blackwell T. K., Moore M. W., Yancopoulos G. D., Suh H., Lutzker S., Selsing E., Alt F. W. Recombination between immunoglobulin variable region gene segments is enhanced by transcription. Nature. 1986 Dec 11;324(6097):585–589. doi: 10.1038/324585a0. [DOI] [PubMed] [Google Scholar]
- Boothby M., Gravallese E., Liou H. C., Glimcher L. H. A DNA binding protein regulated by IL-4 and by differentiation in B cells. Science. 1988 Dec 16;242(4885):1559–1562. doi: 10.1126/science.3144043. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Coffman R. L., Ohara J., Bond M. W., Carty J., Zlotnik A., Paul W. E. B cell stimulatory factor-1 enhances the IgE response of lipopolysaccharide-activated B cells. J Immunol. 1986 Jun 15;136(12):4538–4541. [PubMed] [Google Scholar]
- Coffman R. L., Savelkoul H. F., Lebman D. A. Cytokine regulation of immunoglobulin isotype switching and expression. Semin Immunol. 1989 Sep;1(1):55–63. [PubMed] [Google Scholar]
- Collier D. A., Griffin J. A., Wells R. D. Non-B right-handed DNA conformations of homopurine.homopyrimidine sequences in the murine immunoglobulin C alpha switch region. J Biol Chem. 1988 May 25;263(15):7397–7405. [PubMed] [Google Scholar]
- Coutelier J. P., van der Logt J. T., Heessen F. W., Warnier G., Van Snick J. IgG2a restriction of murine antibodies elicited by viral infections. J Exp Med. 1987 Jan 1;165(1):64–69. doi: 10.1084/jem.165.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didier D. K., Schiffenbauer J., Woulfe S. L., Zacheis M., Schwartz B. D. Characterization of the cDNA encoding a protein binding to the major histocompatibility complex class II Y box. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7322–7326. doi: 10.1073/pnas.85.19.7322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelman F. D., Katona I. M., Urban J. F., Jr, Snapper C. M., Ohara J., Paul W. E. Suppression of in vivo polyclonal IgE responses by monoclonal antibody to the lymphokine B-cell stimulatory factor 1. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9675–9678. doi: 10.1073/pnas.83.24.9675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isakson P. C., Puré E., Vitetta E. S., Krammer P. H. T cell-derived B cell differentiation factor(s). Effect on the isotype switch of murine B cells. J Exp Med. 1982 Mar 1;155(3):734–748. doi: 10.1084/jem.155.3.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida N., Ueda S., Hayashida H., Miyata T., Honjo T. The nucleotide sequence of the mouse immunoglobulin epsilon gene: comparison with the human epsilon gene sequence. EMBO J. 1982;1(9):1117–1123. doi: 10.1002/j.1460-2075.1982.tb01306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrett E. E., Miller H. R. Production and activities of IgE in helminth infection. Prog Allergy. 1982;31:178–233. [PubMed] [Google Scholar]
- Kohl N. E., Legouy E., DePinho R. A., Nisen P. D., Smith R. K., Gee C. E., Alt F. W. Human N-myc is closely related in organization and nucleotide sequence to c-myc. Nature. 1986 Jan 2;319(6048):73–77. doi: 10.1038/319073a0. [DOI] [PubMed] [Google Scholar]
- Lennon G. G., Perry R. P. C mu-containing transcripts initiate heterogeneously within the IgH enhancer region and contain a novel 5'-nontranslatable exon. Nature. 1985 Dec 5;318(6045):475–478. doi: 10.1038/318475a0. [DOI] [PubMed] [Google Scholar]
- Lutzker S., Alt F. W. Structure and expression of germ line immunoglobulin gamma 2b transcripts. Mol Cell Biol. 1988 Apr;8(4):1849–1852. doi: 10.1128/mcb.8.4.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutzker S., Rothman P., Pollock R., Coffman R., Alt F. W. Mitogen- and IL-4-regulated expression of germ-line Ig gamma 2b transcripts: evidence for directed heavy chain class switching. Cell. 1988 Apr 22;53(2):177–184. doi: 10.1016/0092-8674(88)90379-0. [DOI] [PubMed] [Google Scholar]
- Nelson K. J., Haimovich J., Perry R. P. Characterization of productive and sterile transcripts from the immunoglobulin heavy-chain locus: processing of micron and muS mRNA. Mol Cell Biol. 1983 Jul;3(7):1317–1332. doi: 10.1128/mcb.3.7.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida Y., Miki T., Hisajima H., Honjo T. Cloning of human immunoglobulin epsilon chain genes: evidence for multiple C epsilon genes. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3833–3837. doi: 10.1073/pnas.79.12.3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul W. E., Ohara J. B-cell stimulatory factor-1/interleukin 4. Annu Rev Immunol. 1987;5:429–459. doi: 10.1146/annurev.iy.05.040187.002241. [DOI] [PubMed] [Google Scholar]
