Abstract
Genetic modification is critically enabling for studies addressing specification and maintenance of cell fate, however methods for engineering modifications are inefficient. We demonstrate a rapid and efficient recombination system in which an inducible, floxed cre allele replaces itself with an incoming transgene. We target this inducible cassette exchange (ICE) allele to the HPRT locus, and demonstrate recombination in murine embryonic stem (ES) cells and primary cells from derivative ICE mice. Using lentivectors, we demonstrate recombination at a randomly integrated ICE locus in human ES cells. To illustrate the utility of this system, we insert the myogenic regulator, Myf5, into the ICE locus in each platform. This enables efficient directed differentiation of mouse and human ES cells into skeletal muscle, and conditional myogenic transdetermination of primary cells cultured in vitro. This versatile tool is thus well suited to gain-of-function studies probing gene function in the specification and reprogramming of cell fate.
Keywords: ES cells, cassette exchange recombination, conditional gene expression
Introduction
The isolation of mouse and human embryonic stem cells from blastocyst embryos [1-3] has enabled the modeling of early embryonic developmental events and new strategies for tissue engineering. Many studies, especially those probing specification and maintenance of cell fate and mechanisms of differentiation, require genetic modification, and would be facilitated by robust methods to modify cells for conditional gene expression. Because every locus differs in its own unique expressability, particularly the differential level of openness or extent to which it is subject to silencing within different lineages, there are obvious advantages to targeting to a known and defined locus, especially when comparing the effects of expressing two different genes. Homologous recombination, commonly performed in murine ES cells [4, 5] is very difficult to implement in other cell types including human ES cells [6], and although routine in the murine system, suffers from low frequencies (10−6) of recombination and comparatively high frequencies of random integration. Chimeric zinc finger nucleases [7] dramatically improve rates of homologous recombination but the exposure of the genome to such nucleases may result in off-target mutations [8]. Here we describe a system that enables high-efficiency cassette-exchange replacement of a doxycycline-inducible floxed cre transgene with a new transgene of interest. The system, referred to as inducible cassette exchange (ICE), enables high efficiency integration of genes of interest into cells bearing a single copy ICE locus. We have created platforms for this recombination system in murine ES cells, primary cells derived from ICE mice, and human ES cells, and apply this tool to probe the acquisition and stability of cell fate.
Materials and Methods
Generation of A2Lox.cre mES cells
Mouse embryonic stem (ES) cells were cultured on irradiated MEFs in DMEM / 15% FBS, penicillin/streptomycin (P/S, Gibco), 2 mM glutamax (Invitrogen), nonessential amino acids, 0.1 mM β-mercaptoethanol, and 100 U/mL LIF (Peprotech). For EB differentiation, ES cells were trypsinized, and re-plated in differentiation medium (IMDM/15% FBS, 200 μg/mL transferrin (Sigma), 4.5 mM monothiolglycerol (MTG, Sigma), 50 μg/mL ascorbic acid (Sigma), and 2 mM glutamax) for 30 min to allow MEFs to adhere. Nonadherent cells (105) were plated as a cell suspension in low adherence dishes on a slowly rotating shaker. To generate A2Lox.cre ES cells, the HPRT 5′ repair/targeting plasmid [9] carrying the cassette exchange TRE-2loxP-Δneo inducible target locus [10] was digested with XhoI and ligated to an XhoI-SalI fragment bearing the cre transgene from pSalk-cre [11]. 20 μg of SalI-linearized DNA was electroporated into 6×106 A17 mES cells, and selection in ES medium with HAT supplement (Invitrogen) was initiated 24 hours later.
Generation of iGFP and iMyf5 mES cell lines
To generate derivative inducible mES cell lines, A2Lox.cre mES cell lines were exposed to 500 ng/mL doxycycline for 24 hours, trypsinized, counted, and 2×105 cells were electroporated (Amaxa nucleofector 96-well shuttle, solution VHPH-1001, waveform program 96-CG-104) with 4 μg of p2Lox-mMyf5 or p2Lox-EGFP plasmid, and plated on neo-MEFs (Specialty Media). 24 hours after plating, selection was initiated in 300 μg/mL G418 (Gibco) and maintained for 10 days. Colonies were picked at day 8 and replated on neo-MEFs for expansion.
For myogenic differentiation, iMyf5 mES cells were differentiated as EBs for 2 days, then attached to gelatin-coated plates in EB differentiation medium. In the initial 4 days, cells were cultured in DMEM / 10% FBS with 500 ng/mL doxycyline. They were then switched to DMEM / 2% horse serum (HS) with 500 ng/ml doxycyline and cultured for an additional 4 days.
Generation of iDsRed2 and iMyf5 kidney cell lines
ICE mice were derived at the UT Southwestern Transgenic Core Facility by blastocyst injection of ZX1 mES cells, an ICE mES cell line related to A2Lox.cre but with an improved TRE promoter [12]. Mice were housed in a pathogen-free environment and cared for under the guidance of the UT Southwestern and University of Minnesota IACUCs. Primary kidney cells were obtained from collagenase I-treated, minced whole kidney pieces from male ICE mice. These were allowed to attach in DMEM / 10% FBS and P/S at 37°C, enabling constituent cells to spread over the surface of a 6-well dish. Cells were then passaged by trypsinization at 80% confluence. p2Lox-DsRed2 and -Myf5 were introduced by electroporation with an Amaxa nucleofector 96-well shuttle (mouse ES: solution VHPH-1001, waveform program 96-CG-104; mouse primary cells: VPI-1002 waveform program U-012). For the quantification of recombination, cells were passaged twice and induced overnight with 500 ng/mL doxycycline. For the derivation of iMyf5 kidney cells, two days post-nucleofection, selection was initiated in 75 μg/mL G418 for 4 days, then brought to 100 μg/mL and maintained over a period of 20 days. During selection, cells were passaged at 80% confluence. For myogenic differentiation, iMyf5 primary kidney cells were induced with 500 ng/mL doxycycline and cultured on gelatin coated plates in myogenic medium (DMEM / 20% FBS, 10 ng/mL bFGF and 10−7 M Dexamethasone) for 8 days. When the culture reached 100% confluency, medium was replaced with DMEM / 2% HS with 500 ng/mL doxycycline for an additional 4 days.
Lentivirus production
Lentiviral supernatant was produced in 293T cells cultured in DMEM/10% FBS. Lentiviral plasmid constructs, packaging construct pHR8.2deltaR and envelop plasmid pCMV-VSV-G were co-transfected using FUGENE HD (Roche). Medium was changed after 24 hours and the viral supernatant was collected at 48 hours post-transfection. Filtered supernatant (0.45 μm) was spiked with polybrene (4 μg/mL) and applied to cells plated at low density one day before infection. Spin-infection was performed at 2000 g at 33 °C for 90 min in a Sorval Legend RT centrifuge. Cells were incubated for additional 3 hours at 37 °C / 5% CO2 at which time the supernatant was replaced with fresh medium.
Generation of A2Lox.cre.I hES cells
H9 hES cells were cultured in mTESR (Stemcell Technologies) supplemented with P/S (Gibco) on growth factor-free matrigel (BD Biosciences) treated dishes and passaged using Accumax (Millipore) with Rock inhibitor (Enzo). Cells were transduced twice with high titre supernatants of Lenti-rtTA [13], a lentivector bearing rtTA under the Ubiquitin C promoter (a modified version of FUGW [14] in which rtTA2SM2 from pUHDrtTA2SM2 [15] was introduced downstream of the UbiC promoter) to generate derivative H9:rtTA hES cells. These cells were then transduced with 2Lox.cre-ires-GFP [13] lentivector over a dilution series of supernatant. Cells were pulsed with 500 ng/mL doxycycline, and bulk sorted from a dilution with < 5% transduction, to bias for single copy integration events. Sorted cells were grown to 50% confluence, pulsed with doxycycline and sorted a second time to remove clones in which the integration was silenced. When these cells reached 50% confluence, they were then single cell sorted into 96-well dishes and clonal cell lines expanded. A split from each clonal cell line was tested for levels of GFP-inducibility, and the best clones tested for recombination. Clone H9-2Lox.cre.I was selected for all following experiments.
Generation of H9imCherry and H9iMyf5 hES cells
H9-2Lox.cre.I cells were treated with 500 ng/mL doxycycline for 24 hours, harvested and 5 batches of 2×105 cells were nucleofected with 4 μg p2Lox-mCherry, 4 μg p2lox-DS-RED2 or p2Lox-hMyf5-ires-mCherry using an Amaxa 96-well nucleofector shuttle using VHPH-5003 solution for H9 cells and the hES cell waveform protocol, 96-CB-150. For quantification of recombination, cells were passaged twice and then induced overnight with 500 ng/mL Doxycycline. To generate imCherry or iMyf5 derivatives, cells were expanded to 50% confluence on a T75 flask and induced with 200 ng/mL doxycycline overnight. The following day, red fluorescent cells were sorted and plated on a 6-well dish. Secondary and tertiary enrichment sorts were performed in the same way.
Cells were differentiated by plating 4×105 cells/mL in low-adherence dishes in mTESR medium for two days to allow aggregates to form. Medium was then changed to growth factor-free mTESR (Stemcell Technologies) supplemented with 10 ng/mL hBMP4, 3 ng/mL hActivinA, 10 ng/mL hbFGF (all from Peprotech) for 4 days. BMP4 and ActivinA were then withdrawn and bFGF reduced to 5 ng/mL hbFGF for the next 4 days. At this stage, EBs were plated in adherent dishs to allow attachment in myoblast medium: DMEMF12 supplemented with 20% FBS, P/S, 10 ng/ml hbFGF, 10−7 M Dexamethasone, 0.1 mM β-mercaptoethanol. After 2 days of attachment, adherent cells, referred to hereafter as mesenchymal cells, were collected by trypsinization and expanded for up to 6 passages. For myogenic differentiation, mesenchymal cells were maintained in doxycycline from the point of attachment on. Terminal differentiation was induced by switching myoblast medium for DMEM / 2% HS for 6 days.