- Paul W. E. Pleiotropy and redundancy: T cell-derived lymphokines in the immune response. Cell. 1989 May 19;57(4):521–524. doi: 10.1016/0092-8674(89)90121-9. [DOI] [PubMed] [Google Scholar]
- Radbruch A., Sablitzky F. Deletion of Cmu genes in mouse B lymphocytes upon stimulation with LPS. EMBO J. 1983;2(11):1929–1935. doi: 10.1002/j.1460-2075.1983.tb01681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman P., Li S. C., Alt F. W. The molecular events in heavy chain class-switching. Semin Immunol. 1989 Sep;1(1):65–77. [PubMed] [Google Scholar]
- Rothman P., Lutzker S., Cook W., Coffman R., Alt F. W. Mitogen plus interleukin 4 induction of C epsilon transcripts in B lymphoid cells. J Exp Med. 1988 Dec 1;168(6):2385–2389. doi: 10.1084/jem.168.6.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu A., Takahashi N., Yaoita Y., Honjo T. Organization of the constant-region gene family of the mouse immunoglobulin heavy chain. Cell. 1982 Mar;28(3):499–506. doi: 10.1016/0092-8674(82)90204-5. [DOI] [PubMed] [Google Scholar]
- Stavnezer-Nordgren J., Sirlin S. Specificity of immunoglobulin heavy chain switch correlates with activity of germline heavy chain genes prior to switching. EMBO J. 1986 Jan;5(1):95–102. doi: 10.1002/j.1460-2075.1986.tb04182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavnezer J., Radcliffe G., Lin Y. C., Nietupski J., Berggren L., Sitia R., Severinson E. Immunoglobulin heavy-chain switching may be directed by prior induction of transcripts from constant-region genes. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7704–7708. doi: 10.1073/pnas.85.20.7704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavnezer J., Sirlin S., Abbott J. Induction of immunoglobulin isotype switching in cultured I.29 B lymphoma cells. Characterization of the accompanying rearrangements of heavy chain genes. J Exp Med. 1985 Mar 1;161(3):577–601. doi: 10.1084/jem.161.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton R. E., Boothroyd J. C. Evidence for trans splicing in trypanosomes. Cell. 1986 Nov 21;47(4):527–535. doi: 10.1016/0092-8674(86)90617-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas B. J., Rothstein R. Elevated recombination rates in transcriptionally active DNA. Cell. 1989 Feb 24;56(4):619–630. doi: 10.1016/0092-8674(89)90584-9. [DOI] [PubMed] [Google Scholar]
- Tian S. S., Faust C. Rearrangement of rat immunoglobulin E heavy-chain and c-myc genes in the B-cell immunocytoma IR162. Mol Cell Biol. 1987 Jul;7(7):2614–2619. doi: 10.1128/mcb.7.7.2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonegawa S. Somatic generation of antibody diversity. Nature. 1983 Apr 14;302(5909):575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- Vitetta E. S., Ohara J., Myers C. D., Layton J. E., Krammer P. H., Paul W. E. Serological, biochemical, and functional identity of B cell-stimulatory factor 1 and B cell differentiation factor for IgG1. J Exp Med. 1985 Nov 1;162(5):1726–1731. doi: 10.1084/jem.162.5.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter E., Krawinkel U., Radbruch A. Directed Ig class switch recombination in activated murine B cells. EMBO J. 1987 Jun;6(6):1663–1671. doi: 10.1002/j.1460-2075.1987.tb02415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancopoulos G. D., Alt F. W. Developmentally controlled and tissue-specific expression of unrearranged VH gene segments. Cell. 1985 Feb;40(2):271–281. doi: 10.1016/0092-8674(85)90141-2. [DOI] [PubMed] [Google Scholar]
- Yancopoulos G. D., Blackwell T. K., Suh H., Hood L., Alt F. W. Introduced T cell receptor variable region gene segments recombine in pre-B cells: evidence that B and T cells use a common recombinase. Cell. 1986 Jan 31;44(2):251–259. doi: 10.1016/0092-8674(86)90759-2. [DOI] [PubMed] [Google Scholar]
- Yancopoulos G. D., DePinho R. A., Zimmerman K. A., Lutzker S. G., Rosenberg N., Alt F. W. Secondary genomic rearrangement events in pre-B cells: VHDJH replacement by a LINE-1 sequence and directed class switching. EMBO J. 1986 Dec 1;5(12):3259–3266. doi: 10.1002/j.1460-2075.1986.tb04637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan D., Vitetta E. S. Structural studies of cell surface and secreted IgG in LPS-stimulated murine B cells. Mol Immunol. 1983 Apr;20(4):367–375. doi: 10.1016/0161-5890(83)90018-4. [DOI] [PubMed] [Google Scholar]