Immunofluorescence and Western Blotting
Cells were cultured on gelatin coated coverslips, fixed with 4% paraformaldehyde for 20 min, permeabilized by 0.3%Triton X-100 for 30 min and blocked by 10% goat serum for 1 hour at room temperature. Primary mouse anti-MyoD (BD Biosciences c# 554130) or mouse anti-sarcomeric myosin (MF20, Developmental Studies Hybridoma Bank) were incubated in PBS / 2% goat serum at 4 °C overnight, followed by secondary goat anti-mouse Cy3- or Alexa-555-conjugated antibodies (Sigma, and Invitrogen, respectively) at room temperature for 45 min. Cells were counterstained using 4′,6-diamidino-2-phenylindole (DAPI, Invitrogen) to visualize nuclei and mounted in “Immu-Mount” (Thermo Scientific, Pittsburg, USA). Images were acquired on an Axio Observer.Z1 microscope (Zeiss). For Western Blotting, cells were cultured on gelatin coated plates and protein extracted by using RIPA buffer. Primary mouse anti-GAPDH (HRP conjugated) was used to detect specific band of 36 kD. Primary mouse anti-Myf5 (SC-302 clone C-20), and secondary Goat anti-Rabbit-HRP conjugated were used to detect Myf5 specific band at 35kD. Primary mouse anti-MyoD (BD Biosciences c# 554130), mouse anti-Myogenin (BD Bioscience c# 556358), mouse anti-M-Cadherin (BD Bioscience c# 611100), mouse anti-MyHC (DSHB F1.652S) and secondary anti-mouse HRP conjugated were used to detect specific band of 45 kD, 34 kD, 130 kD, 180 kD respectively.
Quantitative Real Time RT-PCR (qRT-PCR)
Total RNA was extracted with Trizol (Invitrogen) and cDNA was generated using 1 μg DNase-treated RNA with oligo-dT primers and TermoScript (Invitrogen). PCR was performed using TaqMan Real Time PCR premixtures on a 7500 RTPCR System from Applied Biosystems. For muscle related genes pre-made probes were purchased from Applied Biosystems: Myod1 Mm00440387_m1, Myf5 Mm00435125_m1, Myog Mm00446194_m1, Mck Mm0432556_m1, Desmin Mm00802455_m1, m-Cad Mm00483183_m1 Gapdh Mm99999915_g1. For Human probes: MYOD1 Hs00159528_m1, MYF5 Hs00271574_m1, MYOG Hs01072232_m1, DESMIN Hs00157258_m1, GAPDH Hs99999905-m1. All reactions were performed at least in triplicate and the data was analyzed by 7500 System Software (Applied Biosystems). The difference in threshold cycle number between transcripts of interest and GAPDH was used to determine the abundance of transcript relative to GAPDH (Delta-CT method).
Statistical analyses
All experiments were done at least 3 times. Data shown for Real Time PCR are the mean ± STDEV. Difference between means was compare by the two-tailed Student test (GraphPad Prism 5) and was considered significantly different at P < 0.05.
Results
Generation of inducible cassette exchange (ICE) murine ES cells
The genomic region upstream of HPRT has been demonstrated to be a site at which transgenes express reliably [9, 16-18], presumably due to HPRT being a housekeeping gene, active in all cell types and therefore embedded in constitutively open chromatin. We have targeted this site with a doxycycline-inducible cre transgene flanked by heterologous, self-incompatible loxP sites (Figure 1A) in A17 mES cells, a derivative of E14Tg2a [19] in which rtTA has been inserted into the Rosa26 locus [20]. Downstream of the floxed cre is Δneo, a G418 resistance gene lacking a start codon and promoter [21]. These cells are referred to as A2Lox.cre. Treating cells with doxycycline causes cre to be expressed, rendering the cells competent for recombination. When transfected with p2Lox, a plasmid bearing the same heterologous loxP sites, a cassette exchange recombination replaces cre with a gene of interest from the incoming plasmid. Because the orientation of the loxP sites is inverted on the plasmid relative to the chromosome, the plasmid in its entirety (except for the short sequence between the loxP sites) is inserted. At the upstream loxP site, a gene of interest is brought under the regulation of the doxycycline-inducible promoter, while at the downstream loxP site, a PGK promoter and a start codon are spliced in frame with the Δneo gene. This results in reversion to G418 resistance and selection for precise integration [21].
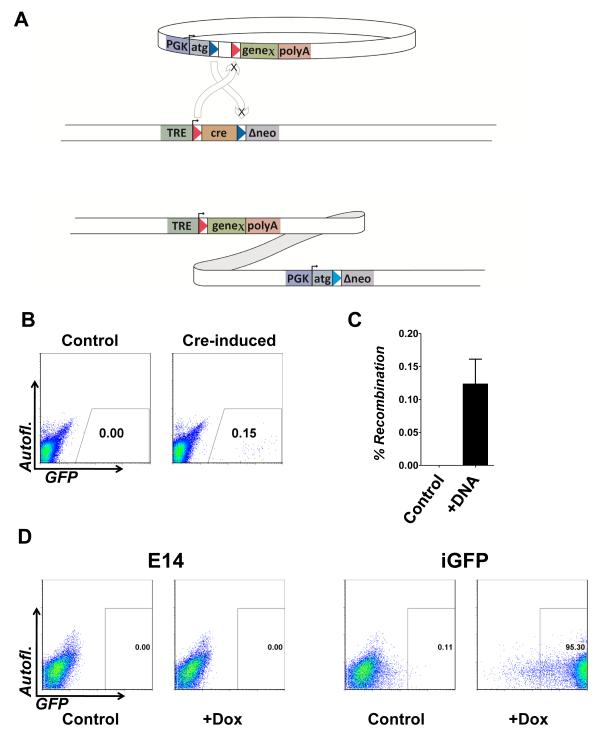
Figure 1. Inducible cassette exchange recombination in A2Lox.cre mES cells.
(A) The incoming plasmid is shown above the ICE target locus, which is integrated at a unique site in the genome (5′ of HPRT in the mouse ES cells). At the ICE target locus, cre is flanked by heterologous loxP sites, and is downstream of a doxycycline (tetracycline)-responsive promoter (TRE). Arrows indicate recombination between homologous loxP sites. The heterologous loxP sites on the incoming plasmid are in the opposite orientation relative to their cognates on the chromosome, therefore recombination catalyzes the integration of the entire plasmid. Cre is exchanged for the incoming gene of interest, rendering that gene doxycycline-inducible in the derivative recombinants. Selection is enabled by correction of the Δneo gene which acquires a PGK promoter, Kozak translational consensus and ATG.
(B) FACS analysis of A2Lox.cre ES cells following recombination in the absence of selection. Cells were either not treated with doxycycline (left) or given a 24-hour pulse of doxycycline (right) prior to nucleofection. After recovery, cells were re-induced with doxycycline to visualize recombinants.
(C) Measured frequency of recombination. n=3, p < 0.0049.
(D) FACS analysis of a derivative inducible-GFP (iGFP) cell line and control (E14) exposed to doxycycline.
To measure the efficiency of ICE recombination in mES cells, we treated A2Lox.cre cells with doxycycline for 24 hours to induce cre expression, or kept untreated cells as controls. We then nucleofected both with p2Lox bearing GFP. After several days of growth in the absence of doxycycline, cells from both arms were treated with doxycycline for 48 hours. In cells nucleofected with p2Lox-GFP but not previously exposed to doxycycline, no integrants were observed, however in cells previously pulsed with doxycycline to induce cre, a recombinant population was observed and the frequency of recombination was determined to be > 1/1000 (the frequency of GFP+ cells, Fig. 1B,C). This is approximately three orders of magnitude more efficient than homologous recombination. By subjecting the treated population to G418 selection, individual colonies can be isolated, and these now show inducible expression, both in the ES cell stage, as well as when differentiated for several days as embryoid bodies (EBs, Fig. 1E).
Directing mesoderm differentiation with myogenic regulatory factors
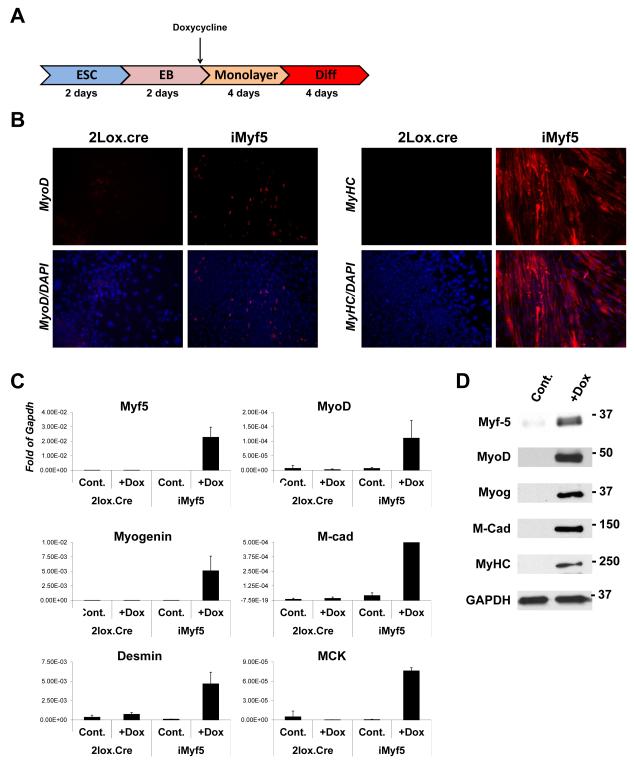
In order to evaluate the usefulness of this system in probing mechanisms of cell fate determination, we sought to derive a developmental lineage that is poorly represented in conventional mES in vitro differentiation. The skeletal myogenic lineage is very difficult to derive from mES cells, most likely because of a deficiency in the inductive signals that initiate somitogenesis in paraxial mesoderm [22]. In order to test the ability of this inducible system to direct the lineage choice of differentiating cells, specifically to direct cells into the myogenic lineage, we generated a new inducible mES cell line using this system: iMyf5 (for inducible Myf5). Myf5 and its related paralog MyoD, display significant genetic redundancy in early myogenic development [23], however Myf5 is temporally and genetically upstream of MyoD [24, 25], thought to be directly induced by Pax3 in cells of the hypaxial somite [26], and is downregulated following myogenic specification. We differentiated iMyf5 cells in serum suspension (EB) culture for 2 days, conditions under which mesoderm is potently induced. EBs were then allowed to attach, and doxycycline was applied from this time point onwards, to induce Myf5 expression in early mesodermal progenitors as soon as they emerged (Fig. 2A). In the presence of Myf5 induction, cells with MyoD+ nuclei were observed, indicating myogenic commitment, whereas in control cells lacking Myf5 induction, cells were uniformly MyoD-negative (Fig. 2B). After 4 days of monolayer culture, cells were exposed to myogenic differentiation medium (2% horse serum, conditions that promote myotube formation from myoblasts) for an additional 4 days. In the absence of Myf5 induction, no cell fusion was observed and myosin heavy chain (MyHC) expression was absent, however in cells exposed to Myf5, large numbers of multinucleated MyHC+ myotubes formed (Fig. 2B). Differentiated cells were evaluated for myogenic gene expression by qRTPCR. As expected, Myf5 was robustly expressed in doxycycline-treated cells, and cells exposed to Myf5 for 8 days expressed the downstream myogenic regulatory factors, MyoD and Myogenin. In addition, various structural gene markers of terminal myogenic development were observed (Fig. 2C, D). These data demonstrate the utility of the system for probing the activity of genes that regulate early events in murine embryonic development, in this case showing that Myf5 expression is sufficient to promote the myogenic commitment of nascent mesoderm and establishing a novel and rapid method of differentiating mES cells into skeletal muscle tissue.
Figure 2. Modification of ICE mES cells with Myf5, and efficient myogenesis in vitro.
(A) Schematic overview of mouse myogenic differentiation protocol.
(B) Immunostaining for MyoD or sarcomeric myosin heavy chain (MHC) in progeny of iMyf5 mES cells or control parental A2Lox.cre mES cells at the final stage of differentiation (in 2% horse serum) in the presence or absence of doxycycline.
(C) Myogenic gene expression measured by qRTPCR in the same cells.
(D) Myogenic protein expression measured by Western Blotting in the same cells.
ICE recombination in human ES cells
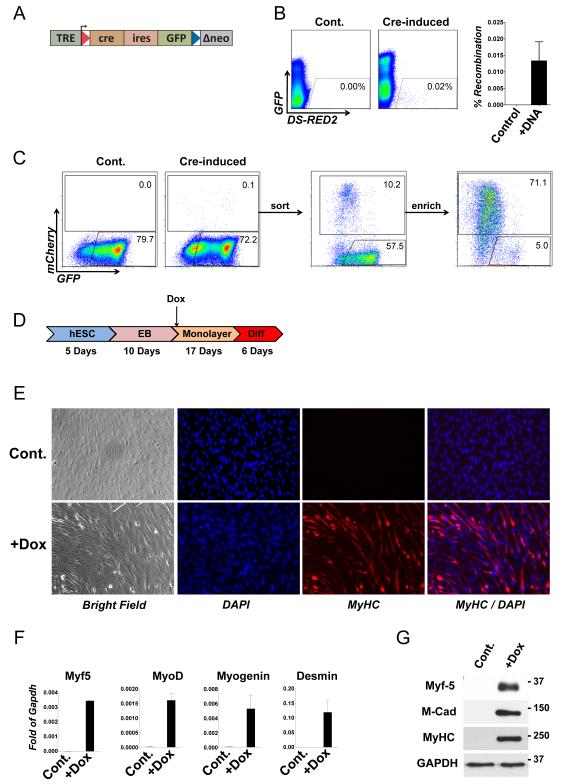
In order to facilitate similar studies in a human system, we sought to derive a human embryonic stem cell with an ICE target locus. We elected to randomly integrate ICE loci, and select clones with favorable expression characteristics. We therefore used a modified version of the ICE locus in which an ires-GFP reporter follows the Cre gene within the domain flanked by loxP sites (Fig 3A). The entire locus is carried by a lentiviral vector which allows for efficient introduction of the ICE locus. The presence of the GFP reporter allows the isolation of clonal cell lines that have picked up a genomic integration the cassette, and their evaluation to determine which have favorable doxycycline-responsiveness. In addition, following cassette exchange, recombinants can be distinguished from parental cells by the absence of inducible GFP expression. We transduced H9 hES cells [1] with this construct at low titre in order to favor single-copy integration, used a transient pulse of doxycycline to identify integrants, which we sorted and replated. Following expansion of this founder population, we pulsed with doxycycline a second time and single cell sorted to isolate clonal cell lines. We evaluated clones for ICE recombination by nucleofecting each with a plasmid construct bearing mCherry and measuring the frequency of green to red conversions. The best clone, H9.2Lox.cre.I, was karyotyped and selected for further studies. When mCherry was introduced into this clone, a small population of doxycycline-inducible red fluorescent cells among a large background of green fluorescent cells was observed, provided cells had been treated with doxycycline to induce Cre expression just prior to nucleofection. The measured recombination rate was somewhat lower in hES cells than in mouse cells (Fig. 3B). These could be sorted and enriched (Fig. 3C). In cells that were not pre-treated with doxycycline to induce Cre expression, nucleofection with p2Lox-mCherry did not promote the emergence of a red fluorescent population. We then introduced Myf5 into this clone on a plasmid bearing an ires-mCherry reporter, and isolated recombinants by flow cytometry. The derivative recombinant cell line was then differentiated in suspension EB culture for 10 days followed by attachment to gelatin-coated dishes in the presence of doxycycline. In both Myf5-induced and uninduced cultures, a mesenchymal cell population emerged from the attached EBs and expanded over the course of 17 days. Medium was then changed to 2% horse serum to promote differentiation, and at this point a clear difference was observed between the control and induced cultures, with the latter having an abundance of long spindle-shaped cells. After one week in differentiation medium, the Myf5-induced cultures displayed a substantial amount of cell fusion, and multinucleated cells were positive for sarcomeric myosin (Fig. 3E). Analysis of gene expression further indicated myogenic differentiation: as seen with the mES cell cultures exposed to Myf5, the downstream MRFs, MyoD and Myogenin were now co-expressed, as well as the differentiation marker, Desmin (Fig. 3F). Markers of myogenic differentiation were also shown by Western Blotting (Fig. 3G). These genes were not induced in the control cultures. This efficient and conditional skeletal myogenic differentiation highlights the utility of ICE in human ES cells to manipulate genetic pathways controlling lineage specification during differentiation in vitro.
Figure 3. Inducible cassette exchange recombination in H9 hES cells, and efficient myogenesis in vitro.
(A) Schematic of the ICE locus, modified to include an ires-GFP reporter gene.
(B) FACS analysis of doxycycline-induced H9.2Lox.cre.I ES cells (left panel) and control uninduced H9.2Lox.cre.I cells (right panel) after nucleofection with p2Lox-DsRed2. Inducible red fluorescence replaces inducible green fluorescence in the recombinants. Quantification over several replicate experiments is shown at right.
(C) Fluorescent red cells were sorted and enriched.
(D) Scheme of human myogenic differentiation.
(E) Immunofluorescence for sarcomeric myosin heavy chain in H9.iMyf5 cells differentiated in the absence (above) or presence (below) of doxycycline. BF, brightfield.
(F) Myogenic gene expression measured by qRTPCR in the same cells.
(G) Myogenic protein expression measured by Western Blotting in the same cells.
ICE recombination in primary cells in vitro
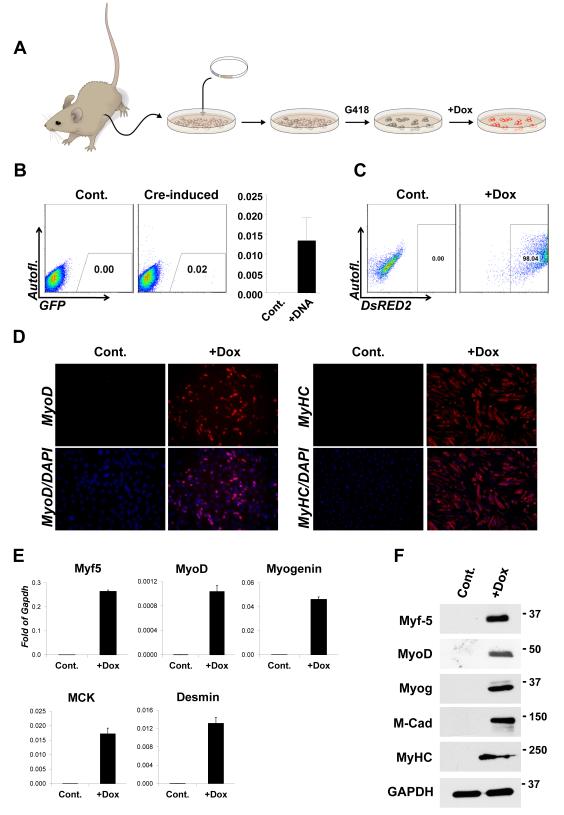
Many applications directed towards probing the stability of cell fate and the feasibility of reprogramming would be facilitated by isogenic methods to compare conditional gene expression in primary cells. However making multiple modifications of ES cells, and taking each cell line through the blastocyst into the germ line to derive new strains would be practical only for a very limited number of constructs. On the other hand, a large number of isogenic modifications could be generated in primary cells from an animal bearing an ICE locus. We therefore derived an ICE mouse strain through blastocyst injection of A2Lox.cre ES cells, and tested the efficiency of recombination in primary cells of these mice. In primary cells derived from the kidneys of ICE mice, the recombination rate, measured by acquisition of doxycycline-inducible dsRed2 protein, was observed to be slightly over 1 in 104 cells (Figure 4B). We then integrated Myf5 and selected a population of G418-resistant cells. Primary cells from the kidney are not myogenic, do not express MyoD, and will not fuse under conditions of growth factor withdrawal (Fig. 4D, control). However, after one week of exposure to Myf5, myogenic potential was acquired by these cells, indicated by nuclear staining for MyoD, and the ability to fuse to form myosin heavy chain-positive myotubes in conventional myoblast differentiation medium (Fig. 4D, doxycycline). Transcriptional and protein analysis of skeletal muscle-specific markers including MyoD, Myogenin, MCK (muscle creatine kinase), Desmin and M-cadherin, (Fig 4E,F) further validate the transdetermination of these kidney-derived cells into the skeletal myogenic lineage upon induction of Myf-5.
Figure 4. ICE mice and respecification of cell fate in primary cells.
(A) Schematic overview of recombination in ICE mice: primary cells are expanded and transfected with p2Lox. Recombinants (indicated in dark beige) are selected with G418 and expanded. When doxycycline is applied, the gene brought in on p2Lox (DsRed2 in the examples shown in B and C, below) is induced.
(B) Recombination in primary kidney cells nucleofected with p2Lox-DsRed2. Controls were not pre-treated with doxycycline to induce cre expression prior to nucleofection. After nucleofection, cells were allowed to recover and expand in the absence of doxycycline, and were later treated with 500 ng/mL doxycycline to visualize recombinants. The histogram at right indicates the average recombination rate over n=3 independent experiments. p=.0161.
(C) FACS for DsRed2 in the cells shown in B after growing out in 150 μg/mL G418.
(D) Immunohistochemistry for MyoD and MHC in Myf5-recombinant kidney cells cultured in 2% horse serum.
(E) Gene expression analysis by qRTPCR for skeletal myogenic markers.
(F) Myogenic protein expression measured by Western Blotting in the same cells.
Discussion
The inducible cassette exchange recombination system is versatile – easily set up in any cellular model system, robust – a cre expression plasmid is not required, cre is expressed at the same level by every cell prior to recombination, and the reaction is self-limiting because after recombination cre is gone (indeed, recombination occurs at levels two or three orders of magnitude above conventional homologous recombination), and rapid – a pure population of derivative inducible gene-expressing recombinants can be obtained within a time frame measured in days. This latter feature is particularly valuable in cells of limited lifespan, such as primary cells, or cells in which high passage numbers are undesirable, such as ES cells.
Whether at a known location, such as upstream of HPRT, or an unknown locus, such as a proviral integration site, all cre-mediated integrations into a given cell type are targeted to the same locus. This reduces variation in expression associated with integration site and copy number differences, and eliminates the likelihood that unwanted genetic changes, such as activation of oncogenes, will occur. These features allow elements of an allelic series, for example a set of different point mutations or deletions within a protein, to be compared to one another directly and phenotypes to be interpreted with confidence.
The conditional doxycycline-inducible nature of the target locus is well suited to gain-of-function experiments. Here, we have focused on determining the effect of a particular transcription factor on the selection of differentiation pathways, or on the acquisition of a novel differentiation pathway. In both the murine and the human system, expression of Myf5 in early mesodermal progenitor cells is an efficient way of promoting the skeletal myogenic lineage. This lineage is particularly difficult to derive from both mouse an human ES cells – although a few examples of skeletal myogenic differentiation of wild-type ES or iPS cells have been reported, these involve complex protocols based on sorting of rare cells [27, 28]. For studies requiring large quantities of early embryonic myogenic progenitors, genetic modification with inducible expression of genes such as Myf5, or its upstream regulators Pax3 and Pax7 [22, 29, 30], are ideal.
Additional applications, for example the modification of IPS cell lines, or the integration of reporter genes at this locus with minimal position effect, are also possible. The ability of ES cells to differentiate into all somatic cell types and the inherent scalability of cultures initiated with immortal cells makes this a powerful system in which to combine discovery of cellular or developmental phenotypes with mechanistic investigations of the role of a developmental regulatory factors.
Acknowledgements
This work was supported by the NIH (grants R01 HL081186, P01 GM081627, U01 HL100407). We thank the Bob and Jean Smith Foundation for their generous support. We thank Cynthia DeKay for graphics.
Footnotes
Author Contributions: Study design: MK. Performed experiments: MI, DB, HF, GB, EM, DR, AM, ZX. Data analysis: MI, DB, MK. Wrote the manuscript: MI and MK.
Disclosure of Potential Conflicts of Interest The authors declare no potential conflicts of interest.
References
- 1.Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic Stem Cell Lines Derived from Human Blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 2.Evans MJ, Kaufman M. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 3.Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc. Natl. Acad. Sci. USA. 1981;78:7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomas KR, Capecchi MR. Site-directed mutagenesis by gene targeting in mouse embryo-derived stem cells. Cell. 1987;51:503–512. doi: 10.1016/0092-8674(87)90646-5. [DOI] [PubMed] [Google Scholar]
- 5.Doetschman T, Gregg RG, Maeda N, et al. Targetted correction of a mutant HPRT gene in mouse embryonic stem cells. Nature. 1987;330:576–578. doi: 10.1038/330576a0. [DOI] [PubMed] [Google Scholar]
- 6.Zwaka TP, Thomson JA. Homologous recombination in human embryonic stem cells. Nat Biotechnol. 2003;21:319–321. doi: 10.1038/nbt788. [DOI] [PubMed] [Google Scholar]
- 7.Porteus MH, Baltimore D. Chimeric nucleases stimulate gene targeting in human cells. Science. 2003;300:763. doi: 10.1126/science.1078395. [DOI] [PubMed] [Google Scholar]
- 8.Radecke S, Radecke F, Cathomen T, et al. Zinc-finger nuclease-induced gene repair with oligodeoxynucleotides: wanted and unwanted target locus modifications. Mol Ther. 2010;18:743–753. doi: 10.1038/mt.2009.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bronson SK, Plaehn EG, Kluckman KD, et al. Single-copy transgenic mice with chosen-site integration. Proc Natl Acad Sci U S A. 1996;93:9067–9072. doi: 10.1073/pnas.93.17.9067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iacovino M, Hernandez C, Xu Z, et al. A conserved role for Hox paralog group 4 in regulation of hematopoietic progenitors. Stem Cells Dev. 2008 doi: 10.1089/scd.2008.0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kyba M, Perlingeiro RCR, Daley GQ. HoxB4 confers definitive lymphoid-myeloid engraftment potential on embryonic stem cell and yolk sac hematopoietic progenitors. Cell. 2002;109:29–37. doi: 10.1016/s0092-8674(02)00680-3. [DOI] [PubMed] [Google Scholar]
- 12.Agha-Mohammadi S, O’Malley M, Etemad A, et al. Second-generation tetracycline-regulatable promoter: repositioned tet operator elements optimize transactivator synergy while shorter minimal promoter offers tight basal leakiness. J Gene Med. 2004;6:817–828. doi: 10.1002/jgm.566. [DOI] [PubMed] [Google Scholar]
- 13.Bosnakovski D, Xu Z, Gang EJ, et al. An isogenetic myoblast expression screen identifies DUX4-mediated FSHD-associated molecular pathologies. EMBO J. 2008;27:2766–2779. doi: 10.1038/emboj.2008.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lois C, Hong EJ, Pease S, et al. Germline transmission and tissue-specific expression of transgenes delivered by lentiviral vectors. Science. 2002;295:868–872. doi: 10.1126/science.1067081. [DOI] [PubMed] [Google Scholar]
- 15.Urlinger S, Baron U, Thellmann M, et al. Exploring the sequence space for tetracycline-dependent transcriptional activators: novel mutations yield expanded range and sensitivity. Proc Natl Acad Sci U S A. 2000;97:7963–7968. doi: 10.1073/pnas.130192197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heaney JD, Rettew AN, Bronson SK. Tissue-specific expression of a BAC transgene targeted to the Hprt locus in mouse embryonic stem cells. Genomics. 2004;83:1072–1082. doi: 10.1016/j.ygeno.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 17.Touw K, Hoggatt AM, Simon G, et al. Hprt-targeted transgenes provide new insights into smooth muscle-restricted promoter activity. Am J Physiol Cell Physiol. 2007;292:C1024–1032. doi: 10.1152/ajpcell.00445.2006. [DOI] [PubMed] [Google Scholar]
- 18.Portales-Casamar E, Swanson DJ, Liu L, et al. A regulatory toolbox of MiniPromoters to drive selective expression in the brain. Proc Natl Acad Sci U S A. 107:16589–16594. doi: 10.1073/pnas.1009158107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hooper M, Hardy K, Handyside A, et al. HPRT-deficient (Lesch-Nyhan) mouse embryos derived from germline colonization by cultured cells. Nature. 1987;326:292–295. doi: 10.1038/326292a0. [DOI] [PubMed] [Google Scholar]
- 20.Wutz A, Rasmussen TP, Jaenisch R. Chromosomal silencing and localization are mediated by different domains of Xist RNA. Nat Genet. 2002;30:167–174. doi: 10.1038/ng820. [DOI] [PubMed] [Google Scholar]
- 21.Fukushige S, Sauer B. Genomic targeting with a positive-selection lox integration vector allows highly reproducible gene expression in mammalian cells. Proc Natl Acad Sci U S A. 1992;89:7905–7909. doi: 10.1073/pnas.89.17.7905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Darabi R, Gehlbach K, Bachoo RM, et al. Functional skeletal muscle regeneration from differentiating embryonic stem cells. Nat Med. 2008;14:134–143. doi: 10.1038/nm1705. [DOI] [PubMed] [Google Scholar]
- 23.Rudnicki MA, Schnegelsberg PN, Stead RH, et al. MyoD or Myf-5 is required for the formation of skeletal muscle. Cell. 1993;75:1351–1359. doi: 10.1016/0092-8674(93)90621-v. [DOI] [PubMed] [Google Scholar]
- 24.Ott MO, Bober E, Lyons G, et al. Early expression of the myogenic regulatory gene, myf-5, in precursor cells of skeletal muscle in the mouse embryo. Development. 1991;111:1097–1107. doi: 10.1242/dev.111.4.1097. [DOI] [PubMed] [Google Scholar]
- 25.Tajbakhsh S, Rocancourt D, Cossu G, et al. Redefining the genetic hierarchies controlling skeletal myogenesis: Pax-3 and Myf-5 act upstream of MyoD. Cell. 1997;89:127–138. doi: 10.1016/s0092-8674(00)80189-0. [DOI] [PubMed] [Google Scholar]
- 26.Bajard L, Relaix F, Lagha M, et al. A novel genetic hierarchy functions during hypaxial myogenesis: Pax3 directly activates Myf5 in muscle progenitor cells in the limb. Genes Dev. 2006;20:2450–2464. doi: 10.1101/gad.382806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mizuno Y, Chang H, Umeda K, et al. Generation of skeletal muscle stem/progenitor cells from murine induced pluripotent stem cells. FASEB J. 2010;24:2245–2253. doi: 10.1096/fj.09-137174. [DOI] [PubMed] [Google Scholar]
- 28.Barberi T, Bradbury M, Dincer Z, et al. Derivation of engraftable skeletal myoblasts from human embryonic stem cells. Nat Med. 2007;13:642–648. doi: 10.1038/nm1533. [DOI] [PubMed] [Google Scholar]
- 29.Darabi R, Pan W, Bosnakovski D, et al. Functional Myogenic Engraftment from Mouse iPS Cells. Stem Cell Rev. 2011 doi: 10.1007/s12015-011-9258-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Darabi R, Santos FN, Filareto A, et al. Assessment of the myogenic stem cell compartment following transplantation of Pax3/Pax7-induced embryonic stem cell-derived progenitors. Stem Cells. 2011;29:777–790. doi: 10.1002/stem.625. [DOI] [PMC free article] [PubMed] [Google Scholar]